Abstract
Background
The effectiveness of high-protein (HP) diets in reducing body weight and adiposity and potentially improving clinical outcomes in heart failure (HF) is not known.
Objective
This feasibility study was conducted to evaluate the impact of 3 dietary interventions on body weight and adiposity, functional status, lipid profiles, glycemic control, and quality of life (QOL) in overweight and obese patients with HF and type 2 diabetes mellitus.
Design
Fourteen patients with HF with a body mass index greater than 27 kg/m2 were randomized to an HP diet, a standard protein diet, or a conventional diet. Data were obtained at baseline and 12 weeks.
Results
There were no significant differences in age (59 ± 10 years), sex (78% male), New York Heart Association class (43% class II, 57% class III), and HF etiology or left ventricular ejection fraction (26 ± 7) between the groups at baseline. Patients on the HP diet demonstrated significantly greater reductions in weight (P = .005), percent body fat (P = .036), total cholesterol (P = .016), triglyceride concentrations (P = .034), and low-density lipoprotein cholesterol (P = .041) and greater improvements in functional status (6-minute walk [P = .010] and VO2 peak [P = .003]), high-density lipoprotein cholesterol (P = .006), and physical QOL scores (P =.022) compared with those on standard protein and conventional diets.
Conclusion
A 12-week HP diet resulted in moderate weight loss and reduced adiposity in a small sample of overweight and obese patients with HF that were associated with improvements in functional status, lipid profiles, glycemic control, and QOL. However, these preliminary findings must be confirmed in studies with more participants and long-term follow-up.
Keywords: heart failure, high-protein diet, quality of life, weight reduction
Obesity and diabetes mellitus (DM) are frequent comorbid conditions in patients diagnosed with heart failure (HF);1 all 3 conditions are prevalent in the US population. Approximately 5 million Americans are affected by HF,2 whereas 23.6 million are affected by DM,3 and 60% of adult Americans are overweight or obese.4 Among the selected group of patients enrolled in chronic systolic HF clinical trials, generally 20% to 30% have DM5 and 28.6% and 38% are overweight (body mass index [BMI], 25.0–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2), respectively.6 Currently, little is known about the effect of obesity and DM on the characteristics, treatment, and outcomes in patients hospitalized for HF. In addition, very little is known about how well guideline-recommended therapies (eg, nutritional management) are being used in patients with HF, obesity, and DM.
The effect of weight loss in patients with HF with increased body mass remains controversial. Because obesity and DM increase the risk of HF, it is attractive to hypothesize that weight loss would be beneficial to patients with HF and obesity, with or without DM. However, several clinical trials involving more than 10,000 patients with HF showed improved outcomes among overweight and obese patients compared with normal or underweight patients, suggesting that weight loss may not be beneficial in these patients7–10; it should be noted that some patients were not in clinical trials intended to demonstrate improved outcomes in overweight/obese patients. On the other hand, some studies suggest that voluntary weight loss may be effective in restoring left ventricular systolic function and regressing eccentric hypertrophy,11 improving functional status,12,13 and reducing cardiovascular risk,12 but results of these studies are not definitive and it is not known whether voluntary weight loss may be desirable in obese patients with HF and DM. Intuitively, weight reduction in obese patients with HF and DM may have the added benefit of improving glycemic control by targeting this triad of comorbidities, thus supporting the argument that testing weight loss interventions in this group may be beneficial.
Despite considerable attempts by obese individuals to lose weight and maintain weight loss, there is currently no scientific consensus on the optimal dietary methods for weight loss and prevention of weight regain. Although most physicians and dietitians recommend conventional methods that emphasize a high-carbohydrate, low-fat intake as recommended by the American Heart Association (AHA),14 the modest weight losses and poor long-term adherence limit its usefulness.15 This has led to a widespread interest in other dietary approaches aimed at reducing weight and excess adiposity.
Although a broad spectrum of alternative approaches has evolved, high-protein (HP) diets have gained significantly more recognition in the medical community. The potential benefits of HP diets can be deduced from the fact that protein is essential in maintaining bodily functions (ie, growth, tissue building, and maintenance) and lean body mass and is the major component of all biologically active molecules in the body.16 Protein is also associated with glucose control, insulin regulation, muscle building, and regulation or increases in metabolism.17 Data in obese patients with DM show that HP diets result in greater reductions in body weight and adiposity and preservation of lean mass compared with conventional diets.18–21 Although data support that protein depletion leads to impaired metabolism, altered immune function, muscle wasting, and death in patients with HF,22 the effects of an HP diet in patients with HF are not known. Therefore, this feasibility study was conducted to compare the effects of an HP, hypoenergetic diet (40% total energy from carbohydrates, 30% from protein, and 30% from fat), a standard protein (SP), hypoenergetic diet (55% total energy from carbohydrates, 15% from protein, and 30% from fat), and a conventional diet (high carbohydrates, low fat, high fiber, with no energy restrictions) on body weight and adiposity in patients with HF and increased BMI greater than or equal to 27 kg/m2 and DM. We hypothesized that patients on the HP diet would show significantly greater reductions in body weight and adiposity than do patients in the SP or conventional diets at 12 weeks, thus resulting in greater improvements in functional status, lipid profiles, glycemic control, and quality of life (QOL).
Methods
Setting and Participants
Patients who were 18 years and older at screening and being treated for HF at a single tertiary outpatient referral center were recruited for the randomized clinical study through the use of flyers posted in the HF clinic. Participants were required to have a BMI of greater than or equal to 27 kg/m2 (an exclusion criterion for transplant eligibility), New York Heart Association (NYHA) class II to III HF, and non-insulin-treated type 2 DM. Participants were excluded if they had been on insulin therapy or if they had significant DM-related complications such as diabetic retinopathy, neuropathy, or proteinuria or a history of clinically significant illness (ie, acute myocardial infarction, sustained arrhythmia, liver, respiratory, gastrointestinal disease, and malignancy) or were pregnant or lactating. Participants were also excluded if they had a history of psychiatric illness, had serum creatinine level greater than 1.5 mg/dL, are currently participating in a supervised weight loss program, and had a previous weight loss of greater than 10% in the last 6 months.
Procedures
The institutional review board of the University of California, Los Angeles, approved the study protocol, and the study was carried out strictly in accordance with ethical standards as outlined by the university. Potential participants completed an initial interview in which the requirements of the study were explained and patient-informed consent was obtained. Before study enrollment, patients received written clearance from their HF specialist. After baseline evaluations, patients were randomly assigned to 1 of the 3 dietary interventions: HP diet, hypoenergetic diet (40% total energy from carbohydrates, 30% from protein, and 30% from fat), SP diet (55% total energy from carbohydrates, 15% from protein, and 30% from fat), and conventional diet. The composition or intake levels of different macronutrients for the SP and conventional diets were the same; both were based on the AHA recommendations for healthy adults.14 However, the SP diet was hypoenergetic and the conventional diet had no energy restrictions.
Patients on the HP and SP diets participated in an intensive 12-week supervised weight loss intervention and were seen by the same dietitian at regular intervals: baseline, 1, 2, 4, 8, and 12 weeks. A member of the patient’s family (eg, spouse) was asked to participate in the counseling sessions. The dietitian provided them with personalized nutrition counseling and support during each of these visits and provided them with a list of resources (ie, list of food items and serving sizes that have taken into account their food, lifestyle, and cultural preferences) and tools (ie, weight chart, food checklists, and diaries) to enhance their adherence to the dietary intervention. The dietitian also reviewed daily dietary checklists that participants were asked to complete and a 3-day food diary with each patient to clarify information and verify portion sizes; this information was used to provide further nutritional advice as needed. The food diaries served as an instrument to determine adherence based on their average daily protein intake. Protein intake greater than or equal to 27 g per day was considered “excellent,” 23 to 26 g per day was considered “very good,” 19 to 22 g per day was considered “good,” 16 to 18 g per day was considered “fair,” and less than or equal to 15 g per day was considered “poor.” Likewise, the participants completed a 2-item survey asking them how easy it was for them to follow the diet (“1” being very difficult and “5” being very easy) and how satisfied they were with the food choices (“1” being very unsatisfied and “5” being very satisfied).
Kilocalorie goals were based on the estimated resting energy expenditure computed using the Harris-Benedict Equation and lean body mass as determined by dual-energy x-ray absorptiometry (DEXA, Hologic 4500A, version 9.03, Hologic Inc, Waltham, Massachusetts). Patients received 1 of 2 standard structured energy-restricted meal plans (1,200 and 1,500 kcal per day). The goal of the meal plan was to provide a total energy intake incorporating a 500- to 800-kcal deficit per day. The recommended pace of weight loss was 0.5 to 1 lbs per week.
Patients randomized to the conventional diet were instructed to consume a diet low in saturated fat and rich in whole grains, vegetables, and fruit as endorsed by the AHA. They were provided with written examples of diet plans that met the recommended dietary guidelines; they did not receive any additional nutritional counseling during the 12-week study period.
Measures
Height and weight measurements were obtained from all patients during their baseline and 12-week visits. Height was measured to the nearest 0.5 cm using a stadiometer. Patients were weighed in clothing without shoes (to the nearest 0.1 kg) using a professional beam scale (model 402KLS, Health-o-Meter, Bridgeview, Illinois). The weighing scale was calibrated before each use following the calibration guidelines provided by the manufacturer. We used the documented heights and weights to compute the BMI, a convenient and increasingly accepted measure of adiposity and its relation to risk.4 The index assesses the amount of total body weight in relation to height and is calculated from the individual’s weight in kilograms divided by the square of the height in meters.23
Body composition was measured using DEXA scan (DEXA, Hologic 4500A, version 9.03, Hologic Inc, Waltham, Massachusetts) at baseline and 12 weeks. DEXA calculates the percentage of lean and fat mass based on measured tissue density and the known density of the 2 tissue types. Abdominal fat mass (measured from the area demarcated by the ribs as the upper border and the iliac crests as the lower border) and waist circumference (measured at the midpoint between the anterior superior iliac crest and the lowest rib) were measured 3 times at baseline and 12 weeks to assess body fat distribution; the average of the 3 measurements was recorded.
Functional status was assessed using cardiopulmonary exercise and 6-minute walk tests. For the former, measurements were obtained from the symptom-limited bicycle exercise test with gas analysis including VO2 peak (highest VO2 observed during exercise), minute ventilation, and anaerobic threshold. 24 A standard 15-W ramp protocol was used. The 6-minute walk test was conducted by asking the patient to walk for 6 minutes at a comfortable pace in a corridor located adjacent to the HF clinic that was measured off in meters. The 6-minute walk distance served as an indicator of exercise tolerance and has been used in numerous clinical trials with high reliability and validity.25
Fasting blood samples were collected in tubes containing either no additives for lipid profiles or sodium fluoride/ethylenediaminetetraacetic acid for glucose measurements. Standard automated methods were used for analyzing lipid profiles (total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglyceride concentrations).
Quality of life was measured using the Minnesota Living with Heart Failure Questionnaire. This disease-specific, 21-item tool asked patients to indicate the extent to which various symptoms they have experienced in the previous month have prevented them from living as they wanted to. The items can be combined to form an overall QOL score, as well as physical health (8 items) and emotional health (5 items) scores. The physical subscale contains items associated with the fatigue and dyspnea of HF. The emotional subscale consists of items such as being worried or feeling down. An additional 8 items included questions about other areas of life affected by HF and are used to compute the overall QOL score.26 Response options are presented as 6-point ordinal scales ranging from 0 (no) to 5 (very much), with a total maximum score of 105 (40 for physical and 25 for emotional health); a lower Minnesota Living with Heart Failure Questionnaire score indicates better health-related QOL.26
Demographic information was collected through a simple self-administered form. The form asked patients about their age, race, marital status, education, current employment status, and annual income. Information pertaining to medical history (eg, NYHA, etiology of HF, left ventricular ejection fraction [LVEF], left ventricular end diastolic diameter [LVEDD], current medications) was obtained through self-reports and medical chart review.
Data Analysis
Data were analyzed using the SPSS for Windows, version 13.0 (SPSS Inc, Chicago, Illinois). The variables of interest are presented as group means (standard deviation) or χ2 test depending on their level of measurement. Baseline and 12-week weights, percent body fat, lean mass, 6-minute walk, VO2 peak, lipid profiles, and QOL were compared among the 3 diet groups using repeated-measures analysis of variance. The changes from baseline (defined as 12-week value-baseline value) and percent change (defined as 100 × (12-week value − baseline value)/baseline value percent) were also obtained. The differences among the diet groups at each time point were examined using a general linear model with the baseline value as a covariate. A value of P < .05 was considered statistically significant.
Results
The current report reflects data from the first 14 patients who consented to participate in the study; recruitment and enrollment are still ongoing. The patients were randomized to the HP (n = 5), SP (n = 5), and conventional diets (n = 4), respectively. Patients had an average (SD) of 58.8 (9.7) years, were predominantly male (78%), were white (71%), were married (71%), had NYHA class II (42.95) or III (57.1%), were HF etiology nonischemic (57.1%), and were with a mean (SD) ejection fraction of 26% (7.3%). There were no significant baseline differences in sex, race, education, and retired or disabled status among the 3 groups at baseline (Table 1). However, differences in marital status were noted; 100% of patients on the HP and 80% in the SP diets were married compared with only 25% of patients on the conventional diet (χ2, 6.405; P = .041). Likewise, there were no significant differences among the 3 groups with regard to NYHA functional class, HF etiology, LVEF, and LVEDD. Rates of angiotensin-converting enzyme inhibitor (93%), β-blocker (86%), loop diuretic (79%), statins (57%), and digoxin (29%) use were also similar in all 3 groups. Body weight and adiposity were comparable among the 3 diet groups at baseline (Table 2).
TABLE 1.
Comparative Baseline Demographic and Clinical Characteristics of Study Participants
| High Protein (n = 5) |
Standard Protein (n = 5) |
Conventional (n = 4) |
P | |
|---|---|---|---|---|
| Age, mean (SD), y | 56.4 (6.6) | 58.6 (13.0) | 62.2 (9.7) | .70 |
| Men, % | 80 | 80 | 75 | .98 |
| White, % | 40 | 80 | 100 | .23 |
| Married, % | 100 | 80 | 25 | .04 |
| Educational level of college or higher, % | 80 | 60 | 75 | .53 |
| Retired/disabled, % | 60 | 100 | 100 | .32 |
| New York Heart Association class II/III, % | 40/60 | 60/40 | 25/75 | .56 |
| Ischemic etiology, % | 20 | 60 | 50 | .41 |
| Left ventricular ejection fraction, mean (SD),% | 27.8 (7.1) | 23.8 (6.8) | 26.6 (9.5) | .71 |
| Left ventricular end diastolic diameter, mean (SD), mm | 62.8 (5.7) | 59.6 (2.5) | 62.5 (4.0) | .47 |
| Hypertension, % | 60 | 60 | 100 | .33 |
| Smoking history, % | 100 | 80 | 25 | .51 |
| Angiotensin-converting enzyme inhibitor (yes), % | 80 | 80 | 75 | .98 |
| β-Blocker (yes), % | 100 | 80 | 100 | .78 |
| Diuretics (yes), % | 100 | 100 | 100 | 1.00 |
| Digoxin (yes), % | 60 | 80 | 75 | .41 |
TABLE 2.
Comparative Baseline Data for Key Study Variables Among Patients on the 3 Diets
| High Protein (n = 5) | Standard Protein (n = 5) | Conventional (n = 4) | P | |
|---|---|---|---|---|
| Weight, kg | 110.8 (11.7) | 99.5 (8.0) | 109.6 (6.3) | .16 |
| Body mass index | 37.3 (1.8) | 35.9 (6.6) | 40.7 (2.3) | .28 |
| Waist circumference | 50.1 (3.5) | 45.4 (3.9) | 50.0 (2.8) | .10 |
| Body fat, % | 44.6 (5.7) | 40.5 (2.0) | 46.6 (4.8) | .14 |
| Lean mass, kg | 54.4 (6.0) | 51.2 (2.6) | 59.3 (9.7) | .22 |
| 6-min walk, ft | 1143.1 (129.2) | 1199.4 (157.8) | 1007.6 (205.1) | .25 |
| Vo2 peak, mL/kg/min | 13.5 (2.4) | 12.7 (2.4) | 10.9 (2.2) | .28 |
| Total cholesterol, mg/dL | 182.6 (30.1) | 168.0 (34.1) | 169.7 (13.8) | .69 |
| Triglycerides, mg/dL | 194.6 (28.3) | 196.2 (48.5) | 217.2.6 (43.7) | .83 |
| HDL-C, mg/dL | 33.4 (10.5) | 34.4 (2.0) | 30.2 (2.9) | .11 |
| LDL-C, mg/dL | 79.2 (28.1) | 77.4 (18.7) | 95.7 (14.3) | .42 |
| HbA1C, % | 6.8 (0.8) | 7.9 (1.0) | 7.25 (1.1) | .72 |
| LHFQ, overalla | 68.5 (19.2) | 73.0 (20.4) | 70.9 (14.5) | .85 |
| LHFQ, physicala | 26.2 (8.5) | 26.8 (9.4) | 30.5 (5.8) | .67 |
| LHFQ, emotionala | 12.6 (6.5) | 14.0 (7.7) | 17.7 (3.2) | .28 |
Abbreviations: HbA1C, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LHFQ, Minnesota Living With Heart Failure Questionnaire.
Values are in mean (SD) unless provided otherwise.
Higher scores indicate greater symptom interference and lower quality of life.
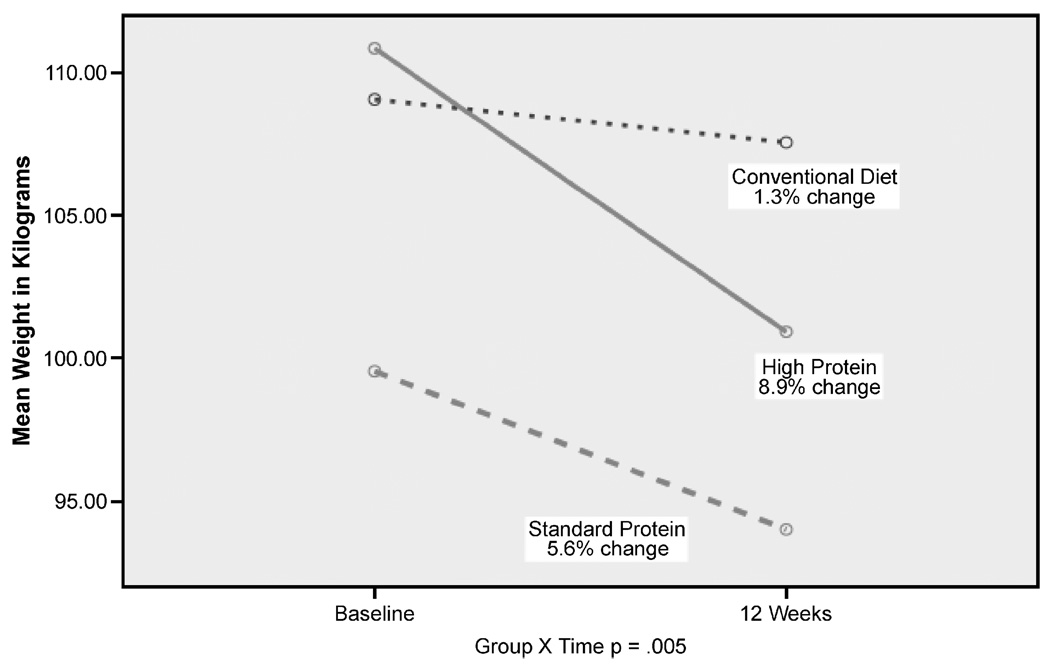
Participants in the HP diet group had significantly greater weight loss compared with the SP or conventional diets (−9.9 vs −5.5 kg vs 1.51 kg, respectively, P < .001). Figure 1 illustrates the weight change between baseline and 12 weeks in patients randomized to the HP, SP, and conventional diets. Patients on the HP diet also demonstrated greater reductions in percent body fat and waist circumference compared with the other 2 diets over time. Table 3 illustrates mean changes in outcomes across 12 weeks and the overall group and time effect for the other clinical outcomes of interest. Study patients in all 3 groups demonstrated significant reductions in hemoglobin A1c and significant improvements in overall QOL over time (baseline to 12 weeks). Between-group differences from baseline to 12 weeks were noted in percent body fat, 6-minute walk test, peak VO2 peak, total cholesterol, triglyceride concentrations, HDL-C, LDL-C, and physical QOL. No differences in lean mass were found among the 3 groups, but a trend toward increased lean mass was noted in the HP group.
FIGURE 1.
Comparison of weight changes in the high protein, standard protein, and conventional diet from baseline to 12 weeks.
TABLE 3.
Mean (SD) Changes in Outcomes From Baseline to 12 Weeks, by Diet and Group and Time
| High Protein (n = 5) |
Standard Protein (n = 5) |
Control Group (n = 4) |
12 Wk | Group × Time | Group Comparisonsa | |||
|---|---|---|---|---|---|---|---|---|
| HP-SP | HP-C | SP-C | ||||||
| Weight, kg | −9.9 (2.0) | −5.6 (0.8) | −1.5 (0.6) | <.001 | .005 | … | …b | …b |
| Waist circumference, cm | −5.9 (1.4) | −2.0 (1.6) | −0.3 (1.5) | <.001 | .002 | … | …b | …b |
| Body fat, % | −2.5 (1.9) | −1.1 (1.9) | −1.2 (2.1) | .110 | .036 | …b | … | … |
| Lean mass, kg | 0.6 (1.0) | −0.3 (0.3) | −2.6 (0.6) | .471 | .180 | … | … | … |
| 6-min walk, ft | 287.3 (69.0) | −12.3 (69.0) | −138.4 (77.1) | .350 | .010 | … | …b | …b |
| VO2 peak, mL/kg/min | 3.1 (1.0) | −0.3 (1.0) | −0.3 (1.1) | .049 | .003 | …b | …b | … |
| Total cholesterol, mg/dL | −35.0 (9.0) | −19.8 (9.0) | 16.5 (10.1) | .056 | .016 | … | …b | … |
| Triglycerides, mg/dL | −66.0 (25.5) | −18.0 (25.5) | 2.0 (28.5) | .076 | .034 | …b | …b | … |
| HDL-C, mg/dL | 15.2 (2.1) | 0.2 (1.8) | −0.3 (2.1) | .069 | .006 | …b | …b | … |
| LDL-C, mg/dL | −4.5 (1.9) | −0.2 (1.9) | 31.3 (2.2) | .075 | .041 | …b | …b | … |
| HbA1c | −0.7 (0.2) | −0.8 (0.2) | −0.5 (0.1) | .001 | .132 | … | … | … |
| LHFQ, overallc | −20.1 (9.5) | −12.2 (4.3) | −5.1 (3.9) | .006 | .046 | … | …b | … |
| LHFQ, physicalc | −5.4 (7.0) | 2.4 (8.9) | 5.7 (2.5) | .050 | .022 | … | …b | … |
| LHFQ, emotionalc | −0.8 (0.5) | −1.0 (0.7) | 0.0 (0.5) | .299 | .187 | … | … | … |
Abbreviations: HbA1C, hemoglobin A1c; HP-C, high proteinYcontrol; HP-SP, high protein–standard protein; LHFQ, Minnesota Living with Heart Failure Questionnaire; SP-C, standard protein–control.
Post hoc analyses (Bonferroni) were done to determine paired group differences.
P < .05 was considered significant.
Higher scores indicate greater symptom interference and lower QOL.
All 14 patients who expressed an interest to participate in this investigation completed the 12-week intensive dietary intervention phase. The registered dietitian indicated that the average adherence rate for the 5 participants randomized to the HP diet was “good” for one, “very good” for two, and “excellent” for two (as assessed from food diaries). Four of the 5 participants in the HP group indicated that following the diet was “easy” and that they were “very satisfied” with the food choices they were given. The fifth participant assigned to the HP group rated the diet as “neither difficult nor easy” to follow and indicated being “satisfied” with the food choices (it was later noted that this participant did not have a person to help him prepare his meals).
Discussion
In the present study, we compared the effects of the 3 diets–HP, SP, and conventional–on body weight and adiposity, functional status, lipid profiles, glycemic control, and QOL in overweight and obese patients with HF and DM. Patients on all 3 diets demonstrated reductions in weight and adiposity after 12 weeks, but participants in the HP diet had significantly greater reductions. We also found that patients on the HP diet who showed modest reductions in body weight and adiposity demonstrated greater improvements in functional status, lipid profiles, and QOL. Although this clinical investigation involved relatively few patients, it is among the first to test different dietary interventions in patients with HF and has produced important findings.
The current study is the first to report the effectiveness of an HP diet in promoting weight loss in overweight and obese patients with HF. The amount of weight loss that we report is appreciably higher compared with weight reductions associated with other interventions in this patient population. A 12-week behavioral program using bibliotherapy and bibliotherapy plus telephone contact was found effective in promoting weight losses of −1.02 and −2.78 kg, respectively, for the 2 groups,27 whereas another report comparing a diet counseling plus Orlistat versus diet counseling alone over 12 weeks showed a weight change of −4.65 versus +4.39 kg for the 2 groups.12 Our group supported the effectiveness of a 6-month home-based exercise program in promoting a weight loss of −6.37 kg in patients assigned to the intervention versus −0.33 kg for patients in the control group.13 The positive effect of an HP diet on weight loss that we observed in our sample has been consistently observed in patients with DM.19,21 The mechanisms that support greater weight losses associated with higher protein diets are still unclear, but researchers speculate that greater weight losses may in some way be related to greater energy expenditure in patients who consume higher protein diets.28
Patients who received the SP diet also showed greater weight reductions after the 12-week intervention compared with patients assigned to the conventional diet. The macronutrient composition of the SP and conventional diets was both based on the AHA recommendations for healthy adults, but patients on the SP diet were seen by a nutritionist at regular intervals during the 12-week program, whereas patients on the conventional diet were not, clearly delineating the important role that social support and counseling play in dietary interventions to promote weight reduction.
We also found reductions in adiposity across all 3 diets. However, the percent body fat loss observed in patients on the HP diet was greater compared to their counterparts. More importantly, patients on the HP diet also showed increased amount of lean mass compared with patients on the SP and conventional diets who showed reductions in lean mass. Although changes in lean mass did not reach statistical significance, the findings were trending in the expected direction to support the hypothesis that HP diets preserve lean tissue in overweight and obese patients with HF. Since the goal of preserving lean mass is currently the mainstay in the treatment of worsening degrees of HF, the absence of further loss of lean mass among patients on the HP diet is noteworthy. Future studies involving a larger sample are warranted to determine the metabolic advantages of HP diet over other diets.
The data also show that patients on the HP diet demonstrated significantly greater improvements in functional status as reflected by better performance on the 6-minute walk test and higher Vo2 peak. The improvements we found on the 6-minute walk test for patients in the HP group (287 ft) were higher compared with improvements observed after weight loss associated with a similar intervention lasting 12-weeks and examining the effectiveness of diet counseling plus Orlistat (150 ft)12 and a 6-month home-based exercise program also observed by our group (198 m). The improvements in Vo2 peak in response to weight loss observed in overweight and obese patients with HF on the HP diet are a novel finding. Improvements in functional status can be attributed to the loss of adiposity and the diuretic effect from reduced carbohydrate intake and its effect on sodium and water loss16; therefore, it is possible that improvements in functional status of patients on the HP diet are in response to the hemodynamic improvements of reducing volume overload.
Patients on the HP diet also demonstrated significantly greater improvements in lipid profiles (reduced total cholesterol, triglyceride, and LDL-C and increased HDL-C). Similar improvements in lipid profiles have been observed among patients with DM receiving an HP diet.20,21 Greater improvements in lipid profiles observed in patients on HP diet compared with SP or conventional diets are attributed to greater reductions in body weight and adiposity. However, concerns have been raised regarding the long-term impact of HP diets on lipid profiles. Specifically, since HP diets are usually high in fats, the question arises whether long-term adherence to an HP diet will actually elevate total cholesterol and LDL-C. Although patients in the HP diet were encouraged to increase their protein intake, they were instructed to use more plant sources than animal sources of protein, which minimizes this potential risk. Nevertheless, the long-term effects of an HP diet on lipid profiles and overall metabolic derangements warrant further investigation.
The positive effects of short-term weight loss on QOL in overweight and obese individuals have been documented in the obesity literature29 and confirmed by data from the current study that showed improvements in overall and physical QOL at the end of the 12-week dietary intervention in which there was moderate weight loss. Fontaine and colleagues29 found a weight loss average (SD) of 8.6 (2.8) kg over a 13-week study after a lifestyle modification treatment program that was associated with significantly higher QOL scores relative to baseline on physical functioning and overall QOL. Although different interventions were tested compared to the current study, the association of weight loss with improvements in physical functioning and QOL in both studies is noteworthy in patients who experienced functional decline and poor QOL as a consequence of HF.
Because of the small number of study participants and lack of information on length of time with HF, DM, and obesity, our results must be considered preliminary. However, despite the small sample size, we were able to observe large effect sizes ranging from 1.70 to 3.51 (80% power, ∝ 0.050) for several of the key variables of interest we measured. These findings support the hypothesis that an HP diet is more effective than SP and conventional diets in reducing weight and adiposity and improving functional status, lipid profiles, and QOL. The use of 2 control groups also strengthens the validity of our findings because, in essence, we compared the HP diet with another hypoenergetic diet and with a conventional diet or the “true” control group.
The effectiveness of the diet in improving left ventricular function secondary to weight loss remains inconclusive. Our data did show that the patients in the HP group demonstrated improvements in LVEDD that was not observed in patients on SP or conventional diets. However, LVEF was not significantly different among the 3 diet groups after the 12-week intervention. Although improvements in left ventricular structure and in systolic and diastolic function after weight reduction were previously found in morbidly obese patients,30 our study did not have the power to detect significant differences in these parameters, except for a significant reduction in left ventricular size. The possible benefits of weight reductions on left ventricular structure and function in future studies may provide us with better insight to support the use of HP diet for improving left ventricular systolic function and consequently reducing HF severity.
Clinical Pearl
This feasibility study suggests that a 12-week dietary intervention with high protein content shows promise in potentially reducing adiposity and improving functional status, lipid profiles, and quality of life without adversely affecting lean body mass in overweight and obese patients with heart failure.
These results open the possibility for planning larger studies to assess the long-term effects of a high protein diet on anthropometric measures, body composition, disease progression, and cardiovascular risk factor reduction in this sub-group of patients with heart failure.
Conclusions
The results of this feasibility study suggest that a 12-week dietary intervention with HP content shows promise in being able to reduce adiposity and improve functional status, lipid profiles, glycemic control, and QOL while preserving lean body mass in overweight and obese patients with HF. Most participants in the HP group stated that the diet was easy to follow and that they were very satisfied with the food choices. These results open the possibility for planning larger studies to assess the long-term effects of an HP diet on anthropometric measures, body composition, disease progression, and cardiovascular risk factor reduction in this subgroup of patients with HF. Although both the HP and SP diets clearly have the potential to help reduce excess adiposity, it is unclear whether weight loss can be sustained, whether there are adverse metabolic consequences, whether the diets are suitable for long-term use in terms of safety and palatability, and whether there will be favorable effects on clinical outcomes. These intriguing findings should be examined in a larger scale prospective randomized clinical trial sufficiently powered to assess clinical outcomes. Although no adverse effects of an HP diet were noted in this 12-week study, the long-term impact on renal function, in particular for patients with HF and DM, warrants further research. Further work is needed to refine dietary interventions with regard to macronutrient and micronutrient composition, develop interventions that ensure optimal adherence and attract a diversity of patients, and address mechanisms underlying the benefits of an HP diet. Until that time, overweight and obese patients with HF should be provided with support to pursue healthier dietary patterns and regular physical activity.
Acknowledgments
This research was partially supported by an intramural grant and by the University of California School of Nursing.
Footnotes
Individual author contributions
Lorraine S. Evangelista was responsible for the overall conduct of the study and writing of the manuscript–no conflict of interest.
David Heber provided his expertise in clinical nutrition during data collection and analyses and writing of the entire article–no conflict of interest.
Zhaoping Li assisted with patient recruitment and follow-up during their participation in the study. She provided insights for the discussion section of the study–no conflict of interest.
Susan Bowerman was the nutritionist who saw the patients enrolled in the study. She helped refine the Methods section of the article–no conflict of interest.
Michele A. Hamilton was the cardiology expert who referred patients to the study and served as the liaison between the investigators, patients, and other cardiologists–no conflict of interest.
Gregg C. Fonarow provided consultation for overall study conduct as it relates to the care and management of patients with heart failure. He was the senior investigator on the research team–no conflict of interest.
Contributor Information
Lorraine S. Evangelista, School of Nursing, University of California, Los Angeles.
David Heber, University of California Center for Human Nutrition, Los Angeles.
Zhaoping Li, University of California Center for Human Nutrition, Los Angeles.
Susan Bowerman, University of California Center for Human Nutrition, Los Angeles.
Michele A. Hamilton, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles.
Gregg C. Fonarow, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles.
REFERENCES
- 1.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics—2007 Update. Dallas, TX: AHA; 2007. [Google Scholar]
- 3.National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 Fact Sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2008. [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–359. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;154:277. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108 927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Horwich TB, Fonarow G, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J. 2005;26:5–7. doi: 10.1093/eurheartj/ehi055. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Milani RV. Obesity and cardiovascular disease: the Hippocrates paradox? J Am Coll Cardiol. 2003;42:677–679. doi: 10.1016/s0735-1097(03)00784-8. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Mosterd A, Cost B, Hoes A. The prognosis of heart failure in the general population: the Rotterdam Study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 12.Beck-da-Silva L, Higginson L, Fraser M, Williams K, Hadda H. Effect of Orlistat in obese patients with heart failure: a pilot study. Congest Heart Fail. 2006;11:118–123. doi: 10.1111/j.1527-5299.2005.03827.x. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista LS, Doering LV, Lennie T, et al. Usefulness of a home-based exercise program for overweight and obese patients with advanced heart failure. Am J Cardiol. 2006;97:886–890. doi: 10.1016/j.amjcard.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Krauss RM, Eckel RH, Howard B, et al. AHA dietary guidelines Revision 2000; a statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 15.Miller E, Erlinger T, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart Trials. Curr Atheroscler Rep. 2006;8:460–465. doi: 10.1007/s11883-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 16.St Jeor ST, Howard B, Prewitt TE, Bovee V, Bazzarre TL, Eckel RH. Dietary protein and weight reduction a statement for health care professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the AHA. Circulation. 2001;104:1869–1874. doi: 10.1161/hc4001.096152. [DOI] [PubMed] [Google Scholar]
- 17.Jeor ST, Ashley JM. Dietary strategies: issues of diet composition. In: Fletcher G, Grundy S, Hayman L, editors. Obesity: Impact on Cardiovascular Disease. New York, NY: Futura Publishing Co., Inc.; 1999. pp. 233–246. [Google Scholar]
- 18.Brinkworth G, Noakes M, Keogh J, Luscombe N, Wittert G, Clifton P. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2004;28:661–670. doi: 10.1038/sj.ijo.0802617. [DOI] [PubMed] [Google Scholar]
- 19.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Sargrad KR, Homko C, Mozzoli M, Boden G. Effect of high protein vs. high carbohydrate intake on insulin sensitivity, body weight, hemoglobin A1c, and blood pressure in patients with type 2 diabetes mellitus. J Am Diet Asso. 2005;105:573–580. doi: 10.1016/j.jada.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.McAuley K, Hopkins C, Smith K, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- 22.Lennie T. Nutritional recommendations for patients with heart failure. J Cardiovasc Nurs. 2006;21:261–268. doi: 10.1097/00005082-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Aronne LJ. Treating obesity: a new target for prevention of coronary heart disease. Prog Cardiovasc Nurs. 2001;16:98–106. doi: 10.1111/j.0889-7204.2001.00589.x. 115. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow G, Stevenson LW, Walden J, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G. Use of the six-minute walk test as an outcome measure in clinical trials in chronic heart failure. Heart Fail. 1987;3:211–217. [Google Scholar]
- 26.Rektor TS, Johnson G, Dunkman B. Evaluation by patients with heart failure of the effects of enalapril compared with hydralazine plus isosorbide dinitrate on quality of life, V-HeFT II. Circulation. 1993;87:71–77. [PubMed] [Google Scholar]
- 27.Park TL, Perri MG, Rodrique JR. Minimal intervention programs for weight loss in heart transplant candidates: a preliminary examination. Prog Transplant. 2003;13:284–288. doi: 10.1177/152692480301300408. [DOI] [PubMed] [Google Scholar]
- 28.Schoeller DA, Buchholz AC. Energetics of obesity and weight control: does diet composition matter? J Am Diet Assoc. 2005;105:24–28. doi: 10.1016/j.jada.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 30.Alpert MA, Terry BE, Muleker M, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effects of weight loss. Am J Cardiol. 1997;80:736–740. doi: 10.1016/s0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]