Obesity is associated with increased risk for insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease, atherogenic dyslipidemia, and cardiovascular disease (1). Recent studies indicate that the body protects itself from weight loss by lowering energy expenditure (2,3). Both energy consumption and energy expenditure are regulated by hormones from a number of organs that act on the brain, as well as neural signals emanating from the brain itself. The adipocyte-derived protein, leptin, is an adiposity signal that reduces food intake by activating leptin receptors in the hypothalamus (4,5). Gut-derived hormones, such as cholecystokinin (CCK) and ghrelin, act in concert with central mechanisms to regulate food intake (6). Other hormones and substrates, including insulin and fatty acids, modulate satiety and hunger by crossing the blood–brain barrier and signaling metabolic pathways in the hindbrain and hypothalamus (7).
Lifestyle modification is the initial intervention for obesity, with emphasis on reducing calorie intake and increasing physical activity; pharmacotherapy may be indicated for certain cardiovascular and metabolic risk factors (8). Weight-loss pharmacotherapy is recommended when BMI is ≥27 in the presence of obesity-related risk factors and when BMI is ≥30 in the absence of associated risk factors (1,9). This review focuses on the link between the biology of the cannabinoid receptor type 1 (CB1 receptor) system and body-weight regulation, as well as clinical data from studies of the first CB1 receptor antagonist, rimonabant.
THE ENDOCANNABINOID SYSTEM
The endocannabinoid system (ECS) affects multiple metabolic pathways in the brain and other organs (10). The transmembrane CB receptors were cloned in the early 1990s, followed shortly thereafter by the discovery of endogenous ligands, now known as endocannabinoids (11,12). Three general types of cannabimimetic compounds have been described: herbal CBs, which occur uniquely in the cannabis plant (Cannabis sativa); endogenous CBs (or endocannabinoids), which are produced in the brain and peripheral tissues; and synthetic CBs, which are functionally similar compounds synthesized in the laboratory (7).
The ECS comprised CB receptors, the endocannabinoids, and the enzymes involved in endocannabinoid synthesis and inactivation (13,14). Arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG), the most-studied endocannabinoids, bind to and activate CB1 and/or CB2 receptors (13,14). 2-AG is pharmacologically different from anandamide in terms of (i) its ability to bind both CB1 and CB2 receptors with similar affinity and (ii) its full agonist activity at CB1 receptors, where anandamide acts as a partial agonist (15). Hemopressin, a novel bioactive peptide derived from the α1-chain of hemoglobin, has recently been identified as a CB1 receptor selective antagonist (16). There are other newly proposed endocannabinoids, for which a physiological role has not yet been established.
Preclinical studies demonstrate that anandamide and 2-AG are present in the uterus, pancreas, liver, gastrointestinal tract, and adipose tissue as well as in the brain (17,18) and both circulate in the blood. The CB1 receptor is expressed in the brain and in a number of peripheral tissues such as adipose tissue, liver, skeletal muscle, the gastrointestinal tract, and pancreas (Table 1) (7,19). A second CB receptor, the CB2 receptor, is expressed in the spleen and tonsils as well as in B cells, monocytes, and T cells, indicating a role in immune function (20,21).
Table 1.
Human tissues and organs expressing the CB1 receptor gene
| Central nervous system | Genitourinary/reproductive | Gastrointestinal | Other |
|---|---|---|---|
| • Brain | • Kidney | • Ileum | • Adipose |
| • Spinal cord | • Placenta | • Liver | • Lung |
| • Prostate | • Stomach | • Skeletal muscle | |
| • Testis and sperm | • Pancreas | • Spleen | |
| • Uterus |
Anandamide and 2-AG are long-chain polyunsaturated fatty acid by-products formed via calcium- and phospholipid-dependent pathways (22). CB receptors are G protein–coupled receptors (18). In peripheral tissues, activation of CB1 receptors (whether via endocrine, paracrine, or autocrine means) leads to inhibition of adenylate cyclase with corresponding inactivation of the protein kinase A phosphorylation pathway, and stimulation of mitogen-activated protein kinase (13,14,17). Stimulation of these cytoplasmic kinases could lead to changes in the expression of target genes. In neurons, endocannabinoids act as retrograde neuromodulators (23). Following CB1 receptor activation, anandamide and 2-AG are degraded rapidly by enzymatic hydrolysis (13,14).
THE ECS AND THE CENTRAL NERVOUS SYSTEM
CB1 receptors are one of the most abundant neuromodulatory receptors in the brain (23,24). The receptors are localized mainly on presynaptic nerve terminals and appear to mediate endocannabinoid-dependent suppression of γ-aminobutyric acid and glutamate release from CB1 receptor–containing nerve terminals in many areas of the brain and especially in the hippocampus and cerebellum (24). CB1 receptor activation is also thought to mediate the rapid inhibition of the stress response in the brain by glucocorticoids (25).
The CB1 receptor is also an integrated component of the networks controlling appetite and food intake in the hypothalamus, which along with the hindbrain, controls caloric intake and regulates energy balance (5,7). Administration of 2-AG into the shell subregion of the nucleus accumbens (a limbic forebrain area implicated in eating motivation) induces short-term hyperphagia in rats, and brain endocannabinoid levels are increased during food deprivation (26). Pharmacologic blockade of the CB1 receptor with SR141716 (rimonabant), a selective CB1 receptor antagonist, reverses this effect, and when administered by itself systemically, rimonabant decreases food intake in both lean and obese animals, whether they are freely feeding or food restricted (27,28).
A hyperactive ECS has been demonstrated in several animal models of obesity. Obese mice with deficient leptin signaling (db/db and ob/ob mice) have higher levels of endocannabinoids in the hypothalamus compared with lean mice (28). Administration of CB1 receptor antagonists reduced food intake and body-weight gain in obese Zucker rats and normal mice (29–32). However, CB1 receptor blockade had no effect on food intake in CB1 receptor knockout mice (33).
The CB1 receptor is also highly expressed in mesolimbic dopamine reward circuits within the brain that process perceptions associated with pleasure/palatability and appetite/incentive stimuli (34). It remains unclear, however, whether ECS activity regulates qualitative aspects of feeding behavior. As recently reviewed (34,35), feeding studies in rimonabant-treated rats have yielded discrepant results when consumption of standard chow is compared to that of highly palatable diets. In one study, adult male Sprague-Dawley rats were fed a high-fat diet (45% kcal from fat), a high-carbohydrate diet (67% kcal from carbohydrate), or standard chow. SR141716 treatment was associated with significantly reduced food intake, which was comparable among rats fed the three different diets (36). By contrast, another study found that the reduction of caloric intake in SR141716-treated rats was specific to a decrease in palatable food (37). In the latter study, which used adult female Sprague-Dawley rats and a paradigm in which the palatable diet was given as a dessert after a meal, there was no significant difference in the consumption of chow between the control and SR141716 treatment groups. However, compared with vehicle-treated rats, rats treated with SR141716 had significantly reduced caloric intake only of the more palatable dessert. SR141716 was recently reported to reduce the increase of dopamine elicited by consumption of palatable food in mesolimbic reward areas of the brain, suggesting a possible mechanism for the action of this CB1 receptor antagonist (38). Another recent report implicated CB1 receptors in the parabrachial nuclei in endocannabinoid-induced stimulation of palatable food intake (39). Parabrachial infusions of 2-AG stimulated acute intake of a high-fat/high-sucrose diet and of pure fat or sucrose but not of standard rodent chow, and this effect was blocked by CB1 receptor antagonism (39). Finally, inhibiting fatty acid amide hydrolase (FAAH), the enzyme that breaks down 2-AG, stimulates the intake of palatable foods (40).
THE ECS AND PERIPHERAL TISSUES
CB1 receptor knockout mice have a lean phenotype and are resistant to diet-induced obesity (41,42). In pair-feeding studies, wild-type mice were fed the same amount of food consumed by CB1 receptor knockout mice. Pair-fed wild-type mice became significantly heavier than CB1 receptor knockout mice consuming the same quantity of food (Figure 1) (41,42). Results from other pair-feeding studies suggested that the decreased body weight in SR141716-treated rats with diet-induced obesity was entirely accounted for by the reduction in food intake (43,44). Pair-feeding studies are important, because they help to determine whether factors other than reduced food intake contribute to the lean phenotype of CB1 receptor knockout mice. Of note, adipocytes and hepatocytes express CB1 receptors and ECS activation increases lipid storage and synthesis in adipose tissue and liver (17,29,45). Importantly, the expression of CB1 receptors both centrally and peripherally (Table 1) provides a mechanistic basis for multiple effects of a single-receptor blockade.
Figure 1.
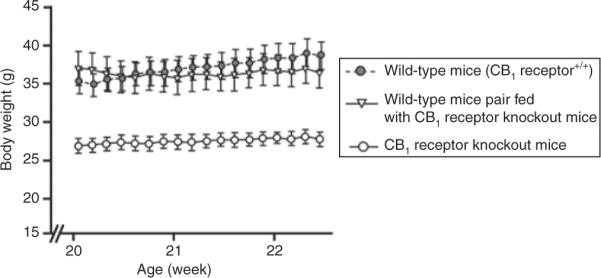
Cannabinoid receptor type 1 (CB1) receptor knockout mice sustain a reduced body weight vs. pair-fed wild-type mice. Each data point represents the mean ± s.e.m. of six mice for each group. P < 0.005 for CB1 receptor knockout mice vs. wild-type mice at each data point. P < 0.005 for CB1 receptor knockout mice vs. pair-fed wild-type mice at each data point. Adapted from Cota et al. (41).
THE ECS AND THE GASTROINTESTINAL TRACT
Gut-derived hormones, such as CCK and ghrelin, act in concert with central mechanisms to influence eating behavior (6), and there is evidence that the ECS interacts with many of them to promote increased caloric intake (46). During feeding, CCK is secreted and interacts with CCK receptors on afferent terminals of the vagus nerve to reduce meal size (6), and reduced ECS activity may mediate the induction of satiety by CCK. Consistent with this, levels of CB1 receptor mRNA on vagal afferent neurons projecting into the region of duodenum where CCK is secreted are decreased in rats fed ad libitum and increased in food-deprived rats (47). Feeding previously fasted rats or else administrating CCK was associated with decreased levels of CB1 receptor mRNA in the same vagal afferent neurons (46).
Exogenous administration of ghrelin increases food intake and adiposity (48) and the ECS appears to mediate the stimulatory effects of ghrelin on food intake (49,50). Kola et al. (49) observed that ghrelin failed to induce an orexigenic effect in CB1 receptor knockout mice. Furthermore, administration of ghrelin increased the endocannabinoid content of the hypothalamus in wild-type mice, an effect that was blocked by pretreatment with SR141716. By contrast, there was no effect of ghrelin on hypothalamic endocannabinoid levels in CB1 receptor knockout mice (49).
THE ECS AND ADIPOSE TISSUE
Adipose expresses CB1 receptors and is a peripheral target of the ECS. The reduction in fat mass associated with CB1 receptor antagonism in animals may be attributed in part to decreased proliferation of preadipocytes (51). Stimulation of CB1 receptors in mouse 3T3 F442A and human preadipocytes increased the expression of peroxisome proliferator–activated receptor-γ as well as the amounts of lipid droplets, two markers of adipocyte differentiation (52). In addition, endocannabinoids were found to activate peroxisome proliferator–activated receptor-γ via CB1 receptors (45,52). These data suggest that activation of CB1 receptors and peroxisome proliferator–activated receptor-γ may be coupled to promote early stages of adipocyte differentiation (53).
Jbilo et al. (54) found that CB1 receptor antagonism with rimonabant reversed the morphological changes in mouse white adipose tissue produced by diet-induced obesity. White adipocytes of rimonabant-treated mice on a high-fat diet were significantly smaller in diameter (by 67%) than fat cells of control animals on the high-fat diet, and slightly smaller (~10%) than those of mice fed a standard low-fat chow (54). In another study, treatment of mouse adipocytes with a CB1 receptor agonist (HU-210) stimulated adipocyte differentiation—an effect that was blocked by coincubation with rimonabant (52). Moreover, levels of 2-AG were shown to be significantly elevated in epididymal fat of diet-induced obese mice (52). These findings are all consistent with a role for 2-AG in the regulation of fat storage, and taken together, they demonstrate that CB1 receptor antagonism may contribute to a lean phenotype. Recent studies in animals and in human subjects demonstrated that CB1 receptor antagonism increased energy expenditure. Compared with control animals, rats treated with 3 and 10 mg/kg rimonabant had an increase in O2 consumption of 18 and 49%, respectively, after 3 h (55). There did not appear to be changes in the rate of carbohydrate or fat oxidation, and factors other than physical activity appeared to contribute to the increase in O2 consumption (55). Addy et al. (56) measured the effect of the CB1 receptor antagonist taranabant on resting energy expenditure in 17 overweight or obese subjects. Compared with placebo, the peak resting energy expenditure 2–5 h after single-dose treatment with 12 mg taranabant was increased significantly by 6% (95% confidence interval 1.01–1.10; P = 0.011) (56). The 12-mg dose of taranabant appeared to increase the rate of fat metabolism, as evidenced by a significant decrease in the mean respiratory quotient compared with placebo (56).
Clinical and population studies support the role of an active ECS in human overweight and obesity (57,58). For example, the enzyme FAAH, which catalyzes the hydrolysis of anandamide (13,14,18), is expressed in human adipocytes (59). It is plausible, therefore, that the presence of a genetic malfunctioning of FAAH and a subsequently impaired degradation of endocannabinoids might underlie the persistent elevated levels of endocannabinoids that are associated with obesity. Indeed, subjects with a relatively common missense polymorphism in the FAAH gene have approximately half the FAAH enzymatic activity of normal subjects (60), and the median BMI in subjects homozygous for this FAAH polymorphism is greater than that of subjects with a heterozygous or normal genotype. However, the role of FAAH polymorphisms in obesity requires further investigation, as a second study found no relationship between FAAH polymorphisms and obesity (61). On the other hand, single polymorphisms of the gene encoding the CB1 receptor (CNRI) are also associated with a higher body weight and increased waist circumference and subscapular skinfold thickness in men (62). Other clinical studies have identified a positive association between elevated plasma endocannabinoid levels and higher BMI (59,63). Matias et al. (45) found that visceral adipose tissue from obese subjects contained significantly higher levels of 2-AG than visceral fat from nonobese volunteers, thus paralleling the findings in mice with diet-induced obesity. Di Marzo et al. found that reduction of body weight and accompanying reduction of waist circumference are associated with a decrease in plasma 2-AG (53b) (58).
THE ECS AND THE LIVER
Human obesity is associated with hepatic steatosis or fatty liver—which results from the accumulation of ectopic fat in hepatocytes (17,29,64)—and there is strong evidence that the ECS also plays a role in hepatic fibrosis (65). Osei-Hyiaman et al. (29) reported that wild-type mice, but not CB1 receptor knockout mice, develop fatty liver despite similar levels of caloric intake from a high-fat diet. In that study, CB1 receptor activation was associated with increases in the expression of sterol regulatory element–binding protein-1c and its downstream enzymes, acetyl-Coenzyme A carboxylase-1, and fatty acid synthase (FAS) (29). Thus, there appear to be liver-specific effects of CB1 receptor blockade that may influence hepatic lipogenesis and/or lipoprotein secretion.
More recently, Jeong et al. demonstrated that ethanol (ETOH)-induced steatosis in mice was mediated specifically by hepatic CB1 receptors (66). CB1 receptor knockout mice were pair fed with wild-type mice and both groups of mice were also fed ETOH. The wild-type mice, but not the CB1 receptor knockout mice, developed fatty liver and hepatocellular damage. Importantly, liver-specific CB1 receptor knockout mice, which lack functional CB1 receptors in hepatocytes but express normal CB1 receptor levels in other tissues, were also resistant to ETOH-induced fatty liver. These data suggest that hepatic CB1 receptors are involved in the development of ETOH-induced hepatic steatosis (66). Similar findings were observed for diet-induced hepatic steatosis using hepatocyte-specific CB1 receptor knockout mice (67).
It is likely that both central nervous system and peripheral components of ECS biology are involved in metabolic homeostasis. In fact, given the widespread distribution of CB1 receptors in the brain, and their involvement in so many regulatory and control systems, it may be desirable to develop formulations of CB1 receptor antagonists that do not enter the brain and hence do not exert undesirable side effects (68). Early attempts to accomplish this are currently underway, as such compounds would presumably circumvent development of the depressive-like symptoms associated with CB1 receptor antagonists that do enter the brain such as rimonabant and taranabant.
THE ECS AND GLUCOSE METABOLISM
Obesity may impair glucose homeostasis, particularly when it is associated with fat accumulation in the abdominal cavity, liver, or skeletal muscle compartments (69). Overweight subjects with type 2 diabetes and hyperglycemia reportedly have significantly higher plasma endocannabinoid levels compared with those of age- and BMI-matched normoglycemic subjects (41). Cell-culture experiments from the same report demonstrated that a high-glucose concentration is associated with increased endocannabinoid levels. By contrast, selective blockade of the CB1 receptor ameliorated abnormalities in glucose and insulin metabolism in mice fed a high-fat diet (70). In another study in mice, Liu et al. (71) found that the rate of glucose uptake by isolated soleus muscle was significantly increased in mice treated with rimonabant for 7 days compared with control mice, although it is unclear whether this effect was independent of changes in caloric intake and/or body weight. CB1 receptor antagonism is associated with increased expression of genes that are involved in glucose metabolism. Expression of phosphofructokinase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglycerate mutase was increased in adipose from diet-induced obese mice treated with rimonabant compared with diet-induced obese control mice (54). The importance of hepatic CB1 receptors in regulating insulin resistance and glucose intolerance was confirmed by Osei-Hyiaman et al. (67). Those investigators observed that impaired glucose tolerance and insulin sensitivity in diet-induced obese mice were attenuated in diet-induced obese mice that lacked functional CB1 receptors specifically in hepatocytes (67).
Adiponectin, a protein hormone secreted primarily from adipocytes (72), inhibits both the expression of gluconeogenic enzymes and the rate of gluconeogenesis (73). CB1 receptor stimulation decreased adiponectin expression in adipocytes (45) while conversely, the treatment of mouse adipocytes with rimonabant was associated with significantly increased levels of adiponectin mRNA compared with control cells (32). Moreover, CB1 receptor antagonism with rimonabant increased adiponectin levels in both diet-induced obese mice (74) and genetically obese rats; effects associated with favorable changes in serum insulin and glucose levels (32,74). Although no association was observed between expression of the CB1 receptor gene in subcutaneous or omental fat and levels of adiponectin, or between CB1 receptor expression and plasma adiponectin, in one report of obese and nonobese subjects (75), a significant correlation was observed between plasma levels of adiponectin and of 2-AG in another (57). Furthermore, by using mice lacking adiponectin, it was recently reported that rimonabant improves insulin sensitivity via adiponectin-dependent as well as adiponectin-independent mechanisms (76).
Clinical Trials of a CB1 Receptor Antagonist
Rimonabant is the first in a new class of agents that are selective CB1 receptor antagonists (77). The Rimonabant in Obesity (RIO) program consisted of four phase III randomized controlled trials (RIO-Europe, RIO-Lipids, RIO-North America, and RIO-Diabetes). A total of 6,635 obese (BMI ≥30 kg/m2) or overweight (BMI ≥27 kg/m2) adult men and women were randomized to receive double-blind treatment with rimonabant 5 or 20 mg/day or placebo, together with diet and/or lifestyle therapy, for 1 or 2 years (78–81). All subjects were instructed to follow a hypocaloric diet (600 kcal/day deficit). Subjects in the RIO-Diabetes and RIO-Lipids trials had type 2 diabetes or untreated dyslipidemia, respectively. The RIO program was conducted in >300 centers in North America, Europe, South America, and Australia. Results from the RIO program were consistent: weight loss after 1 year of treatment with rimonabant 20 mg/day was significantly greater compared with lifestyle therapy alone (Table 2). The average placebo-subtracted changes from baseline body weight ranged from 3.9 to 5.4 kg (82). Weight loss with 5 mg/day of rimonabant was not different from placebo.
Table 2.
Mean placebo-subtracted 1-year weight loss with rimonabant
| Trial | Weight loss (kg) |
|---|---|
| RIO-Europe | 4.7 |
| RIO-Lipids | 5.4 |
| RIO-North America | 4.7 |
| RIO-Diabetes | 3.9 |
RIO, Rimonabant in Obesity.
Adapted from Gadde and Allison (82).
After adjustment for body-weight loss, rimonabant 20 mg appeared to have a direct effect on increasing high-density lipoprotein–cholesterol (HDL-C) and adiponectin and reducing triglycerides due to direct CB1 receptor antagonism on peripheral tissues (83). Decreases independent of body-weight loss were also seen for hemoglobin A1c in obese patients with type 2 diabetes and for fasting insulin in obese patients without diabetes. Regression analysis of change in HDL-C, triglycerides, adiponectin, and hemoglobin A1c vs. body weight at 1 year by analysis of covariance suggested that 45–57% of the effects of rimonabant 20 mg/day could not be explained by the observed weight loss (Table 3). Human pair-feeding studies are needed to confirm the degree to which these effects are independent of weight loss.
Table 3.
Summary of results for primary analysis of metabolic parameters with and without adjustment for body-weight (wt) loss, mean (s.e.m.)
| Parameter | Overall treatment effect, β1 | Effect independent of wt loss, β | %Overall effect not explained by wt, β/β1 (%) |
|---|---|---|---|
| HDL-C (%) | 8.0 (0.6) | 3.6 (0.6) | 45 |
| P < 0.001 | P < 0.001 | ||
| Triglycerides (%) | −14.0 (1.4) | −6.5 (1.4) | 46 |
| P < 0.001 | P < 0.001 | ||
| HbA1c (%) | −0.67 (0.007) | −0.37 (0.007) | 55 |
| P < 0.001 | P < 0.001 | ||
| Fasting insulin (μU/ml) | −2.74 (0.48) | −1.34 (0.51) | 49 |
| P < 0.001 | P = 0.018 | ||
| Adiponectin (μg/ml) | 1.5 (0.2) | 0.85 (0.21) | 57 |
| P < 0.001 | P < 0.001 |
HDL-C, high-density lipoprotein-cholesterol.
Data from ref. 83.
In the RIO-North America study, subjects who received rimonabant 20 mg/day during year 1 and were switched to placebo during year 2 regained weight back to the baseline level, whereas those who continued to receive rimonabant 20 mg/day maintained their weight loss (78). In RIO-Lipids, rimonabant 20 mg/day was associated with significant weight loss, reduction in waist circumference, increase in HDL-C, and reduction in plasma triglycerides (Figure 2). In addition, treatment with rimonabant (20 mg/day for 1 year) resulted in a significant increase in plasma adiponectin level compared with subjects in the placebo group (79). Clinical trials with other types of weight-loss pharmacotherapy have also demonstrated changes in some metabolic parameters. Treatment with orlistat, a reversible inhibitor of lipases, was associated with reduced levels of low-density lipoprotein–cholesterol, presumably due to fecal fat loss induced by the drug (84). Treatment with sibutramine, a norepinephrine and serotonin reuptake inhibitor, was associated with an increased HDL-C, which may have been partially independent from the effects of weight loss (85).
Figure 2.
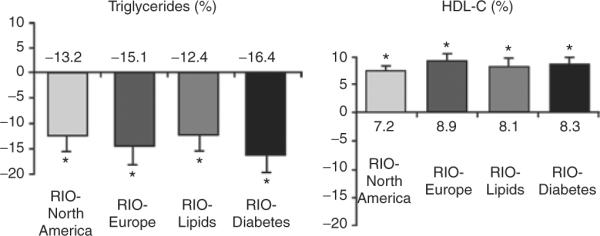
Rimonabant in Obesity (RIO) study: placebo-subtracted changes in atherogenic plasma lipoproteins. Data from the intent-to-treat population, last observation carried forward. From Pi-Sunyer et al. (78); Després et al. (79); Scheen et al. (80); Van Gaal et al. (81). HDL-C, high-density lipoprotein-cholesterol.
In RIO-Diabetes, rimonabant treatment led to significant decreases in body weight and improvements in glycemic control compared with placebo (Table 3) (80). The favorable change in mean HbA1c was observed in subjects receiving metformin or sulfonylureas, suggesting that rimonabant may have additive effects to standard, background oral antidiabetic therapy.
The overall withdrawal rate due to adverse events in the RIO studies was 13.8% of patients receiving rimonabant 20 mg and 7.2% receiving placebo (83). Adverse events leading to study discontinuation over 1 year of treatment in subjects treated with rimonabant 20 mg/day included depressive disorders, nausea, anxiety, and dizziness.
Rimonabant 20 mg/day was associated with about a twofold increase in the risk of psychiatric adverse events (86), including anxiety, depressed mood, and sleep disturbances (Table 4). Subjects treated with rimonabant 20 mg/day reported significantly more serious adverse effects (87), most commonly depression and anxiety. It is important to note that individuals with a history of severe depression (defined as depression leading the patient to be hospitalized, or having two or more recurrent episodes of depression, or a history of suicide attempt) or other psychiatric disorders, or who had recently used antidepressant medications, were excluded from the RIO studies.
Table 4.
Relative risk of psychiatric adverse event—rimonabant 20 mg vs. placebo
| RIO study | RR |
|---|---|
| RIO-Europe | 1.5 |
| RIO-Lipids | 2.5 |
| RIO-North America | 1.7 |
| RIO-Diabetes | 2.1 |
RIO, Rimonabant in Obesity; RR, relative risk.
Data from ref. 86.
Results from two clinical studies with taranabant were reported at the 2008 meeting of the European Association of Atherosclerosis (88,89). In the 12-week study, treatment with taranabant significantly increased weight loss and reduced waist circumference at doses of 2 and 4 mg/day (88). Psychiatric adverse events were reported in 18.1, 27.5, and 31.3% in the placebo, 2-, and 4-mg/day groups, respectively. In a 1-year report from an ongoing 2-year single-blind, placebo-controlled study, 2,502 obese adult subjects completed 52 weeks of treatment (89). Compared with placebo, treatment with taranabant significantly increased weight loss, reduced waist circumference, reduced plasma triglycerides, and increased HDL-C at doses of 2 and 4 mg/day. Study discontinuations due to psychiatric adverse events were 4.6, 8.9, and 12.5% for the placebo (n = 417), 2-mg/day (n = 414), and 4-mg/day (n = 415) groups, respectively (89). The doses of taranabant that were efficacious at reducing body weight in both of these studies produced psychiatric adverse events to a similar extent to those observed in the RIO trials (88,89).
Data from preclinical and human postmortem studies are equivocal with regard to the effect of CB1 receptor antagonism and emotional responses to stress (reviewed in Gadde and Allison) (82). Additional studies are needed to determine the potential effects of rimonabant treatment on adverse events in different patient populations. In particular, clinical studies are needed to determine the risk–benefit profile of rimonabant with regard to psychiatric adverse events. Based on the data available, the Food and Drug Administration ruled against approval of rimonabant due to lack of safety data in people with depression (86). By contrast, rimonabant was approved by the European Medicines Agency in June 2006 (90). However, the European Medicines Agency later recommended that rimonabant was contraindicated in patients with ongoing major depression and in patients being treated with antidepressants (90,91), and most recently, the European Medicines Agency recommended sanofi-aventis to stop the sales of rimonabant. The company complied and also terminated their ongoing trials, including CRESCENDO (Comprehensive Rimonabant Evaluation Study of Cardiovascular Endpoints and Outcomes), a cardiovascular outcomes trial (ClinicalTrials.gov identifier NCT00263042) (92). Shortly afterward, both Merck and Pfizer announced that they were ending development of their CB1 receptor antagonists because of its adverse effects.
The effects of antiobesity drug treatments on cardiovascular morbidity and mortality are not yet known. An ongoing trial, SCOUT (Sibutramine Cardiovascular Outcomes Trial) will assess the effects of sibutramine on cardiovascular events and mortality (ClinicalTrials.gov identifiers NCT00234832). STRADIVARIUS (Strategy to Reduce Atherosclerosis Development Involving Administration of Rimonabant—The Intravascular Ultrasound Study) was a randomized, double-blind, placebo-controlled trial comparing rimonabant 20 mg/day vs. placebo in 839 patients with coronary disease, abdominal obesity, and the metabolic syndrome. There was no significant effect of rimonabant treatment on the change in percent atheroma volume (primary efficacy variable). However, the percentage change in normalized total atheroma volume (secondary efficacy variable) decreased by 2.2 mm3 (95% confidence interval −4.09 to −0.24) in the rimonabant group and increased by 0.88 mm3 (95% confidence interval −1.03 to 2.79) in the placebo group (P = 0.03) (93). Of note, when subjects were divided into subgroups with plasma triglycerides above and below the median level of 140 mg/dl, there was a significant benefit of rimonabant on the primary end point of percent atheroma volume in the group with triglycerides above the median, suggesting possible greater benefit in subjects who were more typical of metabolic syndrome patients (94). However, the latter analysis was one of 14 post hoc subgroup analyses, of which only one other was positive.
Summary
The ECS affects food intake, energy balance, and both lipid and glucose homeostasis. All of these processes, when excessive or perturbed, may contribute to cardiovascular and metabolic risk factors. The ECS appears to modulate metabolic pathways through the actions of the CB1 receptor, which is expressed in a number of organs and tissues involved in energy balance, glucose homeostasis, and lipid storage. Preclinical studies suggest that the clinical effects of rimonabant may involve antagonism of CB1 receptors not only in the brain, but also in liver and adipose tissue, leading to reduced lipid storage and enhanced glucose sensitivity. Additional studies are needed to differentiate the metabolic effects of CB1 receptor antagonism that are independent of weight loss. The efficacy of rimonabant for the management of obesity and related risks was demonstrated in the RIO program; here, rimonabant 20 mg/day consistently produced greater weight loss and improvements in glucose and lipid profiles than placebo. Future studies, such as determining the phenotypes of different types of tissue-specific CB1 receptor knockout mice, use of compounds with selective central or peripheral actions, and clinical trials with a pair-feeding paradigm, will provide important details about how ECS biology is linked to the clinical effects of CB1 receptor antagonism.
ACKNOWLEDGMENTS
H.N.G. and S.C.W. take responsibility for the integrity of the manuscript. Editorial support for this manuscript was provided by Scientiae, LLC, and sanofi-aventis US. Critical revision of the manuscript for intellectual content was done by H.N.G. and S.C.W. The sponsor was permitted to review the manuscript and suggest changes, but the authors made final decisions on the content. Funding for this article was provided by a grant from sanofi-aventis US to Scientiae, LLC.
DISCLOSURE H.N.G. has received grant/research support from Pfizer, Reliant, Takeda, and sanofi-aventis, and has been a consultant for Abbott, Amylin, Bristol-Myers Squibb, GlaxoSmithKline, Isis, Merck, Merck/Schering-Plough, Metabasis, Pfizer, Reliant, Roche, Sanofi-aventis, and Takeda. S.C.W. has received honoraria and has served on the speakers' bureaus for sanofi-aventis and Merck.
REFERENCES
- 1.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 2.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, Ishida-Takahashi R, Villanueva EC, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 5.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 6.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. doi: 10.1159/000115362. [DOI] [PubMed] [Google Scholar]
- 8.Pi-Sunyer FX. Use of lifestyle changes treatment plans and drug therapy in controlling cardiovascular and metabolic risk factors. Obesity (Silver Spring) 2006;14(Suppl 3):135S–142S. doi: 10.1038/oby.2006.293. [DOI] [PubMed] [Google Scholar]
- 9.Aronne LJ, Thornton-Jones ZD. New targets for obesity pharmacotherapy. Clin Pharmacol Ther. 2007;81:748–752. doi: 10.1038/sj.clpt.6100163. [DOI] [PubMed] [Google Scholar]
- 10.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 11.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 14.Placzek EA, Okamoto Y, Ueda N, Barker EL. Membrane microdomains and metabolic pathways that define anandamide and 2-arachidonyl glycerol biosynthesis and breakdown. Neuropharmacology. 2008;55:1095–1104. doi: 10.1016/j.neuropharm.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiura T, Kodaka T, Nakane S, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 16.Heimann AS, Gomes I, Dale CS, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1101–G1104. doi: 10.1152/ajpgi.00057.2008. [DOI] [PubMed] [Google Scholar]
- 18.Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Juan-Picó P, Fuentes E, Bermúdez-Silva FJ, et al. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium. 2006;39:155–162. doi: 10.1016/j.ceca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 22.Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura Y, Fukaya M, Maejima T, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring) 2006;14(Suppl 5):259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- 26.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombo G, Agabio R, Diaz G, et al. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 28.Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 29.Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavon FJ, Bilbao A, Hernández-Folgado L, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21. Neuropharmacology. 2006;51:358–366. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Gary-Bobo M, Elachouri G, Gallas JF, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 32.Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 33.Wiley JL, Burston JJ, Leggett DC, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Mathes CM, Ferrara M, Rowland NE. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R67–R75. doi: 10.1152/ajpregu.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melis T, Succu S, Sanna F, et al. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett. 2007;419:231–235. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 39.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dipatrizio NV, Simansky KJ. Inhibiting parabrachial fatty acid amide hydrolase activity selectively increases the intake of palatable food via cannabinoid CB1 receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1409–R1414. doi: 10.1152/ajpregu.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 43.Thornton-Jones ZD, Kennett GA, Benwell KR, et al. The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav. 2006;84:353–359. doi: 10.1016/j.pbb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- 45.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 46.Viveros MP, de Fonseca FR, Bermudez-Silva FJ, McPartland JM. Critical role of the endocannabinoid system in the regulation of food intake and energy metabolism, with phylogenetic, developmental, and pathophysiological implications. Endocr Metab Immune Disord Drug Targets. 2008;8:220–230. doi: 10.2174/187153008785700082. [DOI] [PubMed] [Google Scholar]
- 47.Burdyga G, Lal S, Varro A, et al. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 49.Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gary-Bobo M, Elachouri G, Scatton B, et al. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol. 2006;69:471–478. doi: 10.1124/mol.105.015040. [DOI] [PubMed] [Google Scholar]
- 52.Pagano C, Pilon C, Calcagno A, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92:4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
- 53.Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol. 2008;20(Suppl 1):124–129. doi: 10.1111/j.1365-2826.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- 54.Jbilo O, Ravinet-Trillou C, Arnone M, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- 55.Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W. Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 2008;32:863–870. doi: 10.1038/ijo.2008.3. [DOI] [PubMed] [Google Scholar]
- 56.Addy C, Wright H, Van Laere K, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 58.Di Marzo V, Côté M, Matias I, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- 59.Engeli S, Böhnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH) Int J Obes (Lond) 2005;29:755–759. doi: 10.1038/sj.ijo.0802954. [DOI] [PubMed] [Google Scholar]
- 61.Jensen DP, Andreasen CH, Andersen MK, et al. The functional Pro129Thr variant of the FAAH gene is not associated with various fat accumulation phenotypes in a population-based cohort of 5,801 whites. J Mol Med. 2007;85:445–449. doi: 10.1007/s00109-006-0139-0. [DOI] [PubMed] [Google Scholar]
- 62.Russo P, Strazzullo P, Cappuccio FP, et al. Genetic variations at the endocannabinoid type 1 receptor gene (CNR1) are associated with obesity phenotypes in men. J Clin Endocrinol Metab. 2007;92:2382–2386. doi: 10.1210/jc.2006-2523. [DOI] [PubMed] [Google Scholar]
- 63.Monteleone P, Matias I, Martiadis V, et al. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–1221. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- 64.Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1101–G1104. doi: 10.1152/ajpgi.00057.2008. [DOI] [PubMed] [Google Scholar]
- 65.Muñoz-Luque J, Ros J, Fernández-Varo G, et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008;324:475–483. doi: 10.1124/jpet.107.131896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Osei-Hyiaman D, Liu J, Zhou L, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kunos G, Osei-Hyiaman D, Bátkai S, Sharkey KA, Makriyannis A. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120(2 Suppl 1):S3–S8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29–S32. [DOI] [PubMed] [Google Scholar]
- 70.Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 71.Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- 72.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 73.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poirier B, Bidouard JP, Cadrouvele C, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 75.Lofgren P, Sjolin E, Wahlen K, Hoffstedt J. Human adipose tissue cannabinoid receptor 1 gene expression is not related to fat cell function or adiponectin level. J Clin Endocrinol Metab. 2007;92:1555–1559. doi: 10.1210/jc.2006-2240. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe T, Kubota N, Ohsugi M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284:1803–1812. doi: 10.1074/jbc.M807120200. [DOI] [PubMed] [Google Scholar]
- 77.Boyd ST, Fremming BA. Rimonabant—a selective CB1 antagonist. Ann Pharmacother. 2005;39:684–690. doi: 10.1345/aph.1E499. [DOI] [PubMed] [Google Scholar]
- 78.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 79.Després JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 80.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 81.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 82.Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation. 2006;114:974–984. doi: 10.1161/CIRCULATIONAHA.105.596130. [DOI] [PubMed] [Google Scholar]
- 83.Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl 2):S229–S240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- 84.Bray GA. Etiology and pathogenesis of obesity. Clin Cornerstone. 1999;2:1–15. doi: 10.1016/s1098-3597(99)90001-7. [DOI] [PubMed] [Google Scholar]
- 85.Finer N, Pagotto U. The endocannabinoid system: a new therapeutic target for cardiovascular risk factor management. Br J Diabetes Vasc Dis. 2005;5:121–124. [Google Scholar]
- 86.Food and Drug Administration [Accessed 9 August 2007];FDA Briefing Document. NDA 21-888. Zimulti (rimonabant) tablets, 20 mg. Sanofi Aventis. Advisory Committee, 13 June 2007. < http://www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4306b1-fda-backgrounder.pdf>.
- 87.Curioni C, André C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006:CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carr R, Erondu N, Gonzalez C, et al. Effects of taranabant, a novel cannabinoid 1 receptor (CB-1R) inverse agonist, on weight reduction in obese patients over 12 weeks. Atherosclerosis Suppl. 2008;9:209. [Google Scholar]
- 89.Carr R, Gantz I, Erondu N, et al. A two-year study to assess the efficacy, safety, and tolerability of taranabant in obese patients: 52 week results. Atherosclerosis Suppl. 2008;9:12. [Google Scholar]
- 90.European Medicines Agency recommends acomplia must not be used in patients on antidepressants or with major depression. Medical News Today, 21 July 2007. < http://www.medicalnewstoday.com>.
- 91.European Medicines Agency . The committee for medicinal products for human use: summary of product characteristics (acomplia) Approved 19 July 2007. [Google Scholar]
- 92.Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7:961–962. doi: 10.1038/nrd2775. [DOI] [PubMed] [Google Scholar]
- 93.Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- 94.Di Marzo V. Play an ADAGIO with a STRADIVARIUS: the right patient for CB1 receptor antagonists? Nat Clin Pract Cardiovasc Med. 2008;5:610–612. doi: 10.1038/ncpcardio1319. [DOI] [PubMed] [Google Scholar]
- 95.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]


