Abstract
Cartilage oligomeric matrix protein/thrombospondin 5 (COMP/TSP5) is a major component of the extracellular matrix (ECM) of the musculoskeletal system. Its importance is underscored by its association with several growth disorders. In this report, we investigated its interaction with aggrecan, a major component of cartilage ECM. We also tested a COMP/TSP5 mutant, designated MUT3 that accounts for 30% of human pseudoachon-droplasia cases, to determine if the mutation affects function. Using a solid-phase binding assay, we have shown that COMP/ TSP5 can bind aggrecan. This binding was decreased with MUT3, or when COMP/TSP5 was treated with EDTA, indicating the presence of a conformation-dependent aggrecan binding site. Soluble glycosaminoglycans(GAGs)partially inhibited binding, suggesting that the interaction was mediated in part through aggrecan GAG side chains. Using affinity co-electrophoresis, we showed that COMP/TSP5, in its calcium-replete conformation, bound to heparin, chondroitin sulfates, and heparan sulfate; this binding was reduced with EDTA treatment of COMP/TSP5. MUT3 showed weaker binding than calcium-repleted COMP/TSP5. Using recombinant COMP/TSP5 fragments, we found that the “signature domain” could bind to aggrecan, suggesting that this domain can mediate the interaction of COMP/TSP5 and aggrecan. In summary, our data indicate that COMP/TSP5 is an aggrecan-binding protein, and this interaction is regulated by the calcium-sensitive conformation of COMP/TSP5; interaction of COMP with aggrecan can be mediated through the GAG side chains on aggrecan and the “signature domain” of COMP/TSP5. Our results suggest that COMP/TSP5 may function to support matrix interactions in cartilage ECM.
Cartilage oligomeric matrix protein/thrombospondin 5 (COMP4/TSP5) is a pentameric extracellular matrix protein that is the fifth member of the thrombospondin (TSP) family (1, 2). COMP/TSP5 is abundantly expressed in the chondrocyte extracellular matrix, and is also found in bone, tendon, ligament, synovium, and blood vessels (3–8). Immunohistochemistry studies of cartilage have revealed specific temporal and spatial distribution patterns of COMP/TSP5. In fetal cartilage, COMP/TSP5 exists in the pericellular matrix of all cartilage, especially in the growth cartilage adjacent to the primary ossification center (9, 10). In adult articular cartilage, COMP/TSP5 is found mostly in interterritorial matrix, with stronger staining in the deeper zone (10–12).
Although the physiological function of COMP/TSP5 is still unclear, its functional importance is implicated by its association with several pathological conditions. The expression pattern and level are altered in patients with arthritis. Compared with normal adult cartilage where a higher level of expression is in the deeper cartilaginous zones, there is decreased COMP/TSP5 expression in the mid and deeper zones and stronger expression in the superficial fibrillated cartilage in osteoarthritic (OA) and rheumatoid arthritic (RA) patients (11). Quantitative analysis shows a net loss of COMP/TSP5 from arthritic cartilage as compared with normal cartilage. Furthermore, COMP/TSP5 protein that remains in the arthritic cartilage shows increased degradation as compared with the mostly intact COMP/TSP5 extracted from normal cartilage (11). Similar patterns of degradation products are also detected in serum and synovial fluids of OA and RA patients as well as patients with other forms of inflammatory joint diseases (11, 13). COMP/TSP5 levels and degradation products in serum and synovial fluid have been proposed to be used as indicators for cartilage destruction in arthritic conditions (11, 13, 14).
COMP/TSP5 mutations cause two human skeletal dysplasias: pseudoachondroplasia (PSACH), and multiple epiphyseal dysplasia (MED/EDM1) of the Fairbanks and Ribbing types (15–18) (OMIM entries 177170 and 132400). PSACH and MED/EDM1 differ in severity with the former presenting as a severe growth deficiency affecting cartilage and bone development. Both conditions are associated with gait abnormalities, and patients require hip replacement in the second or third decade of life. Marked dilation of the rough endoplasmic reticulum in growth plate chondrocytes has been observed ultra-structurally (19). COMP/TSP5, type IX collagen, aggrecan, and possibly link protein have been reported to be retained in the large rER cisternae (20–24). In this investigation, we have studied the function of MUT3, a COMP/TSP5 single amino acid deletion (D469del) mutation accounting for 30% of the PSACH patients.
Although immunohistochemical data and COMP/TSP5 mutations in PSACH and EDM1 suggest that COMP/TSP5 is important in cartilage, its precise functional role is currently unclear. Cartilage ECM contains three classes of proteins: collagens, proteoglycans, and other noncollagenous proteins including COMP/TSP5 (25). Of these major components of cartilage, COMP/TSP5 can interact with type II and type IX collagens in a zinc-dependent fashion via its C-globe region (26–28). Through this interaction, COMP/TSP5 is thought to play a direct role in regulating collagen fibril formation and maintaining collagen network integrity.
Structurally, COMP/TSP5 shares some of the structural domains that are also found in the other TSP proteins (1, 2). It has the coiled-coil region and interchain disulfide bonds responsible for pentamer formation, four type 2 repeats, seven highly conserved type 3 repeats that consist of 13 potential calcium binding loops, and a C-terminal globular domain (C-globe region) (1, 2). COMP/TSP5 lacks the two domains that function as heparin binding sites within TSP1, and it has been reported previously that COMP/TSP5 did not bind to heparin or purified proteoglycans (26, 29, 30).
We and others (28, 31) have shown previously that COMP/ TSP5 is a calcium-binding protein, and that COMP/TSP5 structure depends on its calcium binding ability. Mutations in the type 3 calcium binding repeats affect its ability to fold correctly into the mature conformation. Thus, calcium binding is important for maintaining the proper structure of COMP/ TSP5. According to sequence comparison and structural analysis, the deleted amino acid residue in MUT3 D469 is involved in calcium coordination, and deletion of this residue is expected to interfere with the calcium binding capacity and disrupt the calcium-sensitive conformation of COMP/TSP5 (32). In deed, the mutant protein has been shown to possess decreased calcium binding capacity with altered conformation of the molecule (31). Furthermore, we have shown that the calcium-sensitive conformation of COMP/TSP5 affects its function to support chondrocyte cell attachment through integrins (33). In addition, we have noticed during our initial effort to purify COMP/TSP5 from conditioned medium in its native calcium replete conformation that it bound to heparin-Sepha-rose. This observation prompted us to examine whether COMP/TSP5 could interact with other glycosaminoglycans (GAGs) and GAG-containing proteoglycans, including aggrecan. We postulated that in previous studies when COMP/TSP5 was isolated from tissues, chelation of calcium with EDTA during the purification procedures may have rendered the purified COMP/TSP5 in a calcium-depleted conformation. This led to results that may not reflect the function of COMP/TSP5 in cartilage extracellular matrix which is in a calcium replete environment (1, 29, 30, 34). Therefore, in this study, we have re-assessed the function of COMP/TSP5 purified in the presence of calcium and investigated whether COMP/TSP5 can interact with one of the major components of cartilage, aggrecan.
MATERIALS AND METHODS
Proteins and Reagents
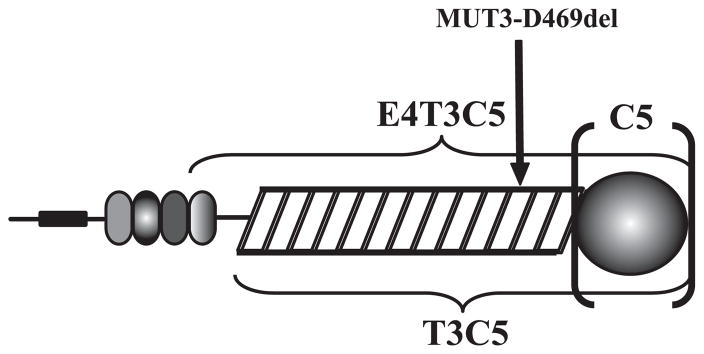
COMP/TSP5 and MUT3 were produced and purified as reported previously (31). Recombinant COMP/TSP5 fragments were produced in Drosophila S2 cells as described in detail under supplemental data. These fragments included: 1) the last type 2 repeat, the type 3 repeats, and the C-terminal domain (E4T3C5), 2) the type 3 repeats and the C-terminal domain (T3C5), or 3) just the C-terminal domain (C5) (Fig. 1). Aggrecan and monoclonal antibody against chondroitin sulfate were purchased from Sigma. Monoclonal antibody against keratin sulfate I22 was from Developmental Studies Hybridoma Bank (Iowa City, IA). Monoclonal antibody against aggrecan core protein is from R&D Systems (Minneapolis, MN). GAGs used for ACE experiments included porcine intestinal heparin, porcine skin dermatan sulfate (DS), bovine corneal keratan sulfate (KS), shark cartilage chondroitin sulfate (chondroitin-6-sulfate; C6S), bovine tracheal chondroitin sulfate (chondroitin-4-sulfate; C4S) from Sigma, and bovine kidney heparan sulfate (HS) from ICN.
FIGURE 1. Schematic presentation of recombinant COMP/TSP5 fragments.
Recombinant fragments of COMP/TSP5 include E4T3C5 that spans the last type 2 repeat (oval modules), the type 3 repeats (rectangles), and the C-terminal domain (globe), and T3C5 that includes the type 3 repeats and the C-terminal domain, and C5, which is composed of just the C-terminal domain. The site of mutation that gives rise to MUT3 (D469del) is also indicated.
Affinity Co-electrophoresis (ACE) and Solid-phase Binding Assays
ACE assay was carried out as described before (35–38). The detailed method is given under supplemental data. For the solid-phase binding assay, purified COMP/TSP5 was coated at 5 μg/ml on Immulon II 96-well plates (Thermo Lab-systems) in HEPES-buffered saline (HBS: 10 mM HEPES pH 7.2, containing 5 mM KCl and 140 mM NaCl) with 2 mM CaCl2 (HBS/C) at 4 °C overnight. Extra binding sites were blocked with heat-inactivated BSA (HI-BSA). Aggrecan at various concentrations in HBS/C was added to the COMP/TSP5-coated wells and incubated at 22 °C for 2 h with gentle agitation. After extensive washing with HBS/C, the monoclonal antibody CS-56 against chondroitin sulfate (Sigma) was added to wells, followed by horseradish peroxidase-conjugated goat anti-mouse IgG (ICN). The steps above were performed with buffers containing 2 mM Ca2+. Chromogenic substrate ABTS was added to the wells, and color development was recorded on a microplate reader (Tecan). Wells coated with HI-BSA, but otherwise treated the same, were considered as background. To investigate the binding of COMP/TSP5 fragments to aggrecan, COMP/TSP5 at 7.5 μg/ml, or its fragments at equal molar concentrations, were coated, and the binding assay was performed as above, except that monoclonal antibody against keratin sulfate was used. Where the interaction of aggrecan with COMP/ TSP5, depleted of calcium ions, was studied, after coating of COMP/TSP5 in the presence of HBS/C, the wells were incubated with HBS containing 5 mM EDTA (HBS/E) for 30 min at 22 °C, followed by extensive washing with HBS/C. Other steps were carried out as described above. For competition studies, after HI-BSA blocking, the protein-coated wells were incubated with various GAG solutions at 0.5 mg/ml in HBS/C for 60 min at 37 °C. The plates were then washed with HBS/C and continued with aggrecan incubation and development as described above.
RESULTS
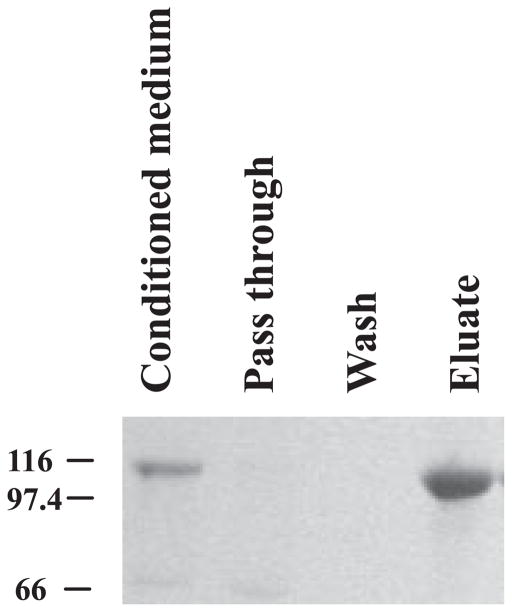
In our initial attempt to purify COMP/TSP5 from the conditioned medium, we found that COMP/TSP5 in the serum-free conditioned medium can be retained quantitatively by heparin-Sepharose (Amersham Biosciences). COMP/TSP5 remained bound on the heparin-Sepharose column with a wash solution of TBS containing 0.20 M NaCl, 2 mM CaCl2 in 10 mM Tris, pH 7.4. This indicates that COMP/TSP5 can bind to heparin under physiological salt concentration. COMP/TSP5 can be eluted from the column with TBS/C containing 0.55 M NaCl (Fig. 2).
FIGURE 2. Binding of COMP/TSP5 to heparin-Sepharose.
Serum-free COMP/TSP5-conditioned medium was loaded on to a heparin-Sepharose column. Unbound material was collected as flow-through. After binding, the column was washed with TBS/C containing 0.15 M NaCl. The bound material was eluted with TBS/C containing 0.55 M NaCl.
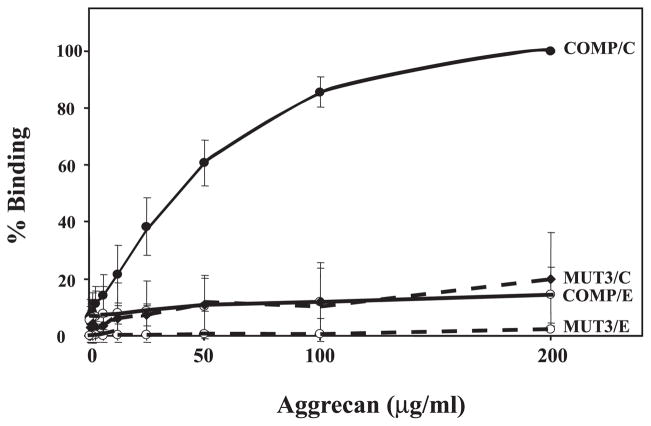
The binding to heparin suggests the presence of functional GAG binding sites in COMP/TSP5. Because aggrecan, one of the major components of cartilage, have abundant GAG side chains of chondroitin sulfate (~100 side chains/core protein) and keratan sulfate (~50 side chains/core protein), we investigated the interaction of COMP/TSP5 with aggrecan using a solid-phase binding assay. Direct binding of aggrecan to COMP/TSP5 was demonstrated using an enzyme-linked immunosorbant assay with COMP/TSP5 in its native calcium-replete state. Using this assay, we showed that aggrecan can bind to COMP/TSP5 in a concentration-dependent manner (Fig. 3). Binding became saturated at aggrecan concentrations of ~100 μg/ml. Positive binding of aggrecan to COMP/TSP5 was shown with both antibodies against the GAG side chains of chondroitin sulfate (shown in Fig. 3) or keratin sulfate (see below, Fig. 6), or against the core protein (not shown). This suggests that the whole aggrecan molecule, but not merely the side chains, bind to COMP/TSP5.
FIGURE 3. Binding of COMP/TSP5, and MUT3 to aggrecan.
The solid-phase binding assay was carried out as described under “Materials and Methods” using antibody against CS. COMP/TSP5 or MUT3 was coated in the presence of calcium. Half of the samples were kept in the calcium-replete conformation while the other half was treated with EDTA before adding aggrecan. Maximal binding of COMP/TSP5 to aggrecan in the presence of calcium ions was set as 100%. Data are expressed as % maximal binding. Shown are the average data of three experiments.
FIGURE 6. Binding of COMP/TSP5 fragments to aggrecan molecule.
The solid-phase binding assay was carried out as described in the legend to Fig. 3 except that antibody against KS was used in this experiment. Proteins were coated in the presence of 2 mM CaCl2. Shown are the average ±S.D.
We have previously shown that COMP/TSP5 is a calcium-binding protein and that calcium binding affects its conformation (28, 31). In addition, mutations in the type 3 calcium binding repeats affect its ability to fold correctly into the mature conformation, and its ability to support chondrocyte attachment (33). Thus, calcium binding is important for maintaining the proper structure and function of COMP/ TSP5. To determine whether the conformation of the type 3 calcium binding repeats could affect the binding of COMP/ TSP5 to aggrecan, and to determine whether the mutation found in MUT3 affects this function, we have used COMP/ TSP5 with calcium chelated from the molecule and MUT3 for the binding assay. When we chelated calcium ions from COMP/TSP5 with EDTA after coating, aggrecan binding to COMP/TSP5 was greatly diminished (Fig. 3). These data suggested an aggrecan binding site in COMP/TSP5 that was also sensitive to its calcium binding-dependent conformation. In agreement, MUT3 showed greatly reduced interaction with aggrecan in this solid-phase binding assay, regardless of whether MUT3 was in the calcium-replete or calcium-depleted conformation (Fig. 3).
Our data showing COMP/TSP5 binding to heparin indicated a functional GAG binding capacity of COMP/TSP5. We therefore investigated whether COMP/TSP5 could bind to other GAGs directly. To evaluate whether COMP/TSP5 is a GAG-binding protein, and to determine whether the conformation of the type 3 calcium binding repeats could affect the functions of COMP/TSP5, we tested the interaction of COMP/TSP5 with common GAGs found in cartilage, tendon, and ligament in the presence of either calcium or EDTA. ACE was used to quantify the binding of COMP/TSP5 to heparin and other GAGs derived from various tissue sources, including chondroitin sulfates from cartilage. The results of these analyses are presented in Table 1, and representative ACE gel images are shown in Fig. 4. In the presence of 2 mM Ca2+, COMP/TSP5 bound low molecular weight heparin with an average Kd value of 88.5 nM. This is similar to the Kd value previously determined for the binding of heparin to human platelet TSP1 (41 nM) (39). This was in agreement with our observation that COMP/ TSP5 could be purified from conditioned medium using heparin-Sepharose.
TABLE 1. Binding of COMP/TSP5 to glycosaminoglycans.
ACE was carried out and values of Kd measured as described in the supplemental data. Where data are reported as greater than a given value, this indicates that no electrophoretic retardation was observed at any protein concentration. An estimate of the minimum value of Kd was made based on the assumption that the protein concentration [P]tot required for just detectable electrophoretic retardation of GAG should occur at a value of [P]tot/Kd that exceeds the highest value of [P]tot/Kd at which no retardation was observed when heparin was used (0.04 for COMP/TSP5). Thus, minimum values are 25 times the highest concentration tested for COMP/ TSP5.
| GAG | COMP, Kd in 2 mM Ca2+ | COMP, Kd in 5 mM EDTA | MUT3, Kd in 2 mM Ca2+ | MUT3, Kd in 5 mM EDTA |
|---|---|---|---|---|
| Heparin | ||||
| Porcine intestine <6 kDa | 73, 104 nM | 477 nM | 121, 134 nM | 404 nM |
| C4S | ||||
| Bovine trachea | 530, 636 nM | –a | 1.04 μM | – |
| C6S | ||||
| Shark cartilage | 325, 481 nM | >10 μM | 546, 604 nM | >12 μM |
| DS | ||||
| Porcine skin | 325, 346 nM | – | 504, 621 nM | – |
| HS | ||||
| Bovine kidney | 1.2 μM | – | >12 μM | – |
| KS | ||||
| Bovine cornea | >10 μM | – | >12 μM | – |
A dash indicates that the value was not determined.
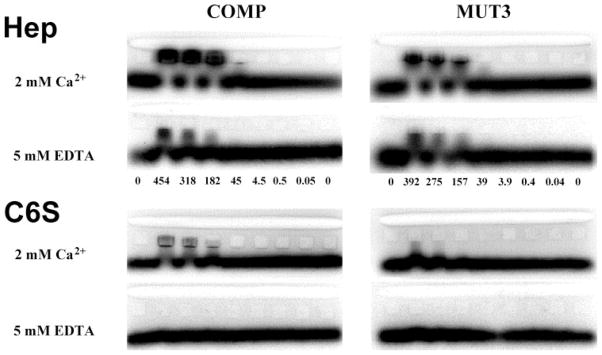
FIGURE 4. Affinity co-electrophoresis of COMP/TSP5 with heparin and chondroitin 6-sulfate.
ACE was carried out as described under supplemental data. Proteins were in solutions containing 2 mM CaCl2, or 5 mM EDTA as indicated in the figure. Concentrations of COMP/TSP5 or MUT3 embedded in each lane are indicated between the gels in nM. Radiolabeled heparin or C6S was loaded into the slot lane at the top of the gel. Where there is an interaction of the protein with the labeled GAGs, the migration of the GAGs is retarded during electrophoresis. The Kd values are calculated as described in the text as a function of the protein concentration and the electrophoretic mobility shift of GAGs in each lane.
To better ascertain potential roles of COMP/TSP5 in relation to GAGs and proteoglycans that are present in cartilage, tendon, and ligament, heparan sulfate, keratan sulfate, and three chondroitin sulfates (C6S, C4S, and DS) were tested for binding to COMP/TSP5 in the presence of 2 mM calcium using ACE (Table 1). Our data revealed that COMP/TSP5 bound to the three chondroitin sulfates with comparable affinities, with average Kd values ranging from 336 to 583 nM, while binding to HS was weaker (1.2 μM). COMP/TSP5 did not bind to keratan sulfate (Table 1).
To further determine whether the conformation of calcium binding was important for GAG binding, COMP/TSP5 was tested for binding to heparin and C6S in the presence and absence of calcium (Fig. 4 and Table 1). Fig. 4 shows four representative ACE gels, comparing binding of heparin and C6S in 2 mM calcium and in 5 mM EDTA. The Kd values measured from these gels are included in Table 1. These results demonstrated that depletion of calcium reduced COMP/TSP5 affinity for both heparin and C6S. These data suggest the existence of a conformation-dependent CS binding site in the type 3 repeats and/or C-terminal domain of COMP/TSP5.
To determine if the PSACH mutation in the type 3 calcium binding repeats affects GAG binding, we measured the binding of MUT3 to the various GAGs in the presence of 2 mM calcium or 5 mM EDTA (Table 1 and Fig. 4). MUT3 was able to bind heparin with a Kd value of 128 nM in the presence of calcium. MUT3 also bound chondroitin sulfates, albeit more weakly than did COMP/TSP5, with average Kd values ranging from 563 nM to 1.04 μM. MUT3 exhibited no discernible binding to HS, or keratan sulfate. Treatment of MUT3 with EDTA also decreased its affinity for heparin by ~3-fold, and abolished its binding to C6S (Fig. 4 and Table 1).
The above data suggest that COMP/TSP5 can bind to aggrecan, probably through its chondroitin sulfate, but not its keratin sulfate side chains. To ascertain that the COMP/TSP5 interaction with the GAG side chains did contribute to COMP/TSP5 binding to aggrecan, we next tested the effect of GAGs on the binding of COMP/TSP5 with aggrecan. Both C4S and C6S were able to inhibit the binding of COMP/TSP5 to aggrecan (Fig. 5). In addition, heparin, which was shown in our ACE assay to be able to bind COMP/TSP5, also inhibited the binding of COMP/TSP5 to aggrecan (Fig. 5). The level of inhibition obtained with heparin, C6S and C4S paralleled the affinity of COMP/TSP5 for these GAGs as measured by ACE (Table 1).
FIGURE 5. Competition of GAGs for aggrecan binding to COMP/TSP5.
The solid-phase binding assay was carried out as described in the legend to Fig. 3 with COMP/TSP5 coated in the presence of calcium. GAGs at a final concentration of 0.5 mg/ml were incubated with coated COMP/TSP5 before aggrecan was added to the plate. The binding was expressed as % binding of COMP/TSP5 to aggrecan without any GAG competitors.
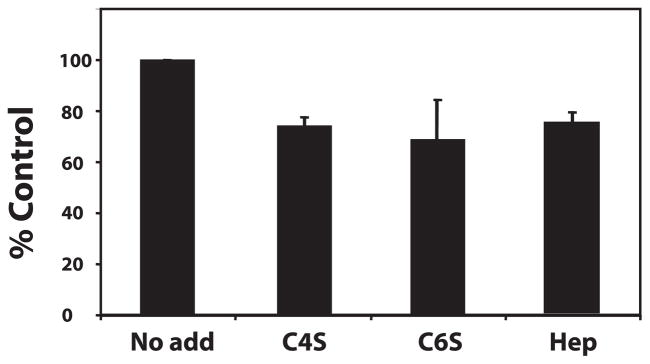
To determine which domain of COMP/TSP5 was responsible for aggrecan binding, we have expressed recombinant COMP/TSP5 fragments covering the various domains of this molecule. These included a fragment containing the fourth EGF repeat and the type 3 calcium binding and the C-globe domains (E4T3C5), a fragment containing the type 3 and the C-globe domains (T3C5), and a fragment containing the C-globe alone (C5, Fig. 1). These fragments were used in the solid-phase binding assay, in comparison to full-length COMP/TSP5. Our results showed that all these fragments, in addition to the full-length molecule, were able to bind to aggrecan, with the best binding obtained with the C-globe domain alone (Fig. 6). To probe whether this was caused by higher coating efficiency of the C-globe region to the wells, we have used an anti-V5 epitope and an anti-polyhistidine antibody for a direct ELISA assay, and found that C-globe fragment was not preferentially coated over the other fragments containing the type 3 repeats (results not shown). To further investigate the interaction of C5 with aggrecan, we determined if this interaction was sensitive to the presence of calcium ions, and if this interaction can be also mediated through aggrecan side chains. Using solid-phase ELISA assay, we found that chelating calcium ions with EDTA after coating of C5 onto the plate did not affect the binding. When GAGs, including heparin, C4S, or C6S were included in the assay, they were able to partially inhibit the binding of aggrecan to C5, with ~25% inhibition (Fig. 7).
FIGURE 7. Competition of GAGs for aggrecan binding to C5 of COMP/ TSP5.
The solid-phase binding assay was carried out as described in the legend to Fig. 3 with C5 coated in the presence of calcium at 2.5 μg/ml, which is equivalent in molar concentration to COMP/TSP5 at 7.5 μg/ml. GAGs at a final concentration of 0.5 mg/ml were incubated with coated C5 before aggrecan was added to the plate. The binding was expressed as % binding of aggrecan to C5 without any GAG competitors.
We also used heparin-Sepharose affinity chromatography to characterize T3C5 and C5 binding to GAGs. T3C5 bound to and was retained on heparin-Sepharose column. While the majority of COMP/TSP5 was eluted from the column at 0.55 M NaCl (Fig. 2), T3C5 could be eluted with 0.25 M NaCl (data not shown). The decrease in apparent affinity for heparin may be due to the fact that COMP/TSP5 is pentameric and T3C5 is monomeric. The C5 recombinant protein did not bind heparin-Sepharose (data not shown).
DISCUSSION
COMP/TSP5 is an extracellular matrix protein that is abundant in the musculoskeletal system including cartilage, tendon, ligament, and synovium. However, despite intensive studies on COMP/TSP5 and the fact that COMP/TSP5 is associated with several pathological conditions, its function in connective tissue remains uncertain. In our previous studies, we have shown that COMP/TSP5 is a calcium-binding protein, and its conformation is influenced by the amount of calcium bound by the protein (31). Because COMP/TSP5 under physiological conditions exists in an environment where the concentrations of calcium are in the mM range, we assessed the functions of COMP/TSP5 purified in a calcium replete conformation. We then asked if chelation of calcium ions affects COMP/TSP5 function. In our previous studies, we have shown that COMP/TSP5 can support chondrocyte attachment, and this function is sensitive to the conformation conferred by calcium binding to the molecule (33).
In cartilage, three groups of molecules, collagens, proteoglycans, and noncollagenous proteins interact with each other to form a highly organized and specialized connective tissue matrix. This matrix and the embedded chondrocytes are responsible for maintaining the health and conveying the physical and biomechanical properties of the tissue. Thus, articular cartilage function is dependent on the highly organized ECM structure. COMP/TSP5 is one of the major noncollagenous proteins in the cartilage ECM. It constitutes up to 1% of cartilage wet weight. Whereas elevated COMP/TSP5 levels in serum and synovial fluid are associated with some conditions of OA and RA, generally, a decreased amount of COMP/TSP5 remains in the cartilage (11, 13, 14). The site of expression is mostly in the superficial fibrillated cartilage of OA and RA patients compared with expression in the deeper zones of normal cartilage (11). Fragments generated by degradation of COMP/TSP5 have also been detected in serum, synovial fluid, and cartilage in these patients. These data suggest that the correct expression and distribution of COMP/TSP5, and inherently the correct interactions with its binding partners in the matrix may be important to the homeostasis of cartilage. It has been shown that COMP/TSP5 can interact with collagen, an abundant component of cartilage (26–28). In this study, we found that COMP/TSP5 interacts with the other major component of cartilage ECM, aggrecan. It is possible, based on our data and the data demonstrating the interaction of collagens, that due to its pentameric structure, COMP/TSP5 can serve to mediate interactions between different matrix molecules, and help to organize the cartilage matrix which can ultimately regulate the load bearing function of cartilage. Indeed, the expression of COMP/TSP5 seems to correlate with the function of tissues to support load. Articular cartilage, which experiences higher loading, expresses higher level of COMP/TSP5 (5 mg/g wet weight) than rib (0.26 mg/g wet weight) and tracheal cartilages (0.04 mg/g wet weight) that are not loaded as heavily (40). During mouse limb development, COMP/TSP5 expression coincides with the elaboration of a weight-bearing chondrocyte matrix (10). Tendons receiving the most load have the highest levels of COMP/TSP5 in the same animal (8). In a foal whose unilateral forelimb was rendered non-weight bearing, COMP/ TSP5 was expressed at considerably higher levels in the meta-carpophalangeal joint articular cartilage and digital flexor tendon in the weight-bearing limb as compared with the non-weight-bearing limb (8). The above observations suggest that in vivo, COMP/TSP5 is synthesized in response to load, and its presence may be necessary for cartilage and tendon to resist load.
In the course of this study, several important observations were made regarding the functions of the wild-type COMP/ TSP5. First, we have shown, for the first time, that COMP/TSP5 in its native conformation can bind aggrecan. Aggrecan is one of the major components of cartilage ECM. The ability of articular cartilage to resist compression is primarily due to the presence of aggrecan aggregates. The high density of fixed negative charge of the glycosaminoglycan chains draws water into the tissue, resulting in a large osmotic pressure (25). The balance between the osmotic pressure of the proteoglycans and the tension in the collagen fibers results in a highly specialized connective tissue well suited for bearing compressive loads. Cartilage tensile stiffness and strength, on the other hand, is determined primarily by the collagen network. Results from other laboratories have also shown that COMP/TSP5 can bind to the other major components of cartilage ECM collagens types II and IX (26–28). Through binding to these two major components, it is possible that COMP/TSP5 may function to organize cartilage ECM.
We have further shown that the “signature domain” (T3C5) can mediate the interaction of COMP/TSP5 to aggrecan. The x-ray crystallographic structure of the “signature domain” of TSP2 indicates that the type 3 repeats and the C-globe region fold together to form a single structural domain in the presence of calcium (32). Interestingly, our results show that the C-globe (C5) alone displays a high level of aggrecan binding that can be greater than intact COMP/ TSP5 (Fig. 6), and this binding was not affected by EDTA treatment of coated C5. By contrast aggrecan binding to the full-length COMP/TSP5 was the strongest when the molecule was in the calcium replete conformation. In addition, the interaction of COMP/TSP5 with aggrecan appears to involve the chondroitin sulfate side chains; however, the interaction of C5 with aggrecan is only slightly inhibited by soluble chondroitin sulfate. Finally, C5 does not bind heparin as measured by heparin-Sepharose affinity chromatography. Taken together, the data indicate that the binding of C5 to aggrecan does not accurately reflect the activity of the intact protein and is distinct in terms of mechanism from that of COMP/TSP5. This interpretation suggests that, in the calcium replete form of intact COMP/TSP5, the type 3 repeats mask a cryptic binding site for aggrecan in the C-globe and present a distinct binding site. This site may involve amino acid sequences from the type 3 repeats and/or the C-globe and require the correct folding of the “signature domain.” Unfortunately, we are not able to address the contribution of the type 3 repeats because this domain could not be expressed in our system. Because EDTA treatment of intact COMP/TSP5 diminishes its interaction with aggrecan, it appears that the aggrecan binding site in C5 remains cryptic under these conditions. The results underscore the importance of studying the structure and function of the members of the thrombospondin gene family in the presence of calcium.
In addition to binding to heparin, we report that COMP/ TSP5 also binds to chondroitin sulfates, including chondroitin-4- and -6-sulfates, and dermatan sulfate with comparable affinities. The affinities of COMP/TSP5 for chondroitin-4-and -6-sulfates are similar to those of TSP1 (39), suggesting the presence of physiologically relevant GAG binding sites in COMP/TSP5. Chondroitin sulfates are the major GAGs expressed in cartilage, tendon, and ligament. This suggests that COMP/TSP5 may be a functional chondroitin sulfate proteoglycan-binding protein in these tissues. Indeed, using direct binding assay, we have shown that COMP can bind aggrecan in a concentration-dependent and saturable fashion. Preparations of CSs can inhibit this interaction, indicating that the binding of COMP/TSP5 to aggrecan is at least in part through the interaction of COMP/TSP5 with the GAG side chains. Because commercially available CS preparations were used in this study, it is worth pointing out that these presumably C4S or C6S preparations are enriched in the respective CS only. For example, C4S from Sigma contains ~70% C4S with the remaining balance as C6S. Therefore we cannot definitively decide which CS is responsible for binding to COMP/TSP5. Collectively, our results suggest that COMP/TSP5 is a functional aggrecan-binding protein through interaction with its GAG side chains.
Our results also show that the affinity of full-length COMP/ TSP5 for GAGs is influenced by its calcium-dependent structure. In the presence of EDTA, COMP/TSP5 binding to chondroitin sulfate is abolished. Similarly, after chelating the calcium ions out of COMP/TSP5, binding to aggrecan is abolished. These data suggest the existence of a conformation-dependent CS binding site involving the type 3 repeats and C-globe of COMP/TSP5. In agreement, compared with COMP/TSP5 binding to GAGs in the presence of calcium, the affinity of MUT3 to GAGs was decreased in calcium replete conformation, and the affinity is further decreased when calcium ions are chelated from the molecule. Reflecting the decreased affinity of MUT3 with the GAGs, binding of MUT3 to aggrecan is greatly reduced as compared with the wild-type COMP/TSP5 in the presence calcium.
The fact that MUT3 had weaker binding to aggrecan may provide a mechanistic basis for the observation that mutations in the type 3 calcium binding repeats cause the phenotypes of PSACH and EDM1. In PSACH patients, COMP/ TSP5 is expressed normally in tendon and ligament ECM (7, 22, 23). Our data suggest that the portion of mutant COMP/ TSP5 that is secreted in cartilage, and the mutant COMP/ TSP5 present in tendon and ligament, may be functionally defective in its interaction with extracellular matrix, thus affecting the stability of the tissues. Decreased COMP/TSP5 interaction with GAGs may contribute to phenotypic abnormality in tendon and ligament that have been observed in PSACH patients.
In summary, we have shown that COMP/TSP5 interacts with aggrecan through its “signature domain” and the GAG side chains of aggrecan. We have further demonstrated the importance of calcium binding to the binding of COMP/TSP5 to aggrecan and shown that COMP/TSP5 mutations reduce this interaction. Our studies indicate that COMP/TSP5 has the ability to bind proteoglycans and may function in matrix-matrix interactions within cartilage and other musculoskeletal tissues. Through these interactions, COMP/TSP5 participates in the formation and maintenance of the architecture of the chondrocyte extracellular matrix.
Supplementary Material
Acknowledgments
We thank Mark Duquette for help with protein purification.
Footnotes
The abbreviations used are: COMP, cartilage oligomeric matrix protein; TSP, thrombospondin; ACE, affinity coelectrophoresis; C4S, chondroitin-4-sulfate; C6S, chondroitin-6-sulfate; DS, dermatan sulfate; MED, multiple epiphyseal dysplasia; GAG, glycosaminoglycan; HS, heparan sulfate; KS, keratan sulfate; PSACH, pseudoachondroplasia; PG, proteoglycan; BSA, bovine serum albumin; ECM, extracellular matrix.
This work was supported in part by Grants HL49081 from the NHLBI, National Institutes of Health (to J. L.) and 15955 from Shriner’s Hospital for Children (to J. T. H.).
The online version of this article (available at http://www.jbc.org) contains supplemental data.
References
- 1.Oldberg A, Antonsson P, Lindblom K, Heinegard D. J Biol Chem. 1992;267:22346–22350. [PubMed] [Google Scholar]
- 2.Newton G, Weremowicz S, Morton CC, Copeland NG, Gilbert DJ, Jenkins NA, Lawler J. Genomics. 1994;24:435–439. doi: 10.1006/geno.1994.1649. [DOI] [PubMed] [Google Scholar]
- 3.Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. J Orthop Res. 2000;18:713–720. doi: 10.1002/jor.1100180506. [DOI] [PubMed] [Google Scholar]
- 4.DiCesare PE, Carlson CS, Stollerman ES, Chen FS, Leslie M, Perris R. FEBS Lett. 1997;412:249–252. doi: 10.1016/s0014-5793(97)00789-8. [DOI] [PubMed] [Google Scholar]
- 5.Di Cesare PE, Hauser N, Lehman D, Pasumarti S, Paulsson M. FEBS Lett. 1994;354:237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 6.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegard D. J Biol Chem. 1992;267:6132–6136. [PubMed] [Google Scholar]
- 7.Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 8.Smith RK, Zunino L, Webbon PM, Heinegard D. Matrix Biol. 1997;16:255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 9.DiCesare PE, Morgelin M, Carlson CS, Pasumarti S, Paulsson M. J Orthop Res. 1995;13:422–428. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JM, Heinegard R, McIntosh A, Sterchi D, Barry FP. Matrix Biol. 1999;18:487–497. doi: 10.1016/s0945-053x(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 11.Di Cesare PE, Carlson CS, Stolerman ES, Hauser N, Tulli H, Paulsson M. J Orthop Res. 1996;14:946–955. doi: 10.1002/jor.1100140615. [DOI] [PubMed] [Google Scholar]
- 12.Di Cesare P, Chen F, Moergelin M, Carlson C, Leslie M, Perris R, Fang C. Matrix Biol. 2002;21:461–470. doi: 10.1016/s0945-053x(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 13.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Br J Rheumatol. 1997;36:1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 14.Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. Br J Rheumatol. 1998;37:46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- 15.Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES, Ceklenial JA, Knowlton RG, Cohn DH. Nat Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 16.Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M, Lawler J. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 17.Cohn DH, Briggs MD, King LM, Rimoin DL, Wilcox WR, Lachman RS, Knowlton RG. Ann N Y Acad Sci. 1996;785:188–194. doi: 10.1111/j.1749-6632.1996.tb56258.x. [DOI] [PubMed] [Google Scholar]
- 18.Briggs MD, Chapman KL. Hum Mutat. 2002;19:465–478. doi: 10.1002/humu.10066. [DOI] [PubMed] [Google Scholar]
- 19.Maynard JA, Cooper RR, Ponseti IV. Lab Investig. 1972;26:40–44. [PubMed] [Google Scholar]
- 20.Stanescu R, Stanescu V, Muriel MP, Maroteaux P. Am J Med Genet. 1993;45:501–507. doi: 10.1002/ajmg.1320450420. [DOI] [PubMed] [Google Scholar]
- 21.Hecht JT, Montufar-Solis D, Decker G, Lawler J, Daniels K, Duke PJ. Matrix Biol. 1998;17:625–633. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- 22.Maddox BK, Keene DR, Sakai LY, Charbonneau NL, Morris NP, Ridgway CC, Boswell BA, Sussman MD, Horton WA, Bachinger HP, Hecht JT. J Biol Chem. 1997;272:30993–30997. doi: 10.1074/jbc.272.49.30993. [DOI] [PubMed] [Google Scholar]
- 23.Delot E, Brodie SG, King LM, Wilcox WR, Cohn DH. J Biol Chem. 1998;273:26692–26697. doi: 10.1074/jbc.273.41.26692. [DOI] [PubMed] [Google Scholar]
- 24.Vranka J, Mokashi A, Keene DR, Tufa S, Corson G, Sussman M, Horton WA, Maddox K, Sakai L, Bachinger HP. Matrix Biol. 2001;20:439–450. doi: 10.1016/s0945-053x(01)00148-2. [DOI] [PubMed] [Google Scholar]
- 25.Mow VC, Gu WY, Chen FH. In: Basic Orthopaedic Biomechanics and Mechano-biology. 3. Mow VC, Huiskes R, editors. Lippincott-Raven; Philadelphia: 2005. [Google Scholar]
- 26.Rosenberg K, Olsson H, Morgelin M, Heinegard D. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 27.Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. J Biol Chem. 2001;276:6046–6055. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- 28.Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, Paulsson M, Maurer P. J Biol Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 29.DiCesare PE, Morgelin M, Mann K, Paulsson M. Eur J Biochem. 1994;223:927–937. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- 30.Hauser N, Paulsson M, Kale AA, DiCesare PE. FEBS Lett. 1995;368:307–310. doi: 10.1016/0014-5793(95)00675-y. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Deere M, Hecht JT, Lawler J. J Biol Chem. 2000;275:26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- 32.Carlson CB, Bernstein DA, Annis DS, Misenheimer TM, Hannah BL, Mosher DF, Keck JL. Nat Struct Mol Biol. 2005;12:910–914. doi: 10.1038/nsmb997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. J Biol Chem. 2005;280:32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommarin Y, Larsson T, Heinegard D. Exp Cell Res. 1989;184:181–192. doi: 10.1016/0014-4827(89)90376-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee MK, Lander AD. Proc Natl Acad Sci U S A. 1991;88:2768–2772. doi: 10.1073/pnas.88.7.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim WA, Sauer RT, Lander AD. Methods Enzymol. 1991;208:196–210. doi: 10.1016/0076-6879(91)08014-9. [DOI] [PubMed] [Google Scholar]
- 37.Herndon ME, Lander AD. In: A Laboratory Guide to Glycoconjugate Analysis. Jackson P, Gallagher JT, editors. Birkhauser Verlag AG; Basel, Switzerland: 1997. pp. 379–398. [Google Scholar]
- 38.San Antonio JD, Slover J, Lawler J, Karnovsky MJ, Lander AD. Biochemistry. 1993;32:4746–4755. doi: 10.1021/bi00069a008. [DOI] [PubMed] [Google Scholar]
- 39.Herndon ME, Stipp CS, Lander AD. Glycobiology. 1999;9:143–155. doi: 10.1093/glycob/9.2.143. [DOI] [PubMed] [Google Scholar]
- 40.Hauser N, Geiss J, Neidhart M, Paulsson M, Hauselmann HJ. Acta Orthop Scand. 1995;266:71–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.