Abstract
Purpose
Radiation therapy to the pelvic lymph nodes in high risk prostate cancer is required on several RTOG clinical trials. Based on a prior lymph node contouring project we have shown significant disagreement in the definition of pelvic lymph node volumes amongst GU radiation oncology specialists involved in developing and executing current RTOG trials.
Methods
A consensus meeting was held on October 3, 2007, to reach agreement on pelvic lymph node volumes. Data was presented to address the lymph node drainage of the prostate. Extensive discussion ensued to develop CTV pelvic lymph node consensus.
Results
Consensus was obtained resulting in CT image-based pelvic lymph node CTVs. Based on this consensus the pelvic lymph node volumes to be irradiated include:
distal common iliac
presacral lymph nodes (S1–S3)
external iliac lymph nodes
internal iliac lymph nodes
obturator lymph nodes
Lymph node CTVs include the vessels (artery and vein) and a 7mm radial margin being careful to “carve out” bowel, bladder, bone, and muscle. Volumes begin at the L5/S1 interspace and end at the superior aspect of the pubic bone. Consensus on DVH constraints for OARs were also attained.
Conclusions
Consensus on pelvic lymph node CTVs for radiation therapy to address high-risk prostate cancer was attained and is available as web-based CT images as well as a descriptive format through the RTOG. This will allow for uniformity in evaluating the benefit and risk of such treatment.
Keywords: prostate cancer, pelvic lymph nodes, target volume, IMRT, Radiation OncologyGuidelines
PURPOSE
Pelvic lymph node (LN) radiation was a required part of the treatment for high-risk/locally advanced prostate cancer patients treated on all the prospective randomized trials used to establish the role of hormone therapy and radiation for this patient population. (1,2,3,4) In addition published data suggests that treating these volumes may improve outcomes for prostate cancer patients if the risk of lymph node involvement is significant.(5) Furthermore, there are two open Radiation Therapy Oncology Group (RTOG) trials for high risk prostate cancer patients which require pelvic lymph node radiation therapy.(6,7)
The challenge is that there are significant differences of opinion regarding appropriate pelvic LN volumes to be treated in this cohort of patients. A 2007 survey of RTOG genitourinary cancer experts indicated that significant variability existed amongst a group of radiation oncologists experienced in the treatment of prostate cancer and design of prostate cancer clinical trials.(8) Given the widely adopted use of intensity modulated radiation therapy (IMRT) to treat the pelvic lymph nodes and spare organs at risk (OAR) it is imperative that a consensus be obtained to establish the appropriate nodal volumes for these patients so that the relative safety and merit of such treatment can be established. The purpose of this study was to establish such a consensus.
METHODS
Given the established variability in LN treatment volumes by the RTOG GU Radiation Oncologists a consensus meeting was held on October 3, 2007 sponsored by the RTOG. All physicians/institutions involved in the original contouring project were invited to attend. (8) Data was presented (CL) to address the lymph node drainage of the prostate using prostatic lymphography, extended lymph node dissection, the sentinel lymph node concept, anatomy texts, pelvic MRI imaging, and evaluation of pelvic lymph nodes treated on prior RTOG trials 85-31, 86-10, and 92-02 used to establish the role of hormone therapy and radiation for this high risk population.(1,2,3)
Consensus was obtained via the physicians present with extensive discussion and actual contouring on a CT scan used in the original contouring study.(8) Dose constraints for the organs at risk were also discussed with consensus participants polled regarding the dose constraints they commonly utilize and discussion ensued. Subsequent circulation of dose volume constraints and review of the CT image sets occurred following the meeting to refine the consensus volumes sand dose constraints for the OARs.
RESULTS
Data presented regarding prostatic lymphography(9,10) reveals prostatic lymph node drainage to the internal iliac, external iliac, presacral and common iliac nodes with the main drainage to the internal iliac and presacral lymph nodes.(9)
Extended lymph node dissection data, which generally involved resection of the external and internal iliac and obturator lymph nodes as well as the common and presacral lymph nodes, showed evidence of nodal drainage in multiple nodal sites.(11,12,13,14,15) Often the nodal involvement was not contiguous.(11,15) This data also pointed to the need to address nodes beyond the obturator and external iliac lymph nodes if one wishes to address all potentially involved areas with the presacral nodes as a significant area of node positivity.(11,14,15) Finally all the lymph node dissection data presented pointed to the fact that the more lymph nodes removed the more positive nodes found, and therefore, recommendation from this data was that patients with high risk prostate cancer should undergo extended lymph node dissection.(11–15) The message here is that the pelvic lymph nodes at risk include obturator, external and internal iliac, and presacral nodes.
Sentinel lymph node concept data shows that the process is not only feasible,(16,17,18) but the more recent data(18) reported (results on over 1,000 patients where extended lymph node dissection and positive sentinel lymph nodes) defined metastatic disease well beyond the obturator and external iliac regions alone. In this data there was evidence of lymph node metastasis to the presacral regions also.(18)
Basic anatomy data revealed three main drainage patterns for the prostate:
Cranially to the external iliac lymph nodes
Laterally to the internal iliac lymph nodes
Posterior to the sub-aortic aspect of the presacral lymph nodes S1–S3 (19,20)
Pelvic MRI imaging with lymphotropic nanoparticles shows evidence of lymph node metastasis for advanced prostate cancer in the drainage areas of the common iliac, external and internal iliac, and presacral regions with an interesting lack of positive lymph nodes below the top of the pubic symphysis.(21) Similar MRI nanoparticle data from gynecologic malignancies shows evidence of lymph nodes well posterior to the external iliac vessels and well anterior the internal iliac vessels showing a need to include margins beyond the vessels themselves for adequate pelvic iliac lymph node coverage.(22) In addition this data suggested a minimum 7mm margin around the pelvic blood vessels to cover the involved lymph nodes.
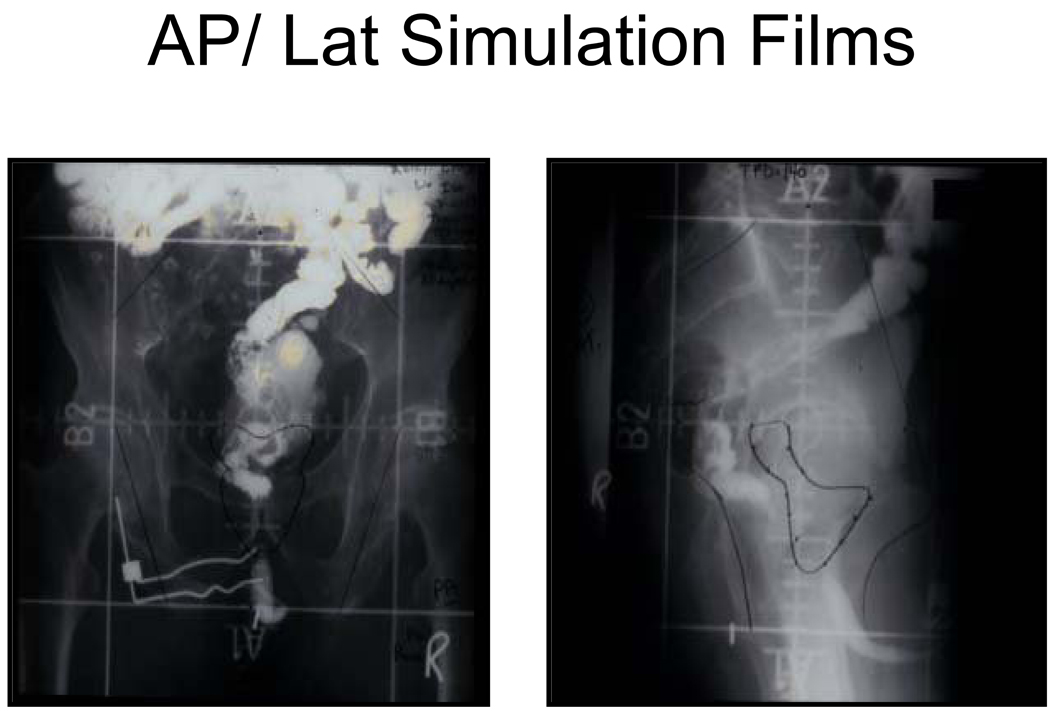
In the interest of consistency in the definition of the pelvic LN regions for IMRT compared to prior techniques ,data from each of the RTOG trials (evaluating the role of hormone therapy and radiation for high risk patients) was reviewed. Previously a four-field box technique was used to address the pelvic lymph nodes.(1,2,3) (Fig 1) The upper border of this technique was at the L4/L5 or L5/S1 interspace and the lower border at the level of the bottom of the ischeal tuberosities. Laterally 2-cm lateral to the true pelvis was recommended. In the lateral fields the anterior border was to include the anterior aspect of the external iliac lymph nodes and the posterior border was to be posterior to the anterior boney sacrum so as to address the presacral lymph nodes superiorly and then to split the rectum inferiorly. These borders were chosen to be sure that the draining pelvic LNs were covered.
Figure 1. AP/ Lat Simulation Films.
Example whole pelvis simulation films for RTOG studies 85-31, 86-10, and 92-02
Review of all the data above and extensive discussions resulted in a consensus being obtained and contours performed on one of the CT scans used in the contouring project. (8) Special focus during the discussion had been placed on the importance of treating the presacral lymph nodes. Review of the data presented resulted in a recommendation to treat the presacral lymph nodes (subaortic only S1–S3). Figure 2 shows representative Clinical Target Volumes (CTV’s) contours drawn on the CT consensus scan. These contours were done by the consensus panel (all co-authors) and a written description was developed as follows:
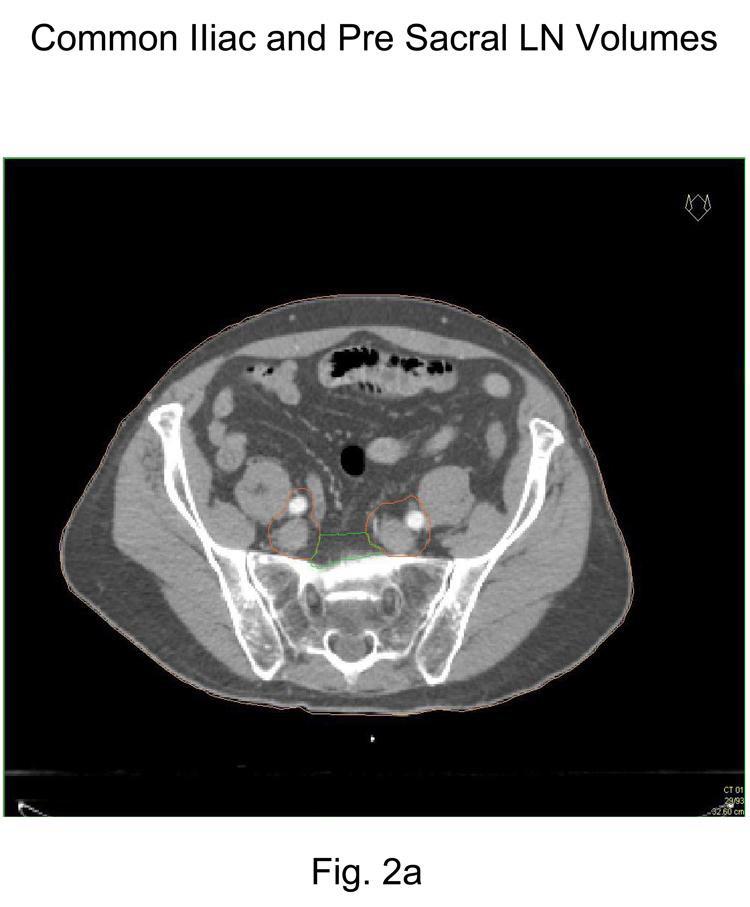
Commence contouring the pelvic CTV lymph node volumes at the L5/S1 interspace (the level of the distal common iliac and proximal presacral lymph nodes) Figure 2a
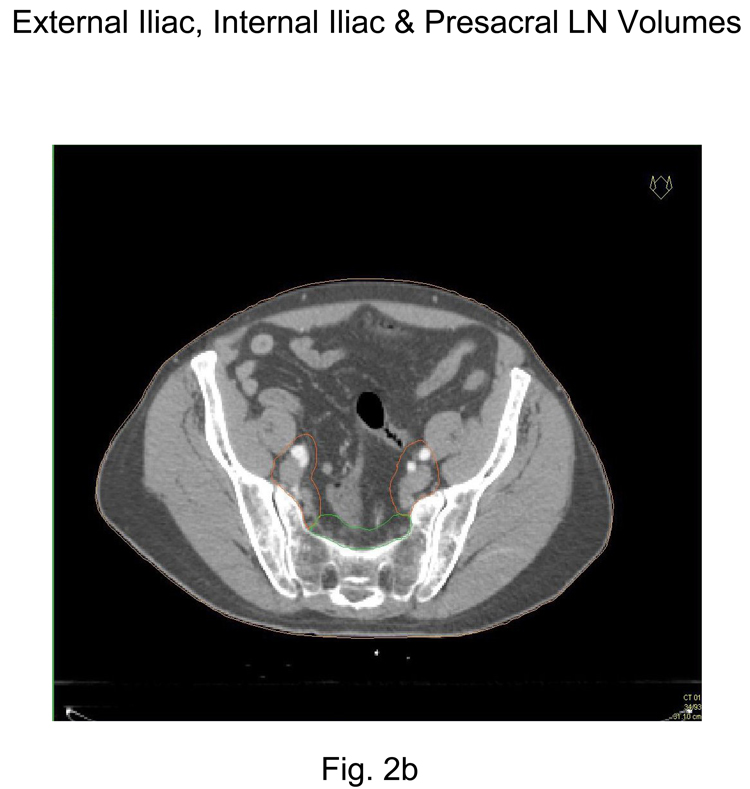
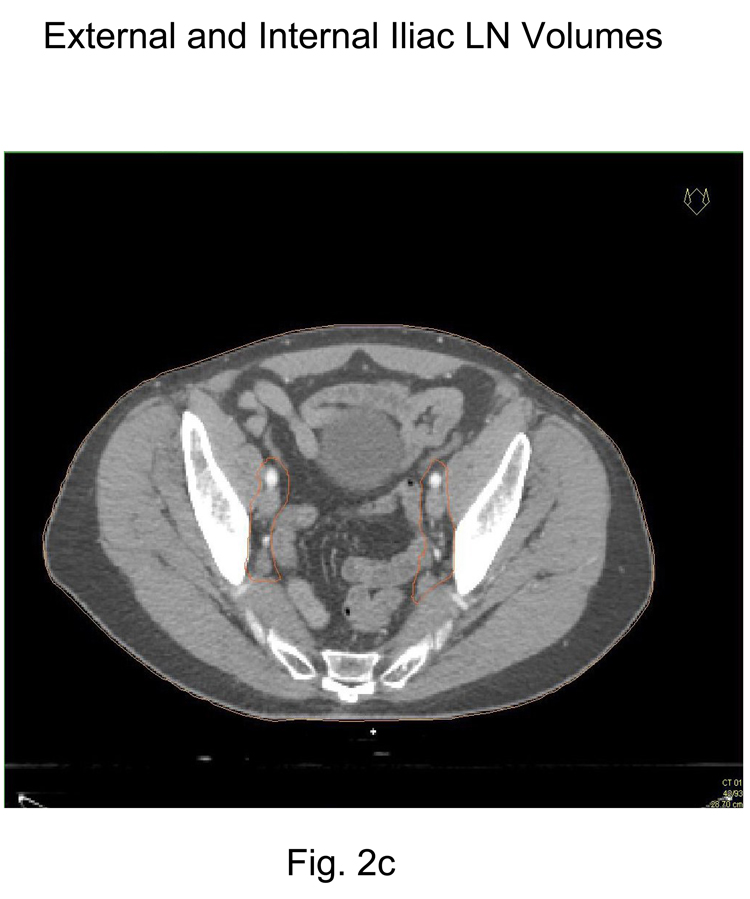
Place a 7-mm margin around the iliac vessels connecting the external and internal iliac contours on each slice, carving out bowel, bladder, and bone. Figure 2b and 2c
Contour presacral lymph nodes (sub-aortic only) S1 through S3, posterior border being the anterior sacrum and anterior border approximately 10 mm anterior to the anterior sacral bone carving out bowel, bladder, and bone. Figure 2a and 2b
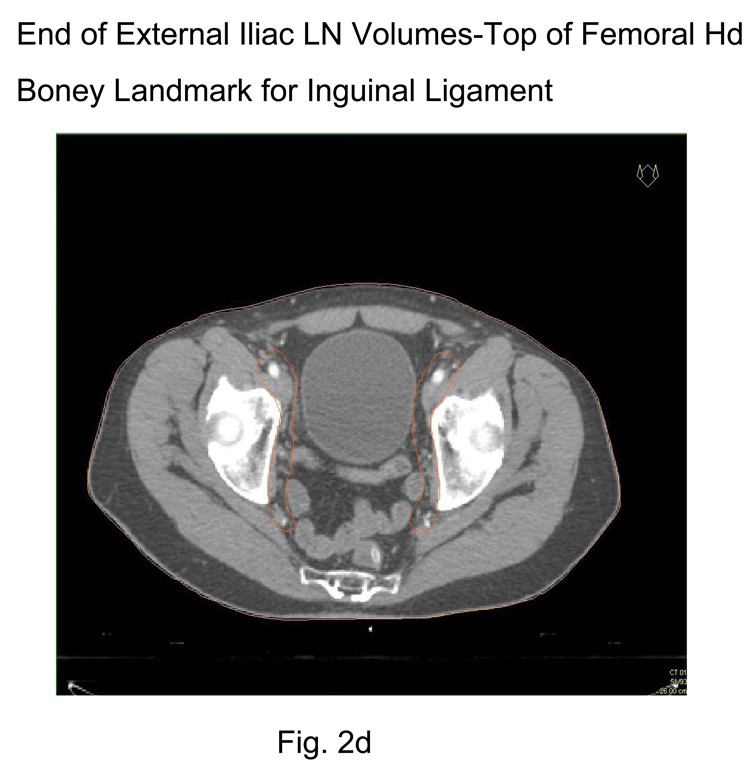
Stop external iliac CTV lymph node contours at the top of the femoral heads (bony landmark for the inguinal ligament) Figure 2d
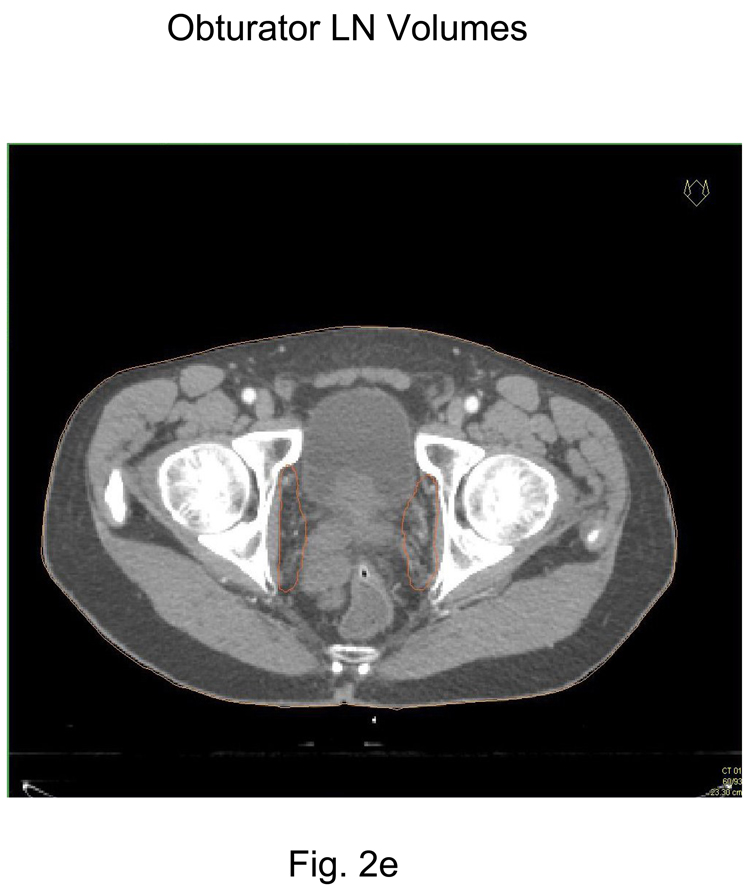
Stop contours of the obturator CTV lymph nodes at the top of the pubic symphysis Figure 2e.
Figure 2.
Representative pelvic lymph node CTV contours from consensus CT:
2a Common Iliac and Presacral CTV lymph node volumes (L5/S1)
2b External, Internal and Presacral CTV lymph node volumes (S1–S3)
2c External and Internal Iliac CTV lymph node volumes (below S3)
2d End of External Iliac CTV lymph node volumes (at top of femoral head – boney landmark for the inguinal ligament)
2e Obturator CTV lymph node volumes (above the top of the pubic symphysis)
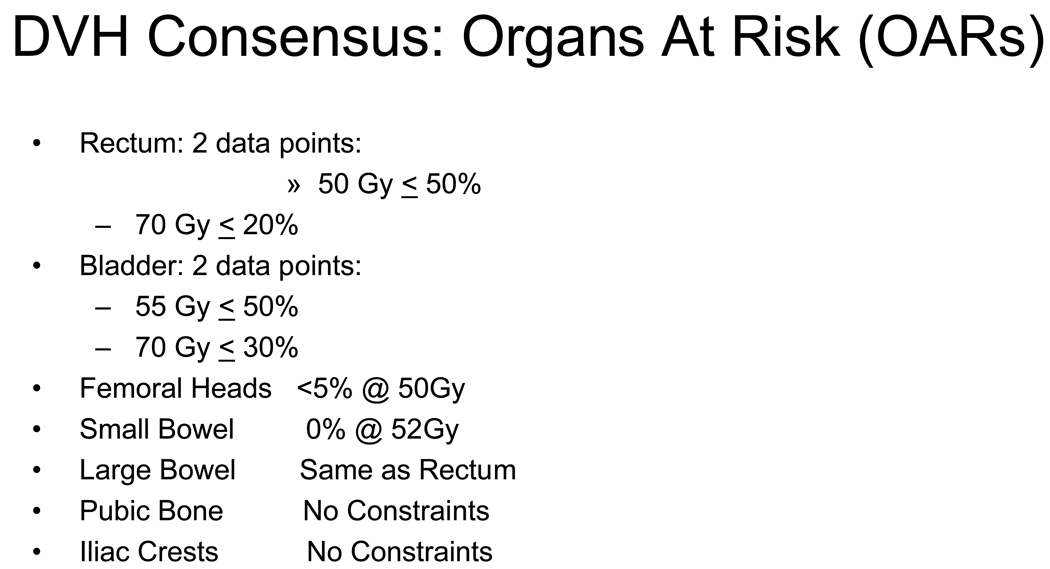
Volume definition and dose constraints for organs at risk (OARs) were also discussed and resulted in a consensus for OAR guidelines. (Figure 3) The rectum should be empty at the time of simulation. Consistent with the RTOG definition of the rectum it is to be outlined from the sigmoid flexure to the bottom of the ischial tuberosities. The bladder should be comfortably full at the simulation. Two dose points were selected for both the rectum and bladder. These dose constraints were based on whole (solid) organ contours. No constraints were identified for the penile bulb and the iliac crests. Large bowel dose constraints were to be the same as the rectum. The small bowel dose limit was 52 Gy and the femoral heads <5 % of their volume at 50 Gy. Dose constraints for all OARs were based on the use of conventional fractionation using a dose per fraction of 1.8 to 2.0 Gy per day.
Figure 3.
Dose Volume Histogram constraints for Organs At Risk (OARs)
CONCLUSIONS
Adenocarcinoma of the prostate is the second most common cause of cancer death in US males with over 27,000 deaths estimated for 2007.(23) Many of these patients will have presented with locally advanced/high-risk disease where regional pelvic lymph node involvement is a reality. The role of radiation therapy in the cure of these patients is evolving, but certainly many can be controlled if not cured with the use of radiation and hormonal manipulation.(12,3,4) These cures are the result of the treatment to the pelvic lymph nodes and prostate in addition to systemic hormone therapy.(1,2,3,4) The exact role of the nodal radiation is still being evaluated yet it remains a requirement for two open RTOG trials, RTOG 05–21 looking at high risk patients and upfront treatment with radiation and hormone therapy plus or minus chemotherapy and for patients with recurrence after surgery (RTOG 05–34). With the advent of IMRT to treat the lymph nodes and spare OARs it becomes imperative that radiation oncologists treat the appropriate lymph node volumes or the relative merit and safety of such treatment can never be appropriately assessed.
Prior to this consensus it was clear that even RTOG GU “experts” did not agree on the lymph node CT volumes to be covered.(8) And other RTOG “experts” such as the gynecologic group have struggled with similar issues. (24) This consensus document is the result of extensive review of the literature to define the lymph nodes at risk, evaluation of lymph nodes treated on critically important recent trials for this high risk population and discussion to attain consensus on appropriate CTV lymph node volumes and dose constraint guidelines for OARs by leading GU radiation oncologists. This consensus document should serve as a guideline for radiation oncologists and is especially important for designing and conducting clinical trials. Utilizing these guidelines will ensure that patients who are at significant risk for pelvic lymph node metastasis and treated with radiation will have the correct lymph nodes addressed. In addition it will help to ensure the safety of such treatment if dose-volume histograms (DVH) suggested in these guidelines for the OARs are consistently followed. As with any set of treatment guidelines individual practioners will have to consider specific patient characteristics. Variability in anatomy and co-morbidities are factors which may need to be considered in specific cases. However, the guidelines presented in this consensus document will be useful to address the merit of pelvic lymph node radiation in high risk patients as it ensures that similar volumes are radiated and will help to avoid the “apples versus oranges” volumes that have existed prior to this consensus.(8)
Acknowledgments
Supported by grants from the National Cancer Institute, CA21661, CA32115, and CA37422.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma long term results of phase III RTOG 85-31. Int J Rad Onc Biol Phys. 2005;61(5):1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, III, Bae K, Speight J, et al. Short Term Neoadjuvant Androgen Deprivation Therapy and External Beam Radiotherapy for Locally Advanced Prostate Cancer: Long Term Results of RTOG 8610 a Phase III Prospective Randomized Trial. Journal of Clinical Oncology. 2008;26(4):585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz E, Bae K, Hanks G, et al. Ten-year follow-up of RTOG 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journ Clin Onc. 2008 May 20;26(15):2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Collette L, Blank L, et al. Long term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study, a phase III randomized trial) Lancet. 2002;360:103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 5.Roach M, III, DeSilvio M, Valicenti R, et al. Whole-pelvis, "mini-pelvis," or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J of Rad Onc, Bio, Phy. 2006;66(3):647–653. doi: 10.1016/j.ijrobp.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 6.RTOG 05-21: A phase III protocol of androgen suppression (AS) and 3D CRT/IMRT vs AS and 3D CRT/IMRT followed by chemotherapy with Docetaxol and Prednisone for localized high risk prostate cancer. See RTOG website http://rtog.org.
- 7.RTOG 05-34: A phase III trial of short term androgen deprivation with pelvic lymph node or prostate bed only adiotherapy (SPPORT) in prostate cancer patients with a rising PSA after radical prostatectomy. See RTOG website http://rtog.org.
- 8.Lawton CA, Michalski J, El-Naqa I, et al. Variation in the definition of clinical target volumes for pelvic node conformal radiaton therapy of prostate cancer. Int J Rad Onc Biol Phy. 2007;69(3) Supplement:S327. doi: 10.1016/j.ijrobp.2008.08.003. Abs #2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavalah NV, ordan WP., Jr Prostatic lymphography. The Journal of Urology. 1979;121:178–181. doi: 10.1016/s0022-5347(17)56712-9. [DOI] [PubMed] [Google Scholar]
- 10.Cerny JC, Farah R, Rian R, Weckstein ML. An evaluation of lymphangiography in staging carcinoma of the prostate. The Journal of Urology. 1975;113:367–370. doi: 10.1016/s0022-5347(17)59483-5. [DOI] [PubMed] [Google Scholar]
- 11.Golimbu M, Morales P, Al-Askari S, Brown B. Extended pelvic lymphadenectomy for prostate cancer. The Journal of Urology. 1979;121:617–620. doi: 10.1016/s0022-5347(17)56906-2. [DOI] [PubMed] [Google Scholar]
- 12.Bader P, Burkhard F, Markwalder R, Studer U. Is a limited node dissection an adequate staging procedure for prostate cancer? The Journal of Urology. 2002;168:514–518. doi: 10.1016/s0022-5347(05)64670-8. [DOI] [PubMed] [Google Scholar]
- 13.Burkhard F, Schumacker M, Thalmann GN, Stude ULr. Is pelvic lymphadenectomy really necessary in patients with a serum prostate-specific antigen level of <10 ng/ml undergoing radical prostatectomy for prostate cancer? BJU International. 2005;95:275–280. doi: 10.1111/j.1464-410X.2005.05280.x. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: High incidence of lymph node metastasis. Journal of Urology. 2002;167:1681–1686. [PubMed] [Google Scholar]
- 15.Allaf M, Palapattu G, Trock G, et al. Anatomical extent of lymph node dissection: Impact on men with clinically localized prostate cancer. The Journal of Urology. 2004;172:1840–1844. doi: 10.1097/01.ju.0000140912.45821.1d. [DOI] [PubMed] [Google Scholar]
- 16.Wawroschek F, Vogt H, Weckermann D, et al. The sentinel lymph node concept in prostate cancer: First results of gamma probe-guided sentinel lymph node identification. European Urology. 1999;36:595–600. doi: 10.1159/000020054. [DOI] [PubMed] [Google Scholar]
- 17.Takashima H, Egawa M, Imao T, et al. Validity of sentinel lymph node concept for patients with prostate cancer. The Journal of Urology. 2004;171:2268–2271. doi: 10.1097/01.ju.0000127735.09469.c4. [DOI] [PubMed] [Google Scholar]
- 18.Weckermann D, Dorn R, Trefz M, et al. Sentinel lymph node dissection for prostate cancer: Experience with more than 1,000 patients. The Journal of Urology. 2007;177:916–920. doi: 10.1016/j.juro.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 19.Netter F. Atlas of Human Anatomy 2nd Edition. 1997. p. 379. [Google Scholar]
- 20.Gil-Vernet JM. Prostate Cancer: Anatomical and surgical considerations. British Journal of Urology. 1996;78:161–168. doi: 10.1046/j.1464-410x.1996.00841.x. [DOI] [PubMed] [Google Scholar]
- 21.Shih H, Harisinghani M, Zietman A, et al. Mapping of nodal disease in locally advanced prostate cancer: Rethinking the clinical target volume for pelvic nodal irradiation based on vascular rather than bony anatomy. Jour Intern Rad Onc Bio & Phys. 2005;63(4):1262–1269. doi: 10.1016/j.ijrobp.2005.07.952. [DOI] [PubMed] [Google Scholar]
- 22.Taylor A, Rockall A, Reznek R, et al. Mapping pelvic lymph nodes: Guidelines for delineation in Intensity Modulated Radiotherapy. Int Jour Rad Onc Bio & Phy. 2005;63(5):1604–1612. doi: 10.1016/j.ijrobp.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society. Cancer Facts and Figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 24.Small W, Jr, Mell LK, Anderson P, et al. Consensus Guidelines for the Delineation of the Clinical Target Volume for Intensity Modulated Pelvic Radiotherapy in the Postoperative Treatment of Endometrial and Cervical Cancer. Int J Radiol Oncol Biol Phys. 2008 June;71(2):428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]