Abstract
The tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) has been studied for chronic disease preventive effects, and is marketed as part of many dietary supplements. However, case reports have associated the use of green tea-based supplements with liver toxicity. We studied the hepatotoxic effects of high dose EGCG in male CF-1 mice. A single dose of EGCG (1500 mg/kg, i.g.) increased plasma alanine aminotransferase (ALT) by 138-fold and reduced survival by 85%. Once-daily dosing with EGCG increased hepatotoxic response. Plasma ALT levels were increased 184-fold following two once-daily doses of 750 mg/kg, i.g. EGCG. Moderate to severe hepatic necrosis was observed following treatment with EGCG. EGCG hepatotoxicity was associated with oxidative stress including increased hepatic lipid peroxidation (5-fold increase), plasma 8-isoprostane (9.5-fold increase) and increased hepatic metallothionein and γ-histone 2AX protein expression. EGCG also increased plasma interleukin-6 and monocyte chemoattractant protein 1. Our results indicate that higher bolus doses of EGCG are hepatotoxic to mice. Further studies on the dose-dependent hepatotoxic effects of EGCG and the underlying mechanisms are important given the increasing use of green tea dietary supplements, which may deliver much higher plasma and tissue concentrations of EGCG than tea beverages.
Keywords: green tea, (-)-epigallocatechin-3-gallate, hepatotoxicity, mouse, oxidative stress
Introduction
(-)-Epigallocatechin-3-gallate (EGCG, Fig. 1) is the most abundant and widely-studied catechin in green tea (Camellia sinensis, Theaceae) (Balentine et al., 1997; Yang et al., 2007). Both green tea and EGCG have been studied extensively for their health beneficial effects, and laboratory data suggest that they might be useful in the treatment and prevention of several chronic diseases including cancer, heart disease, obesity, diabetes, and neurodegenerative diseases (Higdon and Frei, 2003; Khan et al., 2006; Kuriyama et al., 2006; Zaveri, 2006).
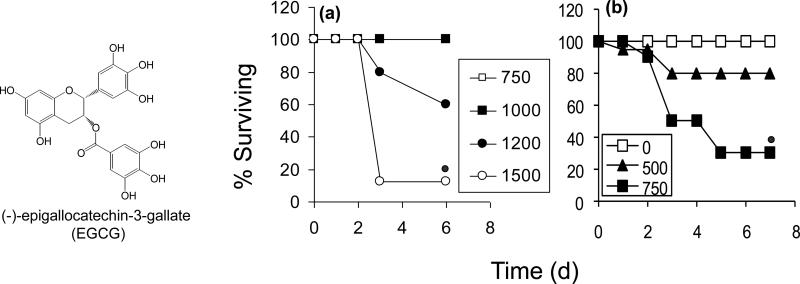
Figure 1.
Effect of oral EGCG on the survival of male CF-1 mice. Oral EGCG caused a time and dose-dependent decrease in survival when give as either a (a) single bolus dose (750 – 1500 mg/kg, i.g.) or as (b) multiple once-daily bolus doses(0 – 750 mg/kg, i.g.). N = 10 – 12 per group.  = p < 0.05 by χ2 test
= p < 0.05 by χ2 test
Green tea enjoys a long history of use as a beverage and is generally regarded as safe. Moreover, numerous human intervention and bioavailability studies using low to moderate doses of green tea preparations or EGCG have reported no serious adverse effects (Lee et al., 2002; Bettuzzi et al., 2006; Chow et al., 2006). Green tea has also been reported to prevent liver toxicity induced by a number of hepatotoxicants in animal models including: 2-nitropropane, carbon tetrachloride, and acetaminophen (Sai et al., 1998; Chen et al., 2004; Oz et al., 2004). Dietary green tea and EGCG have been shown to prevent fatty liver disease in both diet-induced and genetic animal models (Baltaziak et al., 2004; Kuzu et al., 2007; Bose et al., 2008; Bruno et al., 2008)
Recently, however, cases of hepatotoxicity have been associated with consumption of high doses of green tea-containing dietary supplements (10 – 29 mg/kg/d p.o.) (reviewed in (Mazzanti et al., 2009)). In nearly all cases, patients presented with elevated serum alanine aminotransferase (ALT) and bilirubin levels. In some cases, liver biopsies were performed and periportal and portal inflammation were observed. All cases resolved following cessation of supplement consumption. A causative role for the green tea preparations is suggested by the fact that re-injury was observed in some studies when the subject began using the same preparations after symptoms had resolved.
Although in most reports of hepatotoxicity, patients used concentrated extracts or “pill or capsule” dosage forms, there is a report of a 45-yr old man who developed jaundice and elevated serum ALT following consumption of 6 cups/d green tea infusion for 4 months (Jimenez-Saenz and Martinez-Sanchez Mdel, 2006). The underlying reasons for this difference in sensitivity is unclear, but may be related to intra-individual differences in biotransforming enzyme relevant to metabolism of green tea polyphenols.
Laboratory studies of green tea–derived preparations in rodents and dogs have also revealed toxic effects at high doses (Galati et al., 2006; Isbrucker et al., 2006). Oral bolus administration of Teavigo (a green tea polyphenol preparation containing 90% EGCG) or Polyphenon E for 13 or 9 weeks, respectively, to Beagle dogs resulted in dose-dependent toxicity and death. Vomiting and diarrhea were observed throughout both studies. In addition, 500 mg/kg, p.o. Teavigo caused proximal tubule necrosis and elevated serum bilirubin in all dogs treated. Most male dogs (2 of 3) had elevated serum aspartate aminotransferase (AST) levels. Female dogs (2 of 3), but not male dogs, had liver necrosis (Isbrucker et al., 2006). Oral administration of 2000 mg/kg, i.g. Teavigo to rats resulted in 80% mortality (Isbrucker et al., 2006). Histological analysis revealed hemorrhagic lesions in the stomach and intestine. These data suggest that high doses of EGCG can induce toxicity in the liver, kidneys, and intestine. We and others have observed hepatotoxcity of EGCG in mice following intraperitoneal administration of EGCG (Sang et al., 2005; Galati et al., 2006). To our knowledge, there have been no reports of hepatotoxicity in the mouse following oral administration of EGCG. In our previous studies, we observed that mice and humans appear to be more similar to each other than either species is to the rat with regard to EGCG bioavailability and biotransformation (Lambert et al., 2003; Lu et al., 2003a; Lu et al., 2003b). Based on these previous findings and the fact that the mouse is the most widely-used species for studying the disease preventive effects of EGCG and green tea, we decided to study the potential toxicity of EGCG in the mouse.
Pro-oxidative effects may underlie the observed toxicity of high dose EGCG. Although EGCG has been historically regarded as an antioxidant and has strong radical scavenging activity in vitro, there is an increasing body of data to suggest that some of the biological activity of EGCG is due to induction of oxidative stress (Frei and Higdon, 2003; Higdon and Frei, 2003; Rietveld and Wiseman, 2003; Elbling et al., 2005; Hou et al., 2005). For example, we have previously reported that treatment of human esophageal cancer cells with EGCG results in inhibition of epidermal growth factor receptor phosphorylation and protein expression (Hou et al., 2005). Addition of superoxide dismutase, which stabilizes EGCG and prevents EGCG-mediated formation of reactive oxygen species, inhibited this effect, suggesting that it was driven primarily by EGCG-mediated ROS formation rather than by EGCG per se. Galati et al. found that EGCG-induced oxidative stress may play a role in the toxic potential of EGCG (Galati et al., 2006). Following treatment of freshly isolated rat hepatocytes with EGCG, the authors observed a time-dependent increase in cytotoxicity and in reactive oxygen species formation (measured as increased fluorescence). Whether EGCG induces oxidative stress in vivo and whether those actions are related to EGCG-induced hepatotoxicity has not been studied
In the present study, we hypothesized that high oral doses of EGCG would induce hepatotoxicity in the mouse, and that this toxicity was related to EGCG-mediated pro-oxidant effects. Herein, we report the results of these experiments.
Materials and Methods
Chemicals
EGCG (100% pure) was provided by Mitsui Norin Co., Ltd. (Fujieda City, Japan). HPLC grade solvents were purchased from VWR, International (West Chester, PA). All other reagents were of the highest grade commercially available. Dosing solutions of EGCG were prepared in 5% ethanol in water (v/v).
Animals
Male CF-1 mice (7 – 8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA) and allowed to acclimate for at least one week prior to the start of the experiment. The mice were housed 10 per cage in polycarbonate cages with corncob bedding, and maintained in air-conditioned quarters with a room temperature of 20 ± 2°C, relative humidity of 50 ± 10%, and an alternating 12 h light/dark cycle. Animals were given Purina rodent chow (Research Diets, New Brunswick, NJ, USA) and water ad libitum, and fasted prior to treatment. Animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee at Rutgers University.
Treatment and Sample Collection
The toxicity of EGCG was determined after single dose or once-daily dose treatments. For single-dose treatments, mice were fasted overnight and then given a single dose of EGCG (50 – 2000 mg/kg, i.g.). In once-daily dosing experiments, mice were repeatedly dosed with a single daily dose of EGCG (500 or 750 mg/kg, i.g.) for 2 – 7 d depending on the experiment. Prior to each dosing, the mice were fasted from 9 am to 3 pm. In all experiments, food was returned following dosing. The effect of EGCG on survival was assessed by monitoring the mice for seven days following treatment. For single dose experiments, blood was collected from anesthetized animals by cardiac puncture 24 or 48 h after dosing with EGCG. For once-daily dosing studies, blood was similarly collected 24 h after the last dose of EGCG. Plasma was isolated by centrifugation at 500 × g for 15 min and stored at −80°C for later analysis. Livers were collected and quickly washed with PBS. One lobe of the liver was trimmed and fixed in 10% buffered formalin for histopathological analysis; the remaining liver was frozed at −80°C for biochemical analysis.
Determination of Hepatotoxicity
EGCG-induced hepatotoxicity was determined by both biochemical and histopathological methods. Plasma ALT was used as the biochemical marker of hepatotoxicity, and was determined using a commercially-available spectrophotometic assay (λmax = 450 nm) which coupled ALT activity to the disappearance of NADH (Catachem, Inc., Bridgeport, CT). Biochemical determination of hepatotoxicity was confirmed using histopathological methods. Paraffin-embedded liver tissue blocks were cut to produce 6-micron sections which were deparaffinized and stained with hematoxylin and eosin.
Determination of Biochemical Markers Oxidative Stress
Free malonyldialdehyde (MDA) in the liver was determined colorimetrically using a commercially-available kit (Cell Biolabs Inc., San Diego, CA). In brief, liver samples were homogenized with a dounce homogenizer in 5 volumes of T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology Inc., Rockford, IL) containing protease and phosphatase inhibitors. The supernatant was collected after centrifugation at 16,000 g for 10 min. Free MDA levels were determined spectrophotometrically (λmax = 550 nm) as per the manufacturer's instructions.
Plasma levels of 8-isoprostane were determined as a marker of systemic oxidative stress. This was accomplished using a commercially-available ELISA (Caymen Chemical Co., Ann Arbor, MI) according to the manufacturer's protocol.
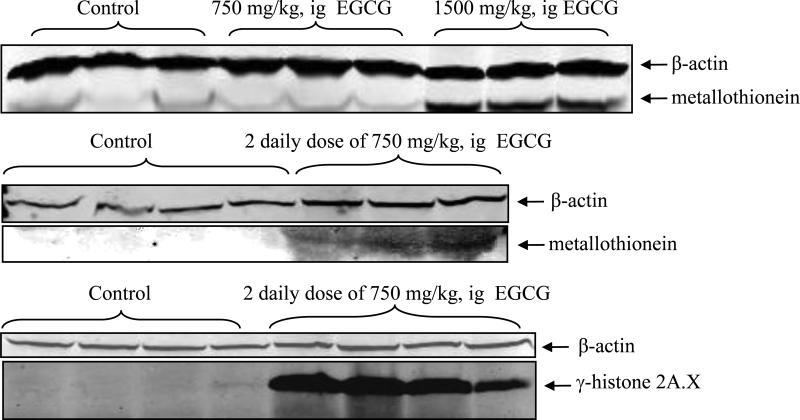
Hepatic expression of metallothionein I/II (MT) and γ-histone 2AX (γH2AX) was measured by western blot as molecular markers of oxidative stress. Tissue extracts were prepared using T-per reagent as described above. After denaturation at 95°C in Laemmli sample buffer, 200 μg protein were subjected to SDS-PAGE (12% or 4-15% gradient, Bio-Rad). Gels were blotted onto polyvinylidene difluoride membranes (Bio-Rad) that were incubated with blocking buffer (Li-Cor Biosciences, Lincoln, NE) for 30 min at room temperature. After washing with tris-buffered saline, the membranes were incubated with fluorescently-tagged secondary antibodies. Fluorescence was detected with an Odyssey Infrared Imaging System (Li-Cor Biosciences). Antibodies against MT and γH2AX were purchased from Assay Designs, Inc. (Ann Arbor, MI).
Immunohistochemical (IHC) Analysis of 4-hydroxynonenal (4-HNE)
Paraffin-embedded liver sections were dewaxed in xylene, and rehydrated in a gradient of ethanol to distilled water. After quenching endogenous peroxidase activity with 3% hydrogen peroxide, tissue sections were incubated in normal sera (10% normal horse serum) to minimize nonspecific binding. Monoclonal 4-HNE antibody (Cosmo Bio Co., LTD, Tokyo, Japan, Cat# MHN-020P, 1:50) was applied at 4°C overnight. Tissue sections were then incubated with secondary biotin conjugated antibody incubation at room temperature for 30 min. An avidin-biotin peroxidase complex (Vector Laboratories, Burlingame, CA, Cat# PK-7200) was then applied, and the staining was visualized with diaminobenzidine (Vector Laboratories, Burlingame, CA, Cat# SK-4100). The sections were counterstained with Mayer's hematoxylin.
Determination of Plasma Cytokines
Plasma levels of interleukin (IL)-6 and monocyte chemoattractant protein 1 (MCP-1) were determined by ELISA using appropriate commercially-available kits (IL-6 from Biosource International Inc., Camarillo, CA; MCP-1 from R&D Systems, Minneapolis, MN) according to the manufacturers’ directions.
LC/MS determination of EGCG-cysteine conjugates in mouse urine
Male CF-1 mice (7 – 8 weeks old, n = 5) were given a single dose of 1500 mg/kg, i.g. EGCG and housed together in a metabolic cage for 24 h. Pooled urine (from n = 5 mice) was collected and combined with ascorbic acid and Na2EDTA to final concentatrations of 2% and 0.05%, (w/v), respectively. Urine samples were frozen at −80°C for later analysis.
Aliquots of urine (10 μL) were combined with an 190 μL 0.4 M phosphate buffer (pH 6.8), 10 μL 20% ascorbic acid (w/v), 1 U sulfatase, and 250 U β-glucuronidase. Samples were incubated at 37°C for 45 min and extracted methylene chloride and ethyl acetate. The ethyl acetate fractions were pooled and dried under vacuum. Samples were reconstituted in 10% aqueous acetonitrile and analyzed by LC/MS. Analysis was carried out with a Finnigan Spectra System equipped with a Surveyour MS pump, a Surveyour refrigerated autosampler plus, and an LTQ linear ion trap mass detector (Thermo-Finnigan, San Jose, CA) equipped with an electrospray ionization (ESI) interface. Separation was achieved using a Gemini C18 column (50 × 2.0 mm, 3 μm particle size, Phenomenex, Torrance, CA) and a binary mobile phase consisting of solvent A (5% aqueous methanol containing 0.2% acetic acid) and solvent B (95% aqueous methanol containing 0.2% acetic acid) with a flow rate of 0.2 mL/min. Gradient conditions have been previously described (Sang et al., 2005). The negative ion polarity mode was set for the ESI ion source with voltage of 15 kV. Nitrogen was used as the sheath gas (flow rate = 30 au) and the auxiliary gas (flow rate = 5 au). Structural information was obtained by tandem mass spectrometry (MS2) through collision-induced dissociation with relative energy of 35%.
Statistical Analyses
Differences in survival were analyzed by χ2 analysis. Differences in biochemical parameters were analyzed by one-way ANOVA with Tukey's multiple comparison post-test or Student's t test as appropriate. P < 0.05 was considered statistically-significant. Data generated in the study appeared to have a normal distribution.
Results
Effect of EGCG on survival
Oral administration of high dose EGCG to CF-1 mice resulted in dose-dependent mortality during the 5 d observation period. Survival was reduced by 85% following treatment with a single dose of 1500 mg/kg, i.g. EGCG (Fig. 1a). Once-daily dosing with EGCG increased the toxic potency of the compound. Whereas 500 and 750 mg/kg, i.g. EGCG were non-toxic following a single dose, once-daily treatment with these doses resulted in a 20% and 75% decrease in survival, respectively (Fig. 1b). Intoxicated mice displayed lethargy, piloerection, and weight loss.
Hepatotoxic effects of EGCG
A time- and dose-dependent increase in plasma ALT levels was observed in CF-1 mice following treatment with high dose oral EGCG. A single dose of EGCG (1500 mg/kg, i.g.) increased plasma ALT levels by 8 – and 108 – fold after 24 and 48 h, respectively, compared to vehicle-treated controls (Fig. 2a). Once-daily dosing with EGCG time-dependently increased plasma ALT levels. The plasma levels of ALT were 6501 U/L when measured 24 h after the last of two consecutive once-daily doses 750 mg/kg, i.g. EGCG compared to 35 U/L at base-line (Fig. 2b).
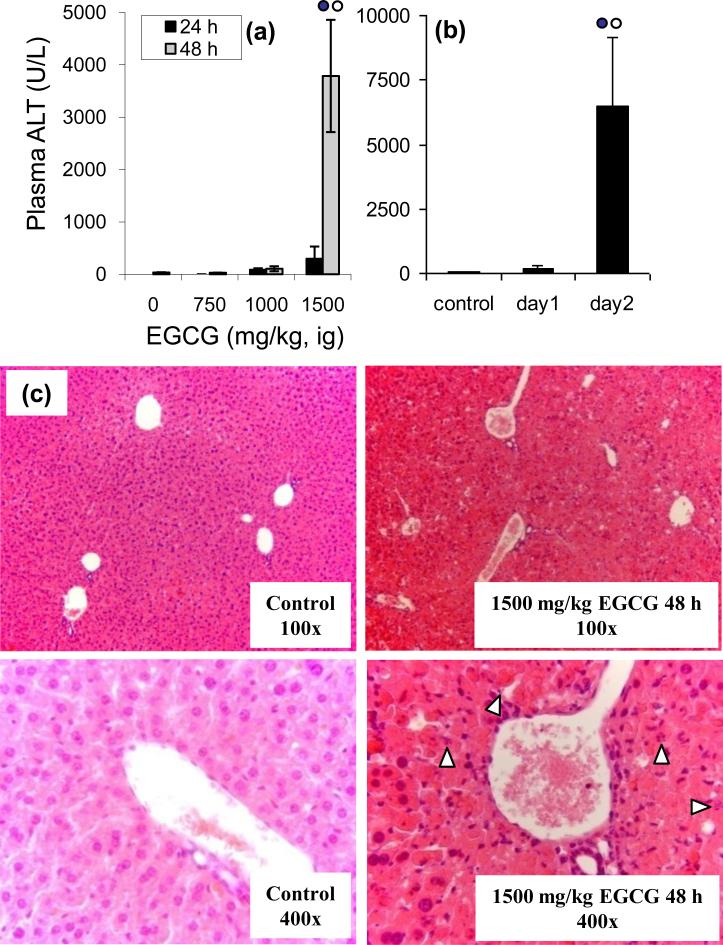
Figure 2.
Biochemical and histopathological analysis of EGCG-mediated hepatotoxicity in male, CF-1 mice. Plasma ALT levels were determined spectrophotometrically following (a) single dose (750 – 1500 mg/kg, i.g.) or (b) multiple once-daily dose (750 mg/kg, i.g.) treatment with EGCG. For mice treated with multiple once-daily doses of EGCG, the plasma was collected 24 h after the last dose. Each bar represents the mean of n = 3 – 8. Error bars represent the SEM.  = p < 0.05 vs other treatment groups and times by one-way ANOVA with Tukey's post-test. Histological analysis of EGCG hepatotoxicity was performed 24 and 48 h after a single dose of 1500 mg/kg, i.g. EGCG. White arrows indicate apoptotic bodies. Micrographs are shown at 100X and 400X magnification.
= p < 0.05 vs other treatment groups and times by one-way ANOVA with Tukey's post-test. Histological analysis of EGCG hepatotoxicity was performed 24 and 48 h after a single dose of 1500 mg/kg, i.g. EGCG. White arrows indicate apoptotic bodies. Micrographs are shown at 100X and 400X magnification.
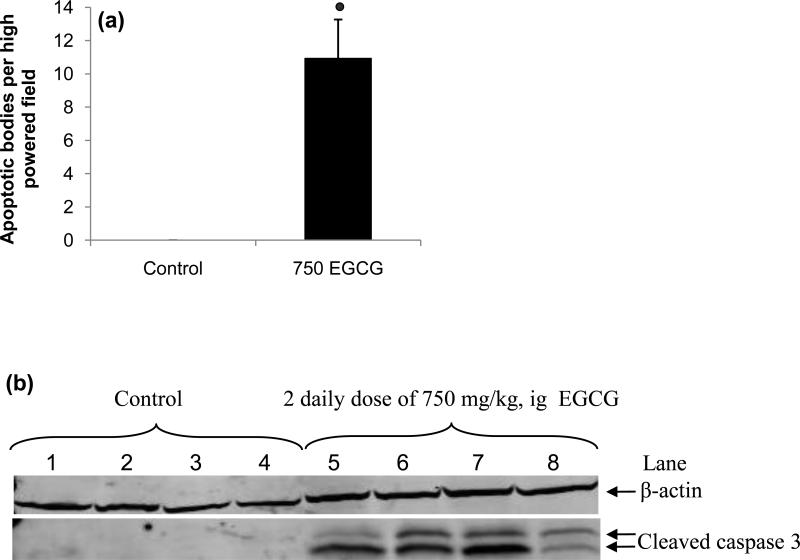
Histopathological analysis of liver samples collected from mice treated with a single dose or two once-daily doses of EGCG showed time dependent morphological changes. In 75% of mice given a single dose of 1500 mg/kg, i.g. EGCG and in 60% of mice given two once-daily doses of 750 mg/kg, i.g. EGCG, moderate to severe hepatic necrosis was observed (Fig 2c). In a smaller number of EGCG treated mice (25% and 40% for 1500 mg/kg, ig EGCG and 750 mg/kg, ig EGCG) only mild necrosis was observed. These differences in histological severity correlated well with plasma ALT levels. In most samples, necrosis was extensively distributed, although in several samples necrotic foci were observed. In addition, apoptotic bodies were observed in 75% of the liver samples from mice treated with two once-daily doses of 750 mg/kg, i.g. EGCG (Fig. 2 and 3a). Induction of apoptosis in these samples was confirmed by Western blot analysis for cleaved caspase-3 (Fig. 3b). Interestingly, caspase 3 cleavage and apoptotic bodies were observed in samples that had only limited histological necrosis and low plasma ALT levels (e.g. lane 8 of Fig. 3b).
Figure 3.
Induction of hepatocyte apoptosis in mice by treatment with high dose oral bolus EGCG. (a) Histopathological analysis revealed an increased number of apoptotic bodies in the livers of CF-1 mice after treatment with two once-daily doses of 750 mg/kg, i.g. EGCG. Bars represent the mean of n = 4 – 5. Error bars represent the SEM.  = p < 0.01 compared to control. (b) Western blot analysis of liver homogenate showed an increase in the levels of cleaved caspase 3 in EGCG-treated mice, but not in vehicle-treated controls.
= p < 0.01 compared to control. (b) Western blot analysis of liver homogenate showed an increase in the levels of cleaved caspase 3 in EGCG-treated mice, but not in vehicle-treated controls.
Oxidative stress induced by EGCG
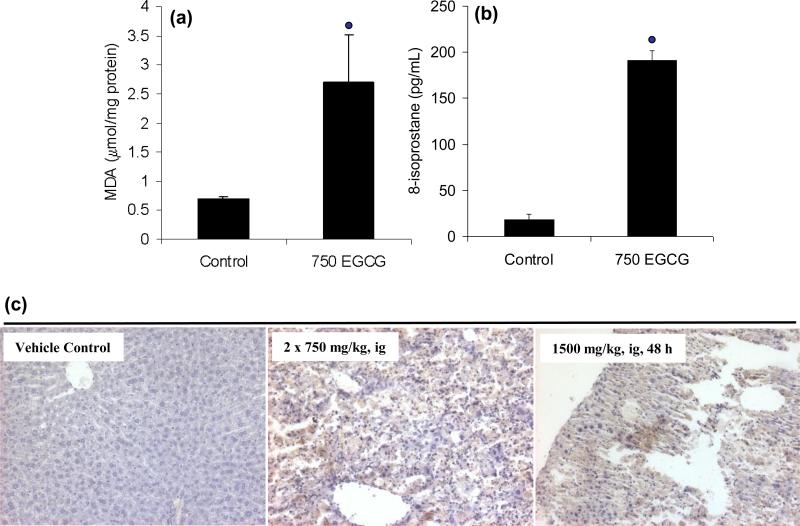
EGCG-mediated hepatotoxicity was associated with increased oxidative stress measured in both the liver and the plasma. Biochemical analysis showed a 3-fold increase in hepatic MDA 24 h after treatment with two once-daily doses of 750 mg/kg, i.g. EGCG, was observed compared to vehicle-treated control mice (Fig. 4). Similarly, plasma levels of 8-isoprostane, a non-enzymatic oxidation product of arachidonic acid, were increased from 18 pg/mL in vehicle-treated control mice to 191 pg/mL in EGCG-treated mice 24 h after two once-daily doses of 750 mg/kg, i.g. EGCG (Fig. 4). IHC analysis of liver samples showed that treatment with either two once-daily doses (750 mg/kg, i.g.) or a single dose of EGCG (1500 mg/kg, i.g.) resulted in increased positive staining for 4-HNE (Fig. 4). This further demonstrated EGCG-mediated increases in lipid peroxidation.
Figure 4.
EGCG-induced lipid peroxidation in male CF-1 mice. (a) Hepatic malonyldialdehyde and (b) plasma 8-isoprostane levels were increased in CF-1 mice after treatment with two once-daily doses of 750 mg/kg, i.g. EGCG. Bars represent the mean of n = 3 – 4. Error bars represent the SEM.  = p < 0.01 compared to control. (c) IHC analysis showed that treatment with a single dose of 1500 mg/kg, i.g. EGCG or two once-daily doses of 750 mg/kg, i.g. EGCG increased staining for 4-HNE. Micrographs are representative of control and EGCG-intoxicated mice, and are at 200X magnification.
= p < 0.01 compared to control. (c) IHC analysis showed that treatment with a single dose of 1500 mg/kg, i.g. EGCG or two once-daily doses of 750 mg/kg, i.g. EGCG increased staining for 4-HNE. Micrographs are representative of control and EGCG-intoxicated mice, and are at 200X magnification.
MT and γH2AX were used as protein markers of oxidative stress. Western blot analysis showed that treatment of CF-1 mice with either a single dose of 1500 mg/kg, i.g. EGCG or two once-daily doses of 750 mg/kg, i.g. EGCG resulted in increased hepatic expression of MT compared to vehicle-treated control mice (Fig. 5). Similarly, there was an increase in hepatic γH2AX levels following treatment with two once-daily doses of 750 mg/kg, i.g. EGCG (Fig. 5).
Figure 5.
EGCG-induced oxidative stress in male CF-1 mice. Hepatic metallothionein I/II protein levels in CF-1 mice were increased by a single dose of 1500 mg/kg, i.g. or two once-daily doses of 750 mg/kg, i.g. EGCG. Hepatic γ-H2AX protein levels in CF-1 mice were increased by two once-daily doses of 750 mg/kg, i.g.
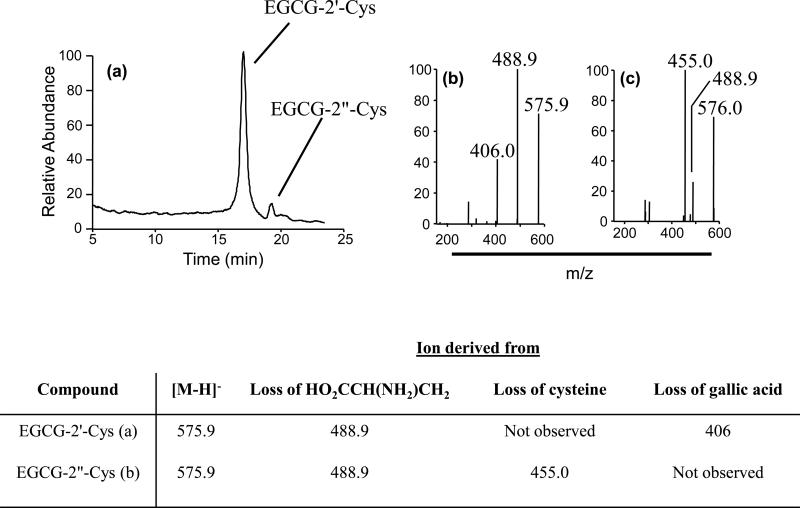
We have previously shown that EGCG-cysteine conjugates arise from the oxidation of EGCG to either a quinone or semi-quinone and subsequent covalent binding of the activated 2’- or 2”- carbon to the sulfhydryl group of cysteine (Sang et al., 2005). These metabolites therefore represent biomarkers for EGCG oxidation. Both EGCG-2’-cysteine and EGCG- 2”-cysteine were detected in the pooled 24 h urine of mice treated with a single dose of 1500 mg/kg, i.g. EGCG (Fig. 6).
Figure 6.
Formation of EGCG-cysteine conjugates in vivo. (a) LC-ESI/MS analysis of the urine from CF-1 mice (pooled from n = 5) treated with 1500 mg/kg, i.g. EGCG revealed the presence of two EGCG cysteine metabolites. MS2 analysis revealed fragmentation patterns consistent with (b) EGCG-2’-cys and (c) EGCG-2”-cys. MS analysis was conducted in the negative mode and structural determination was based on comparison with authentic standards.
Plasma cytokines induced by EGCG
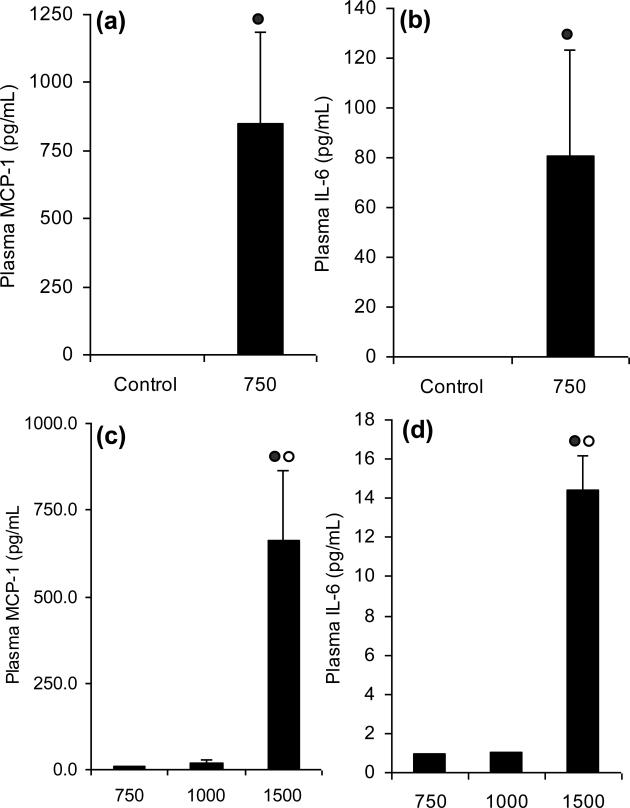
Treatment of CF-1 mice with two once-doses of 750 mg/kg, i.g. EGCG increased plasma levels of IL-6 from undetectable control levels to 80 pg/mL 24 h after the second dose (Fig. 7a). Similarly, plasma MCP-1 levels were increased by 2229% in EGCG-treated mice compared to vehicle-treated controls 24 h after the second dose (Fig. 7b). Similar increases in plasma IL-6 and MCP-1 were observed 48 h after single dose treatment with 1500 mg/kg, i.g. (Fig. 7c and d).
Figure 7.
EGCG-induced hepatotoxicity is associated with increased plasma levels of IL-6 and MCP-1. CF-1 mice were sacrificed (a, b) 24 h after 2 once-daily doses of 750 mg/kg, i.g. EGCG or (c, d) 48 h after a single dose of EGCG (750 – 1500 mg/kg,i.g.). Each bar represents the mean of n = 4 – 8. Error bars represent SEM.  = p < 0.01 compared to the control by Student's t test.
= p < 0.01 compared to the control by Student's t test.  = p < 0.05 vs all other treatment groups by one-way ANOVA with Tukey's post-test.
= p < 0.05 vs all other treatment groups by one-way ANOVA with Tukey's post-test.
Discussion
In the present study, we examined the potential hepatotoxicity of high doses of orally administered EGCG in CF-1 mice. Previous studies by others had reported that EGCG caused toxicity in the liver, kidney and gastrointestinal (GI) tract of Beagle dogs when given at high oral doses (Isbrucker et al., 2006). EGCG also caused GI toxicity in rats, but no hepatotoxicity was observed (Isbrucker et al., 2006). Although high dose EGCG administered by intraperitoneal injection was shown to be hepatotoxic in mice, there are no published reports regarding the hepatotoxicity of orally-administered EGCG in this species (Sang et al., 2005; Galati et al., 2006). In the present study, we assessed the toxic potential of oral EGCG in the mouse.
We observed that EGCG caused a dose and time-dependent decrease in the survival of CF-1 mice when given either as a single dose or as multiple once-daily doses. A total dose of 1500 mg/kg, i.g. was similarly potent whether given as a single dose or as two once-daily doses of 750 mg/kg, i.g (surviving fraction 15% vs. 20%). Interestingly, three or more once-daily doses of 500 mg/kg, i.g. EGCG (giving a total dose of 1500 mg/kg or greater) were less toxic, suggesting that there is a threshold total daily dose between 500 and 750 mg/kg, i.g. above which toxic potential dramatically increases. These results may suggest that at 750 mg/kg, i.g. EGCG damage occurs which cannot be repaired in the time between daily doses and the result is that multiple doses result in cumulative toxicity which does not occur at lower doses. This remains to be further studied.
Our results suggest that hepatotoxicity is the major underlying cause for increased mortality noted after either single dose or multiple daily administration of high dose oral EGCG. We observed increases in both plasma markers of liver damage (i.e. elevated ALT levels) as well as histopathological evidence of liver toxicity. We observed no effects on the kidneys or other major organ systems (data not shown). We also observed increases in plasma levels of IL-6 and MCP-1, which have been related to hepatic response to known hepatotoxicants including acetaminophen (James et al., 2003; Bourdi et al., 2007). The former appears to play a role in exacerbating toxicity, whereas the latter maybe involved in resolving hepatotoxic events. It has been previously reported that high oral dose EGCG (500 mg/kg, i.g., once daily) administered to Beagle dogs caused liver, kidney, and GI toxicity (Isbrucker et al., 2006). The authors reported that toxicity was observed in rats only at higher doses (2000 mg/kg, i.g.) and that the toxicity manifested as hemorrhagic lesions in the stomach and small intestine (Isbrucker et al., 2006). The fact that the mouse and the dog display hepatotoxicity, whereas the rat does not, may be related to the oral bioavailability of EGCG in the different species. Because the bioavailability of EGCG in the rat is relatively low (oral bioavailability = 1.6%), the levels reaching the liver are low and toxicity is confined to the gastrointestinal tract (Chen et al., 1997). In the mouse, where bioavailability is higher (oral bioavailability = 26%), greater concentrations of EGCG reach the liver and hepatotoxicity is observed (Lambert et al., 2003). Although the absolute bioavailability of EGCG in the dog has not been reported, the plasma levels of EGCG following oral administration were approximately 10-fold higher than those observed in the rat following treatment with a similar dose, and likely higher doses reach the liver in the dog (Chen et al., 1997; Isbrucker et al., 2006). Likewise, Isbrucker et al. have reported that the systemic bioavailability of EGCG was lower in fed Beagle dogs than in fasted Beagle dogs (Isbrucker et al., 2006). This decrease in bioavailability correlated with reduced sensitivity to the toxic effects of high dose oral EGCG. Our previous studies have suggested that the bioavailability of EGCG in humans is more similar to that in the mouse than that in the rat: this suggests that the mouse maybe a better predictor of EGCG hepatotoxicity in humans than is the rat (Lambert et al., 2003; Lu et al., 2003a; Lu et al., 2003b).
Our biochemical and IHC studies suggest that EGCG-induced hepatotoxicity in the mouse is related to induction of oxidative stress. This manifests as increased levels of MDA and 4-HNE and increased protein expression of MT and γH2AX in the liver. Plasma levels of 8-isoprostane, a non-enzymatic marker of arachidonic acid oxidation, were also increased by EGCG treatment. This is in agreement with the increasing body of data that suggests that at least some of the biological effects of EGCG are due to induction of oxidative stress (Frei and Higdon, 2003; Higdon and Frei, 2003; Rietveld and Wiseman, 2003; Elbling et al., 2005; Hou et al., 2005). Based on these previous studies and our current data, we suggest that at high doses, EGCG undergoes oxidation in the liver either enzymatically or through a non-ezymatic pathway (e.g. oxidation from reactive oxygen species leaked from the mitochondria) resulting in the formation of a reactive intermediate that can then generate reactive oxygen species, react with cellular macromolecules, or both, resulting in hepatotoxicity.
Based on allometric scaling, the doses of EGCG observed to cause toxicity in the study (500 – 1500 mg/kg) correspond to a dose in humans of 30 – 90 mg/kg assuming a daily requirement of 12 and 2000 kcal for mice and humans, respectively (Schneider et al., 2004). This corresponds to approximately 10.5 – 32 cups of green tea (if each cup is made from 2.5 g green tea leaves in 250 mL). Since normal tea consumption habits are to drink tea one cup at a time over the course of the day rather than as a large bolus dose, the present data do not indicate that tea consumption poses a significant risk for hepatotoxicity. Although at least one case study has reporte hepatotoxicity after consumption of as little as 6 cups/d of green tea infusion (Jimenez-Saenz and Martinez-Sanchez Mdel, 2006). Where a risk may exist, and where case studies have reported toxicity, is when high doses of dietary supplements containing concentrated or purified tea preparations are taken. Based on a search of Internet advertisements, supplements are commercially available with recommended doses of 2 – 12 mg/kg/d EGCG (www.fitnessfire.net, www.progena.com, www.lef.org, www.bulknutrition.com). These doses themselves are not within the apparent toxic range determined in this study, but they are similar to doses of green tea supplements associated with human hepatotoxicity observed in case-reports and the possibility of reaching the toxic threshold by exceeding the recommended dose is not insignificant (Bonkovsky, 2006; Federico et al., 2007). Further, these estimates of safety assume normal basal liver function and do not take into account variations in the genotype of phase II metabolizing enzymes such as catechol-O-methyltransferase, which may affect EGCG bioavailability and increase EGCG exposure (Wu et al., 2003). Further studies on the effects of such polymorphisms or reduced liver function on the toxic potential of EGCG are needed.
In summary, we have observed that high oral doses of the green tea polyphenol, EGCG, are hepatotoxic in mice. This toxicity appears to be related to induction of oxidative stress in the liver. The doses at which toxicity were observed are much higher than those typically delivered by normal tea consumption, but they are more readily achievable in the context of tea-based dietary supplements. Further studies on the mechanisms of EGCG hepatotoxicity and careful monitoring of biomarkers of toxicity in on-going and planned human trials are warranted.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Kathleen R. Roberts, Ba Liu, and Mousumi Bose. This work was supported by the National Institutes of Health [CA125780 to JDL; CA88961 to CSY]; the American Institute for Cancer Research [05A047 to JDL]; and by facilities supported by the National Institute of Environmental Health Sciences Center [grant E05022].
Abbreviations
- 4-HNE
4-hydroxynonenal
- ALT
alanine aminotransferase
- COMT
catechol-O-methyltransferase
- EGCG
(-)-epigallocatechin-3-gallate
- γH2AX
phosphorylated histone 2AX
- IHC
immunohistochemistry
- IL-6
interleukin-6
- LC/MS
liquid chromatography-mass spectrometry
- MCP-1
monocyte chemoattractant protein-1
- MDA
malonyldialdehyde
- MS2
tandem mass spectrometry
- MT
metallothionein I/II
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Critical reviews in food science and nutrition. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- Baltaziak M, Skrzydlewska E, Sulik A, Famulski W, Koda M. Green tea as an antioxidant which protects against alcohol induced injury in rats -- a histopathological examination. Folia Morphol (Warsz) 2004;63:123–126. [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer research. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Annals of internal medicine. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. The Journal of nutrition. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem Res Toxicol. 2007;20:208–216. doi: 10.1021/tx060228l. [DOI] [PubMed] [Google Scholar]
- Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaenously obese mice from hepatic steatosis and injury. Journal of Nutrition. 2008;138:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- Chen JH, Tipoe GL, Liong EC, So HS, Leung KM, Tom WM, Fung PC, Nanji AA. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. The American journal of clinical nutrition. 2004;80:742–751. doi: 10.1093/ajcn/80.3.742. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug metabolism and disposition: the biological fate of chemicals. 1997;25:1045–1050. [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM, Xu MJ, Hsu CH, Ranger-Moore J, Alberts DS. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev. 2006;15:2473–2476. doi: 10.1158/1055-9965.EPI-06-0365. [DOI] [PubMed] [Google Scholar]
- Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, Berger W, Micksche M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb J. 2005;19:807–809. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- Federico A, Tiso A, Loguercio C. A case of hepatotoxicity caused by green tea. Free radical biology & medicine. 2007;43:474. doi: 10.1016/j.freeradbiomed.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. The Journal of nutrition. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Galati G, Lin A, Sultan AM, O'Brien P J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free radical biology & medicine. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical reviews in food science and nutrition. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of Action of (-)-Epigallocatechin-3-Gallate: Auto-oxidation-Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer research. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- Jimenez-Saenz M, Martinez-Sanchez Mdel C. Acute hepatitis associated with the use of green tea infusions. Journal of hepatology. 2006;44:616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer research. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. Jama. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- Kuzu N, Bahcecioglu IH, Dagli AF, Ozercan IH, Ustundag B, Sahin K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. Journal of gastroenterology and hepatology. 2007 doi: 10.1111/j.1440-1746.2007.05052.x. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. The Journal of nutrition. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug metabolism and disposition: the biological fate of chemicals. 2003a;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug metabolism and disposition: the biological fate of chemicals. 2003b;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. European journal of clinical pharmacology. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJ, Chen TS. Diverse antioxidants protect against acetaminophen hepatotoxicity. Journal of biochemical and molecular toxicology. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. The Journal of nutrition. 2003;133:3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- Sai K, Kai S, Umemura T, Tanimura A, Hasegawa R, Inoue T, Kurokawa Y. Protective effects of green tea on hepatotoxicity, oxidative DNA damage and cell proliferation in the rat liver induced by repeated oral administration of 2-nitropropane. Food Chem Toxicol. 1998;36:1043–1051. doi: 10.1016/s0278-6915(98)00073-8. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chemical research in toxicology. 2005;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment--empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–347. doi: 10.1016/j.yrtph.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer research. 2003;63:7526–7529. [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicology and applied pharmacology. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life sciences. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]