Abstract
Research with dopamine D1 receptor antagonists or neuronal inactivating agents suggests dissociable regulation of cocaine-seeking behavior by the rostral and caudal basolateral amygdala. In the present study, discrete infusions of the D1 receptor agonist SKF 81297 (0.0–0.8 μg/side) were compared to the D1 receptor antagonist SCH 23390 (0.0–2.0 μg/side) to demonstrate directly the importance of D1 receptor mechanisms within the rostral and caudal basolateral amygdala for their functional heterogeneity in regulating cocaine-seeking behavior. Under a second-order schedule, cocaine-seeking behavior was studied during maintenance (cocaine and cocaine cues present) and reinstatement (only cocaine cues present). Food-maintained responding examined specificity of maximal behaviorally effective doses of SKF 81297 and SCH 23390. Results demonstrated that the D1 agonist (0.4 or 0.8 μg) increased and D1 antagonist (1.0 μg) decreased cocaine-seeking behavior during maintenance when infused into caudal but not rostral basolateral amygdala. Cocaine intake was not affected by the agonist and was decreased by the antagonist. During reinstatement, the D1 agonist (0.4 μg) increased and D1 antagonist (1.0 μg) decreased cocaine-seeking behavior when infused into rostral but not caudal basolateral amygdala. In tests for behavioral specificity, the above effective doses of SKF 81297 and SCH 23390 used in self-administration experiments did not alter food-maintained responding. However, the 2.0 μg dose of SCH 23390 suppressed drug- and food-maintained responding after infusion into both subregions. Collectively, findings indicate dissociable sensitivity to D1 receptor ligands within the caudal and rostral basolateral amygdala for altering cocaine-seeking behavior under different conditions modeling phases of addiction.
Keywords: Reinstatement, SCH 23390, Second-order schedule, Self-administration, SKF 81297
INTRODUCTION
Clinical findings demonstrate that re-exposure to stimuli previously paired with cocaine or to cocaine itself following a period of abstinence facilitates the onset of craving and relapse (Wallace, 1989; Childress et al., 1999). Examining drug relapse behavior in laboratory animals is accomplished by allowing subjects a fixed period of drug self-administration, later extinguishing drug-reinforced responding, and subsequently assessing the ability of drug-associated stimuli or drug re-exposure to reinstate responding (Shaham et al., 2003). Several studies strongly implicate the basolateral amygdala (BLA) in regulating reinstatement of drug-seeking behavior induced by cocaine-associated stimuli (Meil & See, 1997; Kruzich & See, 2001; Ledford et al., 2003; Yun & Fields, 2003). Moreover, the BLA regulates acquisition and maintenance of drug-seeking behavior emitted under a second-order schedule of cocaine reinforcement (Whitelaw et al., 1996; Kantak et al., 2002).
The role of the BLA in regulating drug-seeking behavior may be more complex than commonly viewed given growing evidence that the BLA is comprised of discrete subregions. Neuroanatomical investigations show that caudal BLA (cBLA) and rostral BLA (rBLA) projection neurons are topographically organized and innervate distinct compartments within the nucleus accumbens (NAc) (Gröenewegen et al., 1991). Research from our laboratory and others have provided evidence for dissociable regulation of drug- or food-seeking behavior by the cBLA and rBLA (Kantak et al., 2002; Alleweireldt et al., 2006; McLaughlin & Floresco, 2007). We demonstrated that in rats trained to self-administer cocaine, lidocaine inactivation of the cBLA attenuated drug-seeking behavior emitted under a second-order schedule of drug reinforcement but not under reinstatement test conditions where the drug-associated stimulus alone was presented. Lidocaine inactivation of the rBLA produced an opposite pattern of results. Factors contributing to subregional differences in the regulation of drug-seeking behavior may be the higher basal level of dopamine and greater number of dopamine-containing neurons in the cBLA than rBLA (Young & Rees, 1998; Brinley-Reed & McDonald, 1999). Differences in magnitude of basal dopamine neurotransmission in the cBLA and rBLA also suggest that there may be differences in dopamine receptor sensitivity within the two BLA subregions. To directly demonstrate the importance of D1 receptor mechanisms within the cBLA and rBLA for dissociable regulation of cocaine-seeking behavior, we used both an agonist and antagonist approach. This approach differs significantly from those used in past research (either dopamine receptor antagonism or neuronal inactivation) to examine functional heterogeneity of the cBLA and rBLA (Kantak et al., 2002; Alleweireldt et al., 2006; McLaughlin & Floresco, 2007). Furthermore, we used a model of maintenance and reinstatement that relies on second-order scheduling of drug delivery and stimulus presentation (Kantak et al., 2002), as research indicates that this type of reinforcement schedule provides a reliable means of investigating neurobiological substrates underlying drug-seeking behavior (Everitt & Robbins, 2000). Importantly, a second-order schedule separates incentive value of cocaine from conditioned cocaine-associated cues, enabling evaluation of the effects of drug self-administration on the reinforcing impact of these cues and assessment of cocaine-seeking under the control of cocaine-associated cues during cue-induced reinstatement (Spealman et al. 1999).
MATERIALS AND METHODS
Subjects
Male Wistar rats [Crl(WI)BR rats, Charles River Breeding Laboratories, Portage, MI], weighing approximately 276–300 g upon arrival, were maintained at 90% of an upwardly adjusting ad libitum body weight throughout the duration of the study by providing 16 g of food per day. Between experimental sessions, rats were allowed unlimited access to water in their home cages. Rats were individually housed in clear plastic cages (43 X 22 X 20 cm) in a temperature- (21–23 °C) and light- (08:00 h on, 20.00 h off) controlled vivarium. Policies and procedures set forth in “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” were followed, as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved all protocols.
Apparatus
Experimental chambers (model ENV-008CT; Med Associates, St. Albans, VT, USA) were each equipped with two response levers positioned 8 cm to the left and right of a center mounted food receptacle and 7 cm from the grid floor. Connected to the food receptacle was a pellet dispenser that delivered 45-mg food pellets (Dustless Precision Pellets; Bio-Serv, Frenchtown, NJ, USA). A white stimulus light was mounted 7 cm above each lever. Each chamber was outfitted with a single channel fluid swivel (Instech Solomon, Plymouth Meeting, PA, USA) and spring leash assembly that were connected to a counterbalanced arm assembly (Med Associates) that allowed the animal to move freely in the chamber. A sound-attenuating cubicle (model ENV-108 M; Med Associates) equipped with a houselight to provide general illumination, a fan to provide ventilation, and an 8 ohm speaker to provide auditory stimuli, enclosed each chamber. Motor-driven syringe pumps (model PHM-100, Med Associates) located immediately outside each sound-attenuating cubicle were used for intravenous drug delivery. A standard personal computer programmed in Medstate Notation™ and connected to an interface (Med Associates) controlled experimental events.
Drugs and Intracranial Infusion Procedures
Drugs used were cocaine hydrochloride (gift from the National Institute on Drug Abuse, Bethesda, MD), the D1-like receptor agonist (+)- SKF-81297 hydrobromide, and the D1-like receptor antagonist (+)- SCH 23390 hydrochloride. Both D1 ligands were purchased from Sigma (St. Louis, MO, USA). Cocaine was dissolved in sterile 0.9% saline solution containing 3 IU heparin/ml to a final concentration of 2.68 mg/ml. For all self-administration sessions, a 1.0 mg/kg unit infusion dose of cocaine was used and delivered intravenously at a rate of 1.8 ml/min. To attain a dose of 1.0 mg/kg, infusion volume was adjusted for body weight, resulting in drug delivery times of 1.2 sec/100 g body weight in individual rats. During saline self-administration sessions, the diluted heparin/saline solution was used.
SKF-81297 was dissolved in sterile 0.9% saline to produce a stock concentration of 1.6 mg/ml or 3.2 mg/ml (12 microinjections total). Lower concentrations were made via serial dilution using sterile 0.9% saline. SCH 23390 was dissolved in sterile 0.9% saline to a produce a stock concentration of 4 mg/ml; the solution was made fresh on testing days immediately before intracranial infusions took place (11 microinjections total). Lower concentrations were made via serial dilution using sterile 0.9% saline. The pH of all solutions for intracranial infusion, including 0.9% saline, was 5.0. Rats received bilateral infusions of an assigned concentration of drug or vehicle (sterile saline) into either the rBLA or cBLA at a rate of 0.59 μl/min immediately prior to each test session. The 28 gauge stainless steel infusion cannula extended 1 mm beyond the guide cannula tip. The infusion cannula was left in place for 1 minute following the infusion.
Surgery and Histology
Rats were anesthetized with an intraperitoneal injection of 90 mg/kg ketamine plus 10 mg/kg xylazine. To enable intravenous delivery of cocaine or saline during self-administration sessions, a catheter made of silicon tubing (inner diameter = 0.51 mm; outer diameter = 0.94 mm) was implanted into the right jugular vein. The catheter ran subcutaneously under the neck, exited through an incision at the top of the head, and was attached to an L-shaped pedestal mount (Plastics One, Roanoke, VA, USA). Subsequent to catheter implantation, 0.1 ml of a solution containing 1.0 mg methohexital sodium (Brevital; King Pharmaceuticals, Bristol, TN, USA) was infused intravenously as needed to maintain anesthesia. After suturing the neck incision, the rat was placed in a stereotaxic frame and bilateral 22 gauge stainless steel guide cannulae (Plastics One) were implanted into the cBLA (n=8; anteroposterior (AP) −3.7 mm, lateral ± 5.0 mm, dorsoventral −8.2 mm) or rBLA (n=9; AP −2.0 mm, lateral ± 4.5 mm, dorsoventral −7.6 mm). Guide cannulae were positioned 1 mm above the intended sites and placements were based on the bregma coordinate system provided by the Swanson (1992) atlas. The guide cannulae, pedestal and three stainless steel anchoring screws were attached to the skull and permanently imbedded in dental cement. Two 28 gauge stainless steel obturators (Plastics One) were used to occlude guide cannulae between infusions. Wounds were treated daily with topical furazolidone powder (Veterinary Products Laboratories, Phoenix, AZ, USA) until healed and rats were allowed one week of recovery from surgery before initiation of the study. Catheters were maintained by daily flushing (Monday-Friday) with 0.1 ml of a 0.9% saline solution containing 3 IU heparin (Baxter Healthcare Corporation, Deerfield, IL, USA) and 6.7 mg Timentin (GlaxoSmithKline, Research Triangle Park, NC, USA). On Fridays, a locking solution consisting of glycerol and undiluted (1000 IU/ml) heparin (3:1) was used to fill the catheter dead space and minimize blockages. This solution remained in catheters until Monday, when it was removed and replaced with the heparin/saline solution prior to the start of behavioral sessions. Additionally, catheters were checked weekly for patency by infusing a 1.0 mg/0.1 ml solution of Brevital intravenously, which produces a rapid temporary loss of muscle tone. A new catheter was implanted into either the left jugular vein or right femoral vein to replace a leaking or nonfunctional catheter.
Upon completion of the studies, rats were given an overdose of sodium pentobarbital and then intracardially perfused with saline and 10% formalin solution. Brains were extracted, post-fixed in 10% formalin for 3–4 days, and then stored in 30% sucrose at 4 °C overnight. 50 μm coronal sections were collected using a cryostat. Sections were then mounted on gelatin-coated slides and stained with thionin to verify bilateral infusion cannulae placements.
Experimental Procedures
Second-order schedule training
Prior to surgery, rats were trained to press a lever under a fixed-ratio 1 (FR1) schedule of food pellet delivery. After rats learned to rapidly press the lever for 50 pellets, right jugular vein catheters and bilateral guide cannulae were implanted. After a week of recovery from surgery, 2 hr cocaine self-administration sessions began. Rats were incrementally trained to self-administer 1.0 mg/kg cocaine under a fixed-interval (FI) 5-min (FR5: S) second-order schedule of drug delivery, as previously described (Kantak et al., 2002). Under the FI 5-min (FR5: S) schedule, every fifth press (FR5) on the active lever during the FI resulted in presentation of a 2-sec conditioned stimulus light (brief CS+) located above the active lever. Responses on the inactive lever were counted separately, but produced no scheduled consequences. For half the rats, the right lever was designated as the active lever and the left lever was designated as the inactive lever. This order was reversed for remaining rats. Intravenous cocaine delivery was contingent upon completion of five responses on the active lever after the 5-min FI elapsed. The light above the active lever remained illuminated for the duration of the infusion as well as for the 20-s timeout period that followed each infusion (prolonged CS+), while the house light was extinguished during the timeout. A 70 db contextual sound stimulus, either an intermittent tone (70 db; 7 kHz: 0.5 sec duration every sec) or continuous white noise (70 db) was counterbalanced across rats and presented for the duration of each session. Baseline training sessions were conducted five days a week during the light phase, and continued until cocaine intake was stable (number of infusions did not deviate by more than 20%) and the number of responses on the inactive lever was no greater than 25 for each session over a 5-day period.
Discrimination Baseline
After rats were trained to self-administer cocaine under a second-order schedule, discrimination training procedures were implemented to provide self-administration training with unique cocaine-associated (S+) and saline-associated (S−) sound and light cues according to methods outlined in Kantak et al., (2002). This aspect of training was included because it facilitates reliable reinstatement of cue-controlled drug-seeking behavior over repeated test sessions without any additional access to cocaine (Weiss et al., 2000). Briefly, rats were given two 1-hr self-administration sessions, separated by one hour, each day during the discrimination phase. For half the rats, cocaine was available for intravenous delivery during the first session of the day followed by intravenous saline delivery during the second session. The order was reversed for remaining rats. During cocaine discrimination sessions, the same contextual cue, either intermittent white noise or tone, that was presented during baseline training sessions was presented throughout the 1-hr drug session. Brief and prolonged CS+ light cues were presented as above. During saline discrimination sessions, the opposite sound stimulus than the one used during drug sessions was presented. To provide a unique visual cue during saline sessions, the stimulus light located above the active lever flashed for the brief CS− presentations during the FI and for the prolonged CS− presentations during saline delivery and 20-sec TO periods. Once rats were successfully discriminating between cocaine and saline, they were tested for effects of SKF 81297 or SCH 23390 (see below) infused bilaterally into the cBLA or rBLA on drug-seeking and drug-taking behavior maintained under a FI 5-min (FR5: S) schedule of cocaine delivery that utilized cocaine-associated contextual and conditioned cues.
Extinction
Following maintenance testing, rats underwent training to extinguish lever responding, as described previously in Kantak et al. (2002). Briefly, rats were placed into the operant chambers in the absence of cocaine or saline. S+ (cocaine-associated) and S− (saline-associated) conditioned light and discriminative contextual cues were also omitted, to avoid reducing the motivational salience of cues. Extinction sessions were 2-hr in duration and continued for at least 10 sessions and until responding on the active lever was less than 10% of the baseline rate for each of three consecutive sessions. Reinstatement testing (see below) commenced immediately following extinction.
Maintenance Testing
Following successful discrimination training between cocaine and saline, bilateral infusions of SKF 81297 (0.0, 0.1, 0.2, 0.4 or 0.8 μg/side; Experiment 1) and SCH 23390 (0.0, 0.5, 1.0 or 2.0 μg/side; Experiment 2) were made into the cBLA or rBLA in a counterbalanced order 5-min prior to a 1-hr cocaine self-administration test session with S+ (cocaine-associated) sound and light cues under the FI 5-min (FR5: S) schedule, wherein cocaine-seeking behavior was maintained by both cocaine availability and cocaine- associated cues. In certain cases, a dose of 1.6 μg/side SKF 81297 was used as a negative drug dose control. Rats underwent maintenance testing following saline or D1 ligand infusion every third day. Discrimination sessions were conducted on intervening days.
Reinstatement Testing
Following maintenance testing and response extinction training, cocaine cue reinstatement testing began. Reinstatement testing took place every third day, and animals remained in their home cages on intervening days. During reinstatement tests, intravenous cocaine or saline were not available for self-administration. In order to assess discriminative control over active lever responding, the first test session always consisted of presenting the S−(saline-associated) cues. The S− contextual sound cue was continuously presented during the 1-hr test session and the CS− light cue was presented according to a FI 5-min (FR5: S) schedule. In this way, the brief CS− would flash for 2-sec following completion of every fifth response during the FI and the prolonged CS− would flash for a length of time equivalent to the infusion duration plus the 20-sec TO period after the FI had elapsed and five responses were completed. Over the next several sessions, S+ (cocaine-associated) cues were presented and SKF 81297 (0.0, 0.1, 0.2, 0.4, or 0.8 μg/side; Experiment 1) and SCH 23390 (0.0, 0.5, 1.0, or 2.0 μg/side; Experiment 2) were bilaterally infused into either the cBLA or the rBLA 5-min prior to testing under the FI 5-min (FR5: S) schedule, wherein cocaine-seeking behavior was maintained by cocaine-associated cues alone. Order of infusions was counterbalanced across rats. Similar to the S− test session, the S+ contextual sound cue was presented throughout the 1-hr reinstatement test sessions, and the CS+ light cue was presented according to an FI 5-min (FR5: S) schedule, as described above.
Food-Maintained Responding
To determine if alterations in drug-seeking behavior after SKF 81297 and SCH 23390 treatments were attributable to nonspecific changes in lever responding, the effects of these D1 ligands on food-maintained responding were examined at the end of the study for each group of rats. No sound or light cues were used during these sessions in which an FR5 schedule of food pellet delivery was used. An FR5 response schedule was used for this control condition to mirror the FR5 response requirement used for drug delivery during self-administration maintenance tests and the FR5 response requirement used for stimulus presentation during reinstatement tests. After baseline responding was stable, rats in the agonist study were first infused with vehicle and then three days later with the same dose of SKF 81297 that generated significant changes in responding during maintenance and reinstatement tests. Thus, the cBLA group received vehicle and 0.8 μg/side and the rBLA group received vehicle and 0.4 μg/side of SKF 81297, administered 5 min before 1-hr test sessions. Also following stable baseline responding, rats in the antagonist study underwent testing with vehicle, 1.0, and 2.0 μg/side SCH 23390 administered 5 min before 1-hr sessions that were each separated by three days of regular food-maintained responding.
Data Analyses
In Experiment 1 (SKF 81297), seven of eight subjects with bilateral cannulae in the cBLA and all nine subjects with bilateral cannulae in the rBLA completed cocaine self-administration and reinstatement phases of the study. One rat subsequently died from the cBLA group and two rats subsequently died from the rBLA group, resulting in group sizes of six and seven, respectively, for the food self-administration phase of the study. In Experiment 2 (SCH 23390), eight subjects with bilateral cannulae in the cBLA and eight subjects with bilateral cannulae in the rBLA completed cocaine self-administration and reinstatement phases of the study. Two rats subsequently died from each of the cBLA and rBLA groups, resulting in group sizes of six for the food self-administration phases of the study.
For each experiment, three dependent measures were calculated: (1) number of active lever responses (2) number of inactive lever responses and (3) number of reinforcers earned (infusions or prolonged CS presentations). Drug-seeking behavior was operationally defined as active lever responding maintained by drug-associated cues either during the second-order schedule of drug delivery (Goldberg et al., 1981) or following response extinction training (Spealman et al., 1999). Baseline measures of performance were based on data averaged over the last three cocaine, saline, and extinction sessions for the cocaine self-administration study, and on data averaged over the last three food pellet sessions for the food self-administration study.
To compare sites for the cocaine study, a series of two-factor ANOVA (site X dose or site X condition, with repeated measures across dose or condition) was used to analyze separately baseline, maintenance and reinstatement test measures. To compare sites for the food study, a series two-factor ANOVA (site X condition, with repeated measures across condition) was used to compare measures taken at baseline to measures taken after vehicle and SKF 81297 (Experiment 1) or after vehicle and SCH 23390 treatments (Experiment 2). Tukey HSD tests were used for post-hoc analyses.
RESULTS
Histology
Bilateral placements were confirmed for all seven rats with cannulae aimed at the cBLA and all nine rats with cannulae aimed at the rBLA in the agonist study (Figure 1A), and for all eight rats with cannulae aimed at the cBLA and all eight rats with cannulae aimed at the rBLA in the antagonist study (Figure 1B). Placements were within 0.5–0.6 mm of the intended position in the AP plane and within the cBLA and rBLA anatomical ranges (Swanson, 1992). These analyses indicate that there was a sufficient separation of cBLA and rBLA placements to delineate the two discrete regions of interest. Cannulae placements also verified that infusions with SKF 81297 and SCH 23390 predominantly encompassed the region of interest. Microscopic examination failed to reveal mechanical damage in either the cBLA or rBLA, other than that generated by insertion of the guide and infusion cannulae. Representative low magnification (2X) photographs of guide and infusion cannulae tracks for cBLA and rBLA placements are shown in Figure 1C. Furthermore, there was no evidence of cell loss following 11–12 microinjections, as illustrated in representative high magnification (20X) photographs in Figure 1D of the microinjection areas of the cBLA (SKF 81297 infusions) and rBLA (SCH 23390 infusions).
Figure 1.
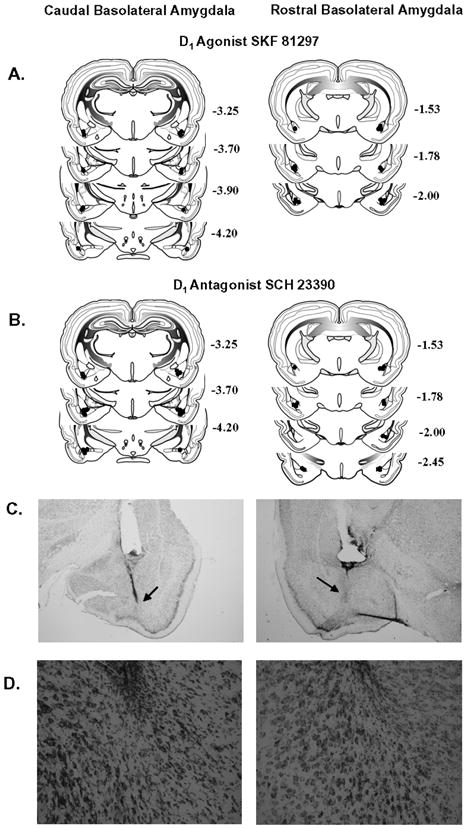
Schematic drawings representing coronal sections of the cBLA (left) and rBLA (right) subregions. Circles indicate the location of the infusion cannula tip in the SKF 81297 study (A) and SCH 23390 study (B). All drawings are based on the atlas of Swanson (1992), with the anterior-posterior references measured from bregma. Each placement is shown at the midpoint of its anterior-posterior extent. (C) Representative low magnification (2X) photographs of guide cannulae placements within the cBLA (left) and rBLA (right); arrows indicate infusion cannulae tracks, and (D) representative high magnification (20X) photographs of microinjection areas within the cBLA (left; SKF 81297 infusions) and rBLA (right; SCH 23390 infusions).
Experiment 1. D1-like agonistSKF 81297
Baseline Self-Administration
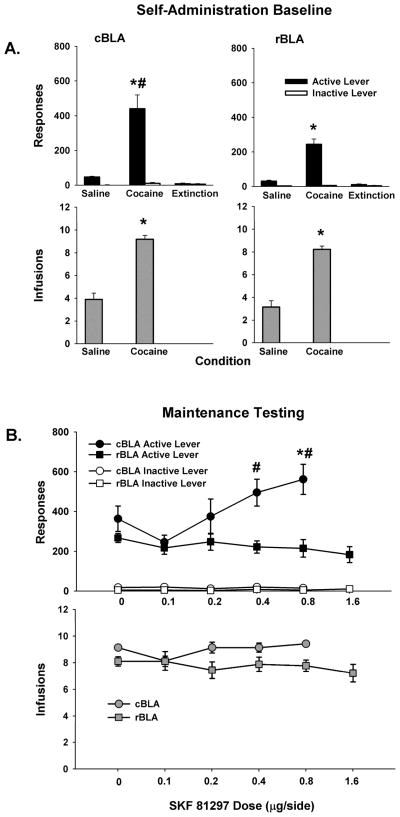
Rats from both groups were able to successfully discriminate cocaine from saline as well as the active from inactive lever prior to commencing maintenance test sessions with the D1-like agonist SKF 81297. Analysis of active lever responses during baseline self-administration sessions revealed significant a main effect of condition (F [2, 28] = 68.5, p < 0.001) as well as a significant interaction of site X condition (F [2, 28] = 6.1, p ≤ 0.01). Post-hoc analyses of the site X condition interaction revealed that active lever responses were significantly greater in the cBLA group than the rBLA group during cocaine baseline sessions (p ≤ 0.01) but not during saline baseline or extinction sessions (Figure 2A, top panels). This site-specific difference during cocaine baseline sessions was caused primarily by a single rat in the cBLA group (subject # 666) that maintained a cocaine baseline rate of responding that was ~ 2-fold greater than the average cocaine baseline for the remaining rats in the cBLA group. Variability generated by this rat, however, did not necessitate transforming data prior to analysis. Inactive lever responses for both groups averaged fewer than 15 across cocaine, saline, and extinction conditions, with no significant differences between sites, over conditions or for the interaction of site X condition (Figure 2A, top panels). As expected, the number of infusions earned during the cocaine baseline condition was significantly greater than the number earned during the saline baseline condition (F [2, 28] = 264, p < 0.001), with no significant differences between sites or for the interaction of site X condition (Figure 2A, bottom panels).
Figure 2.
Baseline behavior during saline and cocaine self-administration sessions and response extinction sessions for cBLA (left) and rBLA (right) groups of subjects, as well as maintenance test session behavior after 0.0–1.6 μg/side SKF 81297 infusions into the cBLA and rBLA. Values are the mean ± SEM number of active and inactive lever responses (A-B, top) and number of infusions earned (A-B, bottom). * p ≤ 0.05 compared to the saline and extinction conditions or compared to 0.0 μg/side SKF 81297, and # p ≤ 0.05 compared to the same condition in the rBLA group.
Maintenance Testing with SKF 81297
During maintenance test sessions, SKF 81297 dose-dependently increased cocaine-seeking behavior after its infusion into the cBLA but not rBLA, without affecting cocaine intake. Analyses of active lever responses revealed significant main effects of site (F [1, 56] = 9.7, p < 0.01) and dose (F [4, 56] = 5.5, p < 0.001), as well as a significant site X dose interaction (F [4, 56] = 6.8, p < 0.01). Post-hoc analyses of the site X dose interaction revealed that active lever responses were significantly greater (p < 0.01) following pretreatment of the cBLA with 0.8 μg/side SKF 81297 compared to the 0.0 μg/side vehicle control dose (Figure 2B, top panel). Active lever responses associated with infusion of other doses of SKF 81297 (0.1, 0.2, and 0.4 μg/side) into the cBLA did not significantly differ from vehicle control. Further post-hoc analyses revealed that there were no changes in active lever responses following pretreatment with 0.1 to 0.8 μg/side SKF 81297 infused into the rBLA compared to vehicle control (Figure 2B, top panel). Comparisons between sites indicated that active lever responses were significantly greater following infusion of 0.4 μg/side (p < 0.001) and 0.8 μg/side (p < 0.001) SKF 81297 into the cBLA relative to infusion of these doses into the rBLA. In order to investigate whether a higher dose of the agonist infused into the rBLA would produce a significant change in active lever responses, rats in this group were additionally given bilateral infusions of 1.6 μg/side SKF 81297. A separate one-factor repeated measures ANOVA indicated no significant changes in active lever responses (p < 0.40) as a function of SKF 81297 dose (0.1–1.6 μg/side and vehicle). Inactive lever responses for both groups averaged 20 or less, with no significant differences between sites, over conditions or for the interaction of site X condition (Figure 2B, top panel).
For the number of cocaine infusions earned during maintenance test sessions, there was a significant main effect of site (F [1, 56] = 9.9, p < 0.01), but not of dose or the interaction of site X dose. Post-hoc testing of the site main effect indicated that cocaine infusions were significantly greater in number in the cBLA group than rBLA group (Figure 2B, bottom panel), but this group difference was less than two on average. Pretreatment with SKF 81297 into either the cBLA or rBLA did not result in significant changes in cocaine infusions compared to vehicle control. Moreover, the highest dose of 1.6 μg/side SKF 81297 infused into the rBLA also failed to alter number of infusions earned (p < 0.68 based on one-factor repeated measures ANOVA analysis (Figure 2B, bottom panel).
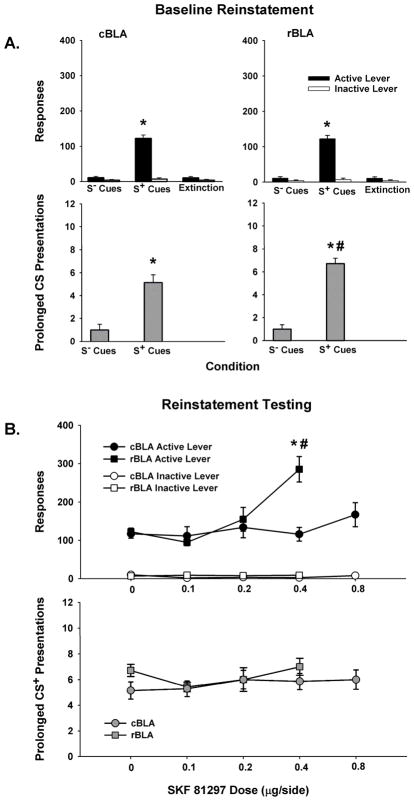
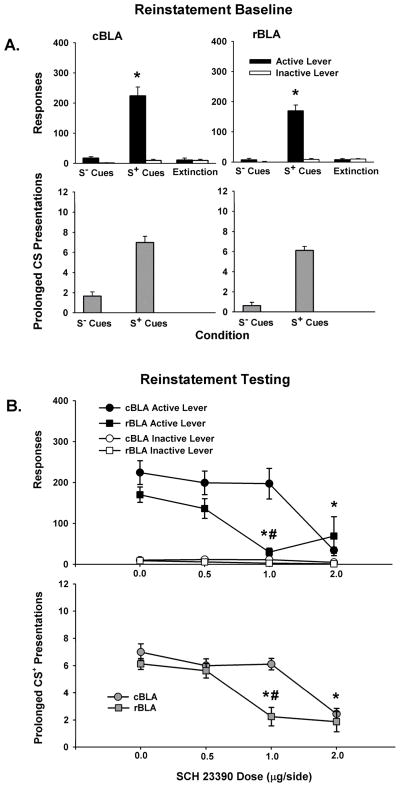
Baseline Reinstatement
Testing with S− (saline-associated) and S+ (cocaine-associated) contextual and conditioned cues revealed that active lever responses were under stimulus control in both groups of rats prior to commencing reinstatement test sessions with the D1-like agonist SKF 81297. For active lever responses during baseline reinstatement sessions, there was a significant main effect of condition (F [2, 24] = 137, p < 0.001), but not of site or a site X condition interaction. Post-hoc testing of the condition main effect indicated that S+ cues reinstated active lever responses significantly (p < 0.001) above levels produced by S− cues and extinction training in both groups (Figure 3A, top panels). Furthermore, active lever responses did not significantly differ between extinction and the S− test condition for either group. Inactive lever responses for both groups averaged 10 or less across extinction, S+ and S− conditions, with no significant differences between sites, over conditions or for the site X condition interaction (Figure 3A, top panels).
Figure 3.
Baseline behavior during S− (saline-associated) and S+ (cocaine-associated) cue reinstatementsessions and response extinction sessions for the cBLA (left) and rBLA (right) groups of subjects, as well as reinstatement test session behavior after 0.0–0.8 μg/side SKF 81297 infused into the cBLA and rBLA. Values are the mean ± SEM number of active and inactive lever responses (A-B, top) and number of prolonged CS or CS+ light presentations earned (A-B, bottom). * p ≤ 0.05 compared to the S− and extinction conditions or compared to 0.0 μg/side SKF 81297, and # p ≤ 0.05 compared to the same condition in the cBLA group.
For number of prolonged CSlight presentations earned during baseline reinstatement sessions, there was a significant main effect of condition (F [1, 12] = 136, p < 0.001) and a significant site X condition interaction (F [1, 12] = 7.7, p < 0.05). Post-hoc testing of the site X condition interaction revealed that the rBLA group earned a significantly greater number of prolonged CS+ light presentations than the cBLA group (p < 0.05), whereas the number of prolonged CS− light presentations earned did not significantly differ between groups (Figure 3A, bottom panels). The group difference in number of prolonged CS+ light presentations was less than two on average.
Reinstatement Testing with SKF 81297
During reinstatement test sessions, SKF 81297 dose dependently increased cocaine-seeking behavior after its infusion into the rBLA but not cBLA. For active lever responses, there was a significant main effect of dose (F [3, 36] = 12.3, p < 0.001) and a significant site X dose interaction (F [3, 36] = 12.0, p < 0.001). Post-hoc testing of the site X dose interaction indicated that while there were no dose-related differences in active lever responses after SKF 81297 infusions into the cBLA, there were significant (p < 0.001) increases in active lever responses after infusion of 0.4 μg/side SKF 81297 into the rBLA compared to infusion of the 0.0 μg/side vehicle control dose (Figure 3B, top panel). Comparisons between sites revealed that following bilateral infusion of 0.4 μg/side SKF 81297, rats in the rBLA group generated a greater number of active lever responses (p < 0.05) than rats in the cBLA group. In order to investigate whether a higher dose of the agonist infused into the cBLA would produce a significant change in active lever responses, rats in this group were additionally given bilateral infusions of 0.8 μg/side SKF 81297. A separate one-factor repeated measures ANOVA indicated no significant change in active lever responses (p < 0.12) as a function of SKF 81297 dose (0.1–0.8 μg/side and vehicle). Inactive lever responses for both groups averaged 10 or less, with no significant differences between sites, over conditions or for the interaction of site X condition (Figure 3B, top panel).
Despite an increase in active lever responses during cue-controlled reinstatement tests by animals in the rBLA group following infusion of the 0.4 μg/side dose of SKF 81297, there was no corresponding change in prolonged CS+ light presentations earned during test sessions (Figure 3B, bottom panel). Analysis revealed no significant effects of site, dose or their interaction for this measure. Furthermore, the highest dose of 0.8 μg/side SKF 81297 infused into the cBLA also failed to alter the number of prolonged CS+ light presentation earned (p < 0.74 by one-factor repeated measures ANOVA analysis of dose in the cBLA group).
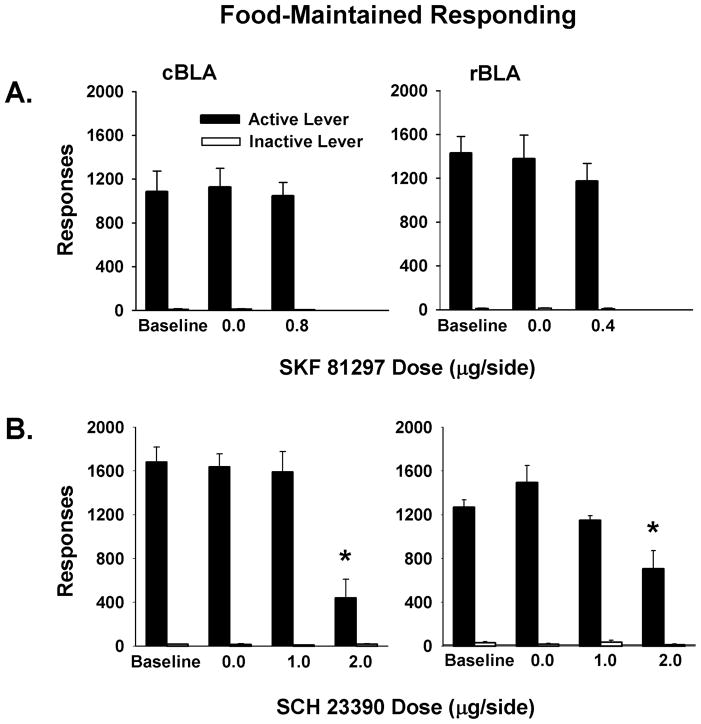
Food-Maintained Responding Following SKF 81297
To determine if the increases in active lever responses after bilateral infusions of the D1-like agonist SKF 81297 were attributable to non-specific changes in lever responding, rats were tested for the effects of SKF 81297 on food-maintained responding. For this test, vehicle (0.0 μg/side) and the maximum behaviorally effective dose of SKF 81297 that altered maintenance or reinstatement of drug-seeking behavior was used (0.8 μg/side for the cBLA group and 0.4 μg/side for the rBLA group). The main and interaction effects of site or drug for active and inactive lever responses were not significant (Figure 4A). Thus, responding maintained by non-drug reinforcement was not affected by doses of SKF 81297 that were maximally effective in increasing responding maintained by cocaine and cocaine-associated stimuli after SKF 81297 infusion into either the cBLA or rBLA.
Figure 4.
Food-maintained responding during baseline and test sessions. (A) Vehicle (0.0 μg/side) and the maximal behaviorally effective doses of SKF 81297 (0.8 μg/side in the cBLA group and 0.4 μg/side in the rBLA group). (B) Vehicle (0.0 μg/side), the site-selective (1.0 μg/side), and the site non-selective (2.0 μg/side) doses of SCH 23390 in the cBLA and rBLA. Values are the mean ± SEM number of active and inactive lever responses. * p ≤ 0.001 compared to 0.0 μg/side SCH 23390.
Experiment 2. D1-like antagonist SCH 23390
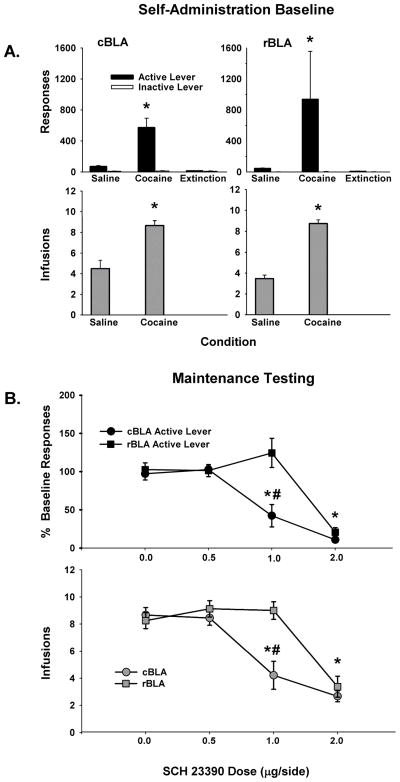
Baseline Self-Administration
Rats from both groups were able to successfully discriminate cocaine from saline as well as the active from inactive lever prior to commencing maintenance test sessions with the D1-like antagonist SCH 23390. Analysis of active lever responses during baseline self-administration sessions revealed a significant main effect of condition (F [2, 28] = 5.38, p < 0.01), as rats emitted significantly more responses during cocaine availability than during saline availability or extinction (p < 0.01) (Figure 5A, top panels). There were no significant differences between sites or for the interaction of site X condition. Inactive lever responses for both groups averaged fewer than 15 across cocaine, saline, and extinction, with no significant differences between sites, over conditions or for the interaction of site X condition. As expected, the number of infusions earned during the cocaine baseline condition was significantly greater than the number earned during the saline baseline condition (F [1, 14] = 117.2, p < 0.001), with no significant differences between sites or for the interaction of site X condition (Figure 5A, bottom panels).
Figure 5.
Baseline behavior during saline and cocaine self-administration sessions and response extinction sessions for cBLA (left) and rBLA (right) groups of subjects, as well as maintenance test session behavior after 0.0–2.0 μg/side SCH 23390 infused into the cBLA and rBLA. Values are the mean ± SEM number of active lever responses and inactive lever responses during baseline self-administration and mean ± SEM number of active lever responses as percent of baseline during maintenance (A-B, top) and number of infusions earned (A-B, bottom). * p ≤ 0.05 compared to the saline and extinction conditions or compared to 0.0 μg/side SCH 23390, and # p ≤ 0.05 compared to the same condition in the rBLA group.
While significant differences between sites were not observed during baseline self-administration with cocaine and saline, or during extinction training, analyses of responding in rats in the rBLA group revealed high variability compared to rats in the cBLA group. The variability in the rBLA group was caused primarily by a single rat (subject # 920) that maintained a cocaine baseline rate of responding that was ~16-fold greater than the average cocaine baseline for the remaining rats in the rBLA group. This high variability necessitated use of percent of baseline data transformation to compare responding of both groups during maintenance test sessions.
Maintenance Testing with SCH 23390
With behaviorally specific doses (see below), SCH 23390 attenuated cocaine-seeking behavior and cocaine intake following infusion into the cBLA but not rBLA during maintenance test sessions. Analyses of active lever responses revealed significant main effects of site (F [1, 42] = 18.6, p < 0.001) and dose (F [3, 42] = 26, p < 0.001), as well as a significant site X dose interaction (F [3, 42] = 6.9, p < 0.001). Post-hoc testing of the site X dose interaction revealed that active lever responses were significantly attenuated (p < 0.001) following pretreatment of both the cBLA and rBLA with 2.0 μg/side SCH 23390 compared to the 0.0 μg/side vehicle control dose (Figure 5B, top panel). Further analyses revealed that active lever responses were attenuated (p < 0.01) following pretreatment of the cBLA but not rBLA with 1.0 μg/side SCH 23390 compared to vehicle control. Active lever responses associated with infusion of 0.5 μg/side SCH 23390 into the cBLA and rBLA did not significantly differ from vehicle control. Comparisons between sites indicated that active lever responses were significantly attenuated following infusion of 1.0 μg/side (p < 0.001) into the cBLA relative to infusion of this dose into the rBLA (Figure 5B, top panel). Inactive lever responses for both groups averaged 15 or less, with no significant differences between sites, across doses or for the interaction of site X dose.
For the number of cocaine infusions earned during maintenance test sessions, there were significant main effects of site (F [1, 42] = 18.8, p < 0.001) and dose (F [3, 42] = 27.2, p < 0.001), as well as a significant site X dose interaction (F [3, 42] = 5.8, p < 0.01). Post-hoc testing of the site X dose interaction revealed that fewer infusions were earned following pretreatment of the cBLA with 1.0 (p < 0.001) and 2.0 μg/side (p < 0.001) SCH 23390 compared to vehicle control (Figure 5B, bottom panel). Number of infusions earned following pretreatment with 0.5 μg/side into the cBLA did not significantly differ from vehicle control. Further post-hoc analyses also revealed that fewer infusions were earned (p < 0.001) following pretreatment of the rBLA with 2.0 μg/side SCH 23390 compared to vehicle control, while pretreatment with the 0.5 and 1.0 μg/side doses SCH 23390 did not significantly alter the number of infusions earned compared to vehicle control (Figure 5B, bottom panel). A comparison between sites revealed that 1.0 μg/side SCH 23390 significantly reduced the number of infusions earned when infused into the cBLA relative to the rBLA (p < 0.05).
Baseline Reinstatement
Testing with saline-associated (S−) and cocaine-associated (S+) contextual and conditioned cues revealed that active lever responses were under discriminative stimulus control in both groups of rats prior to commencing reinstatement test sessions with the D1-like antagonist SCH 23390. For active lever responses during baseline reinstatement test sessions, there was a significant main effect of condition (F [2, 28] = 107.9, p < 0.001), but not of site or the interaction of site X condition. Post-hoc testing of the condition main effect indicated that S+ cues selectively reinstated active lever responding (p < 0.001) above levels produced by S− cues and extinction training in both groups (Figure 6A, top panels). Furthermore, active lever responses did not significantly differ between extinction and the S− test condition for either group. Analyses of inactive lever responses in both groups of rats revealed a significant main effect of condition (F [2, 28] = 7.8, p < 0.01), but not of site or the interaction of site X condition. Comparisons for the condition main effect revealed that more inactive lever responses were made during S+ cue test sessions than S− cue test sessions or extinction sessions (p< 0.01). Inactive lever responses for both groups, however, averaged 10 or less across extinction, S+ and S− conditions (Figure 6A, top panels).
Figure 6.
Baseline behavior during S− (saline-associated) and S+ (cocaine-associated) cue reinstatementsessions and response extinction sessions for the cBLA (left) and rBLA (right) groups of subjects, as well as reinstatement test session behavior after 0.0–2.0 μg/side SCH 23390 infused into the cBLA and rBLA. Values are the mean ± SEM number of active and inactive lever responses (A-B, top) and number of prolonged CS or CS+ light presentations earned (A-B, bottom). * p ≤ 0.05 compared to responding during S− and extinction conditions or compared 0.0 μg/side SCH 23390, and # p ≤ 0.05 compared to the same condition in the cBLA group.
For number of prolonged CS light presentations earned during baseline reinstatement sessions, there was a significant main effect of condition (F [1, 13] = 211.1, p < 0.001), but not of site or the interaction of site X condition. Based on the condition main effect, rats in both the rBLA and cBLA groups earned a significantly (p< 0.001) greater number of prolonged CS+ than CS− light presentations (Figure 6A, bottom panel).
Reinstatement Testing with SCH 23390
With behaviorally specific doses (see below), SCH 23390 attenuated cocaine-seeking behavior following infusion into the rBLA but not cBLA during reinstatement test sessions. Analyses of active lever responses revealed significant main effects of site (F [1, 42] = 5.8, p < 0.05) and dose (F [3, 42] = 12.2, p < 0.001), as well as a significant site X dose interaction (F [3, 42] = 4.9, p < 0.01). Post-hoc testing of the site X dose interaction revealed that active lever responses were significantly attenuated (p < 0.01) following pretreatment of both the cBLA and rBLA with 2.0 μg/side SCH 23390 compared to the 0.0 μg/side vehicle control dose (Figure 6B, top panel). Further analyses revealed that active lever responses were attenuated (p < 0.01) following pretreatment of the rBLA but not cBLA with 1.0 μg/side SCH 23390 compared to vehicle control. Active lever responses associated with infusion of 0.5 μg/side SCH 23390 into the rBLA and cBLA did not significantly differ from vehicle control. Comparisons between sites indicated that active lever responses were significantly attenuated following infusion of 1.0 μg/side (p < 0.001) into the rBLA but not cBLA. Analyses of inactive lever responses in both groups of rats revealed a significant main effect of dose (F [3, 42] = 3.4, p < 0.05), but not of site or the interaction of site X dose. Analysis of the dose main effect revealed that fewer inactive lever responses were made following pretreatment with 2.0 μg/side SCH 23390 into both the cBLA and rBLA compared to vehicle control (p < 0.05). Inactive lever responses for both groups, however, averaged 15 or less across all reinstatement test sessions with vehicle, 0.5, 1.0, and 2.0 μg/side doses SCH 23390 (Figure 6B, top panel).
For number of prolonged CS+ light presentations earned during reinstatement test sessions, there was a significant main effect of site (F [1, 42] = 10, p < 0.01) and dose (F [3, 42] = 28.5, p < 0.001) and a significant site X dose interaction (F [3, 42] = 4.5, p < 0.01).. Post-hoc testing of the site X dose interaction revealed that fewer prolonged CS+ light presentations were earned following pretreatment of the rBLA with both 1.0 μg/side (p < 0.001) and 2.0 μg/side (p < 0.001) SCH 23390 compared to vehicle control (Figure 6B, bottom panel). Number of prolonged CS+ light presentations earned following pretreatment with 0.5 μg/side into the rBLA did not significantly differ compared to vehicle control. Further post-hoc analyses revealed that fewer prolonged CS+ light presentations were earned following pretreatment of the cBLA with 2.0 μg/side SCH 23390 compared to vehicle control (p < 0.001), while pretreatment with the 0.5 and 1.0 μg/side SCH 23390 did not alter the number of prolonged CS+ light presentations earned compared to vehicle control (Figure 6B, bottom panel). Comparisons between sites indicated that the number of prolonged CS+ light presentations earned were significantly attenuated following infusion of 1.0 μg/side SCH 23390 into the rBLA relative to the cBLA (p < 0.001).
Food-Maintained Responding Following SCH 23390
To determine if the decreases in active lever responses after bilateral infusions of the D1-like agonist SCH 23390 were attributable to non-specific changes in lever responding, rats were tested for the effects of SCH 23390 on food- maintained responding using vehicle (0.0 μg/side) and the site-selective 1.0 and site non-selective 2.0 μg/side doses. Analyses of active lever responses during food-maintained responding test sessions revealed a significant main effect of dose (F [2, 20] = 2.4, p < 0.001) but not of site or the interaction of site X dose. Post-hoc testing of the dose main effect indicated that pretreatment with the 2.0 μg/side dose into both cBLA and rBLA decreased food-maintained responding (p< 0.001) but responding was not significantly altered following pretreatment with the 1.0 μg/side dose compared to vehicle control (Figure 4B). Inactive lever responses for both groups averaged 20 or less, with no significant differences between sites, across doses, or for the interaction of site X dose.
DISCUSSION
Functional Heterogeneity of Basolateral Amygdala Subregions
The use of D1 receptor-selective ligands generates insight into specific dopaminergic activity in the rBLA and cBLA that may underlie changes in drug-seeking behavior during different phases of addiction (maintenance vs. reinstatement). This rationale is consistent with previous work strongly implicating dopaminergic mechanisms in the BLA in regulating drug-seeking behavior and stimulus-cue associations (Weiss et al., 2000; See et al., 2001; Di Ciano & Everitt, 2004; Ciccocioppo et al., 2001; Alleweireldt et al., 2006; Berglind et al., 2006). The present findings demonstrate dissociable sensitivity of the rBLA and cBLA to dopamine D1 receptor activation and blockade for regulating drug-seeking behavior under a second-order schedule of cocaine reinforcement and cocaine cue presentation.
Infusion of SKF 81297 into the cBLA dose-dependently increased drug-seeking behavior when cocaine and cocaine cues were present (maintenance), whereas infusion of SKF 81297 into the rBLA dose-dependently increased drug-seeking behavior when only cocaine cues were present (reinstatement). It is noteworthy that increases in drug-seeking behavior were observed without concomitant changes in the number of primary or conditioned reinforcers earned (cocaine or prolonged CS+ light deliveries) after SKF 81297 infusions into the cBLA or rBLA. This parallels previous findings showing that after lidocaine inactivation of the cBLA under cocaine maintenance test conditions and of the rBLA under cocaine cue-controlled reinstatement test conditions, drug-seeking behavior was impaired without concomitant changes in the number of reinforcers earned, further supporting the view that the BLA is not implicated in the control of drug-taking behavior (Kantak et al., 2002: Whitelaw et al., 1996). These findings suggest a selective effect of D1 receptor activation within the cBLA and rBLA on drug-seeking behavior.
The failure of 0.4 and 0.8 μg/side SKF 81297, doses that dissociably increased cocaine-seeking behavior in the cBLA and rBLA during maintenance and cue-induced reinstatement, to alter FR5 food-maintained responding suggests that effects of SKF 81297 were also specific for cocaine and cocaine cue-controlled behavior. It is unlikely that these doses of SKF 81297 failed to increase food-maintained responding simply because of its relatively high baseline response rate, which could mask observing further increases. Notably, previous work has demonstrated that when baseline rates of food-maintained responding were relatively low, infusion of 3.0 μg/side SKF 81297 into the NAc also failed to increase food-maintained responding, even though this same dose of SFK 81297 increased cocaine-seeking behavior (Bachtell et al., 2005).
Regarding our results with SCH 23390, infusion of 2.0 μg/side into both the cBLA and rBLA significantly disrupted drug-seeking behavior during maintenance and reinstatement test sessions, and significantly attenuated food-maintained responding. Thus, these decreases likely reflect non-specific suppression of behavior. However, when cocaine and cocaine cues were present to maintain responding, the infusion of 1.0 μg/side SCH 23390 into the cBLA but not rBLA attenuated cocaine-seeking behavior. This indicates a selective effect of this dose of SCH 233390 within the cBLA during the maintenance phase of testing. In contrast, when only cocaine cues were present to reinstate responding, the infusion of 1.0 μg/side SCH 23390 into the rBLA but not cBLA attenuated cocaine-seeking behavior, indicating a selective effect of this dose of SCH 23390 within the rBLA during the reinstatement phase of testing. Given that 1.0 μg/side SCH 23390 also attenuated the number of cocaine infusions and prolonged CS+ presentations earned in the cBLA and rBLA groups, respectively, it is clear that blockade of D1 receptors in the cBLA and rBLA did not selectively alter drug-seeking behavior. However, the failure of 1.0 μg/side SCH 23390 to significantly alter food-maintained responding suggests that attenuating effects of this dose of SCH 23390 were specific for cocaine and cocaine cue-controlled behavior. Furthermore, because the effects of 1.0 μg/side SCH 23390 on cocaine and cocaine cue-controlled behavior were dissociable with respect to the cBLA and rBLA during maintenance and reinstatement phases of testing, respectively, it is likely that effects of D1 receptor blockade with 1.0 μg/side SCH 23390 were not due to behavioral impairment in general.
It is also necessary to investigate the possibility that the agonist and antagonist-induced alterations in the maintenance and cue-induced reinstatement of cocaine-seeking behavior could be due to other non-specific factors. Testing phase-selective effects of SKF 81297 and SCH 23390 infusions into the cBLA and rBLA make it unlikely that changes in drug-seeking behavior were related to diffusion of drugs to sites outside the cBLA and rBLA or to the use of multiple intracranial infusions. It is also improbable that testing phase-selective effects during one testing phase will influence results in later testing, as SCH 23990 produces rapid regionally selective effects within the first hour of infusion (Caine et al., 1995) and the duration of action for SKF 81297 is also approximately one hour (Schmidt et al., 2006), indicating that effects of the agonist and antagonist are unlikely to persist beyond hour-long test sessions separated by multiple days. Furthermore, a within-subjects experimental design was used wherein rats received multiple counterbalanced doses of SKF 81297 and SCH 23390 during both maintenance and reinstatement phases. Given this design, if multiple infusions were producing damage, then systematic dose-related effects of SKF 81297 and SCH 23390 would not have been obtained. Moreover, damage from repeated infusions would have resulted in uniform changes in responding in both sites, regardless of testing phase and pretreatment drug or dose. A lack of non-specific effects of multiple infusions into these brain areas is supported by our earlier work showing that control rats receiving daily vehicle infusions into the cBLA or rBLA reached criterion in a learning task (10–11 sessions; Kantak et al., 2001) within a similar timeframe as control rats not receiving infusions into these sites (15 sessions; Udo et al., 2004). These findings support our conclusion that the 11–12 infusions of the D1 agonist and antagonist produced site-specific changes in behavior rather than changes related to non-specific damage.
Other investigations on the role of the BLA in regulating cocaine-seeking behavior often did not differentiate between the rostral (AP −1.33 mm to AP −2.85 mm) and caudal (AP −2.45 mm to AP −4.85 mm) subregions. In the examination of studies reporting significant changes in cocaine-seeking behavior during reinstatement testing (e.g., Meil & See,1997; Ledford et al., 2003; Berglind et al., 2006), our analysis of their histology revealed that cannulae placements were predominantly in the rBLA. Meil and See (1997) also examined the effects of their rostrally located BLA lesions on cocaine self-administration, and found no effects. In contrast, Whitelaw et al. (1996) demonstrated that BLA lesioned rats were unable to maintain drug-seeking responses under a second-order schedule of cocaine reinforcement. Examination of their histology revealed that cannulae placements were predominantly in the cBLA. Dissociable involvement of the cBLA and rBLA in regulating behaviors related to cocaine self-administration has been reported previously following D1 receptor blockade (Alleweireldt et al., 2006). It was shown that infusion of SCH 23390 into the cBLA, but not rBLA, selectively increased cocaine self-administration maintained under a variable-ratio schedule, whereas infusion of SCH 23390 into the rBLA, but not cBLA, dose-dependently decreased cocaine-induced reinstatement of drug-seeking behavior. Since a variable-ratio schedule of drug delivery does not differentiate between responding resulting in cocaine intake and responding reflecting cocaine-seeking behavior, it remained unclear if the cBLA and rBLA were dissociable in regulating cocaine-seeking behavior during maintenance and reinstatement phases of testing. Our findings, using an agonist and antagonist approach, provide compelling evidence that D1 receptor mechanisms may contribute to the functional heterogeneity of the cBLA and rBLA in regulating drug-seeking behavior during these different phases of addiction in rats trained to self-administer cocaine (Kantak et al., 2002).
Significance of Functional Heterogeneity within the Basolateral Amygdala
Our findings suggest that functionally distinct parallel circuits may loop through the cBLA and rBLA to separately regulate drug-seeking behavior during maintenance and reinstatement phases of addiction, respectively. Acquisition of responding under a second-order schedule of cocaine reinforcement as well as reinstatement of responding by cocaine-associated cues following a period of extinction training are both thought to reflect goal-directed drug-seeking behavior (Everitt et al., 2007; See et al., 2007). Prolonged responding under a second-order schedule of cocaine reinforcement is thought to reflect habitual drug-seeking behavior (Vanderschuren et al., 2005).
One segment of the neurocircuitry implicated in the control of goal-directed drug-seeking responses is the NAc core (Ito et al., 2004). The NAc core is activated by motivationally salient stimuli serving as conditioned reinforcers (Ikemoto, 2007) and mediates control over behavior by conditioned reinforcers (Parkinson et al., 1999). Neurochemical studies have shown that in the absence of cocaine, cocaine-paired conditioned stimuli selectively increased extracellular levels of dopamine in the NAc core vs. shell (Ito et al., 2000). Pharmacological studies with SKF 81297 have shown that after extinction of the cocaine-paired conditioned stimulus, there was more marked reinstatement of drug-seeking responses after infusion of the D1-like receptor agonist into the NAc core vs. shell (Bachtell et al., 2005). Importantly, neuroanatomical studies have shown that rBLA neurons that project to the NAc preferentially innervate the core compartment (Gröenewegen et al., 1991), which implicates the rBLA as part of the neurocircuitry controlling goal-directed drug-seeking responses. Studies have also shown that the rBLA sends bifurcating projections to the dorsal agranular insular cortex (AId) and NAc core (Shinonaga et al., 1994). Like the rBLA (Kantak et al., 2002) and NAc core (Fuchs et al., 2004), inactivation of the AId results in selective attenuation of cocaine-seeking behavior during cue-controlled reinstatement testing (Di Pietro et al., 2006). Previously, McLaughlin and See (2003) described a circuit for cue-controlled reinstatement of cocaine-seeking behavior that involved the NAc core, dorsal medial prefrontal cortex (dmPFC) and a portion of the BLA that targeted its midpoint along the anterior-posterior plane (midBLA). Interestingly, the midBLA has been shown to send bifurcating projections to the dmPFC and NAc core (Shinonaga et al., 1994). Thus, at least two distinct circuits (rBLA/AId/NAc core and midBLA/dmPFC/NAc core) may exist to regulate goal-directed drug-seeking behavior during reinstatement testing. The necessity for two circuits may relate to the fact that different types of stimuli are processed by the AId and mPFC in the regulation of cocaine-seeking behavior in rats (Di Pietro et al., 2006). Specifically, the AId was important for regulating cocaine-seeking behavior elicited by exposure to cocaine-specific odor, but not sound, cues, whereas the prelimbic region of the mPFC was important for regulating cocaine-seeking behavior elicited by exposure to cocaine-specific sound, but not odor, cues.
There is accumulating evidence that the dorsal striatum is a critical segment of the neurocircuitry underlying habitual drug-seeking behavior (Canales, 2005; Everitt & Robbins, 2005; Vanderschuren et al., 2005; See et al., 2007). Cocaine self-administration under a second-order schedule of reinforcement produces increases in extracellular dopamine that are long-term in the dorsal striatum and short-term in the NAc shell (Ito et al., 2000; Ito et al., 2002). Dopamine activation in the NAc shell by cocaine is thought to abnormally strengthen stimulus-reward and stimulus-response learning (Di Chiara, 1998), the information of which is processed through spiraling connections to the NAc core, which is the gateway from ventral to dorsal striatum (Haber et al., 2000; Ito et al., 2004). Notably, cBLA projections to the NAc predominantly innervate the medial shell compartment (Gröenewegen et al., 1991), which implicates the cBLA as part of the neurocircuitry controlling habitual drug-seeking responses. Neurons of the cBLA are also bifurcating and project to the dmPFC and NAc shell (Shinonaga et al., 1994). Like the cBLA (Kantak et al., 2002), inactivation of the dmPFC results in attenuation of cocaine-seeking behavior during maintenance testing conditions (Di Pietro et al., 2006). Thus, during active cocaine self-administration, dopamine transmission in the cBLA may process information related to stimulus-reward associations that is strengthened by dopamine transmission in the NAc shell to establish habits regulated by the dorsal striatum, which receives descending inputs from the dmPFC (Sesack et al., 1989).
Implications of Dissociable Sensitivity of D1 Receptor Activation within the Basolateral Amygdala
Previous research has proposed a shift between ventral and dorsal striatal control over drug-seeking behavior when responding transitions between goal-directed and habitual states, respectively (Everitt & Robbins, 2005). The transition in behavioral control is thought to depend in part on descending influences of the prefrontal cortex (Everitt et al., 2001). Metabolic mapping studies indicate that when comparing initial goal-directed and chronic stages of cocaine self-administration, the influence of cocaine expands more dorsolaterally and ventromedially within the prefrontal cortex in addition to expanding from ventral to more dorsal areas of the striatum (Porrino et al., 2007). It is possible that the transition between goal-directed and habitual drug-seeking behavior may also depend on changing sensitivity of the rBLA and cBLA to D1 receptor activation.
The potential involvement of D1 receptor activation in the rBLA and cBLA in dissociably mediating the transition between goal-directed and habitual cocaine-seeking behavior may impact future research investigating differences in neural circuitry engaged in regulating cocaine-seeking during different phases of addiction. Dopamine D1 receptor activation is important for both goal-directed actions and habit formation (Wickens et al., 2007) and past research has implicated both the rBLA and cBLA as neural substrates for stimulus-reward learning (Kantak et al., 2001). If, during early acquisition of cocaine-seeking behavior or during its reinstatement following response extinction training, the rBLA is preferentially sensitive to D1 receptor activation, then the transmission of stimulus-reward learning would be through a circuit involving the rBLA, NAc core and cortical sites that are implicated in action-outcome processing. Cocaine-seeking behavior becomes habitual with the presence of repeated cocaine exposure (Vanderschuren et al., 2005). If the presence of repeated cocaine exposure diminishes D1 receptor sensitivity in the rBLA but enhances it in the cBLA, then the transmission of stimulus-reward learning would shift to a circuit involving the cBLA, NAc shell and cortical sites that are implicated in habit formation regulated by the dorsal striatum. As cocaine use continues, this circuit remains active and cocaine-seeking behavior is strengthened and expressed as a habit rather than as a goal-directed action.
Conclusions
The mechanisms underlying changing sensitivity of D1 receptors in the cBLA and rBLA remain to be determined but appear to depend on whether or not cocaine is present during test sessions. Nonetheless, the integration of findings suggests that different therapeutic approaches may be necessary for reducing goal-directed drug-seeking behavior in abstinent cocaine addicts vs. habitual drug-seeking behavior in active cocaine users due to separate neurocircuits involved in regulating behavior during different phases of addiction.
Acknowledgments
The authors thank Lindsay Yager for technical assistance. This project was supported by grant number R01 DA011716 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
ABBREVIATIONS
- AId
dorsal agranular insular cortex
- BLA
basolateral amygdala
- cBLA
caudal basolateral amygdala
- CS
conditioned stimulus
- dmPFC
dorsomedial prefrontal cortex
- FI
fixed-interval schedule
- FR
fixed-ratio schedule
- NAc
nucleus accumbens
- rBLA
rostral basolateral amygdala
- S+
cocaine-associated stimuli
- S−
saline-associated stimuli
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:1–12. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RA. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, McDonald AJ. Evidence that dopaminergic axons provide a dense innervation of specific neuronal subpopulations in the rat basolateral amygdala. Brain Res. 1999;850:127–135. doi: 10.1016/s0006-8993(99)02112-5. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann NY Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT, Goldberg DM. Fixed-ratio responding under second-order schedules of food presentation or cocaine injection. J Pharmacol Exp Ther. 1981;218:271–281. [PubMed] [Google Scholar]
- Gröenewegen H, Berendse H, Meredith G, Haber S, Voorn P, Wolters J, Lohman A. Functional anatomy of the ventral, limbic system-innervated striatum. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: from motivation to action. Wiley; New York: 1991. pp. 19–59. [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance following lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug- associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behavior in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience. 1994;58:389–397. doi: 10.1016/0306-4522(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–226. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Swanson L. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Udo T, Ugalde F, Di Pietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacology (Berl) 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8870. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- Wickens KR, Budd CS, Hyland BI, Arbuthnott GW. Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix. Ann NY Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- Young AM, Rees KR. Dopamine release in the amygdaloid complex of the rat, studied by brain microdialysis. Neurosci Letters. 1998;249:49–52. doi: 10.1016/s0304-3940(98)00390-5. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]