Abstract
Purpose
We conducted a comparative study of Clinical Target Volume (CTV) definition of pelvic lymph nodes by multiple GU radiation oncologists looking at the levels of discrepencies amongst this group.
Methods and Materials
Pelvic CT scans from 2 men were distributed to 14 RTOG GU radiation oncologists with instructions to define CTVs for the iliac and pre-sacral lymph nodes. The CT data with contours was then returned for analysis. In addition, a questionnaire was completed that described the physicians’ method for target volume definition.
Results
Significant variation in the definition of the iliac and presacral CTVs was seen amongst the physicians. The minimum, maximum, mean (SD) iliac volumes (cc) were 81.8, 876.6, 337.6 ± 203 for case 1 and 60.3, 627.7, 251.8 ± 159.3 for case 2. The volume of 100% agreement was 30.6 and 17.4 for case 1 and 2 and the volume of the union of all contours was 1012.0 and 807.4 for case 1 and 2, respectively. The overall agreement was judged to be moderate in both cases (kappa=0.53 (p<0.0001) and kappa=0.48 (p<0.0001). There was no volume of 100% agreement for either of the two presacral volumes. These variations were confirmed in the responses to the associated questionnaire.
Conclusions
Significant disagreement exists in the definition of the CTV for pelvic nodal radiation therapy amongst GU radiation oncology specialists. A consensus needs to be developed so as to accurately assess the merit and safety of such treatment.
Keywords: prostate cancer, pelvic lymph nodes, target volume
PURPOSE
Landmark studies that have demonstrated the benefit of hormone therapy added to radiation therapy have all utilized whole pelvic radiation therapy plus a prostate boost.(1,2,3,4) In addition, other studies have directly suggested that pelvic nodal radiation may improve the outcome in patients with high risk prostate cancer whose risk of lymph node involvement is significant(5,6,7) For these reasons and because current Radiation Therapy Oncology Group (RTOG) trials require treatment of pelvic lymph nodes(8,9) it is essential that a consensus be attempted to define what constraints the proper nodal volumes to be irradiated.
Traditional pelvic radiotherapy techniques encompassed pelvic tissue extensively and adequate dosimetric coverage of nodal volumes was relative insensitive to treatment technique. However, with the development of Intensity Modulated Radiation Therapy (IMRT) techniques to treat only the pelvic lymph nodes (so as to increase protection of juxtaposed normal tissues) the nodal volumes contoured become extremely important so as to insure the appropriate nodes are in fact covered and thus treated. We conducted a comparative study of clinical target volume (CTV) definition of pelvic lymph nodes in the treatment of prostate cancer by multiple genitourinary (GU) radiation oncologists looking at the congruence or levels of discrepancies amongst this group.
METHODS
DICOM intravenous contrast enhanced pelvic CT scans from two men referred for definitive treatment of their prostate cancer were distributed to 14 radiation oncologists with instructions to define CTVs for the iliac and presacral lymph node regions. The GU radiation oncologists were asked to define two nodal CTVs: CTV-iliac and CTV-PS (presacral). CTV iliac was defined as all of the lymph nodes that the oncologist would generally target for a patient with a high risk of pelvic lymph node involvement, but with out the presacral nodes included (whether or not the oncologist would standardly treat them). CTV-PS was to include only the presacral lymph node volume.
In addition a clinical scenario and questionnaire accompanied the CTs which described the two cases of prostate cancer:
Case 1 - a 60-year-old with adenocarcinoma of the prostate clinical stage T3b, Gleason Score 7 (4+3), prostate specific antigen (PSA) 40.
Case 2 – a 60-year-old with adenocarcinoma of the prostate clinical stageT1c, Gleason Score 9, PSA 9.
The oncologists were asked to describe the superior limit of the nodal volumes for each case and whether they would treat the presacral lymph nodes in each case. They were also asked to describe the inferior and anterior limit of the external iliac lymph nodes, the inferior and posterior limit of the internal iliac, and the anterior limit of the presacral lymph node volumes. The questionnaire asked whether the oncologists had dose constraints for their nodal planning target volumes (PTV) such as volume 100% (V100), volume 110% (V110), isodose line 98% (D98), isodose line 95% (D95), isodose line 90% (D90), or others. Finally the oncologists were asked to describe their dose constraints for organs at risk (OAR) including bladder, rectum, femoral heads, penile bulb, small bowel, and large bowel.
The CT data was then returned to Washington University investigators by CD ROM or DICOM export to the ITC. and questionnaires were returned to the Medical College of Wisconsin for collation and analysis by Dr. Lawton.
The contours from each investigator were then imported into an in-house research treatment planning system (CERR). Contours were then compared for agreement by using the Matlab statistical software package.
Several algorithms to measure the level of agreement between physicians were investigated. The commonly used apparent volume overlap was calculated as the average agreement probability by which a voxel is selected by the experts. This was corrected for agreement by chance by using generalized kappa statistics.(10) Kappa statistics assume values between +1 (perfect agreement) through 0 (no agreement above chance) and -1 (complete disagreement). According to Landis and Koch criteria, a kappa value of 0 would indicate poor agreement, 0.01 – 0.20 slight agreement, 0.21 – 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 – 0.80 substantial agreement, and 0.80 – 1.00 almost perfect agreement. (11)
We applied an imputation method based on the Expected Maximum (EM) algorithms for simultaneous truth and performance level estimation (STAPLE) to estimate the “true” CTV contours.(12) In this approach, the consensus contouring decisions at each image voxel are formulated as maximum-likelihood estimates from the observed investigator contours by optimizing sensitivity and specificity parameters of each expert’s determination of anatomical sites that could harbor subclinical disease.
IRB approval was obtained for this study.
RESULTS
Fourteen radiation oncologists returned completed questionnaires and 11 completed CT contouring. Significant disagreements in the definition of iliac and presacral CTVs were seen amongst the physicians as summarized in Table 1. The agreement on the CTV-iliac was relatively better than the CTV-PS (moderate versus fair according to the Landis & Koch criteria). The range in volume definitions was 81.8–876.6 cc CTV-iliac versus 24.3– 157.3 cc CTV-PS in case 1 and 60.3–627.9 cc CTV-iliac versus 26.6–98.8 cc CTV-PS in case 2. Figures 1 and 2 show sample results for case #1 with agreement volumes at different confidence levels for CTV-iliac and CTV-PS, respectively. These two figures show that as the confidence level is increased the agreement volume is decreased. The decrease in volume is monotonic in cases of apparent volume and Kappa estimates but reaches more of a plateau in case of the STAPLE algorithm for intermediate confidence levels. The histogram graphical summary supports previous numerical results of better agreement in the case of CTV-iliac reflected in the relative ‘shallowness’ of the slopes of the different statistical estimates as a function of agreement compared to the CTV-PS case. A sagital image of the presacral contour volumes is shown in Figure 3. A coronal image of the iliac contour volumes is shown in Figure 4.
Table 1.
Pre-consensus statistical analysis of agreement level
| Clinical target volume | STAPLE Estimates | Kappa statistics agreement | |||
|---|---|---|---|---|---|
| Sensitivity | Specificity | ||||
| Case#1 | CTV-iliac | 0.63 ± 0.21 | 0.99 ± 0.03 | 0.53 (p < 0.0001) | “moderate” |
| CTV-PS | 0.41 ± 0.15 | 0.99 ± 0.02 | 0.33 (p<0.0001) | “fair” | |
| Case#2 | CTV-iliac | 0.59 ± 0.21 | 0.99 ± 0.02 | 0.48 (p < 0.0001) | “moderate” |
| CTV-PS | 0.36 ± 0.12 | 0.99 ± 0.01 | 0.29 (p<0.0001) | “fair” | |
Figure 1.
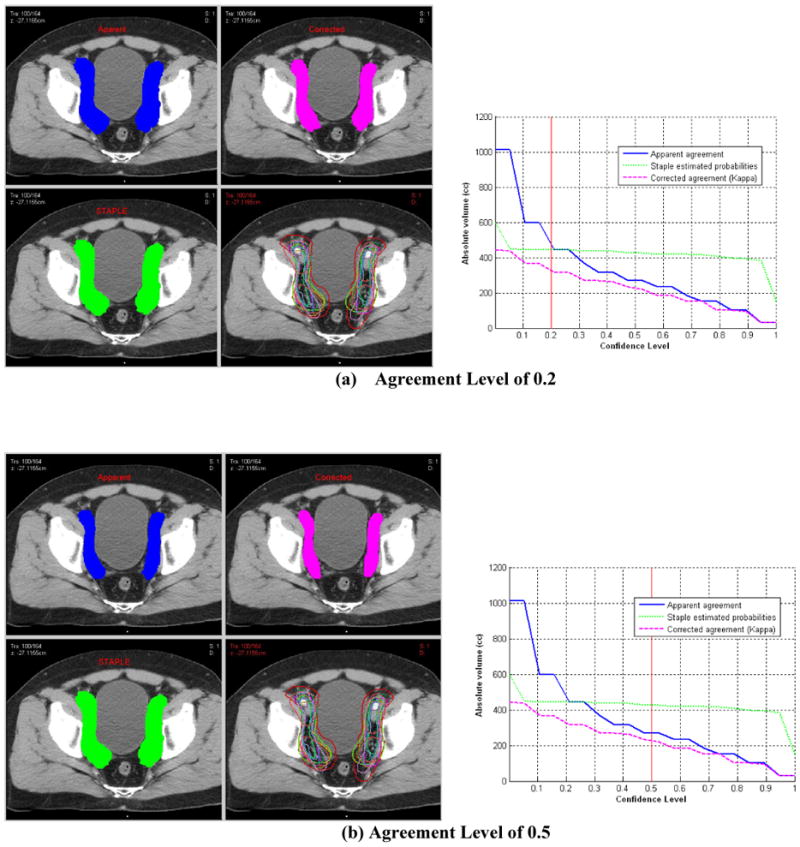
Statistical analysis of prostate CTV-iliac nodes (Case 1) contoured by 11 experts. Left column are transverse view snapshots with multiple panels showing apparent estimated volume (blue), kappa chance corrected volume (pink), staple detected volume (green) original 11 experts contours (bottom right) at different confidence agreement levels (a) Agreement level of 0.2, (b) 0.5, and (c) 0.8. Right column are corresponding graphical summary of estimated volume for each method as a function of confidence agreement level.
Figure 2.

Statistical analysis of prostate CTV-PS nodes (Case 1) contoured by 11 experts. Left column are transverse view snapshots with multiple panels showing apparent estimated volume (blue), kappa chance corrected volume (pink), staple detected volume (green) original 11 experts contours (bottom right) at different confidence agreement levels (a) Agreement level of 0.2, (b) 0.5, and (c) 0.8. Right column are corresponding graphical summary of estimated volume for each method as a function of confidence agreement level.
Figure 3.
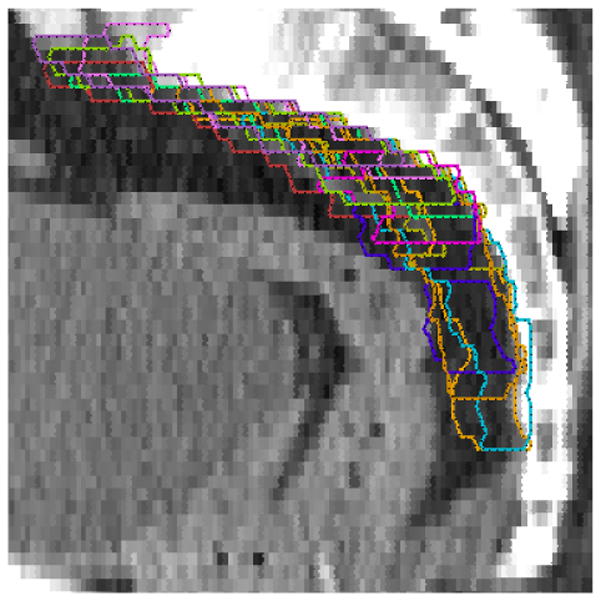
Sagital image of the presacral (PS) lymph node contours
Figure 4.
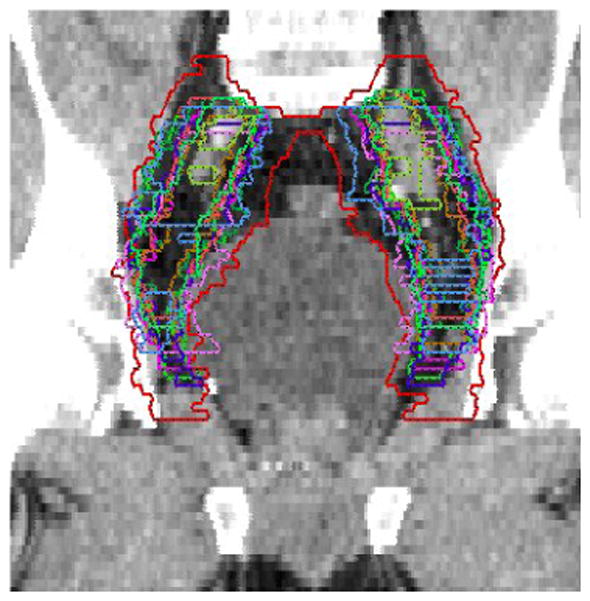
Coronal image of the iliac lymph node contours
These variations were confirmed in the response to the associated questionnaire. The superior limit of the nodal volumes ranges from L4/L5 interspace to the bottom of the sacral iliac joints. Five of the 14 oncologists chose the L5/S1 interspace as the superior limit of the nodal volumes.
The inferior limit of the external iliac lymph nodes ranged from 3-cm superior to the base of prostate down to the level of obturator foramen. The anterior limit of the external iliac lymph nodes ranged from the anterior aspect of the pubic symphysis to 2-cm posterior to the pubic symphysis.
Definition of the inferior limit of the internal iliac nodes ranged from 3-cm above the superior aspect of the sciatic notch to the top of the pubic symphysis with 4 of 14 oncologists choosing the level of the seminal vesicles as the inferior limit. The posterior limit of the internal iliac nodes ranged from no anatomic location identified and basing the inferior limit on the rectal dose volume histogram (DVH) to 1-cm posterior to the internal iliac vessels.
Definition of the anterior limit of the presacral nodes ranged from 3-cm anterior to the bony sacrum to 1.5-cm anterior to the bone with 5 of 14 oncologists choosing 2-cm anterior to the bony sacrum as the anterior limit of these nodes.
Dose constraints for the nodal PTV revealed that 3 of 14 oncologists had no dose constraints, but most of the respondents chose 95% – 98% V100 or the D98 or D95 at 98–100%.
For organs at risk there was also variation:
Bladder – 3 oncologists had no constraints, yet 9 of 14 chose at least 2 dose levels with constraints. One dose level at the 45–50 Gy level where the volume constraints ranged from < 40% to < 60% and a second high dose level of 60–70 Gy where the volume constraint range from < 5% to <25%.
Rectum – all respondents had constraints for the rectum with 12 of 14 having two dose levels with constraints. The first was in the range of 40–60 Gy where the volume constraint ranged from 25% – 50%. The second dose point ranged from 65 – 75 Gy with a volume constraint ranging from 5% – 30%.
Femoral head constraints ranged from none or “minimize” to < 5% exceeding 60 Gy. Eleven oncologists had constraints in the 40 – 50Gy range of < 1% to < 50%.
Penile bulb constraints showed that 7 of 14 of the oncologists had no constraints. For those oncologists with constraints the dose ranged from 45 – 53 Gy with a volume constraint of <40 to < 50%.
Small bowel constraints revealed 4 of 14 oncologists with no constraints. The remaining 10 oncologists had very wide range of constraints from < 1% at 50 Gy to < 10% at 68 Gy.
Large bowel including sigmoid colon constraints range revealed that 6 of 14 oncologists had no constraints. The remaining 8 GU experts showed some variation in constraints ranging from 0 % at 55 Gy to <30% at 53 Gy.
CONCLUSIONS
Pelvic nodal irradiation for high risk/locally advanced adenocarcinoma of the prostate has become a standard based on the radiation therapy plus hormone data from multiple prospective randomized trials.(1,2,3) In addition, it is required as part of two current RTOG trials (05–21 and 05–34).(8,9) Treating the correct lymph node volume appears to affect outcomes.(6) Certainly in the era of IMRT it is imperative that if lymph node volumes are to be treated, we must ensure that the “correct volumes” are covered and appropriate OAR’s are spared in order to address the relative merit and safety of such treatment.
Our data which polled 14 RTOG GU radiation oncologists who design and implement such trials has shown wide and statistically significant variation in the volumes treated. This data shows that a consensus on pelvic lymph node volumes needs to be developed so as to correctly evaluate the benefit and safety of such treatment. Consensus development by RTOG GU “experts” has been sponsored by the RTOG with scientifically data based consensus contours of these nodal volumes forthcoming.
Acknowledgments
This work was supported by NIH U24 grant CA81647, “Advanced Technology QA Center”
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma long term results of phase III RTOG 85–31. Int J Rad Onc Biol Phys. 2005;61(5):1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, III, Bae K, Speight J, et al. Short Term Neoadjuvant Androgen Deprivation Therapy and External Beam Radiotherapy for Locally Advanced Prostate Cancer: Long Term Results of RTOG 8610 a Phase III Prospective Randomized Trial. Journal of Clinical Oncology. 2008;26(4):585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Collette L, Blank L, et al. Long term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study, a phase III randomized trial) Lancet. 2002;360:103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz E, Bae K, Hanks G, Porter A, Grignon D, Brereton H, Venkatesan V, Lawton C, et al. Ten-year follow-up of RTOG 92–02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Onc. 2008 May 20;26(15):2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 5.Lawton C, DeSilvio M, Roach M, III, et al. An Update of the Phase III Trial Comparing Whole-Pelvic (WP) to Prostate Only (PO) Radiotherapy and Neoadjuvant to Adjuvant Total Androgen Suppression (TAS): Updated Analysis of RTOG 94–13, with Emphasis on Unexpected Hormone/Radiation Interactions. Int J Rad Onc Biol & Phys. 2007;69(3):646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roach M, III, DeSilvio M, Valicenti R, et al. Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J of Rad Onc, Bio, Phy. 2006;66(3):647–53. doi: 10.1016/j.ijrobp.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 7.Seaward SA, Weinberg V, Lewis P, et al. Improved freedom from PSA failure with whole pelvic irradiation for high risk prostate cancer. Int J Rad Onc Biol Phys. 1998;42:1055–1062. doi: 10.1016/s0360-3016(98)00282-x. [DOI] [PubMed] [Google Scholar]
- 8.RTOG 05–21: A phase III protocol of androgen suppression (AS) and 3D CRT/IMRT vs AS and 3D CRT/IMRT followed by chemotherapy with Docetaxel and Prednisone for localized high risk prostate cancer. See RTOG website. http://rtog.org.
- 9.RTOG 05–34: A phase III trial of short term androgen deprivation with pelvic lymph node or prostate bed only radiotherapy (SPPORT) in prostate cancer patients with a rising PSA after radical prostatectomy See RTOG website. http://rtog.org.
- 10.Fleiss JL. Statistical methods for rates and proportions. 2. New York: Wiley, J; 1981. [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 12.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004 Jul;23(7):903–21. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]




