Abstract
Tocopherols are lipophilic antioxidants found in vegetable oils. Here, we examined the growth inhibitory effect of a γ-tocopherol-enriched tocopherol mixture (γTmT) against CL13 murine lung cancer cells grown in culture and as subcutaneous tumors in A/J mice. We found γTmT had no effect after 2 d and weakly inhibited the growth of CL13 in culture after 5 d (28% growth inhibition at 80 µM). Dietary treatment with 0.1% and 0.3% γTmT for 50 d inhibited the growth of CL13 tumors in A/J mice by 53.9 and 80.5%, respectively. Histopathological analysis revealed an increase in tumor necrosis compared to control tumors (80% and 240% increase by 0.1% and 0.3% γTmT, respectively). Dietary treatment with γTmT dose-dependently increased γ- (10.0 – 37.6-fold) and δ-tocopherol (8.9 – 26.7-fold) in the tumors of treated mice compared to controls. Dietary treatment with γTmT also increased plasma γ- (5.4 – 6.7-fold) and δ-tocopherol (5.5 – 7-fold). Whereas others have demonstrated the cancer preventive activity of γTmT against mammary and colon cancer, this is the first report of growth inhibitory activity against lung cancer. Further studies are needed to determine the underlying mechanisms for this anticancer activity, and to determine if such activity occurs in other models of cancer.
Keywords: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, lung cancer, tocopherols, tumor necrosis, syngeneic tumor model
1. Introduction
Tocopherols are plant-derived polyphenolic compounds characterized by the presence of chromanol-ring attached to a phytl tail (Fig. 1). These compounds are found in high amounts in vegetable oils commonly found in the human diet: the most abundant tocopherols being α-tocopherol (α-T), β-tocopherol (β-T), γ-tocopherol (γ-T), and δ-tocopherol (δ-T) [1, 2]. These naturally-occurring tocopherols all have an R, R, R stereochemistry, but differ in terms of the number and location of methyl substituents on the chromanol ring. These methyl substituents affect the relative antioxidant activity of the various tocopherols by hindering access to the phenolic hydroxyl group. Although α-T is considered the “Classical Vitamin E” based on studies of fertility maintenance, various studies have shown that γ-T and δ-T have stronger antioxidant and anti-inflammatory activity [3]. This suggests that γ-T and δ-T may have greater cancer preventive activity.
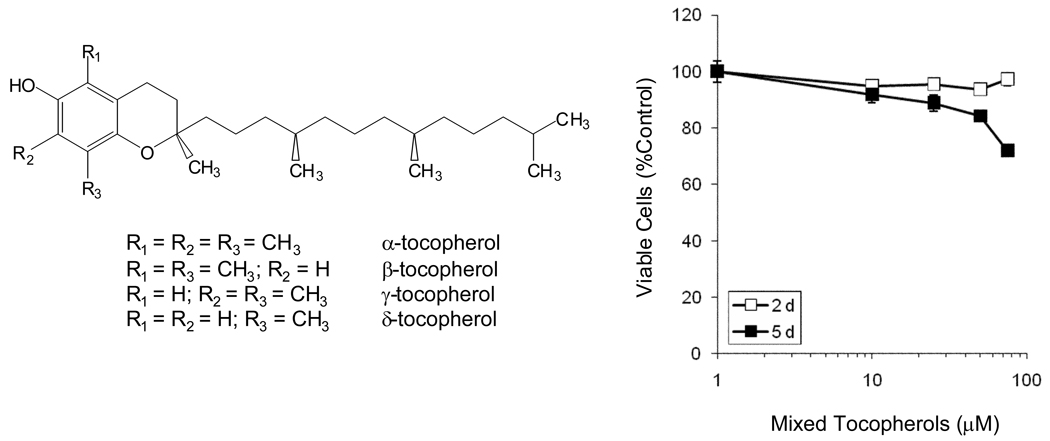
Figure 1.
Structures of the tocopherols and the effect of mixed tocopherols (γTmT) on the growth of CL13 cells in culture. Effects on cell growth and viability were assessed using the MTT assay. Cells were treated for 2 or 5 d with γTmT in serum-complete cell culture medium. Each point represents the mean of n = 18. Error bars represent the SEM. For points were no error bar is visible, this indicates that the error is smaller than the size of the point.
Human intervention and epidemiological studies are mixed with regard to the potential cancer preventive activities of tocopherols. The NHANES I cohort study found no significant correlation between lung cancer risk and α-T [4]. By contrast, a nested case-control study by Mahabir et al., found a significant inverse correlation between increasing intake of α-T, β-T, γ-T, or total tocopherols and lung cancer risk [5]. Although an association between higher blood levels of γ-T and reduced risk of prostate cancer have been found in two nested case-control studies [6], no such association was observed in the Physician's Health Study [7]. In the Women’s Health Study, supplementation with 600 IU α-T every other day had no significant effect on incidence of invasive cancer or cancer death [8].
Recent studies have shown that dietary supplementation with a mixture of tocopherols can prevent tumorigenesis in animal models of mammary and colon cancer [9–11]. In a recent study in our laboratory, we found that dietary treatment of azoxymethane (AOM)/dextran sulfate sodium-treated mice with a 0.3% (w/w) γ-T-rich tocopherol mixture (γTmT) reduced the multiplicity of total colon tumors, adenomas, and adenocarcinomas by 87.5%, 83.3%, and 83.3%, respectively, compared to control-treated mice [11]. Short-term studies in the same model showed that treatment with γTmT decreased markers of oxidative stress and inflammation in the colon and plasma including: prostaglaniding E2, leukotriene B4, and nitrotyrosine [11]. Similar preventive effects were also reported in the N-methyl-N-nitrosourea-induced mammary tumor model in rats and AOM-induced aberrant crypt foci (ACF) model [9, 10]. Treatment with 0.1% γTmT reduced mammary tumor burden and multiplicity by 43.5% and 26.5%, respectively in the former model, whereas induction of ACF by AOM was reduced by 55% compared to control treated rats in the latter model.
Lung cancer is the second most commonly diagnosed cancer and is the leading cause of cancer death in the United States [12]. Although cessation of smoking is the most effective means of preventing the development of lung cancer, there is still significantly elevated risk of developing lung cancer in former smokers, and lung cancer incidence due to occupational exposure and other factors not related to smoking are significant [13, 14]. Although advances have been made in the treatment of various types of cancer, the 5-year survival rate for lung cancer has only slightly improved (13% in 1975 vs 16% in 2006) [15]. Clearly, new strategies are needed to prevent the development of new lung cancer cases and treat cases once they have developed. In the present study, we determined the efficacy of γTmT against CL13 murine lung cancer cells grown in culture and as subcutaneous syngeneic subcutaneous tumors in A/J mice. This cell line was derived from lung adenocarcinomas generated in female A/J mice using the classical 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorgenesis model. The subcutaneous syngeneic tumor model represents an economical method to rapidly establish the efficacy of test compounds and to establish a dose-range prior to undertaking long-term mouse lung carcinogenesis studies. This study represents the first report on the effect of γTmT in an animal model of lung cancer.
2. Materials and Methods
2.1 Chemicals and Diets
γTmT (57% γ-T, 24% δ-T, 13% α-T, and ~0.5% β-T) was obtained from Cognis Corporation (Kankakee, IL). γTmT was formulated into AIN93M basal diet at 0.1% or 0.3% w/w by Research Diets, Inc (New Brunswick, NJ) and stored at 4°C in sealed bags. The tocopherol content of all diets is listed in Table 1. All other chemicals were of the highest purity available and purchased from VWR International (West Chester, PA).
Table 1.
Tocopherol content of the experimental diets
| mg/kg diet |
||||
|---|---|---|---|---|
| Diet | α-T | β-T | γ-T | γ-T |
| AIN93M1 | 79.8 | 0.4 | 24.4 | 7.6 |
| AIN93M + 0.1% γTmT (w/w) | 199.8 | 15.4 | 604.4 | 217.6 |
| AIN93M + 0.3% γTmT (w/w) | 439.8 | 45.4 | 1764.4 | 637.6 |
Tocopherol content in AIN93M diet is due to α-T in the vitamin mix (V10037) which is added to the diet at 1% and soybean oil which is added as 4%. The amounts of tocopherols present in soybean oil are based on previously reported values [24].
2.2 Cell Culture and Cell Viability Assay
CL13 mouse lung adenocarcinoma cells were a gift from Dr. Steven A. Belinsky (Lovelace Respiratory Research Institute, Albuquerque, NM). Cells were maintained in log-phase growth in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 0.1 mg/mL streptomycin. Cells were maintained at 37°C in a 5% CO2 atmosphere with 95% relative humidity.
To determine the growth inhibitory activity of γTmT, CL13 cells were plated in 96-well plates (5 × 103 cells per well) and allowed to attach for 24 h. The medium was replaced with fresh, serum-complete medium containing 0 – 80 µM γTmT. Cells were incubated for 2 or 5 d at 37°C. The medium was removed, the cells were washed once with medium containing no γTmT, and the number of viable cells were determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay [16]. Treatment effect determined by comparison to dimethylsulfoxide (vehicle)-treated controls. Final dimethylsulfoxide concentration was less than 0.1% in all treatments.
2.3 Subcutaneous Tumor Studies
Animal experiments were conducted according to a protocol (91-024) approved by the Institutional Animal Care and Use Committee at Rutgers University (Piscataway, NJ). Female A/J mice (7 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed 10 per cage in air-conditioned quarters with room temperature of 20 ± 2°C, relative humidity of 50 ± 10%, and alternating 12 h light/dark cycle. After a one-week acclimation period, mice were randomized based on weight into the following treatment groups: control (AIN93M diet), 0.1% γTmT (w/w in AIN93M diet), or 0.3% γTmT (w/w in AIN93M) diet. After one week of feeding with experimental diet, each mouse was inoculated subcutaneously with 1 × 106 CL13 cells (in 100 µL PBS) on both rear flanks. Mice had access to food and water ad libitum. Body weight and food consumption were determined weekly. Tumors were measured weekly and volume was calculated using the following formula:
where V is tumor volume, L is the longest and W is the shortest tumor dimension. We elected to begin treatment with γTmT prior to tumor cell inoculation in order to better model cancer prevention rather than cancer therapy. In cancer prevention (or secondary prevention), treatment is begun when only a small population of cancerous or precancerous cells is present, rather than an established tumor mass. All mice were sacrificed by CO2 asphyxiation when the mean tumor volume of the control group reached 400 mm3. Blood was collected by cardiac puncture. Plasma was prepared by centrifugation and frozen at −80°C for later analysis. Tumors were excised and halved. Half of the tumor was snap frozen on dry ice for HPLC analysis of tocopherols levels and the other half was fixed in 10% buffered formalin. Formalin-fixed tumors were dehydrated, embedded in paraffin, and cut into 5 µm sections. Sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) for histopathological analysis. Necrotic areas in tumors were defined as areas composed of either large amounts of dead cell debris or the empty space in the center of tumors formed after cell death. The total tumor area and the necrotic area of each tumor were measured using the Image-pro Plus program and quantified as a percentage.
2.4 HPLC Analysis of Tocopherols
The procedure for the determination of tocopherol levels in plasma was described previously [11]. In brief, 10 µL plasma was combined with 140 µL deionized water and 150 µL ethanol. Fat-soluble materials, including tocopherols, were extracted with hexane. For tumors, 10 mg samples were homogenized with a mechanical dounce homogenizer in 140 µL deionized water and 150 µL ethanol. After homogenization, the supernatant was extracted with hexane. The hexane-extractable material was dried under vacuum and redissolved in 100 µL ethanol for HPLC analysis.
HPLC separation was achieved using a Supelcosil C18 reversed-phase column (150 × 4.6 mm; 5 µm particle size; Bellefonte, PA). An isocratic mobile phase was used consisting of 82% aqueous ethanol containing 20 mM ammonium acetate (pH=4.4). The flow rate was 1.2 mL/min. The eluant was monitored with an ESA 5600A Coulochem electrode array system (ESA Inc. Bedford, MA) with potentials set at 200 mV, 300 mV, 500 mV, and 700 mV. Quantification was accomplished by comparing the peak area of the sample tocopherol peaks with the peak area in reference plasma. The reference plasma was prepared as previously described [11].
2.5 Statistical Analysis
Tumor growth inhibition and body weight were analyzed by repeated measured ANOVA with Bonferroni post-test. Tumor necrosis, and plasma and tumor levels of tocopherols were analyzed by one-way ANOVA with Tukey’s post-test. Significance was achieved at p < 0.05.
3.0 Results and Discussion
3.1 Inhibition of lung cancer cell growth in vitro and in vivo
The effect of γTmT on the growth and viability of CL13 cells in culture was assessed using the MTT assay. Treatment for 2 d with 0 – 80 µM γTmT had no significant effect on the number of viable cells relative to control (Fig. 1). However, treatment for 5 d dose-dependently decreased the number of viable cells, with inhibition of 28% at 80 µM (Fig. 1).
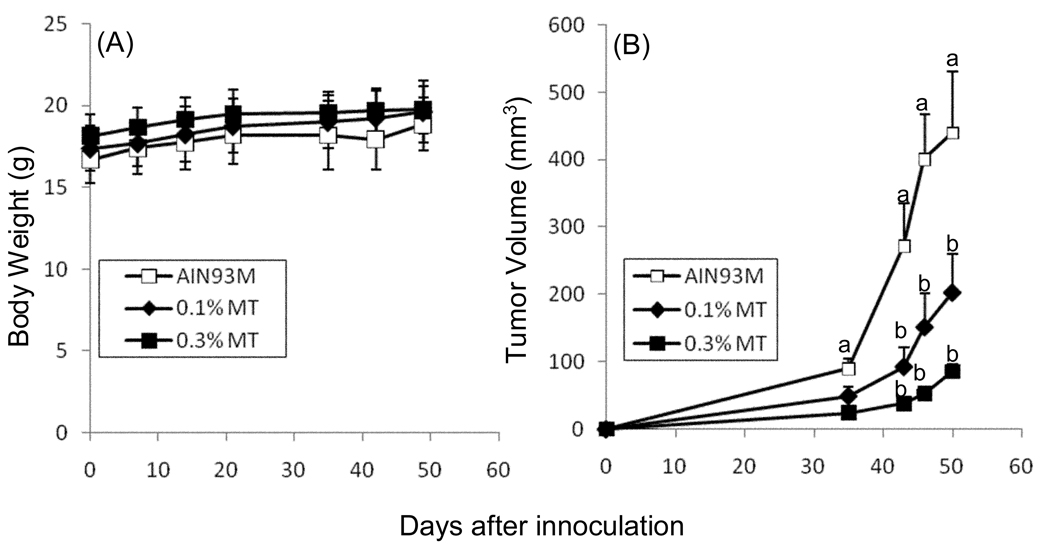
To determine the growth inhibitory effects of γTmT in vivo, we randomized female A/J mice and treated them for 1 week with 0.1% or 0.3% γTmT in the diet. Mice were then implanted subcutaneously with CL13 cells and dietary treatment with γTmT was continued for the duration of the experiment. Treatment with γTmT had no significant effect on the body weight of treated mice compared to mice fed AIN93M diet (Fig. 2A). γTmT dose-dependently inhibited the growth of subcutaneous CL13 tumors in A/J mice (Fig. 2B). γTmT at 0.1% and 0.3% in the diet reduced final tumor volume by 53.9% and 80.5%, respectively, compared to control.
Figure 2.
Effect of γTmT treatment on the growth of subcutaneous CL13 tumors in A/J mice. A/J mice were treated with AIN93M diet supplemented with 0, 0.1% or 0.3% γTmT for one week prior to injection with CL13 cells. Treatment γTmT -supplemented diet continued until sacrifice. Body weight (A) and tumor volume (B) were determined throughout the course of the experiment. Each point represents the mean of n = 14 – 15 mice (for body weight) and 25 – 27 tumors (for tumor volume). Error bars represent the SEM. Data points with different superscripts are significantly different (p < 0.05) by repeated measures analysis of variance with Bonferroni post-test.
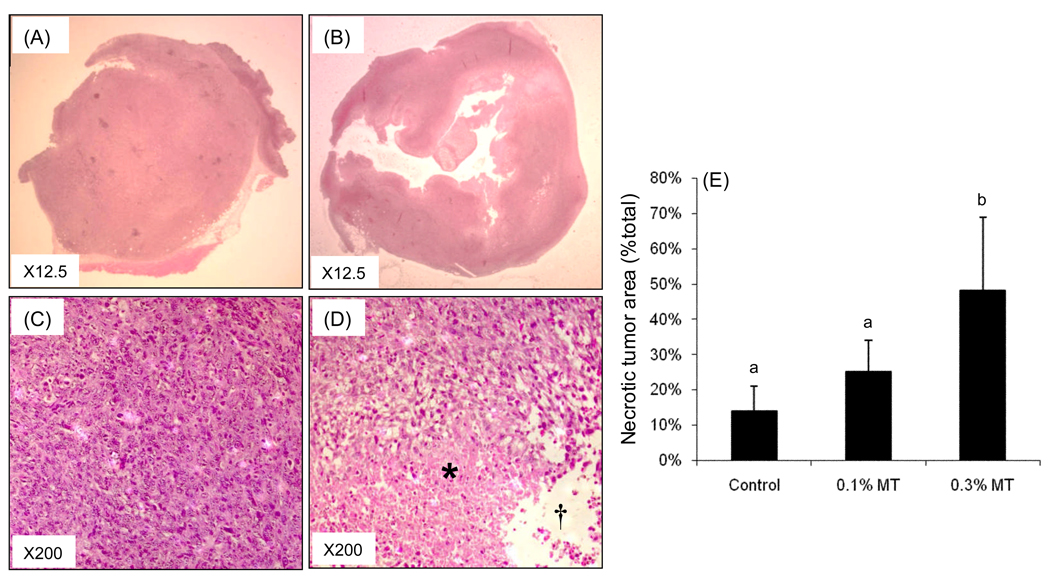
Tumors from control and γTmT-treated animals were sectioned and stained with H&E (Fig. 3). Histopathological analysis revealed that tumors from control-treated mice were uniformly composed of living cells with a small population of dead cells (Fig. 3A and C) By contrast, tumors from γTmT -treated mice had large amounts of tumor cell necrosis (Fig. 3B and D). The necrotic areas were defined as areas composed of either large amount of dead cell debris (labeled as * in Fig.3D) or the empty space in the center of tumors formed after cell death (labeled as † in Fig. 3D). Quantification of the necrotic tumor area as a percent of the total tumor showed that treatment with 0.3% γTmT increased tumor necrosis by 2.4-fold compared to control (Fig. 3E). The underlying mechanisms for this increase in tumor cell necrosis remain unclear. It is possible that γTmT can directly induce cell death, but the results of our in vitro studies with CL13 cells do not support this notion. Rather, our data suggest that γTmT may work by an indirect mechanism such as disruption of tumor cell – stromal cell interactions as has been reported in other studies [17–22]. This possibility remains to be tested in the current model.
Figure 3.
Histopathological analysis of CL13 tumors in A/J mice treated with 0.3% γTmT. Representative tumors from the AIN93M-treated mice (A,C) and 0.3% γTmT-treated mice (B,D) were stained with H&E and the necrotic area of the tumor was quantified by microscopy and normalized to total tumor area in each section (E). Each bar represents the mean of n = 10 tumors. Error bars represent the SD. Bars with different superscript are significantly different (p < 0.05) by one-way ANOVA with Tukey’s post-test.*, † indicate areas defined as tumor necrosis.
3.2 Plasma and tumor levels of tocopherols
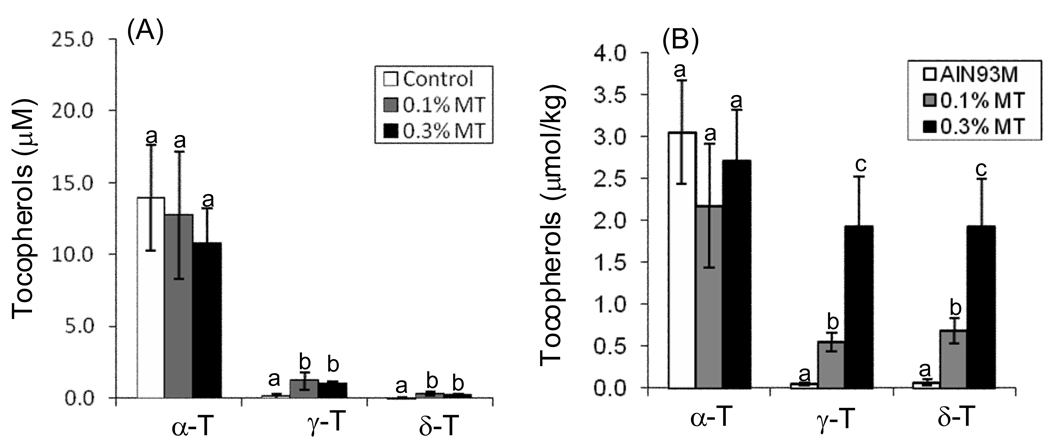
The plasma and tumor levels of individual tocopherols (α-T, γ-T, and δ-T) were determined by HPLC following treatment of tumor-bearing mice for 50 d. Although the diet was supplemented with higher levels of γ-T (57%) and δ-T (24%) than α-T (13%), the plasma and tumor levels of α-T were significantly higher than γ-T and δ-T in this study (Fig. 4). Dietary treatment with γTmT had no significant effect on the levels of α-T in either the plasma (Fig. 4A) or the tumors (Fig. 4B) compared to control. By contrast, treatment with γTmT increased plasma levels of γ-T and δ-T by 5.4 – 6.7-fold and 5.5 – 7.0-fold, respectively, compared to control (Fig. 4A). Dietary γTmT also dose-dependently increased tumor levels of γ-T (10 – 37.6-fold increase) and δ-T (8.8 – 26.7-fold increase) (Fig. 4B).
Figure 4.
Plasma (A) and tumor (B) levels of tocopherols in A/J mice treated with 0.1 or 0.3% γTmT in the diet. The levels of α-T, γ-T, and δ-T were determined by HPLC with electrochemical detection. Each bar represents the mean of n = 7. Error bars represent the SEM. Bars with different superscripts are significantly different (p < 0.05) by one-way ANOVA with Tukey’s post-test.
The apparent contradiction between dietary supplementation with lower levels of α-T compared to γ- or δ-T, but much higher levels of α-T resulting in plasma and tissues has been observed in other studies [10, 11]. It has been reported that hepatic α-T transfer protein (α-TTP), which maintains plasma α-T levels, preferentially binds to α-T over γ-T [23]. The result is a 3-fold greater half-life for α-T compared to γ-T. There is also evidence to suggest that non-α-T tocopherols are metabolized more readily that α-T by cytochromes P450 [23]. The lack of change in α-T after dietary supplementation, again, is probably due to the tight regulation by α-TTP. These data indicate that dietary administration is an effective at increasing tumor levels of tocopherols. It is unclear why the increase in tumor levels of γ-T and δ-T is higher than the increase in plasma levels of these compounds. It is likely that the tocopherols partition into the tumor because of the more non-polar environment of the tissue relative to the plasma.
4.0 Concluding Remarks
In the present study, we observed that dietary administration of γTmT inhibited the growth of CL13 mouse lung adenocarcinoma subcutaneous tumors in A/J mice, even though these compounds were only weakly active against CL13 cells in culture. Given the apparent antitumor activity of γTmT in this short-term study, long-term studies in the NNK-induced carcinogenesis model in A/J mice are warranted. Furthermore efficacy studies should be conducted in other animal models of other tumor types to determine the spectrum of anticancer activity of γTmT.
Acknowledgments
The present study was supported by American Institute of Cancer Research Grant 05A047 (to JDL), NIH grant CA125780 (to JDL), and an NIEHS Pilot Project ES005022-20 (to JDL). The authors wish to thank Harold L. Newmark, D. Sci. (Hon.) for introducing mixed tocopherols to the Department of Chemical Biology at Rutgers University.
Abbreviations
- α-T
α-tocopherol
- ACF
aberrant crypt foci
- AOM
azoxymethane
- β-T
β-tocopherol
- γ-T
γ-tocopherol
- γTmT
mixed tocopherols
- δ-T
δ-tocopherol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Traber MG. In: Modern Nutrition in Health and Disease. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Baltimore, MD: Lippincott, Williams, and Wilkins; 2006. pp. 396–411. [Google Scholar]
- 2.Eitenmiller T, Lee J. Vitamin E: Food Chemistry, Composition, and Analysis. New York, NY: Marcel Dekker, Inc.; 2004. [Google Scholar]
- 3.Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitamins and hormones. 2007;76:1–21. doi: 10.1016/S0083-6729(07)76001-6. [DOI] [PubMed] [Google Scholar]
- 4.Yong LC, Brown CC, Schatzkin A, Dresser CM, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. American journal of epidemiology. 1997;146:231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 5.Mahabir S, Schendel K, Dong YQ, Barrera SL, et al. Dietary alpha-, beta-, gamma-and delta-tocopherols in lung cancer risk. International journal of cancer. 2008;123:1173–1180. doi: 10.1002/ijc.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, et al. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157:335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 7.Gann PH, Ma J, Giovannucci E, Willett W, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 8.Lee IM, Cook NR, Gaziano JM, Gordon D, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutrition and cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 10.Suh N, Paul S, Lee HJ, Ji Y, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutrition and cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 11.Ju J, Hao X, Lee MJ, Lambert JD. A g-tocopherol-rich mixture of tocopherols inhibit colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prevention Research. 2008 doi: 10.1158/1940-6207.CAPR-08-0099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 13.Dubey S, Powell CA. Update in lung cancer 2007. American journal of respiratory and critical care medicine. 2008;177:941–946. doi: 10.1164/rccm.200801-107UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutation research. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Anonymous. American Chemical Society; 2008. p. 18. [Google Scholar]
- 16.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. The Journal of surgical research. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 17.Neuzil J, Swettenham E, Wang XF, Dong LF, et al. alpha-Tocopheryl succinate inhibits angiogenesis by disrupting paracrine FGF2 signalling. FEBS letters. 2007;581:4611–4615. doi: 10.1016/j.febslet.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Grossie B, Bennett M, Mills N, et al. Alpha-tocopheryl succinate (alpha-TOS) modulates human prostate LNCaP xenograft growth and gene expression in BALB/c nude mice fed two levels of dietary soybean oil. European journal of nutrition. 2007;46:34–43. doi: 10.1007/s00394-006-0629-4. [DOI] [PubMed] [Google Scholar]
- 19.Woodson K, Triantos S, Hartman T, Taylor PR, et al. Long-term alpha-tocopherol supplementation is associated with lower serum vascular endothelial growth factor levels. Anticancer research. 2002;22:375–378. [PubMed] [Google Scholar]
- 20.Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T. Anti-angiogenic function of tocotrienol. Asia Pacific journal of clinical nutrition. 2008;17 Suppl 1:253–256. [PubMed] [Google Scholar]
- 21.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. The Journal of biological chemistry. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 22.Miyazawa T, Tsuzuki T, Nakagawa K, Igarashi M. Antiangiogenic potency of vitamin E. Annals of the New York Academy of Sciences. 2004;1031:401–404. doi: 10.1196/annals.1331.057. [DOI] [PubMed] [Google Scholar]
- 23.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free radical biology & medicine. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner K. Increasing gamma- and delta-tocopherols in oils improves oxidative stability. Lipid Technology. 2007;19:229–231. [Google Scholar]