Abstract
The lumbar spinal cord of rats contains the sexually dimorphic, steroid-sensitive spinal nucleus of the bulbocavernosus (SNB). Androgens are necessary for the development of the SNB neuromuscular system, and in adulthood, continue to influence the morphology and function of the motoneurons and their target musculature. However, estrogens are also involved in the development of the SNB system, and are capable of maintaining function in adulthood. In this experiment we assessed the ability of testosterone metabolites, estrogens and non-aromatizable androgens, to maintain neuromuscular morphology in adulthood. Motoneuron and muscle morphology was assessed in adult normal males, sham-castrated males, castrated males treated with testosterone, dihydrotestosterone, estradiol, or left untreated, and gonadally intact males treated with the 5α-reductase inhibitor finasteride or the aromatase inhibitor fadrozole. After 6 weeks of treatment, SNB motoneurons were retrogradely labeled with cholera toxin-HRP and reconstructed in three dimensions. Castration resulted in reductions in SNB target muscle size, soma size, and dendritic morphology. Testosterone treatment after castration maintained SNB soma size, dendritic morphology, and elevated target muscle size; dihydrotestosterone treatment also maintained SNB dendritic length, but was less effective than testosterone in maintaining both SNB soma size and target muscle weight. Treatment of intact males with finasteride or fadrozole did not alter the morphology of SNB motoneurons or their target muscles. In contrast, estradiol treatment was completely ineffective in preventing castration-induced atrophy of the SNB neuromuscular system. Together, these results suggest that the maintenance of adult motoneuron or muscle morphology is strictly mediated by androgens.
Indexing terms: gonadal hormones, motoneurons, dendrites, spinal cord, rat
INTRODUCTION
The spinal cord of the rat contains a sexually dimorphic nucleus, the spinal nucleus of the bulbocavernosus [SNB; also known as the dorsomedial nucleus or DM (Schrøder, 1980)]. In male rats, the SNB consists of approximately 200 motoneurons that innervate the perineal muscle complex consisting of the bulbocavernosus (BC) and levator ani (LA; collectively, BC/LA), as well as the external anal sphincter (Breedlove and Arnold, 1980; Schrøder, 1980; McKenna and Nadelhaft, 1986). The BC/LA muscles attach to the penis and are essential for successful copulation and insemination (Sachs, 1982; Hart and Melese-D’Hospital, 1983). The SNB in mature females, who lack or have greatly reduced perineal musculature (Hayes, 1965; Èihák et al., 1970; Tobin and Joubert, 1991), contains only approximately 60 motoneurons, innervating primarily the external anal sphincter (McKenna and Nadelhaft, 1986). The morphology of SNB motoneurons is also sexually dimorphic, and SNB somata of adult females are typically half as large as those of males (Breedlove and Arnold, 1981; McKenna and Nadelhaft, 1986).
The dimorphisms in the SNB neuromuscular system result from the actions of gonadal steroids, operating both developmentally and in adulthood. During perinatal development, androgens are responsible for the prevention of normally occurring motoneuron death (Nordeen et al., 1985), somal growth (Lee et al., 1989; Goldstein et al., 1990; Goldstein and Sengelaub, 1992), perineal musculature retention (Èihák et al., 1970), and neuromuscular synapse elimination (Jordan et al., 1989a; Jordan et al., 1989b). Conversion of testosterone to estrogenic metabolites does not appear to be involved in this regulation, and treatment with estradiol has no effect on SNB motoneuron survival (Breedlove et al., 1982; Goldstein and Sengelaub, 1990), somal growth (Breedlove et al., 1982; Goldstein and Sengelaub, 1994; Burke et al., 1997; Hebbeler et al., 2002), target muscle retention (Breedlove et al., 1982; Breedlove, 1997), or neuromuscular synapse elimination (Jordan et al., 1995).
By contrast, both androgens and estrogens are involved in dendritic development in the SNB. SNB dendrites typically grow profusely through the first four postnatal weeks (Goldstein et al., 1990). In males castrated seven days after birth, dendrites never grow beyond their precastration lengths, while dendritic lengths of castrates receiving testosterone replacement are equivalent to those of intact males by four weeks of age (Goldstein et al., 1990). Estradiol treatment of castrated male rats partially supports dendritic growth through four weeks of age (Goldstein and Sengelaub, 1994), and the androgen dihydrotestosterone is only capable of fully supporting dendritic growth in castrates when it is administered in combination with estradiol (Burke et al., 1997). Similarly, SNB dendritic growth is supported by ovarian hormones (Hebbeler et al., 2001), and reducing estradiol by inhibiting its aromatization from testosterone reduces dendritic growth (Burke et al., 1999). It appears that estrogens act in the neuromuscular periphery to support this postnatal SNB dendritic growth (Nowacek and Sengelaub, 2006). Local blockade of estrogen receptors at the BC muscle of gonadally intact males results in severely reduced dendritic lengths similar to those seen in castrates, whereas local administration of estradiol to the BC muscle of castrated males supports dendritic growth.
The SNB neuromuscular system remains hormone-sensitive in adulthood, and again, androgenic action dominates. Castration causes a reduction in the penile reflexes mediated by SNB-innervated musculature (Hart, 1967; Davidson et al., 1978), and testosterone replacement prevents (Hart, 1967; Davidson et al., 1978) or reverses (Hart, 1967; Hart et al., 1983) this reduction. Testosterone regulates a variety of SNB motoneuron characteristics, including soma size (Breedlove and Arnold, 1981), dendritic length (Kurz et al., 1986), the number and size of synapses and gap junction plaques (Leedy et al., 1987; Matsumoto et al., 1988a,b), and the expression of a variety of proteins (e.g., ciliary neurotrophic factor receptor α, Forger et al., 1998; N-cadherin; Monks et al., 2001, calcitonin gene-related peptide, Popper and Micevych, 1989; androgen receptors, Matsumoto et al., 1996) and mRNA (e.g., β-actin, Matsumoto et al., 1992, β-tubulin, Matsumoto et al., 1993; gap junction protein connexin 32, Matsumoto et al., 1992; calcitonin gene-related peptide, Popper and Micevych, 1990). Peripherally, testosterone maintains several characteristics of SNB-innervated musculature; these include muscle weight (Wainman and Shipounoff, 1941), muscle fiber (Venable, 1966), neuromuscular junction size (Bleisch and Harrelson, 1989; Balice-Gordon et al., 1990), and acetylcholine receptor number (Bleisch et al., 1982; Bleisch and Harrelson, 1989).
Testosterone appears to be particularly potent in regulating some features of the adult SNB system. Treatment of castrates with the non-aromatizable androgen dihydrotestosterone is not as effective in preventing castration-induced reductions in SNB soma (Fraley and Ulibarri, 2002) or target muscle size (Forger et al., 1992), and estrogens are completely ineffective (Forger et al., 1992; Fraley and Ulibarri, 2002). However, as in development, estrogens have been reported to affect other aspects of the SNB system in adulthood. As with androgens, treatment with estrogens after castration maintains (Johnson and Tiefer, 1974; Meisel et al., 1984) or restores (Södersten, 1973; Södersten and Larsson, 1975) copulatory behavior. The penile reflexes mediated by SNB-innervated musculature are also sensitive to estradiol treatment, maintaining in copula sexual reflexes following castration (O’Hanlon et al., 1981). Treatment of castrates with estradiol maintains EMG activity of the BC during copulation (Holmes and Sachs, 1992) and after peripheral nerve stimulation (Fargo et al., 2003), and this effect can be localized to the neuromuscular periphery (Foster and Sengelaub, 2004). Similarly, estradiol treatment increases glucose-6-phosphate dehydrogenase activity in the SNB target musculature (Knudsen and Max, 1980).
In this experiment we assessed the ability of testosterone metabolites, non-aromatizable androgens and estrogens, to maintain SNB neuromuscular morphology in adulthood. As discussed above, previous studies have assessed the hormonal regulation of SNB soma size and target muscle weight, but the regulation of SNB dendrites by testosterone metabolites in adulthood has not been addressed. Given that non-aromatizable androgens act in combination with estrogens in the development of SNB dendrites, in this study we assessed their potential role in maintaining dendritic morphology in adults.
MATERIALS AND METHODS
Animals
Young adult male rats (Harlan Laboratories, Indianapolis, IN), approximately 11 weeks old at the beginning of the study, were maintained on a 12:12 light/dark cycle with food and water freely available. To assess potential differential effects of gonadal hormones on the maintenance of adult SNB neuromuscular morphology, several manipulations were performed. Four groups of rats were anesthetized with ether, castrated, and implanted subcutaneously with Silastic capsules (3.18 mm, o.d., 1.57 mm, i.d.; Smith et al., 1977) filled with either crystalline testosterone (45 mm; Kurz et al., 1986), dihydrotestosterone (30 mm; Forger et al., 1992), or estradiol (5 mm, in a 1:1 mixture with cholesterol; Argente et al., 1990; Chowen et al., 1990) or left blank. All hormone treatments were six weeks in duration. These forms and dosages of hormone were chosen because they have previously been demonstrated to be effective in the adult SNB neuromuscular system and/or to produce physiological levels of circulating hormone. All hormones were obtained from Steraloids, Inc. (Newport, RI). Four groups of gonadally intact males were also included. One group was anesthetized with ether and implanted subcutaneously with Silastic capsules containing the 5α-reductase inhibitor finasteride (30 mm; Steraloids, Inc., Newport, RI; Frye, 2001; Wright et al., 1999). To assess the potential role of estrogens acting in combination with androgens, we treated another group with the aromatase blocker fadrozole (fadrozole HCl, Ciba-Geigy CGS 16949A; Steele et al., 1987; Bonsall et al., 1992). Males were anesthetized with ether and implanted subcutaneously with Alzet mini-osmotic pumps (Model 2004, Alza Corp., Palo Alto, CA) filled with fadrozole in aqueous solution (0.25 mg/kg/day); pumps were replaced once after 3 weeks. This treatment regime with fadrozole has been demonstrated to inhibit conversion of testosterone to estradiol and suppress male sexual activity (Bonsall et al., 1992). Finasteride and fadrozole treatments were also six weeks in duration. The remaining two groups of intact males were either sham castrated or left untreated (n = 43; 4–6 animals per group, eight groups overall; see Table 1).
Table 1.
Treatment groups by gonadal condition, treatment, and group size (N).
| Gonadal condition | Treatment | N |
|---|---|---|
| Castrated males | testosterone implant | 6 |
| dihydrotestosterone implant | 6 | |
| estradiol implant | 4 | |
| blank implant | 6 | |
| Intact males | finasteride implant | 6 |
| fadrozole mini pump | 4 | |
| sham castration | 5 | |
| normal male (untreated) | 6 |
To confirm the efficacy of fadrozole treatment, the normal and fadrozole-treated males were paired with receptive females on two consecutive days immediately prior to morphological study. Stimulus females were anesthetized with chlorapent (0.3 ml/100 g ip), ovariectomized, and implanted subcutaneously with Silastic capsules filled with estradiol (10 mm). Sexual receptivity was then induced with injections of progesterone (Steraloids, Newport, RI; 1 mg, dissolved in sesame oil) administered 4–6 hours prior to behavioral testing (Bonsall et al., 1992). Tests were conducted 2–4 hours after lights off. Each male was placed in a chamber (44 × 23 × 20 cm high) with a fresh receptive female and videotaped (Sony Hi8) under dim light for 30 minutes. Videotapes were later scored for duration of perivaginal inspection (total sec), latency to first mount attempt (time from introduction to first mount or 1800 sec for nonresponders), number of attempted mounts per test, and inter-mount latency (1800 sec for nonresponders) by an observer blind to experimental condition.
All procedures were performed in accordance with the Indiana University Animal Care and Use Guidelines.
Histochemistry
Horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP; List Biological, Campbell, CA) was used to retrogradely label SNB motoneurons innervating the BC muscle. Previous studies have demonstrated that BHRP labeling permits sensitive detection and quantitative analysis of SNB somal and dendritic morphologies (Kurz et al., 1986; Goldstein et al., 1990; Nowacek and Sengelaub, 2006). After the six weeks of treatment, animals were anesthetized with ether, and the left BC muscle was exposed and injected with BHRP (0.5μL, 0.2% solution). Forty-eight hours after BHRP injection, a period that ensures optimal labeling of SNB motoneurons (Kurz et al., 1986; Goldstein et al., 1990), animals were overdosed with urethane (0.25 g/100 g body weight) and perfused intracardially with saline followed by cold 1% paraformaldehyde/1.25% glutaraldehyde. Presence of implants was confirmed, and lumbar cords were removed, postfixed in the same solution for 5 h, and then transferred to sucrose phosphate buffer (10% w/v, pH = 7.4) overnight for cryoprotection. Spinal cord segments were then embedded in gelatin and frozen-sectioned transversely at 40μm; all sections were collected into four alternate series. For visualization of BHRP, the tissue was immediately reacted using a modified tetramethyl benzidine protocol (TMB; Mesulam, 1982), mounted on gelatin-coated slides, and counterstained with thionin. BC/LA muscles were removed at perfusion and weighed to evaluate potential treatment effects on gross muscle weight.
Motoneuron Somata
The number of BHRP-filled motoneurons was assessed in all sections through the entire rostrocaudal extent of the SNB for all animals. Counts of labeled motoneurons in the SNB were made under brightfield illumination, where somata and nuclei could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed. Estimates of the total number of labeled SNB motoneurons were obtained using the optical disector method outlined by Coggeshall (1992) and a procedure similar to that of West and Gundersen (1990). This method yields an unbiased count of SNB motoneurons (Raouf et al., 2000). Counts were made at 500X under brightfield illumination, and motoneuron somata could be easily visualized in multiple focal planes. BHRP labeled motoneurons were counted as their somata first appeared in focus while focusing through the z axis, and labeled somata in the first focal plane (i.e., “tops”) were not counted. For each animal, counts were derived from sections spaced at 160 μm intervals uniformly distributed through the entire rostrocaudal extent of the SNB. Within each section, all labeled somata within the SNB were counted. Estimates of the total number of labeled SNB motoneurons were then obtained by correcting for the percentage of the tissue sampled. All counts were performed blind to experimental condition.
The cross-sectional soma area of BHRP-labeled motoneurons was measured in an average of 21.2 motoneurons for each animal using a video-based morphometry system (Stereo Investigator, MicroBrightField, Inc., Williston, VT) at a final magnification of 1350×. Soma areas within each animal were averaged for statistical analysis. The optical density of labeled somata was also measured under brightfield illumination to confirm equivalence of BHRP labeling density.
Dendritic Length
For each animal, dendritic lengths in a single representative set of alternate sections were measured under dark-field illumination. Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the SNB dendritic field was assessed in every other section (320μm apart) in three dimensions using a computer-based morphometry system (Neurolucida, MBF Bioscience, Inc., Williston, VT; final magnification 250X) to yield both composite illustrations of the arbor and measurements of individual fiber lengths. All BHRP-labeled fibers were drawn regardless of location, size, or contiguity with labeled cell bodies to ensure a complete assessment of dendritic length. Because the entire rostrocaudal range of the SNB dendritic field in each animal was sampled, this method allows for a complete assessment of SNB dendrites in both the transverse and horizontal planes. Average dendritic arbor per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by two to correct for sampling, then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons (Kurz et al., 1991), but has been shown to be a sensitive and reliable indicator of changes in dendritic morphology during normal development (Goldstein et al., 1990), after hormonal or surgical manipulation (Kurz et al., 1986, 1991; Forger and Breedlove, 1987; Goldstein et al., 1990; Goldstein and Sengelaub, 1994; Goldstein et al., 1996; Hays et al., 1996; Hebbeler and Sengelaub, 2003; Nowacek and Sengelaub, 2006), due to dendritic interactions (Goldstein et al., 1993), or after NMDA receptor blockade (Kalb, 1994; Hebbeler et al., 2002).
To assess potential redistributions of dendrites across treatment groups, for each animal the composite dendritic arbor created in the length analysis was divided using a set of axes radially oriented around the central canal, dividing the spinal cord into twelve bins of 30 degrees each. The portion of each animal’s dendritic arbor per labeled motoneuron contained within each location was then determined.
Dendritic Extent
The comparability of BHRP labeling across groups was assessed by quantifying both the rostrocaudal and radial dendritic extent of SNB arbors. The rostrocaudal extent of the dendritic arbor was determined by recording the distance between the first and last section in which labeled SNB dendrites were present for each animal. In the mediolateral plane, for each animal the maximal radial extent of the dendritic arbor for each section throughout the rostrocaudal extent of the SNB dendritic field was measured using the same set of axes and 30 degree bins used for the dendritic distribution analysis. For each bin, the distance between the central canal and the most distal BHRP-filled process was measured.
All data were collected without prior knowledge of the hypothesis being tested. Statistical analysis consisted of analyses of variance (one-way or two-way with repeated measures) followed by appropriate planned comparisons (Fisher’s Protected LSD) as described below. Digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop.
RESULTS
Sexual Behavior
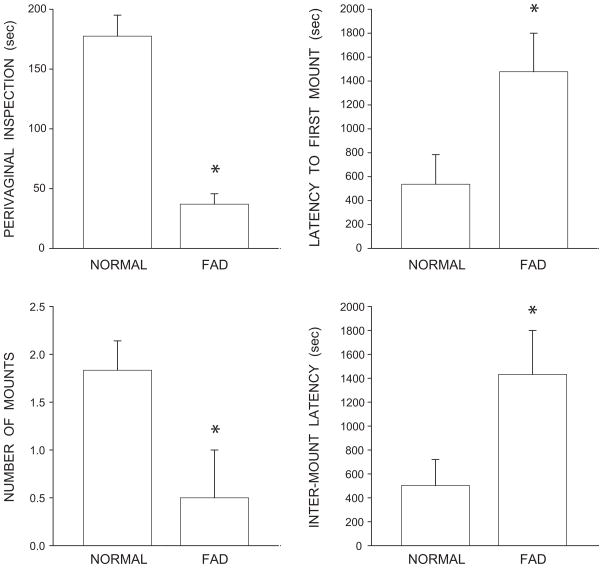
Fadrozole treatment was effective in reducing male sexual behavior (Fig. 1). Perivaginal inspection of stimulus females was reduced by 79% in fadrozole-treated males compared to that of normal males [F(1,8) = 37.25, p < 0.001]. Latency to the first mount attempt was increased by 175% in fadrozole-treated males compared to that of normal males [F(1,8) = 5.50, p < 0.05]. The number of mount attempts was decreased by 73% in fadrozole-treated males [F(1,8) = 5.85, p < 0.05], and the inter-mount latency was increased by 178% in fadrozole-treated males compared to normal males [F(1,8) = 5.43, p < 0.05].
Figure 1.
Copulatory behavior after exposure to sexually receptive females in gonadally intact and fadrozole-treated males, as assessed by perivaginal inspection (top, left), latency to first mount (top, right), number of mounts (bottom, left), and inter-mount latency (bottom, right). Bar heights represent means ± SEM. *Significantly different from normal males.
BC/LA Muscle Weight
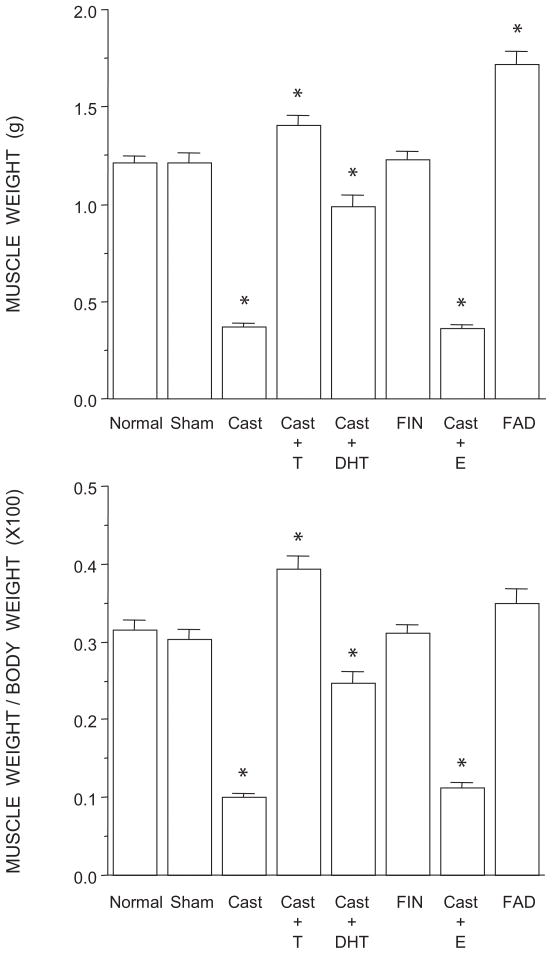
The weights of BC/LA musculature showed a significant effect of group [F(7,35) = 106.82, p < 0.0001; Fig. 2, top]. The weight of the BC/LA muscles in normal males, sham castrates, and finasteride-treated males was typical, and did not differ across groups (LSDs, all n.s.). Muscle weights in testosterone-treated castrates and fadrozole-treated males were increased 15% and 41%, respectively, relative to those of normal males (LSDs, p < 0.003). Muscle weights in castrates and castrates treated with estradiol were on average 69% less than those of normal males (LSDs, p < 0.0001). Castrates treated with dihydrotestosterone had muscle weights that were 17% less than those of normal males (LSD, p < 0.001) but on average 63% more than those of estradiol-treated or untreated castrates (LSDs, p < 0.0001).
Figure 2.
Weights of BC/LA muscles expressed as raw weights (top) and relative to individual body weights (bottom) of normal, sham-castrated, castrated, testosterone (T)-treated castrated, dihydrotestosterone (DHT)-treated castrated, finasteride (FIN)-treated, estradiol (E)-treated, or fadrozole (FAD)-treated males. Bar heights represent means ± SEM. *Significantly different from normal males.
To determine if the altered BC/LA muscle weights reflected a general somatic effect of treatment, we assessed overall body weights across groups and found a significant effect of treatment [F(7,35) = 20.84, p < 0.0001]. Body weights in normal males (385.17 ± 12.36 g; mean ± SEM), sham castrates (401.60 ± 12.64 g), dihydrotestosterone-treated castrates (405.17 ± 5.60 g), untreated castrates (375.17 ± 4.61 g), and finasteride-treated males (396.00 ± 10.02 g) did not differ across groups (LSDs, all n.s.). In contrast, fadrozole-treated males had body weights (493.50 ± 16.18 g) that were 28% more than those of normal males (LSD, p < 0.0001), while those of testosterone- (356.50 ± 7.44 g) and estradiol-treated (318.70 ± 6.42 g) castrates were on average 12% less (LSDs, p < 0.04). After correcting for the differences in body weight (Fig. 2, bottom), differences in BC/LA muscle remained [F(7,35) = 76.56, p < 0.001]. While corrected BC/LA muscle weights in fadrozole-treated males were now not different from those of normal males, sham castrates, or finasteride-treated males, BC/LA muscle weights of testosterone-treated males remained significantly increased (compared to those of normal males; LSD, p < 0.0001). Similarly, corrected muscle weights in castrates and castrates treated with estradiol continued to be significantly decreased relative to those of normal males (LSDs, p < 0.0001), and muscle weights in castrates treated with dihydrotestosterone were still less than those of normal males (LSD, p < 0.001) but more than those of estradiol- or untreated castrates (LSDs, p < 0.0001).
Morphometry
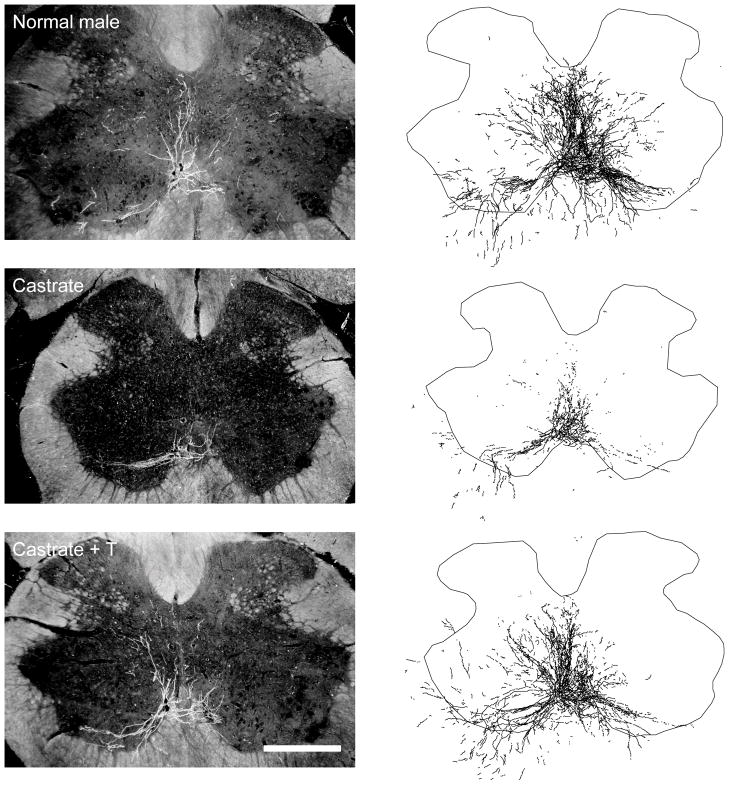
Injection of BHRP into the left BC successfully labeled ipsilateral SNB motoneurons of all animals (76.15 ± 3.21 per animal) in a manner consistent with previous studies (Kurz et al., 1986; Fargo and Sengelaub, 2004a,b; Fargo et al., 2006). SNB motoneurons displayed their characteristic multipolar morphologies, with dendritic arbors projecting ventrolaterally, dorsomedially and across the midline into the area of the contralateral SNB (Figs. 3, 4).
Figure 3.
(left) Dark-field photomicrographs of transverse sections through the lumbar spinal cord of a normal (top), castrated (middle), and testosterone (T)-treated castrated male after BHRP injection into the left BC muscle. Scale bar = 500 μm. (right) Computer-generated composites of BHRP-labeled SNB somata and processes drawn at 320 μm intervals through the entire rostrocaudal extent of the SNB; these composites were selected as they are representative of their respective group average dendritic lengths.
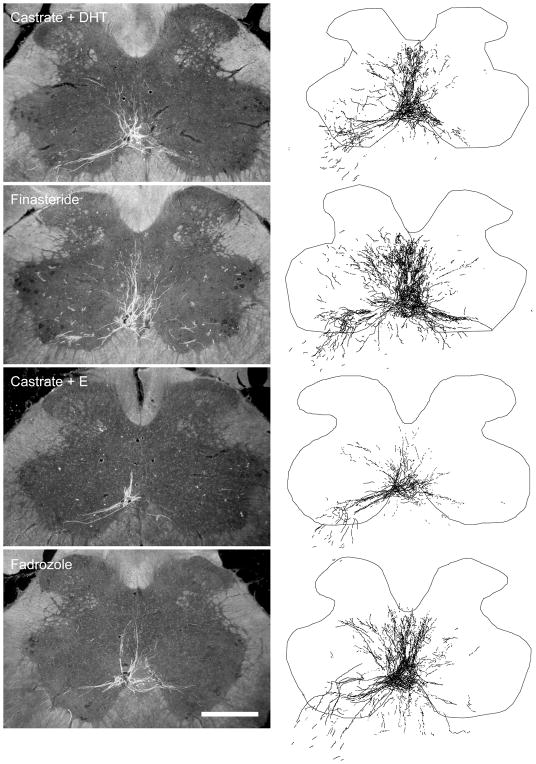
Figure 4.
(left) Dark-field photomicrographs of transverse sections through the lumbar spinal cord of a dihydrotestosterone (DHT)-treated castrated (top), finasteride (FIN)-treated (middle, upper), estradiol (E)-treated castrated (middle, lower) and fadrozole (FAD)-treated male after BHRP injection into the left BC muscle. Scale bar = 500 μm. (right) Computer-generated composites of BHRP-labeled SNB somata and processes, drawn and selected as in Fig. 3.
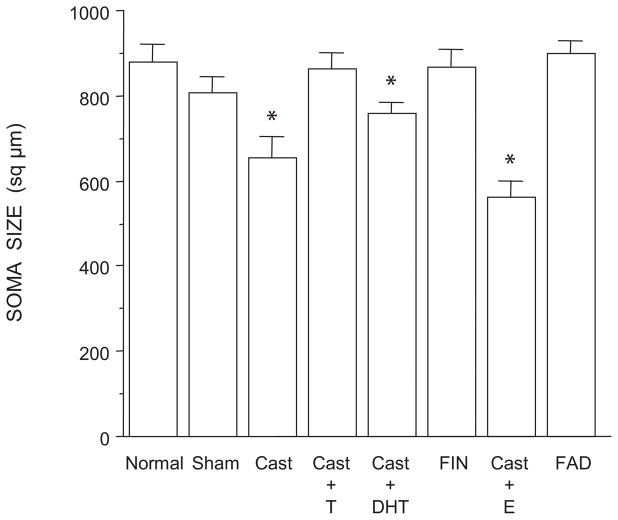
Soma Area
The size of SNB somata differed across groups [F(7,35) = 8.84, p < 0.0001; Fig. 5]. The mean cross-sectional areas of SNB somata in normal males, sham castrates, castrates treated with testosterone, and finasteride- or fadrozole-treated males were typical, and did not differ from each other (LSDs, all n.s.). However, SNB somata in castrates and castrates treated with estradiol were smaller (26% and 30% respectively) than those of normal males [(LSDs, ps < 0.0001), and did not differ from each other (LSD, n.s.). Castrates treated with dihydrotestosterone had SNB somata that were 14% smaller than those of normal males (LSD, p < 0.03) but on average 25% larger than those of estradiol- or untreated castrates (LSDs, p < 0.05).
Figure 5.
Soma areas of SNB motoneurons in normal, sham-castrated, castrated, testosterone (T)-treated castrated, dihydrotestosterone (DHT)-treated castrated, finasteride (FIN)-treated, estradiol (E)-treated, or fadrozole (FAD)-treated males. Bar heights represent means ± SEM. *Significantly different from normal males.
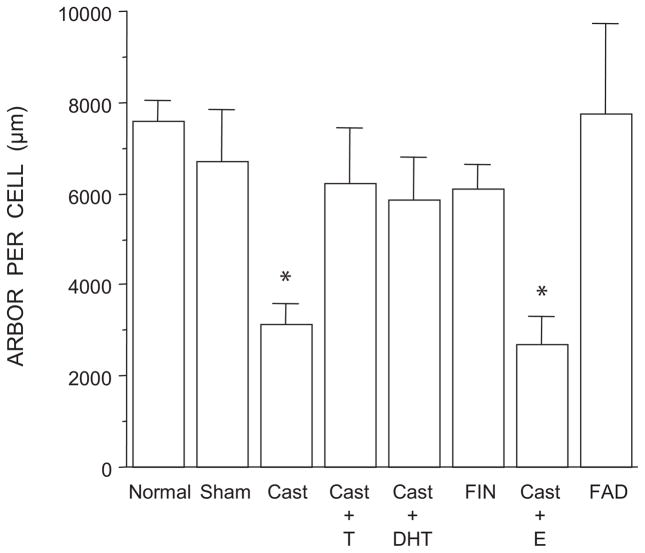
Dendritic Length
The overall length of SNB dendrites differed across groups [F(7,35) = 3.87, p < 0.01; Fig. 6]. SNB dendritic lengths in normal males, sham castrates, castrates treated with either testosterone or dihydrotestosterone, and males treated with either finasteride or fadrozole did not differ from each other (LSDs, all n.s.). However, SNB dendritic lengths in castrates and castrates treated with estradiol were smaller (58% and 64% respectively) than those of normal males (LDSs, p < 0.01) and did not differ from each other (LSD, n.s.).
Figure 6.
Dendritic lengths expressed as length of arbor per labeled motoneuron in normal, sham-castrated, castrated, testosterone (T)-treated castrated, dihydrotestosterone (DHT)-treated castrated, finasteride (FIN)-treated, estradiol (E)-treated, or fadrozole (FAD)-treated males. Bar heights represent means ± SEM *Significantly different from normal males.
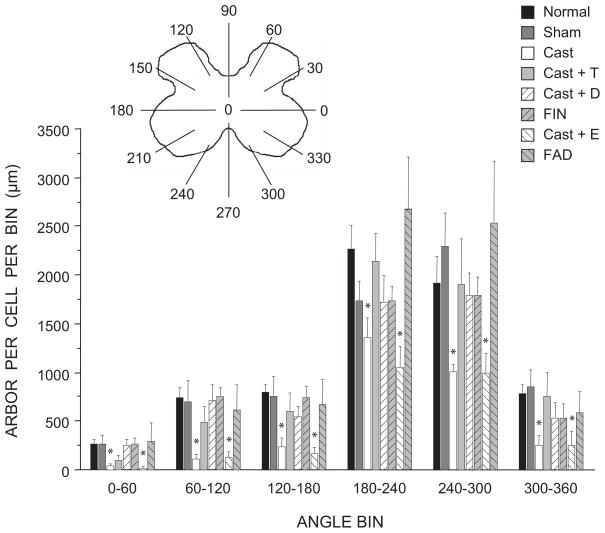
Dendritic Distribution
As previously noted (Goldstein et al., 1993), the SNB dendritic arbor of normal males is radially organized but not uniformly distributed, with over 50% of the arbor concentrated ventrolaterally between 180° and 300° (Fig. 7). The distribution of SNB dendrites showed the typical significant effects of location [F(11,374) = 138.23, p < 0.0001], as well as a main effect of group [F(7,374) = 3.509, p < 0.01]. SNB dendrites in normal males, sham castrates, castrates treated with either testosterone or dihydrotestosterone, and males treated with either finasteride or fadrozole showed the usual non-uniform distribution [F(11,286) = 109.90, p < 0.0001], and there was no evidence of a group difference [F(5,286) = 0.46, n.s.]. Although the non-uniform distribution of dendrites was retained in castrates [repeated measures F(11,110) = 29.99, p < 0.0001] and estradiol-treated castrates [repeated measures F(11,88) = 34.83, p < 0.0001], compared to normal males the amount of dendritic arbor in all locations was reduced [castrates, ranging from 36% to 87% per bin; F(1,110) = 39.99, p < 0.0001; estradiol-treated castrates, 40% to 90% per bin; F(1,88) = 34.83, p < 0.001]. The amount of dendritic arbor in any location did not differ between the castrate and estradiol-treated castrate groups [location by group interaction F(11,88) = 0.57, n.s.].
Figure 7.
(inset) Schematic drawing of spinal gray matter divided into radial sectors for measure of SNB dendritic distribution. Length per radial bin of SNB dendrites in normal, sham-castrated, castrated, testosterone (T)-treated castrated, dihydrotestosterone (DHT)-treated castrated, finasteride (FIN)-treated, estradiol (E)-treated, or fadrozole (FAD)-treated males; for graphical purposes length per radial bin measures have been collapsed into 6 bins of 60° each. Bar heights represent means ± SEM. *Significantly different from normal males.
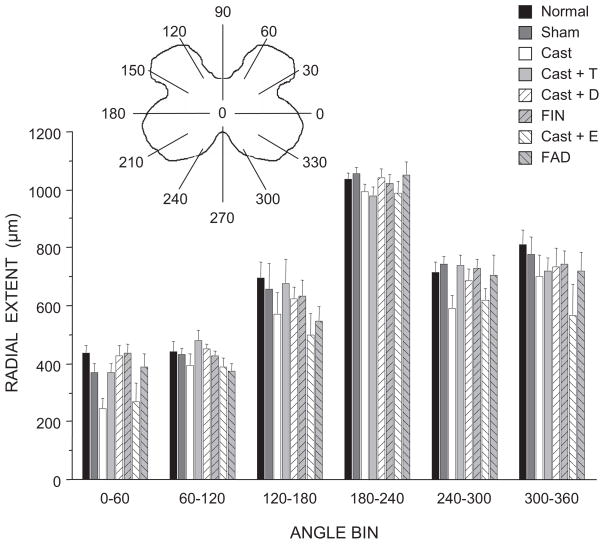
Dendritic Extent
The distance spanned by SNB dendrites throughout the rostrocaudal axis did not differ across treatment groups [F(7,35) = 0.85, n.s.; normal males = 3333 ± 140 μm, sham castrates = 3360 ± 72 μm, castrates = 3307 ± 135 μm, castrates treated with testosterone = 3200 ± 248 μm, castrates treated with dihydrotestosterone = 3440 ± 164 μm, finasteride-treated males = 3520 ± 230 μm, castrates treated with estradiol = 3040 ± 113 μm, fadrozole-treated males = 3080 ± 177 μm]. Similarly, the radial extent of BHRP labeling was typical, and was equivalent across groups [F(7,374) = 1.84, n.s.; Fig. 8] whether measured per radial bin or as an overall average. The overall mean radial extent ranged from 559.37 ± 37.95 μm in castrates treated with estradiol up to a maximum of 694.17 ± 14.82 μm in normal males.
Figure 8.
(inset) Schematic drawing of spinal gray matter divided into radial sectors for measure of SNB radial extent. Radial extents of SNB dendrites in normal, sham-castrated, castrated, testosterone (T)-treated castrated, dihydrotestosterone (DHT)-treated castrated, finasteride (FIN)-treated, estradiol (E)-treated, or fadrozole (FAD)-treated males; for graphical purposes dendritic extent measures have been collapsed into 6 bins of 60° each. Bar heights represent means ± SEM.
DISCUSSION
In adulthood, the morphology of the SNB neuromuscular system is differentially sensitive to testosterone and its metabolites. Consistent with previous reports (Forger et al., 1992; Fraley and Ulibarri, 2002), castration-induced reductions in SNB soma size and target muscle weight were prevented by treatment with testosterone, but only partially attenuated by treatment with its non-aromatizable, androgenic metabolite dihydrotestosterone. By contrast, SNB dendritic length was fully maintained by treatment with either testosterone or dihydrotestosterone after castration, suggesting that there are differences in androgen responsivity across the various elements of the SNB system. Furthermore, treatment of intact males with finasteride or fadrozole did not alter the morphology of SNB motoneurons or their target muscles. Finally, estradiol treatment was completely ineffective in preventing castration-induced atrophy of the SNB neuromuscular system. Together, these results suggest that the maintenance of adult SNB motoneuron and target muscle morphology is strictly mediated by androgens.
BC/LA Muscle Weight
As previously reported (Wainman and Shipounoff, 1941; Dorfman and Kincl, 1963; Vyskocil and Gutmann, 1977), castration of adult males resulted in a significant reduction in corrected BC/LA muscle weight. Treatment with testosterone or its metabolites had varying effects. Testosterone treatment resulted in hypertrophy of BC/LA muscle weight compared to that of gonadally intact males. Given that testosterone implants of this size produce plasma titers in the physiological range (Smith et al., 1977), this hypertrophy is interesting. The superior efficacy of implants over endogenous hormone in supporting androgen-dependent features has been observed previously (e.g., Damassa, 1976;), and may reflect the difference between a given (and fixed) circulating testosterone level from an implant and the same average (but fluctuating) level of hormone in intact males. On the other hand, dihydrotestosterone was only partially effective in maintaining BC/LA muscle weight and estrogen was completely ineffective. In addition, treatment of gonadally intact males with the aromatase inhibitor fadrozole or the 5α-reductase inhibitor finasteride had no effect on BC/LA muscle weight, suggesting that conversion of testosterone to either of its primary metabolites is not necessary for the maintenance of BC/LA muscle weight in adulthood. It should be noted that although the behavioral data indicate that the fadrozole treatment was effective, the lack of a similar positive control for the finasteride treatment leaves the possibility that testosterone conversion to dihydrotestosterone was unaffected. Thus, the lack of effects in the finasteride-treated males should be interpreted with caution. However, in agreement with the muscle weight data previously reported in Forger et al. (1992), these findings do confirm that the maintenance of BC/LA muscle weight in adulthood is strictly androgenic. Previous studies have suggested that androgenic regulation of BC/LA muscle weight occurs locally at the muscle (Rand and Breedlove, 1992; Monks et al., 2004). For example, BC/LA muscles express high levels of androgen receptors (Dube et al., 1976; Tremblay et al., 1977; Monks et al., 2006) and BC muscle weight can be regulated by local administration of testosterone or the anti-androgen hydroxyflutamide directly to the muscle (Rand and Breedlove, 1992). Furthermore, androgens are capable of maintaining BC/LA muscle weight even after denervation (Fishman and Breedlove, 1988). Thus, it is likely that the maintenance of BC/LA muscle weight with testosterone treatment we observed was mediated by androgenic action at the muscle.
In the current experiment, BC/LA muscle weight was better maintained with testosterone than with dihydrotestosterone. This difference could simply reflect the larger implant size used for testosterone treatment, and it is possible that the lower dosage of dihydrotestosterone was not sufficient to maintain BC/LA muscle weight. However, this difference in effectiveness was not seen across all measures, as dendritic length was maintained in castrated males to a similar degree with both testosterone and dihydrotestosterone (see below). Alternatively, it is possible that not all androgens exert equal effects on the BC/LA. The affinity constant for testosterone in the BC/LA muscles is almost four times larger than that of dihydrotestosterone, which suggests that testosterone is the more active steroid in muscles (Dube et al., 1976). The differential effects of testosterone and dihydrotestosterone on BC/LA muscle weight could also be due to differences in the local concentrations of metabolic enzymes in the BC/LA. For example, high levels of 3α-hydroxy steroid dehydrogenase (3α-HSD) are present in striated muscle tissue, and exogenously supplied dihydrotestosterone is quickly converted to inactive metabolites in the BC/LA muscle, whereas testosterone itself is relatively stable (Dionne et al., 1977; Tremblay et al., 1977; Krieg et al., 1974). In addition, 5α-reductase activity is negligible in striated muscles, including the BC/LA (Tremblay et al., 1977), suggesting that conversion of testosterone to dihydrotestosterone does not normally occur there.
SNB Morphometry
Somata
Similar to BC/LA weight, castration resulted in a significant decrease in the size of SNB somata, while treatment with testosterone or its metabolites had varying effect. As in the BC/LA muscles, SNB soma size after castration was most effectively maintained with testosterone and partially so with dihydrotestosterone. This difference in efficacy could again simply reflect the dosages of testosterone and dihydrotestosterone used. However, using equal-sized testosterone and dihydrotestosterone implants, Forger et al. (1992) reported similar results for the effect of androgens on SNB soma size (although in that experiment the difference between testosterone and dihydrotestosterone treatment failed to reach significance). Furthermore, if our attempt to block the conversion of testosterone to dihydrotestosterone was successful, the lack of effect on SNB soma size in finasteride-treated males also suggests that the metabolism of testosterone is not necessary for the maintenance of SNB somal morphology in adulthood.
In contrast, estradiol was completely ineffective in maintaining SNB soma size, and treatment with fadrozole with the goal of blocking the conversion of testosterone to estradiol had no effect. These findings are in good agreement with previous reports (Forger et al., 1992, Fraley and Ulibarri, 2002) demonstrating that gonadal hormone regulation of SNB soma size is strictly androgen-dependent. Previous reports have suggested that the site of action for the androgenic regulation of SNB soma size is the motoneurons themselves. For example, androgen receptors are present in SNB motoneurons (Dube et al., 1976; Breedlove and Arnold, 1980) and treatment of Tfm females with testosterone maintains SNB soma size only in those motoneurons expressing the wild-type, functional AR (Watson et al., 2001). Furthermore, the BC muscle does not appear to be the site of action for the androgenic regulation of SNB somata. For example, testosterone treatment increases SNB size after axotomy (Yang and Arnold, 2000) and local administration of testosterone directly to the BC muscle fails to maintain SNB soma size in castrated animals (Rand and Breedlove, 1995). Finally, if soma size is regulated by hormone action at the motoneurons themselves, the lack of accumulation of radiolabeled estradiol by SNB motoneurons (Breedlove and Arnold, 1980) is consistent with the failure of estradiol treatment to support soma size after castration.
Dendrites
Similar to previous findings (Kurz et al., 1986), castration resulted in a substantial reduction in SNB dendritic length, which was prevented by treatment with testosterone. Unlike the BC/LA muscles or SNB soma size, dihydrotestosterone was also effective in fully maintaining SNB dendritic length after castration. Again, if the finasteride treatment was successful, the lack of effect on SNB dendrites also suggests that the metabolism of testosterone is not necessary for the maintenance of SNB dendritic morphology in adulthood. In contrast, estradiol was completely ineffective in maintaining SNB dendritic length, and treatment with fadrozole with the goal of blocking the conversion of testosterone to estradiol had no effect. These results demonstrate that the maintenance of SNB dendritic morphology in adulthood is strictly androgen-dependent. The site of action for the androgenic regulation of SNB dendritic morphology is not known. However, several reports suggest the BC/LA muscles as a likely possibility. For example, the length of dendritic arbor can be regulated by local application of testosterone or the anti-androgen flutamide directly to the BC/LA (Rand and Breedlove, 1995). Furthermore, axotomy of SNB motoneurons prevents the maintenance of SNB dendrites in testosterone-treated castrated males (Yang et al., 2004). Thus, it is likely that the androgenic effects we observed on SNB dendrites were regulated via the BC/LA muscles.
Previous studies have demonstrated that neither axonal transport of BHRP (Leslie et al., 1991), nor dendritic transport as demonstrated by the rostrocaudal or mediolateral extent of dendritic labeling (Kurz et al., 1991; Goldstein and Sengelaub, 1994) are affected by hormone levels. In the present experiment, the possibility that hormonal differences could affect retrograde transport is an important consideration, as such an artifact could potentially result in apparent alterations in dendritic morphology. No differences in either the rostrocaudal or radial extents of dendrites were observed, indicating that the ability of SNB dendrites to transport BHRP to the most distal, highest order branches was not affected. Thus, we believe the dendritic labeling across groups was comparable and the differences in dendritic lengths we report reflect changes in the amount of dendritic material consequent to hormonal manipulation.
Differential Responses to Hormones in the SNB Neuromuscular System
Our current results demonstrate that after castration testosterone and dihydrotestosterone are equally effective in maintaining SNB dendritic length but not BC/LA muscle weight. Evidence suggests that both of these features of the SNB neuromuscular system are regulated through androgenic action at the target musculature (Rand and Breedlove, 1992, 1995; Yang et al., 2004; Monks et al., 2004). Given that dihydrotestosterone is found in relatively low concentrations in the BC/LA muscles (Tremblay et al., 1977; Bartsch et al., 1983), our current results suggest that SNB dendrites may be more responsive than the BC/LA muscles to the same level of androgen. Alternatively, the failure of dihydrotestosterone to fully support BC/LA muscle weight after castration suggests that there may be different pathways involved in the regulation of BC/LA muscle weight and SNB dendritic morphology.
Our current data support the hypothesis that the relative potency of testosterone and dihydrotestosterone is cell-type specific. This specificity may depend on the steroidogenic enzymes and/or androgen receptor co-factors that are expressed (Tremblay et al., 1977; Martini et al., 1993; Pozzi et al., 2003; O’Bryant and Jordan, 2005; Tetel, 2009). Furthermore, as discussed above, the various elements of the SNB neuromuscular system are likely regulated by androgenic action at different loci. Differential responses to androgens have been observed in the behavioral activation of the SNB system and the maintenance of its morphological features. For example, implantation of a 5 mm silastic capsule containing testosterone is sufficient to maintain male copulatory behavior but ineffective in maintaining SNB soma or nuclear size or BC/LA muscle weight (Raouf et al., 2000). Previous work from our laboratory has shown differential effects of androgens on different components of the SNB system during development. For example, administration of dihydrotestosterone to postnatal castrates results in full masculinization of SNB somal growth; however, SNB dendritic growth is only partially supported by dihydrotestosterone treatment (Goldstein and Sengelaub, 1994). In addition, this difference is not due to a critical role for testosterone’s estrogenic metabolites, as treatment with estradiol alone or in conjunction with dihydrotestosterone has no effect on soma size (Forger et al., 1992). Similarly, in the present study, treatment with the aromatase inhibitor fadrozole with the intent of blocking estradiol synthesis did not affect SNB somal and dendritic morphology or BC/LA muscle weight.
Estrogenic Effects on Dendrites are Limited to Early Development
The SNB neuromuscular system has traditionally been thought of as exclusively androgen-dependent in adulthood and the current data support this conclusion. The failure of estradiol to support adult dendritic morphology stands in sharp contrast to estradiol’s support of SNB dendritic development. Unlike other aspects of SNB development, estrogens appear to be a critical component for the initial growth of SNB dendrites. Treatment of castrated males with estradiol supports SNB dendritic growth through the first 4 postnatal weeks (Goldstein and Sengelaub, 1994), but typically not to the level of testosterone-treated castrates or intact males. Similarly, blocking estrogen receptors by treatment with tamoxifen (Taylor et al., 1995; Nowacek and Sengelaub, 2006), or preventing estradiol synthesis with fadrozole (Burke et al., 1999), during the period of dendritic growth in gonadally intact males results in dendritic lengths that are significantly below those of normal males. This estrogenic influence is apparently limited to the early postnatal period. By seven weeks of age, SNB dendrites become insensitive to estrogens (Goldstein and Sengelaub, 1994; Hebbeler et al., 2001), and this late-developing insensitivity to estrogens persists into adulthood (current results). The transient estrogenic effect on SNB dendrites may be the result of postnatal changes in the developing spinal cord, for example via the decline in NMDA receptor expression (Verhovshek et al., 2005). Taken together, these findings suggest that there are separate pathways/mechanisms mediating the estrogenic effects on SNB morphology during development and the behavioral/muscular activation effects observed in adulthood (O’Hanlon et al., 1981; Holmes and Sachs, 1992; Fargo et al., 2003). Furthermore, the estrogen-sensitive behavioral and muscular activation components in adults do not appear to be mediated by estrogenic effects on the morphological features of the underlying motoneurons and muscles.
Conclusions
Previous research (Forger et al., 1992; Fraley and Ulibarri, 2002) has shown that SNB soma size and BC/LA muscle weight in adulthood is strictly dependent on androgens and that testosterone is more effective in maintaining these structures than dihydrotestosterone. Our current results support and extend these findings by demonstrating that SNB dendritic morphology in adulthood can be maintained by either testosterone or dihydrotestosterone, while estradiol is completely ineffective. Taken together, these findings suggest that differences in androgen responsivity may exist across various elements of the SNB neuromuscular system. In addition, they support the idea that there are hormone-specific pathways at different loci mediating the various morphological and behavioral components of the SNB neuromuscular system. Furthermore, the metabolism of testosterone to estrogens does not appear to be necessary for the maintenance of any of the morphological features of the SNB neuromuscular system in adulthood. Thus, in adulthood, androgens appear to be both necessary and sufficient for the maintenance of SNB neuromuscular morphology.
Acknowledgments
We wish to thank Dr. Cara Wellman and our anonymous reviewers for helpful comments on this manuscript.
Supported by NIH-NICHD HD35315 to D.R.S.
References
- Argente J, Chowen-Breed JA, Steiner RA, Clifton DK. Somatostatin messenger-rna in hypothalamic neurons is increased by testosterone through activation of androgen receptors and not by aromatization to estradiol. Neuroendocrinol. 1990;52:342–349. doi: 10.1159/000125618. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Breedlove SM, Bernstein S, Lichtman JW. Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci. 1990;10:2660–2671. doi: 10.1523/JNEUROSCI.10-08-02660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch W, Knabbe C, Voigt KD. Regulation and compartmentalization of androgens in rat prostate and muscle. J Steroid Biochem. 1983;19:929–937. doi: 10.1016/0022-4731(83)90036-5. [DOI] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson A. Androgens modulate endplate size and Ach receptor density at synapses in rat levator ani muscle. J Neurobiol. 1989;20:189–202. doi: 10.1002/neu.480200403. [DOI] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson AL, Luine VN. Testosterone increases acetylcholine receptor number in the “levator ani” muscle of the rat. J Neurobiol. 1982;13:153–161. doi: 10.1002/neu.480130207. [DOI] [PubMed] [Google Scholar]
- Bonsall RW, Clancy AN, Michael RP. Effects of the nonsteroidal aromatase inhibitor, Fadrozole, on sexual behavior in male rats. Horm Behav. 1992;26(2):240–254. doi: 10.1016/0018-506x(92)90045-w. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Neonatal androgen and estrogen treatments masculinize the size of motoneurons in the rat spinal nucleus of the bulbocavernosus. Cell Mol Neurobiol. 1997;17:687–697. doi: 10.1023/A:1022590104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorskim RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–81. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Burke KA, Kuwajima M, Sengelaub DR. Aromatase inhibition reduces dendritic growth in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1999;38:301–312. [PubMed] [Google Scholar]
- Burke KA, Widows MR, Sengelaub DR. Synergistic effects of testosterone metabolites on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1997;33:1–10. doi: 10.1002/(sici)1097-4695(199707)33:1<1::aid-neu1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Chowen J, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger-RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinol. 1990;52:581–588. doi: 10.1159/000125647. [DOI] [PubMed] [Google Scholar]
- Cihak R, Gutman E, Hanzlikova V. Involution and hormone-induced persistence of the muscle sphincter (levator ani) in female rats. J Anat (Lond) 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15(1):9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Kobashigawa D, Smith ER, Davidson JM. Negative feedback control of LH by testosterone: A quantitative study in male rats. Endocrinol. 1976;99:736–742. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Stefanick ML, Sachs BD, Smith ER. Role of androgen in sexual reflexes of the male rat. Physiol Behav. 1978;21(2):141–6. doi: 10.1016/0031-9384(78)90033-1. [DOI] [PubMed] [Google Scholar]
- Dionne FT, Dube JY, Tremblay RR. Apparent saturability of a 4S dihydrotestosterone-binding protein in rat muscle cytosol: Role of 3α-hydroxysteroid and albumin. Can J Biochem. 1977;55:995–1000. doi: 10.1139/o77-148. [DOI] [PubMed] [Google Scholar]
- Dorfman RI, Kincl FA. Relative potency of various steroids in an anabolic androgenic assay using the castrated rat. Endocrinol. 1963;72:259–266. [Google Scholar]
- Dube J, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Foster AM, Harty MW, Sengelaub DR. Estrogen alters excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J Neurobiol. 2003;56:66–77. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Fargo KW, Iwema CL, Clark-Phelps MC, Sengelaub DR. Androgen-mediated plasticity in an aging sexually dimorphic motor system. Horm Behav. 2006;51:20–30. [Google Scholar]
- Fargo KN, Sengelaub DR. Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J Neurobiol. 2004a;60:348–359. doi: 10.1002/neu.20027. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol. 2004b;469:96–106. doi: 10.1002/cne.10991. [DOI] [PubMed] [Google Scholar]
- Fishman RB, Breedlove SM. Neonatal androgen maintains sexually dimorphic muscles in the absence of innervation. Muscle Nerve. 1988;11:553–560. doi: 10.1002/mus.880110606. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Seasonal variation in striated muscle mass and motoneuron morphology. J Neurobiol. 1987;18:155–165. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- Forger NG, Fishman RB, Breedlove SM. Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav. 1992;26:204–213. doi: 10.1016/0018-506x(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Forger NG, Wagner CK, Contois M, Bengston L, MacLennan AJ. Ciliary neurotrophic factor receptor α in spinal motoneurons is regulated by gonadal hormones. J Neurosci. 1998;18:8720–8729. doi: 10.1523/JNEUROSCI.18-21-08720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AM, Sengelaub DR. Hormone sensitivity of muscle activation in the sexually dimorphic SNB/BC neuromuscular system of the rat. Neurosci Lett. 2004;359:41–44. doi: 10.1016/j.neulet.2004.01.065. [DOI] [PubMed] [Google Scholar]
- Fraley G, Ulibarri C. Long term castration affects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res. 2002;953:265–271. doi: 10.1016/s0006-8993(02)02949-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Inhibition of 5alpha-reductase enzyme or GABA(A) receptors in the VMH and the VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J Endocrinol Invest. 2001;24(6):399–407. [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Kalkbrenner AE, Sengelaub DR. Changes in dendritic morphology of rat spinal motoneurons during development and after unilateral target deletion. Dev Brain Res. 1993;73:151–163. doi: 10.1016/0165-3806(93)90133-u. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Mills AC, Sengelaub DR. Motoneuron development after deafferentation. I. Dorsal rhizotomy does not alter growth in the spinal nucleus of the bulbocavernosus. Dev Brain Res. 1996;91:11–19. doi: 10.1016/0165-3806(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. IV. Masculinization of the spinal nucleus of the bulbocavernosus with testosterone metabolites. J Neurobiol. 1990;21:719–730. doi: 10.1002/neu.480210506. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 1992;326:147–157. doi: 10.1002/cne.903260113. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1994;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Hart BL. Testosterone regulation of sexual reflexes in spinal male rats. Science. 1967;155(767):1283–4. doi: 10.1126/science.155.3767.1283. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-d’Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:802–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hart BL, Wallach SJR, Melese-d’Hospital PY. Differences in responsiveness to testosterone of penile reflexes and copulatory behavior of male rats. Horm Behav. 1983;17:274–283. doi: 10.1016/0018-506x(83)90026-0. [DOI] [PubMed] [Google Scholar]
- Hays TC, Goldstein LA, Mills AC, Sengelaub DR. Motoneuron development after deafferentation: II. Dorsal rhizotomy does not block estrogen-supported growth in the dorsolateral nucleus (DLN) Dev Brain Res. 1996;91:20–28. doi: 10.1016/0165-3806(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Hayes KJ. The so-called ‘levator ani’ of the rat. Acta Endocrinol. 1965;48:337–347. doi: 10.1530/acta.0.0480337. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. Ovariectomy attenuates dendritic growth in hormone-sensitive spinal motoneurons. J Neurobiol. 2001;48:301–314. doi: 10.1002/neu.1059. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Sachs BD. Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Horm Behav. 1992;26:406–419. doi: 10.1016/0018-506x(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Johnson WA, Tiefer L. Mating in castrated male rats during combined treatment with estradiol benzoate and fluoxymesterone. Endocrinol. 1974;95(3):912–915. doi: 10.1210/endo-95-3-912. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. I. Androgen delays the loss of multiple innervation in the levator ani muscle. J Neurosci. 1989a;9:229–238. doi: 10.1523/JNEUROSCI.09-01-00229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. II. Multiple innervation persists in the adult levator ani muscle after juvenile androgen treatment. J Neurosci. 1989b;9:239–247. doi: 10.1523/JNEUROSCI.09-01-00239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Watamura S, Arnold AP. Androgenic, not estrogenic, steroids alter neuromuscular synapse elimination in the rat levator ani. Dev Brain Res. 1995;84:225–232. doi: 10.1016/0165-3806(94)00175-y. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Knudsen J, Max SR. Aromatization of androgens to estrogens mediates increased activity of glucose 6-phosphate dehydrogenase in rat levator ani muscle. Endocrinol. 1980;106:440–443. doi: 10.1210/endo-106-2-440. [DOI] [PubMed] [Google Scholar]
- Krieg M, Szalay R, Voigt KD. Binding and metabolism of testosterone and of 5α-dihydrotestosterone in bulbocavernosus/levator ani (BCLA) of male rats: in vivo and in vitro studies. J Steroid Biochem. 1974;5:453–459. doi: 10.1016/0022-4731(74)90043-0. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol. 1991;22:976–988. doi: 10.1002/neu.480220909. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jordan CL, Arnold AP. Critical period for androgenic regulation of soma size of sexually dimorphic motoneurons in rat lumbar spinal cord. Neurosci Lett. 1989;98:79–84. doi: 10.1016/0304-3940(89)90377-7. [DOI] [PubMed] [Google Scholar]
- Leedy MG, Beattie MS, Bresnahan JC. Testosterone-induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res. 1987;424:386–390. doi: 10.1016/0006-8993(87)91484-3. [DOI] [PubMed] [Google Scholar]
- Leslie M, Forger NG, Breedlove SM. Does androgen affect axonal transport of cholera toxin HRP in spinal motoneurons? Neurosci Lett. 1991;126:199–202. doi: 10.1016/0304-3940(91)90553-6. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Hyodo S. Androgenic regulation of expression of β-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J Neuroendocrinol. 1993;5:357–363. doi: 10.1111/j.1365-2826.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expression of gap junction and b-actin mRNAs in adult rat motoneurons. Neurosci Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Prins GS. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol. 1996;8:53–559. doi: 10.1046/j.1365-2826.1996.04899.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci. 1988a;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Micevych PE, Arnold AP. Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J Neurosci. 1988b;8:4168–4176. doi: 10.1523/JNEUROSCI.08-11-04168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. Organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Meisel RL, O’Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm Behav. 1984;18:56–64. doi: 10.1016/0018-506x(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tracing Neural Connections with Horseradish Peroxidase. Chichester: John Wiley & Sons; 1982. p. 251. [Google Scholar]
- Monks DA, Getsios S, MacCalman CD, Watson NV. N-cadherin is regulated by gonadal steroids in adult sexually dimorphic spinal motoneurons. J Neurobiol. 2001;47:255–264. doi: 10.1002/neu.1033. [DOI] [PubMed] [Google Scholar]
- Monks DA, O’Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: Enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks DA, Kopachik W, Breedlove S, Jordan CL. Anabolic responsiveness of skeletal muscles correlates with androgen receptor protein but not mRNA. Can J Physiol Pharmacol. 2006;84:272–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- Nowacek AS, Sengelaub DR. Estrogenic support of motoneuron dendritic growth via the neuromuscular periphery in a sexually dimorphic motor system. J Neurobiol. 2006;66:962–976. doi: 10.1002/neu.20274. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and – unresponsive motoneurons. Horm Behav. 2005;47:29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- O’Hanlon JK, Meisel RL, Sachs BD. Estradiol maintains castrated male rats’ sexual reflexes in copula, but not ex copula. Behav Neural Biol. 1981;32:269–273. doi: 10.1016/s0163-1047(81)90645-2. [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE. The effect of castration on calcitonin gene-related peptide in spinal motor neurons. Neuroendocrinol. 1989;50(3):338–343. doi: 10.1159/000125243. [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE. Steroid regulation of calcitonin gene-related peptide mRNA expression in motoneurons of the spinal nucleus of the bulbocavernosus. Mol Brain Res. 1990;8:159–166. doi: 10.1016/0169-328x(90)90060-q. [DOI] [PubMed] [Google Scholar]
- Pozzi P, Bendotti C, Simeoni S, Piccioni F, Guerini V, Marron TU, Martini L, Poletti A. Androgen 5-alpha-reductase type 2 is highly expressed and active in rat spinal cord motor neurones. J Neuroendocrinol. 2003;15(9):882–887. doi: 10.1046/j.1365-2826.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992;23:17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf S, Van Roo B, Sengelaub D. Adult plasticity in hormone-sensitive motoneuron morphology: methodological/behavioral confounds. Horm Behav. 2000;38:210–221. doi: 10.1006/hbeh.2000.1620. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscle in penile reflexes, copulation and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone Administration: Peripheral and Intracranial Implants. In: Meyer RD, editor. Methods in Psychobiology. New York: Academic Press; 1977. pp. 259–279. [Google Scholar]
- Södersten P. Estrogen-activated sexual behavior in male rats. Horm Behav. 1973;4(3):247–56. doi: 10.1016/0018-506x(73)90009-3. [DOI] [PubMed] [Google Scholar]
- Södersten P, Larsson K. Lordosis behavior and mounting behavior in male rats: effects of castration and treatment with estradiol benzoate or testosterone propionate. Physiol Behav. 1975;14(2):159–64. doi: 10.1016/0031-9384(75)90160-2. [DOI] [PubMed] [Google Scholar]
- Steele RE, Mellor LB, Sawyer WK, Wasvary JM, Browne LJ. In vitro and in vivo studies demonstrating potent and selective estrogen inhibition with the nonsteroidal aromatase inhibitor CGS 16949A. Steroids. 1987;50(1–3):147–61. doi: 10.1016/0039-128x(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Widows MR, Sengelaub DR. Estrogenic influence and possible site of action in development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. Soc Neurosci Abstr. 1995;21:40. [Google Scholar]
- Tetel MJ. Modulation of steroid action in the central and peripheral nervous systems by nuclear receptor coactivators. Psychoneuroendocrinol. 2009 doi: 10.1016/j.psyneuen.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY, Ho-Kim MA, Lesage R. Determination of rat muscles androgen-receptor complexes with methyltrienolone. Steroids. 1977;29:185–195. doi: 10.1016/0039-128x(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Venable JH. Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat. 1966;119:271–301. doi: 10.1002/aja.1001190206. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Wellman CL, Sengelaub DR. NMDA receptor binding declines differentially in three spinal motor nuclei during postnatal development. Neurosci Lett. 2005;384:122–126. doi: 10.1016/j.neulet.2005.04.080. [DOI] [PubMed] [Google Scholar]
- Vyskocil F, Gutmann E. Anabolic effects of testosterone on the levator ani muscle of the rat. Pfluegers Arch. 1977;371:3–8. doi: 10.1007/BF00580765. [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff GC. The effects of castration and testosterone propionate on the striated perineal musculature in the rat. Endocrinol. 1941;29:975–978. [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: Direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21:1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5α-reductase. Endocrinol. 1999;140:4509–4515. doi: 10.1210/endo.140.10.7039. [DOI] [PubMed] [Google Scholar]
- Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinol. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]