Abstract
Little is known regarding which neural systems regulate dose-related changes in responding maintained by self-administered cocaine. This empirical question is important because elucidating neural systems engaged in this process could provide clues for effectively treating cocaine addiction. It has been suggested that different cocaine doses represent reinforcers of differing magnitudes, implicating the dorsal striatum or orbitofrontal cortex as important. Rats were trained to self-administer 1.0 mg/kg cocaine under a fixed-interval based second-order schedule. Next, cocaine unit doses (0.1–3.0 mg/kg) were each non-systematically available for a 5-day block of sessions. Tests (1-hr) were conducted on day 3 (vehicle) and day 5 (100 μg lidocaine) of each block. Lidocaine inactivation of the lateral dorsal striatum had no effect on dose-related responding or cocaine intake. In contrast, when doses along the ascending limb were available for self-administration, lidocaine inactivation of the lateral orbitofrontal cortex caused reductions in responding and cocaine intake, resulting in overall flattening of dose-response curves. This included reductions during the entire 1-hr test sessions and during the interval immediately following the first cocaine infusion of test sessions. Lidocaine inactivation of the lateral orbitofrontal cortex did not alter responding during the first cocaine-free interval of test sessions, but increased the latency to the first infusion. Collectively, the findings suggest that when the amount of experience with different cocaine unit doses is limited to a few sessions, the lateral orbitofrontal cortex regulates the dose-related effects of self-administered cocaine, likely by processing information pertaining to the reinforcing value of each unit dose.
Keywords: Cocaine, Dorsal striatum, Dose-response, Orbitofrontal cortex, Reinforcer magnitude, Self-administration
Introduction
Many preclinical studies have examined the influence of pharmacological agents on the dose-related effects of self-administered cocaine to evaluate potential pharmacotherapies for cocaine addiction [31]. Despite these advances, little is known regarding which neural systems regulate dose-related changes in responding maintained by self-administered cocaine. This empirical question is important because elucidating which neural systems might be engaged in this process could provide clues for effectively treating cocaine addiction.
A previous investigation demonstrated that nucleus accumbens neurons undergo significant dose-dependent changes in firing rate that are correlated with shifts in behavioral responding when different unit doses are available for self-administration in rats [2]. These findings, however, only partially elucidate the neural systems that might regulate dose-dependent changes in responding, as dose-dependent changes in nucleus accumbens firing occurred only during early “load-up” portion of the self-administration sessions. Insight into additional neural systems stems from literature suggesting that different cocaine unit doses represent reinforcers of differing magnitudes [45]. Studies investigating the neurobiology of reinforcer magnitude have implicated several brain sites, including the dorsal striatum and the orbitofrontal cortex as important in rats [13, 34, 42, 46], monkeys [5, 14, 39, 43] and humans [6, 25, 29]. Thus, either the dorsal striatum or orbitofrontal cortex might be components of the neural circuitry that regulate responding in relation to cocaine dose if different unit doses reflect a change in reinforcer magnitude.
Second-order schedules are particularly useful to ascertain the neural systems that might regulate the dose-related effects of self-administered drugs [7, 35]. With second-order schedules, brief stimuli, serving as conditioned reinforcers, maintain high rates of responding during prolonged drug-free periods. Rate of responding under a second-order schedule is not absolute, but depends on unit dose. Fixed-interval (FI) 5-min based second-order schedules generate dose-related effects that form well defined inverted-U shape curves [e.g., 4, 21, 24], and are therefore amenable to pharmacological analysis. An additional advantage of using a second-order schedule is that factors regulating the strength of responding in relation to unit dose can be evaluated without the possible confounding effects of high cocaine intake that is associated with fixed-ratio (FR) reinforcement schedules (see table 1 in [21]). We used 100 μg of lidocaine to inactivate either the lateral dorsal striatum or lateral orbitofrontal cortex in rats self-administering a full range of cocaine unit doses based on prior work indicating that this dose of lidocaine can modify self-administration of a 1 mg/kg cocaine unit dose under an FI 5-min based second-order schedule [e.g., 19]. Previous studies have used neuronal inactivation procedures to examine the role of the lateral dorsal striatum and lateral orbitofrontal cortex on reinstatement of cocaine-seeking behavior or on self-administration of a single cocaine unit dose in rats [11, 12, 20, 36], but none has examined inactivation of these sites against a full range of cocaine unit doses. If the dorsal striatum or the orbitofrontal cortex is important for regulating responding in relation to cocaine dose, then inactivation of either site by lidocaine is predicted to change the shape and/or position of the cocaine dose-response function.
Methods
Subjects
Male Wistar strain rats (Crl(WI)BR, Charles River Laboratories, Portage, MI, USA) weighing between 275–300 g upon arrival were individually housed in plastic cages (24×22×20 cm) within a temperature (21–23 °C) and light controlled (0800 hours on; 2000 hours off)-controlled vivarium. Rats had ad libitum access to water, and approximately 16g of food were provided each day to maintain body weight at 85–90% of the upwardly adjusting ad libitum body weight. The policies and procedures set forth in the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Academy of Sciences were followed. The Boston University Institutional Animal Care and Use Committee approved this study.
Apparatus
Experimental chambers, as previously described in detail [18], were used. Motor driven syringe pumps (Model PHM-100, Med Associates, Georgia, VT) located outside of each sound-attenuating cubicle were used for drug delivery. A syringe pump (Model 341A, Orion/Sage, Boston, MA) was also used for intracerebral infusions. A standard personal computer programmed in MedState Notation and connected to an interface (Med Associates, Georgia, VT) controlled experimental events.
Drugs
Cocaine hydrochloride (gift from NIDA, Bethesda, MD) was dissolved in a sterile 0.9% saline solution containing 3 IU of heparin/ml. A cocaine unit dose of 1.0 mg/kg (2.68 mg/ml) was used for training, and cocaine unit doses of 0.1 mg/kg (0.27 mg/ml), 0.3 mg/kg (0.81 mg/ml), 0.56 mg/kg (1.51 mg/ml), 1.0 mg/kg (2.68 mg/ml) and 3.0 mg/kg (8.1 mg/ml) were used to determine cocaine dose-response curves. The concentration of cocaine in the 20-ml syringes was varied according to dose to maintain a constant drug delivery time of 1.2 sec/100g body weight. The infusion pump speed was 1.8 ml/min.
Lidocaine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile 0.9% saline to make a 20% (200 mg/ml) solution. A total volume of 0.5 μl, resulting in a lidocaine dose of 100 μg was infused bilaterally at a rate of 0.59 μl/min. The 28-gauge stainless steel infusion cannula extended 1mm beyond the tip of the guide cannula and was left in place for 1 min following the infusion. Sterile 0.9% saline was used as the vehicle control for lidocaine.
Surgery and Histology
Surgical implantation of jugular vein catheters was performed under ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. Upon completion of catheter insertion, 22 gauge guide cannulae (Plastics One, Roanoke, VA) were stereotaxically implanted, according to the atlas of Swanson [37], 1 mm above the lateral dorsal striatum (AP −1.1 mm, ML ± 4.0 mm, DV −3.5 mm) or the lateral orbitofrontal cortex (AP +3.2 mm, ML ± 2.5 mm, DV −4.6 mm). Details of our surgical and post-surgical treatment as well as catheter maintenance and histology are described elsewhere [10]. These coordinates were selected based on studies showing that neurons in the posterior half of the lateral dorsal striatum are important for habit learning [22, 46] and that neurons in the posterior half of the lateral orbitofrontal cortex are important for processing the incentive value of reinforcers [13].
Experimental Procedures
Experiment 1: Inactivation of the Lateral Dorsal Striatum
Prior to surgical implantation of the jugular vein catheter and the lateral dorsal striatal cannulae, food restricted rats (n=10) were autoshaped overnight to lever press for 45 mg food pellets. After 1-week recovery from surgery, cocaine self-administration training began. Training sessions were 2 hr in duration. Rats were trained incrementally to self-administer cocaine under a terminal FI 5-min (FR 5:S) second-order schedule that incorporated contextual sound stimuli and conditioned light stimuli [19]. For half the rats, the right lever was designated as the active lever and continuous white noise (70 db) was used as the contextual sound cue. For the remainder of the rats, the left lever was designated as the active lever and an intermittent tone (70 db; 7 kHz; 0.5sec duration every sec) was used as the contextual sound cue. Delivery of cocaine was contingent on the completion of five responses on the active lever after the FI 5-min elapsed. The light stimulus over the active lever remained illuminated for the duration of the infusion and during a 20-sec timeout (TO) period that followed each infusion. The TO period was signaled by extinguishing the house light. Responding during the infusion or TO periods had no scheduled consequences, but was recorded. During the FI 5-min, the stimulus light over the active lever was presented for 2-sec after every fifth response on the active lever. Training sessions were conducted 5 days a week during the light phase and continued until the number of cocaine infusions did not deviate by more than 10% and responses on the inactive lever were 15% or less of active lever responses per session for a 5-day period in individual animals. Following baseline training, rats received in a non-systematic order a different cocaine unit dose (0.1, 0.3, 0.56, 1.0, and 3.0 mg/kg) for a block of five sessions per dose. The 2-hr sessions on days 1 and 2 of each 5-day block were used to familiarize rats to the specified cocaine unit dose. A 1-hr vehicle test session was always conducted on day 3 to ensure that an intracranial infusion per se did not non-specifically alter the rate of responding maintained by the specified cocaine unit dose. On day 4, no treatments were administered prior to 2-hr dose maintenance sessions, and data from the first hr were used as a no-treatment control test to ensure that there were no carry over effects of an intracranial infusion before conducting 1-hr test sessions with lidocaine on day 5. Vehicle and lidocaine were infused 5-min before testing. Test sessions of 1 hr duration were used because inhibition of nerve conductance by lidocaine disappears after 30–90 min, with higher concentrations of lidocaine, such as that used in the present study, resulting in a time course of inactivation in the high end of this range [26; 27]. An advantage of using lidocaine compared to other forms of inactivation (e.g. mucimol) is that reversibility is complete rather than variable, and duration of inactivation is on the order of minutes rather than hours or days [26]. This feature is particularly beneficial when a within-subjects experimental design is used. Moreover, as fibers of passage are also affected by lidocaine, the functional changes temporarily produced by lidocaine are more akin to those produced by permanent brain site ablations, but without concern for compensatory changes over time [26].
Experiment 2: Inactivation of the Lateral Orbitofrontal Cortex
All procedures were identical to those used in experiment 1 except for cannulae location and number of subjects (n=8).
Data Analyses
Six dependent measures were obtained: active lever responses during 1 hr test sessions, inactive lever responses during 1 hr test sessions, infusions earned during 1 hr test sessions, latency to the first infusion of the test sessions, active lever responses during the first cocaine-free interval of the test sessions, and active lever responses during the interval immediately following the first cocaine infusion of the test sessions. Inclusion of measures obtained at the start of the sessions helps to refine interpretation of the effects of lidocaine inactivation by providing micro-measures of performance [7]. Using SigmaStat V 3.1 statistical software, each dependent measure was analyzed by 2-factor (dose × treatment) repeated measures ANOVA followed by simple main effects tests and/or Tukey HSD post-hoc tests.
Results
Histology
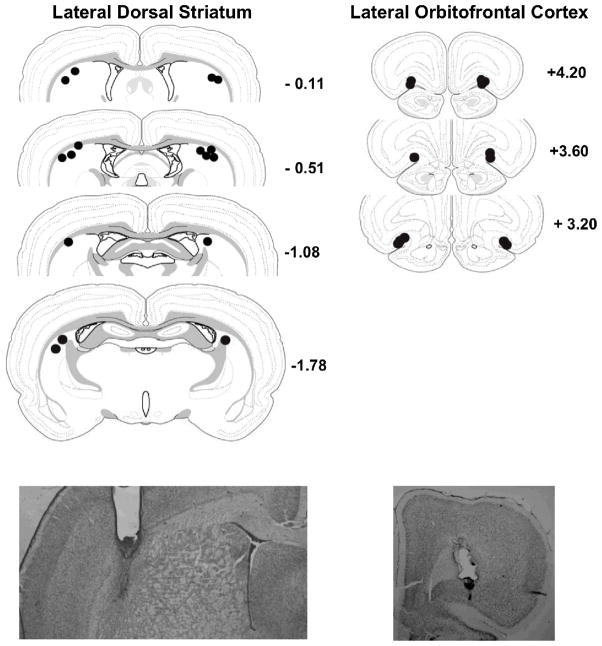
The histology is reported for 8 of 10 rats with lateral dorsal striatal cannulae implants (experiment 1) and for 6 of 8 rats with lateral orbitofrontal cortex cannulae implants (experiment 2). Two rats from experiment 1 had placements outside the region of interest and two rats from experiment 2 dislodged their head mount during the course of testing. Their data are not included in the analyses. Histological verification of placements and functional spread of lidocaine for the remaining rats are depicted in Figure 1 for the lateral dorsal striatum (upper left panel) and lateral orbitofrontal cortex (upper right panel). For both sites, the mid-point of cannulae placements was within 1 mm of the intended stereotaxic coordinate in the antero-posterior plane. As a 0.5 μl volume of lidocaine has an estimated radial spread of 0.49 mm from the infusion site, based on the spherical volume equation [38], the spread of lidocaine in the lateral dorsal striatum was likely confined to a location lateral to the dorsal junction of the internal capsule with the globus pallidus. Some diffusion into the corpus callosum (two rats) and the internal capsule (one rat) was evident based on cannulae placements. The spread of lidocaine in the lateral orbitofrontal cortex was likely confined to a location encompassing layers 1, 2 and 3.
Figure 1.
Upper panels show infusion locations for the lateral dorsal striatum (left) and lateral orbitofrontal cortex (right). Anterior-Posterior levels are measured in mm from bregma. The functional spread of lidocaine in individual rats is depicted by the filled circles, the radius of which is estimated from the spherical volume equation, V= 4/3 π r3 [38]. Lower panels show representative photomicrographs of guide and infusion cannulae tracks for the lateral dorsal striatum (left) and lateral orbitofrontal cortex (right).
Representative photomicrographs of the lateral dorsal striatum (Figure 1, lower left panel) and lateral orbitofrontal cortex (Figure 1, lower right panel) show tracks produced by implantation of 22 ga guide cannulae, which terminated 1 mm above the intended site, and insertion of 28 ga infusion cannulae.
Experiment 1: Inactivation of the Lateral Dorsal Striatum
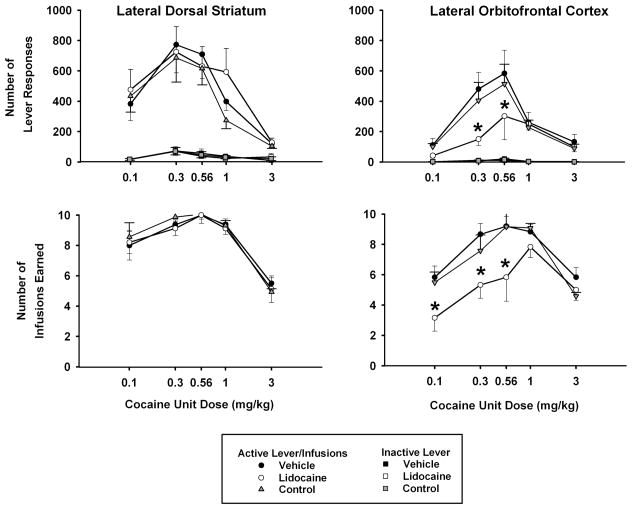
Under the FI 5-min (FR5:S) second-order schedule, cocaine produced an inverted-U shaped dose-response curve (Figure 2, top left panel). Active lever responses peaked at a unit dose of 0.3 mg/kg cocaine for all three treatment conditions, and analyses confirmed that there was a significant main effect of cocaine unit dose [F(4,28) = 8.9, p ≤ 0.001]. There were, however, no significant differences due to treatment or the interaction of dose × treatment. Thus, lidocaine inactivation of the lateral dorsal striatum did not modify the shape or position of the cocaine dose-response curve for active lever responses relative to vehicle treatment or the no-treatment control. Instead, responding was relatively stable over the last 3 days of the cocaine unit dose substitutions, regardless of whether vehicle, no treatment, or lidocaine was administered. Inactive lever responses remained at relatively low levels (15% or less of active lever responses) and showed no significant differences across doses or between treatments (Figure 2, top left panel).
Figure 2.
Effects of lidocaine (100 μg) inactivation of the lateral dorsal striatum (left panels) and lateral orbitofrontal cortex (right panels) on the dose-related effects of self-administered cocaine (x-axis, log scale) for the entire 1-hr test sessions. Values are the mean ± S.E.M. number of active and inactive lever responses (top panels) and infusions earned (bottom panels). * p ≤ 0.05 compared to the corresponding cocaine dose after vehicle treatment or the no-treatment control condition. See text for specific probability values associated with each comparison.
For the number of infusions earned, there was a significant main effect of cocaine unit dose [F(4,28) = 21.9, p ≤ 0.001], with the dose-response functions peaking at a unit dose of 0.56 mg/kg cocaine and forming an inverted U-shape for all three treatment conditions (Figure 2, bottom left panel). As confirmed by lack of a significant treatment main effect or dose × treatment interaction, lidocaine inactivation did not change the shape or position of the dose-response curves for infusions earned relative to vehicle treatment or the no-treatment control.
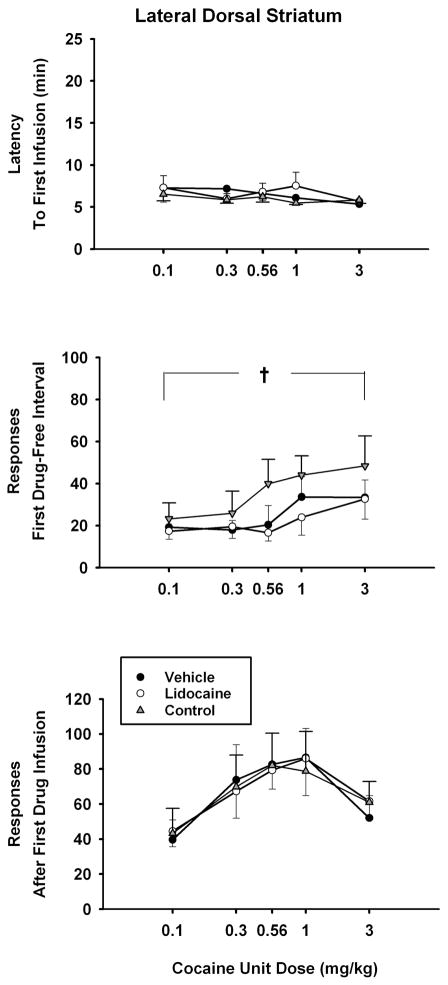
Analyses of the three dependent measures obtained at the beginning of the test sessions revealed no statistical differences between treatments for latency to the first cocaine infusion (Figure 3, top panel); active lever responding during the first cocaine-free interval (Figure 3, middle panel), and active lever responding during the interval immediately after the first cocaine infusion (Figure 3, bottom panel). None of the interactions of dose × treatment were significant for these three dependent measures as well. However, significant differences due to cocaine unit dose were obtained for active lever responding during the first cocaine-free interval of the test session [F(4,28) = 2.8, p ≤ 0.048]. Post-hoc Tukey tests of the dose main effect indicated that lever responding was significantly greater (p ≤ 0.03) when the highest anticipated drug dose (3.0 mg/kg) was compared to the lowest anticipated drug dose (0.1 mg/kg). Significant differences due to cocaine unit dose were obtained also for active lever responding during the interval immediately after the first cocaine infusion of the test session [F(4,28) = 5.0, p ≤ 0.004]. Post-hoc Tukey tests of the dose main effect revealed that responding was significantly greater after the first infusion of 0.3, 0.56 and 1.0 mg/kg unit doses compared to the 0.1 mg/kg unit dose (p ≤ 0.01).
Figure 3.
Effects of lidocaine (100 μg) inactivation of the lateral dorsal striatum on the dose-related effects of self-administered cocaine (x-axis, log scale) for measures obtained at the start of the sessions. Values are the mean ± S.E.M. latency to the first cocaine infusion of the test sessions (top panel), number of active lever responses during the first cocaine-free interval of the test sessions (middle panel), number of active lever responses during the interval immediately following the first cocaine infusion of the test sessions (bottom panel). † p ≤ 0.05 comparing the highest cocaine dose to the lowest cocaine dose across all treatments. See text for specific probability values associated with each comparison.
Experiment 2: Inactivation of the Lateral Orbitofrontal Cortex
Cocaine self-administration under the FI 5-min (FR5:S) second-order schedule produced a more pronounced inverted-U shaped dose-response curve for active lever responses after vehicle treatment and the no-treatment control than after lidocaine inactivation of the lateral orbitofrontal cortex (Figure 2, top right panel). Analysis revealed that the main effect of cocaine unit dose [F(4,20) = 9.9, p ≤ 0.001], main effect of treatment [F(2,10) = 5.2, p ≤ 0.029] and the dose × treatment interaction [F(8,40) = 2.4, p ≤ 0.03] were significant. Post-hoc Tukey tests of the interaction indicated that there were significantly fewer active lever responses for the 0.3 and 0.56 mg/kg cocaine unit doses after lidocaine inactivation compared to both vehicle treatment and the no-treatment control (p ≤ 0.03). Further testing within the vehicle treatment and the no-treatment control with simple main effects tests revealed that active lever responses significantly varied as a function of cocaine unit dose [vehicle: F(4,20) = 8.9, p ≤ 0.001; no-treatment control: F(4,20) = 7.2, p ≤ 0.001], whereas comparisons within the lidocaine treatment with a simple main effects test revealed that active lever responses did not significantly vary as a function of cocaine unit dose. This suggests lidocaine inactivation of the lateral orbitofrontal cortex caused a relative flattening of the cocaine dose-response function for active lever responses by shifting the ascending limb downward. Inactive lever responses remained at relatively low levels (7% or less of active lever responses) and showed no significant differences across doses or between treatments (Figure 2, top right panel).
For the number of infusions earned, there was a significant main effect of cocaine unit dose [F(4,20) = 11.9, p ≤ 0.001], with the dose-response function forming an inverted U-shape more so after the vehicle treatment and the no-treatment control than after lidocaine inactivation (Figure 2, bottom right panel). There was a significant main effect of treatment [F(2,10) = 11.0, p ≤ 0.003] and a nearly significant dose × treatment interaction [F(8,40) = 2.1, p ≤ 0.055] for infusions earned. Post-hoc Tukey tests of the interaction indicated that there were significantly (p ≤ 0.03) fewer infusions earned of the 0.1, 0.3 and 0.56 mg/kg cocaine unit doses after lidocaine inactivation compared to both the vehicle treatment and the no-treatment control. Further testing within the vehicle treatment and the no-treatment control with simple main effects and post-hoc Tukey tests revealed that the infusions earned significantly varied as a function of cocaine unit dose [vehicle: F(4,20) = 9.2, p ≤ 0.001; no-treatment control: F(4,20) = 10.2, p ≤ 0.001], with the 0.3, 0.56 and 1.0 mg/kg unit doses producing a greater number of infusions than the 0.1 and 3.0 mg/kg unit doses (p ≤ 0.05). In contrast, although testing within the lidocaine treatment with a simple main effects test also revealed dose-related differences in infusions earned [F(4,20) = 4.1, p ≤ 0.013], the only significant dose difference revealed by a post-hoc Tukey test was between the 1.0 and 0.1 mg/kg unit doses (p ≤ 0.006). There were no other significant pair-wise comparisons among the unit doses. These findings suggest that lidocaine inactivation of the lateral orbitofrontal cortex produced a relative flattening of the inverted-U shape dose-response function for infusions earned by shifting the ascending limb downward.
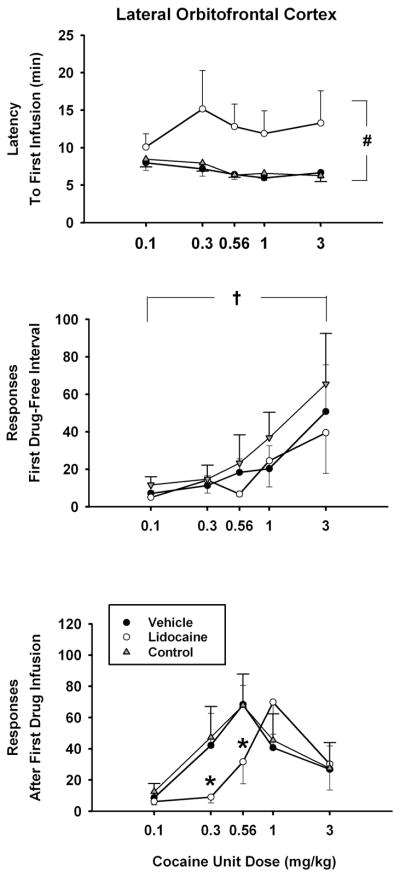
Analysis of latency to the first cocaine infusion of the test sessions (Figure 4, top panel) revealed significant differences due to treatment [F(2,10) = 11.9, p< 0.002], but not cocaine unit dose nor the interaction of dose × treatment. Post-hoc Tukey tests of the treatment main effect indicated that the latency was significantly longer for all anticipated doses of cocaine after lidocaine inactivation than after vehicle treatment or the no-treatment control (p ≤ 0.005). Analysis of responding during the first cocaine-free interval of the test sessions (Figure 4, middle panel) revealed significant differences due to cocaine unit dose [F (4, 29) = 3.5, p< 0.025]. Post-hoc Tukey tests of the dose main effect indicated that lever responding was significantly greater (p ≤ 0.024) when the highest anticipated drug dose (3.0 mg/kg) was compared to the lowest anticipated drug dose (0.1 mg/kg). The main effect of treatment and the interaction of dose × treatment were not significant for this measure, indicating that lidocaine inactivation of the lateral orbitofrontal cortex did not impact the ability of the cocaine-paired stimulus to maintain drug-seeking responses when presented before the first cocaine infusion of the session. Analysis of responding during the interval immediately after the first cocaine infusion of the test sessions (Figure 4, bottom panel) showed a significant effect of cocaine unit dose [F(4,20) = 7.0, p < 0.001] as well as a significant dose × treatment interaction [F(8,40) = 2.4, p < 0.03]. Post-hoc Tukey tests of the interaction showed that compared to the vehicle treatment and the no-treatment control, lever responses associated with lidocaine inactivation were significantly less (p ≤ 0.05) after the first infusion of the 0.3 and 0.56 mg/kg cocaine unit doses. Overall, except for the highest dose tested, the dose-response curve was shifted rightward.
Figure 4.
Effects of lidocaine (100 μg) inactivation of the lateral orbitofrontal cortex on the dose-related effects of self-administered cocaine (x-axis, log scale) for measures obtained at the start of the sessions. Values are the mean ± S.E.M. latency to the first cocaine infusion of the test sessions (top panel), number of active lever responses during the first cocaine-free interval of the test sessions (middle panel), number of active lever responses during the interval immediately following the first cocaine infusion of the test sessions (bottom panel). # p ≤ 0.05 comparing the lidocaine treatment to the control treatments across all doses; † p ≤ 0.05 comparing the highest cocaine dose to the lowest cocaine dose across all treatments; * p ≤ 0.05 compared to the corresponding cocaine dose after vehicle treatment or the no-treatment control condition. See text for specific probability values associated with each comparison.
Discussion
Responding Maintained Under a Second-Order Schedule
Under control conditions with the FI 5-min (FR5:S) second-order schedule, self-administration of cocaine produced well-defined inverted-U shaped dose-response curves. Responding peaked at a cocaine unit dose of either 0.3 or 0.56 mg/kg, replicating earlier findings [4, 21]. A feature shown in the present experiments and by Arroyo and colleagues [1] is that lever responding during the first cocaine-free interval increased monotonically as the anticipated cocaine dose increased, thus providing a straightforward measure of the reinforcing value of cocaine. The peak effect occurred at the highest anticipated cocaine doses (3.0 mg/kg in the present study and ~1.5 mg/kg (0.5 mg/rat) in [1]). Other work has demonstrated that under a second-order schedule of cocaine delivery, responding during the first cocaine-free interval is of smaller magnitude than responding during the interval immediately following the first cocaine infusion of the session [7]. As shown here using an FI 5-min (FR5:S) second-order schedule and in [1] using an FI 15-min (FR10:S) second order schedule, the augmentation of responding during the second interval depended on cocaine unit dose as well. In [1], pronounced increases in responding during the second interval relative to the first cocaine-free interval were measured with cocaine unit doses of ~0.2 mg/kg (0.083 mg/rat) and ~0.75 mg/kg (0.25 mg/rat). In experiment 1 (dorsal striatum manipulations) augmentation during the second interval was measured with cocaine unit doses of 0.3, 0.56 and 1.0 mg/kg, and in experiment 2 (orbitofrontal cortex manipulations) augmentation during the second interval was measured with cocaine unit doses of 0.3 and 0.56 mg/kg. In contrast, self-administration of the highest cocaine unit dose (with ~1.5 mg/kg in [1] and with 3.0 mg/kg in the present study) attenuated this increase. It should be noted that monotonic dose-related changes in responding were observed during the second interval in [1], with pronounced rate-decreasing effects observed at the highest cocaine unit dose tested (~33% of peak levels of responding). In contrast, bitonic dose-related changes in responding were observed during the second interval in the present study, with modest rate-decreasing effects observed at the highest cocaine unit dose tested (~77% of peak levels of responding). These differences are likely a result of a using a wider range of cocaine unit doses in the present study coupled with procedural differences for the second-order schedule including having a cocaine-free interval of shorter duration (5 min vs. 15 min), a smaller FR requirement for cocaine delivery and drug cue presentation (5 vs. 10) and an opportunity for earning a greater number of infusions during daily sessions (11 vs. 5). Since lengthening the duration of the FI and/or decreasing the number of available cocaine infusions during a session can change the shape and position of the cocaine dose-response curve [35], the minor differences between the present study and the work of Arroyo and colleagues [1] for measures obtained at the start of the sessions are readily explained.
Like Arroyo and colleagues [1], we speculate that under a second-order schedule, the degree to which drug-seeking responses are increased by an anticipated drug dose may be an index of that dose’s reinforcing value. We suggest further that the degree to which drug-seeking responses are augmented or maintained by the delivery of the first infusion under an FI 5-min (FR5:S) second-order schedule also may provide an index of a dose’s reinforcing value, given that pronounced rate-decreasing effects were not produced by cocaine unit doses in the range of 0.1 to 3.0 mg/kg. Consequently, analyses of the control data suggest that a 0.1 mg/kg unit dose of cocaine may have low reinforcing value because drug-seeking responses during the interval before and after its first infusion on tests days 3 and 4 were relatively low. Unit doses of 0.3 and 0.56 mg/kg may have intermediate reinforcing values because drug-seeking responses after their first infusion on test days 3 and 4 were augmented while drug-seeking responses before their first infusion on test days 3 and 4 were relatively low. Lastly, unit doses of 1.0 and 3.0 mg/kg may have high reinforcing value because drug-seeking responses on test days 3 and 4 were at peak levels or near peak levels before and after their first infusion. As previous research has shown that information about the value of reinforcers is processed predominantly by orbitofrontal cortex neurons [39, 43], manipulation of this site is expected to have a significant effect on these measures of reinforcer value relative to manipulation of the dorsal striatum.
Role of the Orbitofrontal Cortex
After lidocaine inactivation of neurons in the lateral orbitofrontal cortex, the dose-response curves for cocaine intake and lever responding were shifted downward along the ascending limb, resulting in an overall flattening of the inverted-U shape curves for the 1-hr test sessions. A reduction in responding has been reported in other studies in which the lateral orbitofrontal cortex was lesioned in rats prior to acquisition of cocaine self-administration under a second-order schedule [11] or inactivated in rats undergoing cue-induced reinstatement following a period of explicit extinction training [12]. In the present study, dose-related responding during the first cocaine-free interval was not altered by lidocaine inactivation of the lateral orbitofrontal cortex on day 5. When drug doses were anticipated by the rat during the first cocaine-free interval on day 5, their likely expectation was that the same cocaine dose as on the prior four days would be available, and so, the magnitude of responding did not change for the first interval. It was not until these rats actually experienced a cocaine infusion on day 5 that responding changed.
The rightward shift in the dose-response curve for responding during the interval immediately following the first infusion of the test sessions supports the view that lidocaine reduced the reinforcing value of the first infusion of cocaine. Rats reacted to the first infusion of 0.3, 0.56 and 1.0 mg/kg unit doses as if these doses were lower in magnitude than they actually were (as if the doses were 0.1, 0.3 and 0.56 mg/kg, respectively). The reaction to the first infusion of 3.0 mg/kg was not altered. Notably, past research has indicated that animals respond differentially for reinforcers when their value is high vs. low [28]. Lesions of the orbitofrontal cortex cause rats and monkeys to lose their ability to distinguish between reinforcers of high and low value [28]. Differences in reinforcer value may therefore form the basis for differences in the magnitude of responding in relation to cocaine unit dose under normal conditions. As rats continued to self-administer cocaine at all unit doses, it is clear that lidocaine inactivation of the lateral orbitofrontal cortex did not prevent cocaine from being reinforcing. Instead, inactivation of this site may have lowered the reinforcing value of the first cocaine infusion (unless a high dose was available), which subsequently reduced the ability of rats to distinguish between reinforcers of high and low value (responding was statistically similar across the entire dose range) after their repeated delivery during the test session.
Role of the Dorsal Striatum
Lidocaine inactivation of neurons in the lateral dorsal striatum, shown previously to disrupt stimulus-response habit learning [22], produced no significant changes in the dose-related effects of self-administered cocaine, either for the entire 1-hr sessions or for the measures obtained at the start of the sessions. The lack of sensitivity of the lateral dorsal striatum to lidocaine inactivation in the present study is at odds with some earlier reports showing that the lateral dorsal striatum is engaged to regulate responding maintained by self-administered cocaine and stimuli paired with cocaine [36, 41]. A possible basis for these divergent findings may be related to a critical procedural difference between studies. In these past studies, responding was made habitual prior to manipulation of the lateral dorsal striatum. This was accomplished either by providing a single cocaine unit dose for a prolonged period of time under a second-order schedule or by providing a period of abstinence without the use of explicit extinction training following prolonged self-administration training with a single cocaine unit dose under a fixed-ratio schedule. In the design of the present study, responding may not have been habitual because each cocaine unit dose was available in a non-systematic order for a block of only 5 sessions per dose following initial training. It has been established that dorsal striatal-dependent habit learning is acquired slowly, taking a large number of daily sessions for rats to maintain a habit-based strategy [30]. Moreover, as explicit extinction training is a process that serves to suppress habitual responding [36], it is noteworthy that in an earlier investigation in which rats were trained to self-administer cocaine under a second-order schedule and then exposed to explicit extinction training, active lever responses induced by cocaine-paired discrete and contextual cues were not altered following inactivation of the lateral dorsal striatum [20]. In contrast, when the lateral dorsal striatum was inactivated following prolonged self-administration of 1 mg/kg cocaine under a second-order schedule, active lever responses were altered in a manner consistent with a reduction in the cocaine unit dose, i.e., responding was significantly elevated [20]. A similar trend was observed in the present study whereby after lidocaine inactivation of the lateral dorsal striatum, responding was elevated in rats self-administering the dose of cocaine for which they had the most experience (the 1 mg/kg training dose). Observing only a trend for group differences at the 1 mg/kg cocaine unit dose likely reflects the fact that this dose was not consistently the first dose that was tested after initial training. This may have resulted in responding that, overall, was less habit-like than if testing with 1 mg/kg consistently followed initial training. Perhaps a better way to conduct future studies to eliminate all habitual responding before initiating dose substitution testing would be to either engage the rats in explicit response extinction training first [36] or to not test responding maintained by the training dose. Collectively, the findings suggest that when the amount of experience with different cocaine unit doses is limited to just a few sessions for each dose, the lateral dorsal striatum may not be a critical substrate for regulating responding in relation to cocaine unit dose.
Specificity of Effects
Before drawing conclusions, it is necessary to explore the possibility that the lidocaine-induced reductions in the dose-related effects of self-administered cocaine were due to non-specific factors. Some may argue that the use of 10 intracranial infusions is excessive and leads to non-specific damage and changes in behavior. This argument can be countered by the representative photomicrographs, which show no non-specific damage beyond that produced by implantation of a 22 ga guide cannula and insertion of a 28 ga infusion cannula. Moreover, the use of 10 intracranial infusions per se does not account for reduced responding because significant effects were found following manipulation of the lateral orbitofrontal cortex but not the lateral dorsal striatum. Furthermore, although similarities between the vehicle and no-treatment control dose-response functions per se can not be used as evidence against cellular damage having an effect, the shape and position of these control curves are similar to those previously determined under a second-order schedule without directly manipulating the brain [21]. A lack of a non-specific effect of multiple infusions into these brain sites is supported also by our earlier findings showing a similar time course for acquisition of a learning task in rats either given daily vehicle infusions into the lateral dorsal striatum vs. no treatment (15–16 sessions to acquire the win-stay task; [22, 40]) or given daily vehicle infusions into the lateral prefrontal cortex vs. no treatment (9–11 session to acquire the odor-guided delayed win-shift task; [9, 23]). Together, these findings with multiple vehicle infusions suggest that if multiple lidocaine infusions produce a change in behavior, then this change is due to temporary neuronal inactivation rather than due to non-specific damage.
Another possibility is that lidocaine produced non-specific reductions in motor activity after inactivating the lateral orbitofrontal cortex to cause reductions in lever responding. However, if lidocaine were producing non-specific reductions in motor activity, then responding during the first cocaine-free interval of the test session would have been significantly reduced and there would not have been an increase in responding as the anticipated drug dose increased. Others have shown normal levels of locomotor activity after inactivation or lesions of the lateral orbitofrontal cortex [12]. Lastly, it is important to mention that lesions or inactivation of brain sites neighboring the lateral orbitofrontal cortex (e.g., the medial orbitofrontal cortex and agranular insular prefrontal cortex) do not cause reductions in responding maintained by self-administered cocaine [10, 12]. Thus, it is unlikely that the reductions in the dose-related effects of self-administered cocaine were due to diffusion of lidocaine to sites outside the lateral orbitofrontal cortex.
There are, however, two aspects of the effects of lidocaine inactivation of the lateral orbitofrontal cortex that warrants further consideration. Firstly, the latency to the first cocaine infusion was significantly longer across all unit doses relative to control latencies. This finding suggests that lidocaine inactivation of the lateral orbitofrontal cortex may have decreased the motivation to seek and take cocaine [8], which could account for the observed changes in the dose-related effects of cocaine. Using a progressive ratio schedule of food pellet delivery, it has been demonstrated that dopamine D1 and D2 receptor blockade of the posterior portion of the lateral orbitofrontal cortex reduced the breakpoint, indicating a reduction in the motivation to obtain a reinforcer [3]. A decreased motivation to seek and take cocaine, however, can not account entirely for the effects of lidocaine inactivation to the lateral orbitofrontal cortex. If only motivation were affected, then significant reductions in responding would have occurred as well during the first interval, as shown following dopamine D3 receptor blockade [8]. Moreover, once the first cocaine infusion was earned, dose-related changes in responding were still observed during the second interval, albeit the curve was shifted rightward. If low motivation were the only factor, then dose-related changes during the second interval would have been eliminated. It is more likely that following lidocaine inactivation of the lateral orbitofrontal cortex, the initial motivation to respond was low, regardless of cocaine unit dose. Once responding began during the first interval, presentation of the highly motivating cocaine-paired conditioned stimulus re-engaged the rats in lever responding. The higher the anticipated drug dose, the greater the magnitude of responding, suggesting that the reinforcing value of the cocaine-paired conditioned stimulus was not altered by lidocaine inactivation of the lateral orbitofrontal cortex. However, following the first infusion, responding was controlled conjointly by the reinforcing value of the cocaine-paired conditioned stimulus and by cocaine. Since the reinforcing value of the cocaine-paired conditioned stimulus was not altered by lidocaine inactivation of the lateral orbitofrontal cortex, the reductions in the seeking and taking of cocaine could reflect a reduction in the reinforcing value of cocaine itself, unless a high cocaine dose is provided.
Secondly, as discussed above, the reinforcing value of the cocaine-paired conditioned stimulus measured during the first cocaine-free interval was not altered by lidocaine inactivation of the lateral orbitofrontal cortex. This finding contrasts with previous work demonstrating that lesions of the orbitofrontal cortex cause rats to no longer exhibit a change in conditioned responding to a visual conditioned stimulus following unconditioned stimulus devaluation [14]. These findings suggest that the orbitofrontal cortex processes the value of conditioned reinforcers rather than just unconditioned reinforcers like cocaine. However, it has been established also that learning based on the acquired reinforcer properties of second-order conditioned stimuli is relatively insensitive to devaluation [17]. Learning acquired on such a basis might be relatively immune to changes in the value of the original unconditioned stimulus [14].
Conclusions
Evidence is presented here to suggest that when the amount of experience with different cocaine unit doses is limited to a few sessions, the lateral orbitofrontal cortex regulates the dose-related effects of self-administered cocaine likely by processing information pertaining to the reinforcing value of each unit dose. As the lateral orbitofrontal cortex is thought to regulate value of reinforcers in general [33], this regulation is unlikely specific for cocaine. Nonetheless, it is hypothesized that this regulation involves a neural circuit encompassing the orbitofrontal cortex [11], nucleus accumbens core [17] and basolateral amygdala [44], which are neurosubstrates implicated in controlling responding during the early phase of cocaine addiction and the early phase of relapse. This suggests that therapeutic efforts aimed at devaluing cocaine reinforcement in cocaine addicts (e.g., alternative reinforcer therapy; [15]) may only be successful during the early phase of cocaine addiction and during early phase of relapse. When behavior becomes habitual, it is insensitive to outcome devaluation [16]. The present findings leave open the possibility that when responding becomes habitual, the lateral dorsal striatum may be more dominantly engaged to regulate cocaine-maintained responding [36, 41]. Such a mechanism implicates a transition from limbic system control to dorsal striatal/basal ganglia control over responding, a process supported by metabolic mapping studies in animals self-administering cocaine for varying lengths of time [32]. Thus, treatment of habitual drug-seeking behavior may be best served by an approach that aims to reduce the size of cocaine reinforcement in cocaine addicts (e.g., peripheral blocker therapy; [18]).
Acknowledgments
This project was supported by grant number R01DA011716 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- 1.Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacol. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- 2.Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther. 1996;277:385–393. [PubMed] [Google Scholar]
- 3.Celtin T, Freudenberg F, Füchtemeier M, Koch M. Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neurosci Lett. 2004;370:114–117. doi: 10.1016/j.neulet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Collins SL, Kantak KM. Neuronal nitric oxide synthase inhibition decreases cocaine self-administration behavior in rats. Psychopharmacol. 2002;159:361–369. doi: 10.1007/s00213-001-0935-8. [DOI] [PubMed] [Google Scholar]
- 5.Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 6.Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacol. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 9.Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- 10.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 11.Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol. 2001;85:2477–2489. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- 15.Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- 17.Holland, Rescorla 1975 [Google Scholar]
- 18.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 19.Kantak KM. Vaccines against drugs of abuse: A viable treatment option? Drugs. 2003;63:341–352. doi: 10.2165/00003495-200363040-00001. [DOI] [PubMed] [Google Scholar]
- 20.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacol. 2002;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- 22.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacol. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 23.Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance following lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- 24.Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacol. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- 25.Katz JL, Goldberg SR. Second-order schedules of drug injection: implications for understanding reinforcing effects of abused drugs. Adv Subst Abuse. 1991;4:205–223. [Google Scholar]
- 26.Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. NeuroImage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 27.Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 29.Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 31.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 32.Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacol. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- 33.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3662. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Salinas JA, White NM. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behav Neurosci. 1998;112:812–826. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- 36.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacol. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 37.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacol. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 38.Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 39.Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 41.Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacol. 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- 42.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8870. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Duuren E, Lankelma J, Pennartz CM. Population coding of reward magnitude in the orbitofrontal cortex of the rat. J Neurosci. 2008;28:8590–8603. doi: 10.1523/JNEUROSCI.5549-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 45.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacol. 1996;127:213–224. [PubMed] [Google Scholar]
- 46.Winger G, Woods JH, Galuska CM, Wade-Galuska T. Behavioral perspectives on the neuroscience of drug addiction. J Exp Anal Behav. 2005;84:667–681. doi: 10.1901/jeab.2005.101-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]