Abstract
Introduction
If a caregiver does not have access to a refrigerator, the South African National Department of Health advises that stavudine adult capsule formulations be employed using the off-label ‘opened capsule’ dosing method. The accuracy of this dosing method has not previously been validated.
Aim
To assess the accuracy of the off-label opened capsule method for stavudine dosing in infants and children. In addition, we assessed the relative ease of dispersion of generic and original capsule preparations in water to determine which preparations, if any, are suitable for the off-label opened capsule dosing method.
Method
We evaluated 10 Zerit (Bristol-Myers Squibb), 5 Stavudine (Aspen), and 5 Stavir (Cipla) capsules. Each capsule was dispersed in 30 ml water, creating 20 separate solutions. Timed dispersion of each generic was compared with that of the original (Zerit). Each solution was then centrifuged to remove sediment, and the concentration of active drug (mg/ml) was analysed by high-performance liquid chromatography.
Results
The ease of dispersion of the contents of Aspen Stavudine capsules was equivalent to that of Zerit, and resulted in a mean recovery of active drug from solution of over 97%, confirming the accuracy of this dosing method. The contents of Stavir capsules, however, were extremely difficult to disperse in water despite prolonged agitation; consequently, the recovery of active drug from the solution was reduced.
Conclusion
The accuracy of the off-label opened capsule dosing method for stavudine is acceptable. There is no need to instruct caregivers to include sediment in the aliquot given to the infant. However, studies that confirm adequate bioavailability and efficacy are needed. In addition, it is important to avoid supplying generic capsules the contents of which do not disperse easily in water, as this may lead to a significant reduction in the amount of active drug that a child receives.
Paediatric clinicians are often forced to use medicines in an off-label manner, as pharmaceutical companies tend to avoid the expense of developing paediatric formulations of established adult medicines unless the market demand is high. Although the USA’s Food and Drug Administration (FDA) and European Medicines Agency (EMEA) now mandate that a paediatric plan be put in place for essential medicines, paediatric formulations are still often not appropriate. A prime example is the Aluvia paediatric tablet (100/25 mg) that contains a dose that is too large for most young children. The alternative of using adult formulations in an off-label manner leads to questions around dosing accuracy, bioavailability, efficacy and safety. Studies are therefore under way to investigate the bioavailability of cut or crushed Aluvia tablets in children (Dr Brookie Best, Center for AIDS Research, University of California, San Diego, USA – personal communication).
Stavudine is an important component of the first-line antiretroviral regimen used in the public sector in South Africa.1 The proprietary paediatric liquid formulation of stavudine (Zerit; Bristol-Myers Squibb) requires a specific volume (202 ml) of purified water for reconstitution. Once reconstituted, the solution has to be refrigerated at 4°C to maintain stability,2 and is only stable for 1 month when refrigerated. Adult stavudine capsules, however, are stable at room temperature for 2 years.3 In an attempt to overcome the logistical problems associated with supplying the proprietary solution to children in rural areas, the Department of Health recommended that caregivers without access to a refrigerator should instead open a stavudine capsule and mix the contents in a specific volume of tap water.1 Caregivers are to mix the contents of a capsule in 5 ml water and then withdraw the required fraction of the solution (usually 2.5 ml) with a syringe. If there is any delay, the caregiver is advised to re-agitate the mixture carefully to re-suspend the sediment that collects at the bottom of the container, before drawing up the aliquot for the child. This administration method has been termed the ‘opened capsule’ dosing method.
There are no published data on the accuracy of the opened capsule dosing method advocated by the South African Department of Health for stavudine dosing in infants and children. The opened capsule method is off-label. Manufacturers do not recommend that capsules be opened or made into a suspension in water before consumption.2,3 The contents of stavudine capsules do not dissolve completely, and rapidly form a sediment at the bottom of the container. There is no guarantee that half the volume of such a solution reliably contains half the dose. The accuracy of this practice needs to be assessed. In addition, as generic products are widely used in the public programme, the ease of dispersion of the various generic capsule preparations should be independently compared.
The aim of this study was to assess the accuracy of the off-label opened capsule dosing method for stavudine in infants and children. In addition, we assessed the relative ease of dispersion of generic and original capsule preparations in water to determine which preparations, if any, are suitable for off-label opened capsule dosing.
Method
We compared two of the commercially available generic adult stavudine capsules (Aspen Stavudine and Cipla Stavir ) with the original preparation Zerit (Bristol-Myers Squibb). We evaluated 10 Zerit, 5 Aspen Stavudine and 5 Stavir capsules. The generic capsules were obtained from the Western Cape Department of Health antiretroviral depot. The Stavir capsules were from lot X70669 (expiry date 06/2009). The Zerit capsules were purchased commercially by the Children’s Infectious Diseases Clinical Research Unit (KID-CRU) pharmacy, Tygerberg Children’s Hospital.
Each capsule was stated to contain 30 mg of active drug, and was dispersed in 30 ml water, thus creating 20 separate solutions. Rather than use the technical dispersion method for capsules described in the British Pharmacopoeia,5 we intentionally used the manual dispersion technique taught to caregivers in public sector clinics. However, we used a larger volume of water than described in the National Department of Health guidelines to avoid amplifying the effect of any inaccuracy from the manual technique, ensuring that the recovery of active drug from each solution could be fairly compared. All solutions were made up by the same investigator using the same standardised method. Timed dispersion of each generic was compared with the original (Zerit). Each solution was then centrifuged, and the concentration of active drug (mg/ml) was analysed by high-performance liquid chromatography (HPLC). Statistical analysis was performed by JMP statistics software, version 7.0 (SAS Corporation, California).
Laboratory method
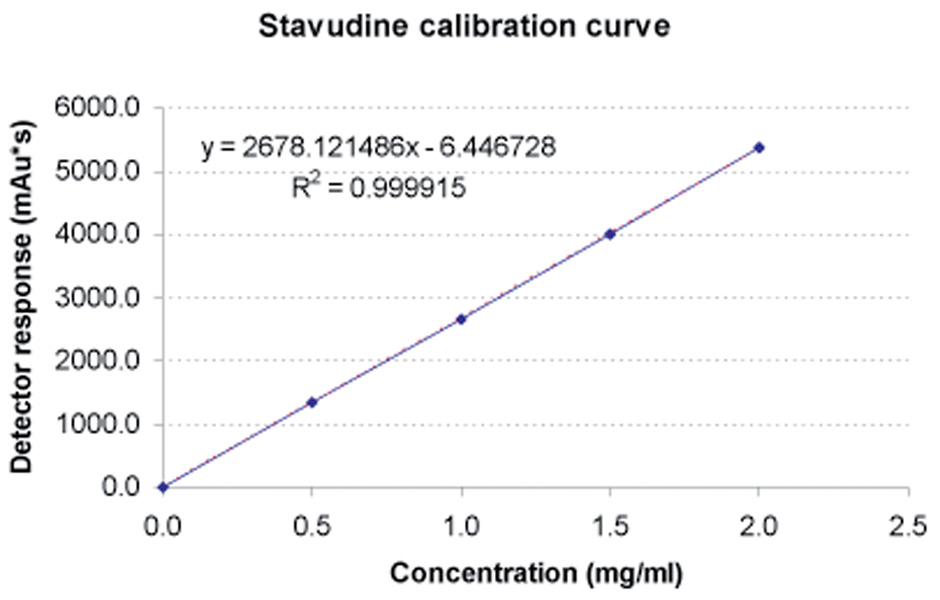
Undissolved particulate matter was separated from the solution by centrifuging and was then discarded. The stavudine-containing samples were analysed using a Hewlett Packard 1100 series high-performance binary liquid chromatograph (Agilent Technologies, Waldbronn, Germany) with an Eclipse (XDB-C18) Zorbax analytical column (15 mm × 4.6 mm ID, 5 micron particle size). This was preceded by a 30 mm × 2.1 mm ID C18-guard column (40 micron particle size). The temperature was maintained at 40°C and the flow rate at 1 ml/min. The mobile phase consisted of a mixture of two solvents: A (50 mM KH2PO4, pH 5.42) and B (acetonitrile:isopropanol 4:1 (v/v)). The following gradient was used: 0 – 1 minute = isocratic at 5% B; 1 – 7.5 minutes = linear increase to 15% B; 7.5 – 10 minutes = isocratic at 15% B. All reagents used for the mobile phase were HPLC grade (E Merck, Darmstadt, Germany) and were filtered through a 0.45 micron filter to remove particular matter. De-ionised water was used for the preparation of all aqueous solution and standards. Two hundred µl of the sample was diluted with 800 µl of de-ionised water to avoid detector overload and to ensure that detector response remained within linear range of the calibration curve. Aliquots of 5 µl from each sample were injected onto the column. The variable wave detector was set at 266 nm. The retention time of stavudine was 4.92 minutes, with a total run time of 7.5 minutes. Three minutes was allowed for the column to stabilise at 5% B before each subsequent injection. Recording and integration of the peaks were performed by means of an Agilent Chem Station (Agilent Technologies, Santa Clara, California). Spiked standards were prepared from pure stavudine compound (Aspen Laboratories, batch # 13961 V), and were randomly included in each batch over the expected concentration range. The calibration curve of triplicate samples was found to be linear over the entire concentration range (R2=0.999915) (Fig. 1). The limits of quantification were 0.5 – 2 mg/ml.
Fig. 1.
Calibration curve for pure stavudine compound. The dotted line represents the best-fit linear curve (y=2678.121488 x −6.446728, R2=0.999915).
Results
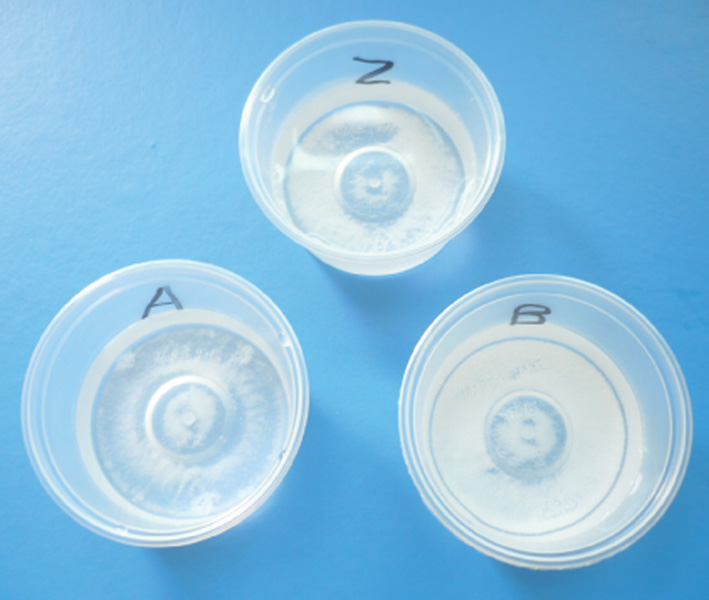
All three capsule preparations produced a sediment that rapidly collected at the bottom of the container when the contents of a capsule were mixed in water (Fig. 2).
Fig. 2.
Incomplete dissolution of stavudine capsules in water (Z = Zerit, A = Aspen Stavudine, B = Stavir).
The Medicines Control Council requires that the content of active ingredient in any medicinal preparation should lie within 10% of the label claim.4 The contents of Zerit capsules dispersed easily with minimal agitation, resulting in a mean recovery of active drug from solution of over 97% (Table I). The ease of dispersion of the contents of Aspen Stavudine capsules was equivalent to that of Zerit, resulting in a mean recovery of active drug from solution of over 97% (Table I). The minor difference in recovery of active drug from solution when comparing Zerit with Aspen Stavudine was not statistically significant (p=0.8272), suggesting that these two drugs are likely to be equivalent when given to children using the off-label opened capsule administration method.
Table I.
Comparison of the dispersibility and active drug content of mixtures made with original and generic brands of stavudine capsules using the ‘opened capsule’ dosing method
| Zerit A | Aspen Stavudine | Stavir | |
|---|---|---|---|
| (N=10) | (N=5) | (N=5) | |
| Obtained stavudine concentration in the constituted solution (mg/ml) | |||
| (mean ± SD) | 0.971±0.022 | 0.974±0.018 | 0.938±0.070 |
| Expected stavudine concentration in the constituted solution (mg/ml) | 1.000 | 1.000 | 1.000 |
| Stavudine recovered from the solution (%) (mean ± SD) | 97.12±2.18 | 97.39±1.86 | 93.77±6.96 |
| Percentage deviation from the expected concentration (%) | −2.86 | −2.61 | −6.23 |
| Time taken for complete dissolution of stavudine capsule in water (s) | 18 | 18 | >65 |
SD = standard deviation; expected stavudine concentration in the constituted solution should yield an ideal solution; percentage stavudine recovered from the solution was the ratio of obtained to expected concentrations.
The contents of Stavir capsules were extremely difficult to disperse in water despite prolonged agitation. Their contents appeared to be partially hydrophobic, floating persistently on the meniscus of the sample. Consequently, recovery of active drug was reduced (Table I). However, because of the small sample size, the difference in recovery of active drug from solution when comparing Zerit with Stavir did not reach statistical significance (p=0.1730).
Discussion
The lack of appropriate paediatric antiretroviral formulations is a global problem.6,7 The use of liquid formulations may not be feasible in rural areas, as they are heavy and difficult to transport, have a short shelf-life, and may require refrigeration. Paediatric clinicians are often forced to employ an adult formulation in rural areas where the logistic obstacles to supplying a liquid formulation are prohibitive. Therefore, where suitable, solid paediatric antiretroviral formulations are not available, the World Health Organization (WHO) has recommended that off-label use of adult formulations should be investigated.8
When a drug is employed in an off-label manner in children, the clinical implications should be carefully considered. The dosing accuracy, bioavailability, efficacy and safety of using an adult formulation in children all require investigation. In addition, off-label use of adult formulations requires manipulation that may affect the dosage, such as cutting adult tablets or emptying the contents of capsules into water and then administering a specific volume. These activities are time-consuming and may affect adherence negatively. However, once tested, the off-label use of adult formulations in children can be extremely effective, as has become well established in fields such as paediatric cardiology.9
In our study, we found that, when the contents of a stavudine capsule were dispersed in water, practically all the active drug was found in the supernatant; by inference, only a negligible amount remains in the visible sediment. It is probable that the sediment is composed only of the pharmacologically inactive excipients of the capsule formulation, such as magnesium stearate. This information is important to caregivers, who do not need to re-suspend sediment when giving the solution to their infant. It is therefore unnecessary to instruct caregivers to agitate the mixture before withdrawing the aliquot to be given to the child. This important finding will help to simplify a technique that is at best difficult for elderly caregivers, who struggle with the dexterity and precision needed for this method.
We found a significant difference in the ease of dispersion of the capsule contents of the two generic capsule preparations, which may lead to a significant reduction in the amount of active drug that a child receives when Stavir capsules are supplied to caregivers. However, the opened capsule dosing method is off-label, and our data do not permit comment on the absorption of active drug from intact capsules of any of the preparations tested. There is a need to perform a pharmacokinetic study to confirm that stavudine administered by the opened capsule method is bioequivalent to a proprietary formulation approved by regulatory agencies.
In the future, paediatric fixed-drug combination (FDC) antiretroviral formulations will greatly accelerate the roll-out of antiretrovirals to children in rural resource-limited settings. However, no paediatric antiretroviral FDCs have as yet been registered in South Africa. Until FDCs become widely available in the public sector, clinicians should use the best available data when prescribing adult antiretroviral formulations in an off-label manner. However, studies that confirm adequate bioavailability and efficacy are needed.
Conclusion
Although the method of dispersing the contents of a capsule in water is off-label, and not condoned by the manufacturers of any of the three preparations, our findings suggest that the accuracy of the off-label opened capsule dosing method for stavudine dosing is acceptable. We have also shown that there may be no need to instruct caregivers to include sediment in the aliquot given to the infant. However, studies that confirm adequate bioavailability and efficacy are needed. Finally, it is important to use generic capsules whose contents disperse easily in water, as it may otherwise lead to a significant reduction in the amount of active drug that a child receives.
Acknowledgments
The authors thank Aspen Pharmacare for the donation of pure stavudine compound, which was necessary for calibration of laboratory equipment. The authors are indebted to Mr Chris Muller for statistical advice. The authors also thank the National Department of Health Research Reference Committee for funding, which formed part of that committee’s Operational Plan for Comprehensive HIV and AIDS Care, Treatment and Management for South Africa.
Footnotes
The authors declare no commercial or other association that could pose a conflict of interest.
Contributor Information
Steve Innes, KID-CRU (Children’s Infectious Diseases Clinical Research Unit), Tygerberg Academic Hospital, W Cape.
Marlize Smuts, KID-CRU (Children’s Infectious Diseases Clinical Research Unit), Tygerberg Academic Hospital, W Cape.
Mark F Cotton, KID-CRU (Children’s Infectious Diseases Clinical Research Unit), Tygerberg Academic Hospital, W Cape.
Heiner Seifart, Division of Pharmacology, Faculty of Health Sciences, Stellenbosch University, Tygerberg, W Cape.
Bernd Rosenkranz, Division of Pharmacology, Faculty of Health Sciences, Stellenbosch University, Tygerberg, W Cape.
References
- 1.Meyers T, Eley B, Loening W. Guidelines for the Management of HIV-infected Children, National Department of Health, South Africa. 1st ed. Pinetown: Jacana Media Publishers; 2005. p. 82. [Google Scholar]
- 2.Package insert for BMS Zerit. Bristol-Myers Squibb Co; 2004. [Google Scholar]
- 3.Package insert for Aspen Stavudine. Aspen Pharmacare; 2006. [Google Scholar]
- 4.Medicines Control Council. Pretoria: Medicines Control Council; Medicines Control Council Pharmaceutical and Analytical Guideline, no. 2.02: section 3.6.5. 2004
- 5.British Pharmacopoeia Commission. London: TSO (The Stationery Office); British Pharmacopoeia 2008. 2008
- 6.American Academy of Pediatrics Policy Statement. Increasing antiretroviral drug access for children with HIV infection. Pediatrics. 2007;119(4):838–845. doi: 10.1542/peds.2007-0273. [DOI] [PubMed]
- 7.WHO Paediatric Recommendations. [accessed 12 June 2009];HIV Treatment Bulletin. 2007 8(1, 2) http://www.i-base.info/htb/v8/htb8-1-2/WHO.html.
- 8.WHO HIV/AIDS Department. Applications for the 15th WHO model list of essential medicines. [accessed 12 June 2009]; http://archives.who.int/eml/expcom/children/Comments/emlc/HIV_WHO_comments.pdf.
- 9.Pasquali SK, Hall M, Slonim AD, et al. Off-label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circ Cardiovasc Qual Outcomes. 2008;1:74–83. doi: 10.1161/CIRCOUTCOMES.108.787176. [DOI] [PubMed] [Google Scholar]