Abstract
Mesophyll conductance to CO2 (gm) limits carbon assimilation and influences carbon isotope discrimination (Δ) under most environmental conditions. Current work is elucidating the environmental regulation of gm, but the influence of gm on model predictions of Δ remains poorly understood. In this study, field measurements of Δ and gm were obtained using a tunable diode laser spectroscope coupled to portable photosynthesis systems. These data were used to test the importance of gm in predicting Δ using the comprehensive Farquhar model of Δ (Δcomp), where gm was parameterized using three methods based on: (i) mean gm; (ii) the relationship between stomatal conductance (gs) and gm; and (iii) the relationship between time of day (TOD) and gm. Incorporating mean gm, gs-based gm, and TOD-based gm did not consistently improve Δcomp predictions of field-grown juniper compared with the simple model of Δ (Δsimple) that omits fractionation factors associated with gm and decarboxylation. Sensitivity tests suggest that b, the fractionation due to carboxylation, was lower (25‰) than the value commonly used in Δcomp (29‰) and Δsimple (27‰). These results demonstrate the limits of all tested models in predicting observed juniper Δ, largely due to unexplained offsets between predicted and observed values that were not reconciled in sensitivity tests of variability in gm, b, or e, the day respiratory fractionation.
Keywords: Carbon isotope discrimination, Farquhar model, internal conductance, Juniperus, mesophyll conductance, stomatal conductance
Introduction
Low mesophyll conductance of CO2 from substomatal cavities to sites of carboxylation (gm) can reduce the partial pressure of CO2 (pCO2) at the site of carboxylation, limit photosynthesis (A), and affect carbon isotope discrimination (Δ) (Farquhar et al., 1989; Niinemets et al., 2009). gm varies on numerous time scales in response to environmental drivers, from rapid variation in response to changes in intercellular [CO2] (Flexas et al., 2007; Vrábl et al., 2009) to shifts in response to temperature (Bernacchi et al., 2002), water stress (Galmés et al., 2007; Grassi et al., 2009), light gradients (Piel et al., 2002; Monti et al., 2009), and others (for reviews, see Flexas et al., 2008; Warren, 2008a). The responses of gm to environmental drivers, however, are not universal (Tazoe et al., 2009). Scaling relationships between gm and photosynthetic capacity have been shown (Evans and von Caemmerer, 1996; Le Roux et al., 2001; Ethier et al., 2006) and challenged (Warren and Adams, 2006). Similarly, a correlation between gm and gs has been demonstrated in several species (Loreto et al., 1992; Lauteri et al., 1997; Flexas et al., 2002; Hanba et al., 2003; Ethier et al., 2006; but see Bunce, 2009), and is intriguing because of the potential for high frequency modelling of gs and subsequent estimates of gm. Recurrent diurnal patterns in gm could also provide a simple method of accounting for variation in mesophyll conductance within carbon exchange models. Studies of diurnal gm are limited (Bickford et al., 2009; Grassi et al., 2009) but open up the possibility of establishing a relationship between time of day and variation in mesophyll conductance that could be used as a dynamic model parameter. Mesophyll conductance has also been recognized as an important factor influencing the 13C/12C ratio of leaf material (δ13CL; Le Roux et al., 2001; Hanba et al., 2003; Warren and Adams, 2006) and ecosystem respiration (δ13Cresp; Ogée et al., 2003; Cai et al., 2008) which has implications for interpreting water use efficiency and terrestrial carbon exchange, among other applications. Δ is a strong regulator of δ13CL and δ13Cresp (Bowling et al., 2008), and therefore a better understanding of gm in leaf-level predictions of discrimination may improve interpretation of δ13C signals from multiple sources. Studies testing the role of gm in Δ predictions are limited, but suggest (Wingate et al., 2007) and demonstrate (Le Roux et al., 2001; Bickford et al., 2009) that the influence of gm was important.
Δ is influenced by numerous environmental and physiological regulators and is well correlated with key physiological indicators. The ratio of intercellular to ambient pCO2 (pi/pa) is a physiological parameter that succinctly describes the variability in the pCO2 gradient driven by A and stomatal conductance (gs), and its linear relationship with Δ has been widely observed over the last three decades (Farquhar et al., 1982a, 1989; Brugnoli and Farquhar, 2000). pi/pa is integral to two models of Δ: a comprehensive model that incorporates fractionation factors associated with diffusion, carboxylation, and decarboxylation processes (Δcomp; Farquhar et al., 1982b); and a simplified version of Δcomp that omits fractionation factors associated with decarboxylation activity and much of the diffusive pathway (Δsimple; Farquhar et al., 1982b). The parsimonious Δsimple evolved from the same theoretical work as Δcomp (Farquhar et al., 1982b) and gained wide usage primarily because of its simplicity and power in explaining observations of Δ, but also because the effects of decarboxylation activity and gm were thought to be negligible in predicting Δ.
Mechanistic models are used to predict Δ across a variety of temporal and spatial scales, where variation is driven by pi/pa interacting with key model parameters (Farquhar et al., 1982b). In addition to pi/pa, the key drivers of Δsimple include: (i) the carboxylation term, b, that represents net fractionation associated with phosphoenolpyruvate (PEP) carboxylase and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco); and (ii) the fractionation associated with diffusion in air and through stomata (a; 4.4‰) (Farquhar et al., 1989). Theory suggests the Rubisco carboxylation fractionation may be between 25‰ and 30‰ (Tcherkez and Farquhar, 2005) and is supported by recent measurements of Rubisco fractionation near 27‰ in tobacco (Nicotiana tabacum; McNevin et al., 2007). b is typically estimated at ∼27‰ in Δsimple, which is ∼2‰ lower than most measurements of the Rubisco fractionation in C3 plants (∼29‰; Roeske and O'Leary, 1984) due to the influence of PEP carboxylase activity and omitted fractionation factors (Farquhar and Richards, 1984; Gessler et al., 2008).
The comprehensive mechanistic Δ model incorporates the factors discussed above plus fractionation associated with CO2 diffusion, including gm, and decarboxylation activity. As previously discussed, gm is dynamic and may influence Δ by restricting diffusion from substomatal cavities to the chloroplast. The influence of day respiration (Rd), its associated fractionation factor (e), and fractionation associated with photorespiration (f) was thought to be negligible in early studies of gm and Δ (Evans et al., 1986; von Caemmerer and Evans, 1991). Recent evidence suggests, however, that these may be non-negligible variables (Ghashghaie et al., 2003; Tazoe et al., 2009), with f values ranging from ∼7‰ to 13‰ (Tcherkez, 2006; Lanigan et al., 2008) and e thought to be around −6‰ (Ghashghaie et al., 2003). Rd is difficult to measure and not well understood, but existing studies demonstrate inhibition of the respiration rate under illuminated conditions (Tcherkez et al., 2005) and biochemical differences between Rd and dark respiration (R; Tcherkez et al., 2008, 2009). Similarly, e is very difficult to estimate and no direct leaf-level measurements currently exist in the literature. Consequently, e is frequently estimated based on the dark respiration fractionation (ed; Ghashghaie et al., 2001; Tcherkez et al., 2003; Barbour et al., 2007), though the similarity, if any, of the isotope effects in R and Rd are not yet well understood (Tcherkez et al., 2008).
In this study a tunable diode laser absorption spectroscope (TDL) coupled to infra-red gas analysers (IRGAs) was used to measure gm and Δ of Juniperus monosperma (Engelm.) Sarg. (juniper) trees at high frequency on days representative of the growing season at a high elevation semi-arid field site in 2007. The objectives of this study were to (i) measure the diurnal variation of gm; (ii) quantify the relationship between diurnal gm and (a) gs and (b) time of day (TOD); (iii) assess model sensitivity to variation in e and b; (iv) measure the diurnal variation in Δ and examine the relationship between Δ and environmental and physiological drivers; and (v) assess the performance of Δcomp, when fitted with diurnally variable gm, compared with predictions from Δsimple.
Materials and methods
The study was conducted on 1 June 2007, 20 June 2007, 19 July 2007, and 23 August 2007 on Mesita del Buey near Los Alamos, NM, USA (elevation 2140 m) at a field site described in Breshears (2008) and Bickford et al. (2009). Precipitation at the site was 156.2 mm between May and August 2007, but was 65.5 mm in the January–April period preceding measurements.
Leaf gas exchange measurements
Two simultaneous measurements of leaf gas exchange were collected: (i) on the crowns of three mature juniper trees (jambient) which were rotated between ∼06:00 h and 18:00 h on each day with measurements conducted maintaining the chamber environment similar to ambient conditions; and (ii) on an adjacent mature juniper tree (jmanipulate) measured continuously throughout each day but subjected to light manipulations. Measurements were occasionally interrupted by rainfall, and did not resume until foliage was dry. Among the three rotational trees comprising jambient, leaf gas exchange and 13C discrimination were measured in response to ambient conditions. For both jambient and jmanipulate, temperature regulation in the chamber block was engaged when leaf temperature (TL), measured by energy balance, was ≥35 °C. Incoming irradiance in jmanipulate was manipulated by using a plastic shade to reduce incident light by ∼50% once or twice per hour to regulate net photosynthetic rate (A; μmol m−2 s−1) and assess the impact of irradiance on gm. Shading was maintained for 15–25 min intervals within each hour across the diurnal measurement period. Natural variation in irradiance occurred during both shaded and unshaded periods, and contributed to a wide range of A and light intensity. While all light manipulations were performed on one tree (jmanipulate), different groups of leaves were measured over the course of each day and across the season: two groups on 1 June, three on 20 June, two on 19 July, and three on 23 August.
Leaf gas exchange was measured by providing buffered air, via two 50.0 l volumes, to two LICOR 6400 portable photosynthesis systems (IRGAs; LI-COR Biosciences Inc., Lincoln, NE, USA); one IRGA was used to measure jambient and the other to measure jmanipulate. Each IRGA was fitted with a conifer chamber (LI-COR 6400-05), and incoming and outgoing gas streams were plumbed to a TDL (TGA100A, Campbell Scientific Inc., Logan, UT, USA) for measurement of the [12C16O2] and [13C16O2] within each gas stream. Lines connecting each IRGA and the TDL were of different lengths, resulting in different lag times, and the 33 s and 50 s lag between the two IRGAs and the TDL were accounted for when summarizing data between the instruments. To ensure high data quality for all Δ measurements and subsequent model testing, a priori criteria were established to filter error-prone data. These filtering criteria included ensuring that the difference in [CO2] of the gas entering and exiting the leaf chamber was >30 μmol mol−1, that the difference in entering and exiting δ13C was ≥1 ‰, and that ξ was <10 (see below for explanation of the ξ ratio). Leaf area within the conifer chamber ranged between 29.7 cm2 and 49.3 cm2. Instrument precision was previously determined to be 0.06‰ over 1 h periods (Bickford et al., 2009). Three minute TDL measurement cycles were used where each calibration tank (see below) was measured for 40 s, of which the last 10 s were used to calculate the means for both isotopologues, and 25 s for each of the four measurement inlets, of which the last 15 s were used for calculating concentrations. Details of the instrument coupling and measurement cycle calibration follow procedures described in Bickford et al. (2009).
Working standard (WS) calibration tanks spanning the range of expected [CO2] measurements used to calibrate each measurement cycle were (mean ±SE) 548.7±0.04 μmol mol−1 (12C16O2): 5.9±0.0005 μmol mol−1 (13C16O2): 2.2±0.0001 μmol mol−1 (12C18O16O) for the high WS tank; and 347.3±0.3 μmol mol−1 (12C16O2): 3.7±0.003 μmol mol−1 (13C16O2): 1.4±0.001 μmol mol−1 (12C18O16O) for the low WS tank during 1 June, 20 June, and 19 July measurements. The [CO2] of a new high WS calibration tank used in the 23 August measurements was measured as 535.9±0.3 μmol mol−1 (12C16O2): 5.8±0.003 μmol mol−1 (13C16O2): 2.2±0.001 μmol mol−1 (12C18O16O), while the low WS tank was the same as described above. All WS calibration tanks were calibrated for 4 h monthly against WMO-certified tanks that were filled and δ13C calibrated at the Stable Isotope Lab of the Institute for Arctic and Alpine Research, a cooperating agency of the Climate Monitoring division of the National Oceanic and Atmospheric Administration's Earth Research Laboratory. The [CO2] of the WMO-traceable tanks used in this study were, for the high tank, 539.57 μmol mol−1 (12C16O2): 5.93 μmol mol−1 (13C16O2): 2.21 μmol mol−1 (12C18O16O); and for the low tank, 339.43 μmol mol−1 (12C16O2): 3.76 μmol mol−1 (13C16O2): 1.40 μmol mol−1 (12C18O16O). Measurements of [CO2] occasionally exceeded the lower span of the WS calibration tanks (maximum deviation: 42.6 μmol mol−1), but post-hoc tests of the TDL demonstrated a linear measurement response beyond the lowest range of CO2 values observed in this study (Bickford et al., 2009).
Pre-dawn leaf water potential (Ψw) was measured using a Scholander-type pressure bomb (PMS Instruments Co., Corvallis, OR, USA) on six mature juniper trees near the study trees on 23 May, 27 June, 25 July, and 23 August 2007. Soil water content was measured at depths of 0.02–0.3 m using 11 neutron probes (503DR Hydrophobe Neutron Moisture Probes, Campbell Pacific Nuclear, Inc., Pacheco, CA, USA) at 2 week intervals between 23 May and 9 August 2007.
Model parameterization
The study tested whether variable gm improved model predictions of Δobs in jambient using a comprehensive model of Δ (Δcomp; Farquhar et al., 1982b),
| (1) |
where ab, aw, and bs represent the fractionation factors associated with CO2 diffusion through the leaf boundary layer (2.9‰), water (0.7‰), and fractionation attributed to CO2 entering solution (1.1‰). The variables pa, ps, pi, and pc represent pCO2 (Pa) in the chamber surrounding the leaf, at the leaf surface, in the intercellular spaces, and at the sites of carboxylation, respectively. Γ*, Rd, k, f, and e represent the CO2 compensation point in the absence of day respiration (Pa), day respiration rate (μmol m−2 s−1), carboxylation efficiency (μmol m−2 s−1 Pa−1), and fractionations associated with photorespiration and day respiration (‰), respectively.
Parameters pa, ps, pi, and pc were calculated by incorporating atmospheric pressure in Los Alamos (∼79 kPa) with mole fraction measurements of [CO2]; pc was estimated as pc=pi−A/gm (Farquhar and Sharkey, 1982). Rd was estimated at 1.5 μmol m−2 s−1 based on reported measurements of dark respiration in juniper (Bickford et al., 2009), k was calculated as A/pc for each 3 min cycle, and Γ* was calculated based on the expanded TL expression presented in Brooks and Farquhar (1985) that incorporates data from Jordan and Ogren (1984). The photorespiratory, f, and day respiratory, e, fractionations were estimated at 11.6‰ (Lanigan et al., 2008) and −3‰, respectively. e has often been estimated based on the dark respiration fractionation, and previous work suggests juniper exhibits a 2–3‰ dark respiration fractionation (Bickford et al., 2009). Recent evidence demonstrates biochemical shifts between light and dark respiration that may influence the isotopic signature of respired CO2 (Tcherkez et al., 2008), but currently there are no data in the literature providing estimates of the offset between day and dark respiratory fractionation at the leaf level. Because uncertainty in e and b could contribute to model uncertainty, tests were performed to evaluate the sensitivity of Δcomp to variation in each, and model predictions were compared with Δobs. In these sensitivity tests Δcomp was fitted with a gm=1.72 μmol m−2 s−1 Pa−1 (Δc.mean) and both Δc.mean and Δsimple were tested against all Δobs values (n=552), where Δsimple is:
| (2) |
and b is equal to 27‰ to account for omitted fractionation factors (Farquhar and Richards, 1984).
Δ and diurnal gm
Leaf carbon isotope discrimination (Δobs) was calculated from TDL-generated data:
| (3) |
where δe and δo equal the δ13C of the entering and outgoing chamber gas streams, respectively, and ξ equals ce/(ce–co) where ce and co are the [CO2] of the gas entering and exiting the leaf chamber, respectively. gm was estimated in jmanipulate leaf gas exchange and isotopic data using the point-based method (Evans et al., 1986),
| (4) |
where predicted discrimination (Δpred) is Δsimple with b=29‰. The estimate of the fractionation attributed to decarboxylation activities, Δef, was calculated as,
| (5) |
All components of Δef were parameterized as described for Δcomp. gm estimates that fell below zero were excluded, and this occurred when Δpred <Δobs. Measurement error in Δobs and gm incorporated instrument error for both total CO2 concentration and isotopic composition, and this uncertainty was propagated through analyses of gm using a bootstrapping approach described in Bickford et al. (2009). Point-based estimates were used to quantify gm in three different ways for model testing. First, a mean gm was calculated from all gm estimates (gm.mean; 1.72 μmol m−2 s−1 Pa−1). Secondly, a regression was fitted between TOD and gm measured within each day. The TOD and gm data were pooled across dates, analysed using least squares regression, and the resulting expression was used to estimate gm (gm.TOD). Thirdly, each gm estimate was transformed from expression in partial pressure (μmol CO2 m−2 s−1 Pa−1) to a flux density (mol CO2 m−2 s−1) by multiplying gm by the ambient pressure (∼79 kPa) which increased each gm value by 21.1%. The stomatal conductance to CO2 (gsc; mol CO2 m−2 s−1) was calculated as stomatal conductance to H2O (gsw) divided by 1.6 to account for differences in diffusivity between water vapour and CO2 (Farquhar and Sharkey, 1982). The transformed gm values were then compared with gsc data using linear regression, and the linear expression describing the relationship was used to estimate gm (gm.gs). To ensure the analysis of the relationship between gm and TOD or gsc was robust, a priori criteria for gm uncertainty were established. When the uncertainty in each point gm estimate, presented here as 1 SE, exceeded 0.10×gm that point gm estimate was excluded from regression analysis. Means testing was computed using the Tukey–Kramer honestly significant differences test (P <0.05 level) unless indicated otherwise. All statistical tests were performed in R (version 2.9.1; R Core Development Team, 2009).
Δcomp was parameterized in three ways for intermodel testing by calculating Δcomp using gm.mean (Δc.mean), gm.TOD (Δc.TOD), and gm.gs (Δc.gs). All three variations of Δcomp along with Δsimple were tested against Δobs. Model performance was evaluated using model bias and the root mean squared error (RMSE) as test statistics. Both were calculated from residuals where all models conformed to a slope of 1 and intercept of 0 (i.e. residuals=model prediction−Δobs). The mean of these residuals represents model bias, while the standard deviation of the residuals represents the RMSE (Bickford et al., 2009).
Results
Diurnal gm
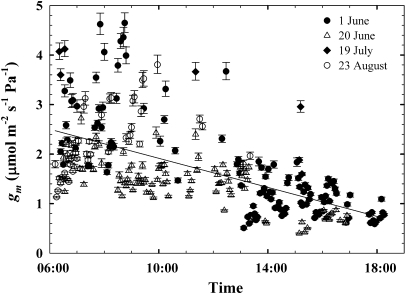
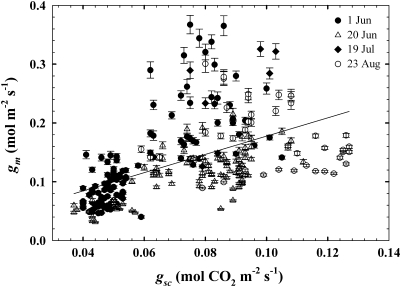
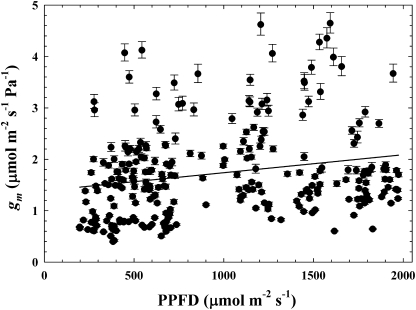
gm ranged between 0.4 and 4.6 μmol m−2 s−1 Pa−1 in jmanipulate across the four measurement days and generally declined across the morning to late day period (Fig. 1). Mean gm was not different between 1 June (mean ±SE=1.69±0.09 μmol m−2 s−1 Pa−1) and 20 June (1.44±0.05 μmol m−2 s−1 Pa−1), but was higher on 19 July (3.13±0.42 μmol m−2 s−1 Pa−1) and 23 August (2.22±0.10 μmol m−2 s−1 Pa−1; P <0.05). There was a significant relationship between gsc and gm (r2=0.27; P <0.0001; Fig. 2) and TOD and gm (P <0.0001). The linear expression gm= −3.52TOD+3.38 described the TOD–gm relationship (r2=0.37, F=154.6). The relationship between photosynthetic photon flux density (PPFD) and gm was weak, but significant (r2=0.05, P=0.0004; Fig. 3).
Fig. 1.
Significant diurnal variation in mesophyll conductance to CO2 (gm) across the four measurement dates (P <0.0001; r2=0.37). Mean gm was not different between 1 June and 20 June, but was higher on 19 July and 23 August (P <0.05; Tukey's HSD). Error bars represent 1 SE.
Fig. 2.
The relationship between stomatal conductance to CO2 (gsc) and mesophyll conductance (gm) across all four measurements dates (gm=1.55gsc+0.022; P <0.0001, r2=0.27). Error bars represent 1 SE.
Fig. 3.
The relationship between photosynthetic photon flux density (PPFD) and gm (r2=0.05, P=0.0004). Error bars represent 1 SE.
Δobs, physiological, and environmental parameters
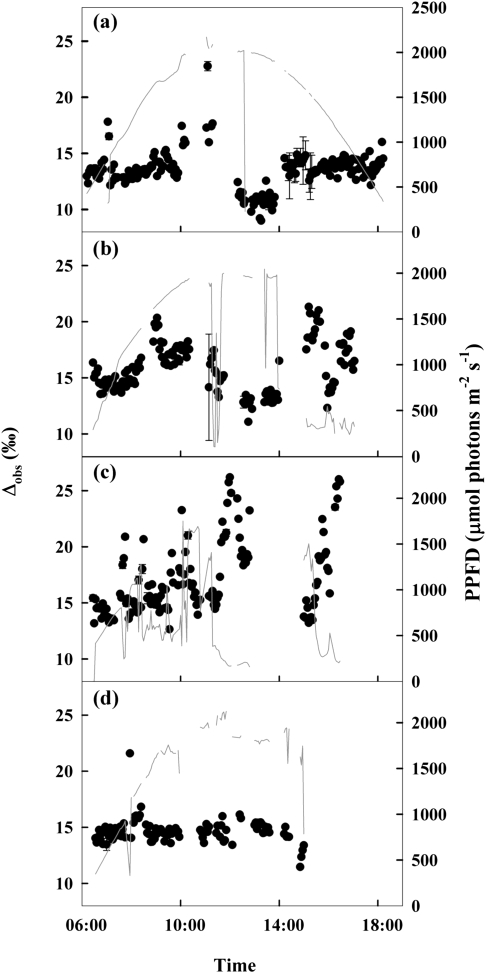
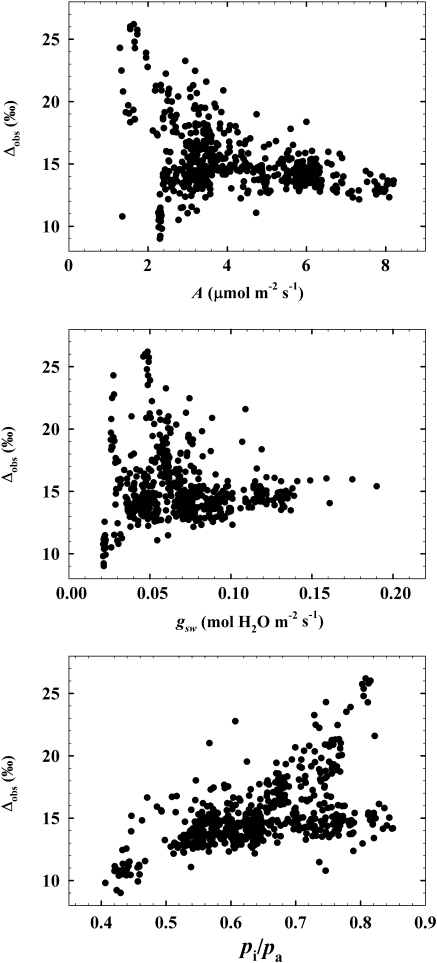
Mean Δobs in jambient was 13.5±0.1‰ on 1 June, 15.9±0.2‰ on 20 June, 17.0±0.2‰ on 19 July, and 14.7±0.1‰ on 23 August. Δobs was significantly different between all dates (P >0.05; Fig. 4). When pooled across months, some physiological parameters exhibited significant but weak linear relationships with Δobs, including A (P <0.0001, r2=0.13, F=80.7) and pi/pa (P <0.0001, r2=0.29, F=225.9), but not gsw (P=0.24, r2=0.0006, F=1.3; Fig. 5). A was higher on 23 August compared with 20 June, but was not significantly different among other dates (P >0.05; Table 1); gsw was similar on 1 June and 19 July, but was different on all other days (P ≤0.05; Table 1).
Fig. 4.
Diurnal variation in carbon isotope discrimination (Δ; filled circles) and photosynthetic photon flux density (PPFD; grey line) on 1 June (A), 20 June (B), 19 July (C), and 23 August 2007 (D). The abrupt shifts in Δ mid-day on 1 June can be attributed to variation among trees, but variation seen on other dates results from plant environmental response. Error bars represent 1 SE.
Fig. 5.
The relationship between observed discrimination (Δobs) and net photosynthetic rate (A), stomatal conductance to H2O (gsw), and the ratio of partial pressure of CO2 in intercellular spaces and the atmosphere around the leaf (pi/pa). When pooled across months these parameters exhibited significant linear relationships with Δobs, including A (P <0.0001, r2=0.13) and pi/pa (P <0.0001, r2=0.29), but not gsw (P=0.24, r2=0.0006).
Table 1.
Mean diurnal net photosynthetic rate (A; μmol m−2 s−1), stomatal conductance to H2O (gsw; mol m−2 s−1), and vapour pressure deficit (VPD; kPa), each reported with 1 SE, and number of observations each day
| A | SE | gsw | SE | VPD | SE | Observations | |
| 1 June | 4.34 a,b | 0.15 | 0.06 a | 0.002 | 2.86 a | 0.04 | 182 |
| 20 June | 3.97 a | 0.09 | 0.07 b | 0.001 | 2.17 b | 0.04 | 138 |
| 19 July | 4.07 a,b | 0.13 | 0.06 a | 0.002 | 2.31 b | 0.06 | 134 |
| 23 August | 4.54 b | 0.12 | 0.11 c | 0.003 | 1.22 c | 0.03 | 98 |
Different letters denote significant differences between dates (P ≤0.05; Tukey's honestly significant differences test).
There were weak but significant relationships between Δobs and TL on 19 July (P=0.006, r2=0.05, F=7.81) but not other dates (P ≥0.05). Mean TL was 31.8±3.43 °C (mean ±SD) across all dates. There were also weak but significant relationships between Δobs and vapour pressure deficit (VPD) on each day except 23 August (P ≤0.03), and when VPD data were pooled across months (P <0.0001, r2=0.05). VPD was significantly higher on 1 June and lower on 23 August compared with other days (P ≤0.05), but was similar on the remaining days (P >0.05; Table 1). Finally, there was a weak but significant linear relationship between Δobs and PPFD across all dates (P <0.0001, r2=0.16). Soil water content at 200 mm over the study period ranged from a high of 19.2% on 23 May to a low of 12.0% on 25 July, before recovering to 13.9% on 9 August. Ψw measured in nearby juniper trees (n=6) was highest early in the season at −0.62±0.06 MPa (23 May) and then declined to −2.1±0.2 MPa (27 June) and −3.4±0.33 MPa (25 July) before increasing to −2.75±0.34 MPa (23 August). The relationship between Ψw and Δobs was not significant (P=0.15, r2=0.75).
Model performance
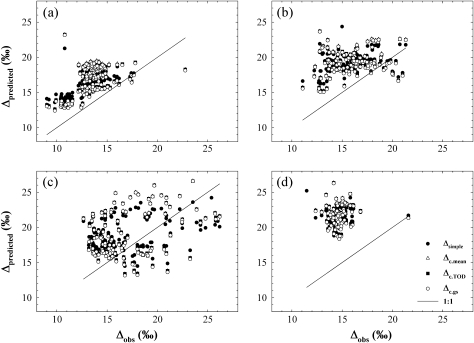
Δcomp did not consistently outperform Δsimple, and the reductions in Δcomp model bias observed over most of the study varied little with different parameterizations of gm. Δsimple exhibited lower RMSE on 1 June and 23 August, and across the pooled measurements dates (Table 2, Fig. 6), but also exhibited higher model bias on most dates (P <0.0001, paired t-test). All three variations of Δcomp showed comparable RMSE, and the differences in error were within 0.05‰ of one another. Model bias was significantly greater than zero in predictions of Δobs from all four models on all dates (P <0.0001 for all, paired t-test). A primary conclusion from Table 2 is that all models overpredicted Δ by at least 1‰, and that the limited improvements in predictions of Δ by incorporating gm were small compared with the bias between Δobs and Δcomp, which averaged 3.6‰ across the study.
Table 2.
Summary of model prediction tests of observed discrimination, where the values in bold highlight the lowest RMSE (‰) best performing model in each month and across the study
| Model | 1 June | 20 June | 19 July | 23 August | Whole study | |||||
| Bias | RMSE | Bias | RMSE | Bias | RMSE | Bias | RMSE | Bias | RMSE | |
| Δc.mean | 3.20 | 1.65 | 3.56 | 1.35 | 1.45 | 1.62 | 7.17 | 1.55 | 3.57 | 2.42 |
| Δc.TOD | 3.18 | 1.61 | 3.55 | 1.36 | 1.45 | 1.62 | 7.20 | 1.52 | 3.56 | 2.42 |
| Δc.gs | 3.05 | 1.66 | 3.45 | 1.36 | 1.32 | 1.63 | 7.06 | 1.57 | 3.44 | 2.43 |
| Δsimple | 3.78 | 1.33 | 3.73 | 1.43 | 1.59 | 1.96 | 6.97 | 1.32 | 3.80 | 2.30 |
Δsimple predictions showed the lowest RMSE across the study, but exhibited higher model bias (‰) across the whole study compared with all three parameterizations of Δcomp (P <0.0001).
Fig. 6.
Model tests of observed discrimination (Δobs) on 1 June (A), 20 June (B), 19 July (C), and 23 August 2007 (D). Four models were tested against Δobs including the simple model of discrimination (Δsimple; filled circles), the comprehensive model of discrimination using a mean mesophyll conductance (gm) of 1.72 μmol m−2 s−1 Pa−1 (Δc.mean; open triangles), the comprehensive model of discrimination using a gm estimated from the regression between diurnal gm and time of day (TOD) (Δc.TOD; filled squares), and the comprehensive model of discrimination using a gm estimated from the regression describing the relationship between stomatal conductance of CO2 and gm (Δc.gs; open circles). Δpredicted represents discrimination predictions of any of the four models. On two dates Δc.mean or Δc.TOD performed best, but on other dates and across the whole study Δsimple exhibited the lowest model error. These results support the use of Δsimple to predict leaf-level diurnal carbon discrimination of field-grown juniper.
Sensitivity tests showed reduced model bias and RMSE in Δc.mean when e and b were set to moderate and low values, respectively (compare Tables 2 and 3). Model bias increased 31% as e shifted from more positive (−1‰) to more negative (−6‰) values when b was set at 29‰. Model error, however, showed the lowest values when e was −3‰. Across tested e values the use of lower b values in Δc.mean consistently reduced model bias and error. Error changed minimally when Δc.mean was parameterized with e= −3‰ and b=29‰, and gm was decreased to 0.172 μmol m−2 s−1 Pa−1 (bias=1.66, RMSE=2.46) or increased to 17.2 μmol m−2 s−1 Pa−1 (bias=3.76, RMSE=2.43) compared with a gm=1.72 μmol m−2 s−1 Pa−1 (bias=3.57, RMSE=2.42), though model bias did decline 54% at the lowest gm value (P <0.0001). Δsimple showed an 85% reduction in model bias and a 4.7% reduction in error when fit with b=22‰ instead of b=27‰ (Table 3). Excluding 19 July, all variations of Δcomp and Δsimple overestimated Δobs by 3–7‰, as determined by model bias, though accounting for the variance, as in the RMSE term, reduced total error to between 1.3‰ and 2.4‰ on individual days. Using RMSE as the metric, the best fit to Δobs using Δc.mean was with e= −3‰ and b=25‰ (RMSE=2.25), but that fit was still poorer than predictions from Δsimple where b=22‰ (RMSE=2.19).
Table 3.
Results from sensitivity tests where the parameters representing the day respiration fractionation (e; ‰) and fractionation during carboxylation (b) were adjusted in the comprehensive model of carbon discrimination where gm was held constant at 1.72 μmol m−2 s−1 Pa−1 (Δc.mean; Equation 1), and b was adjusted in the simplified version of carbon discrimination (Δsimple; Equation 2)
| Δc.mean | Δsimple | |||||
| e (‰) | b (‰) | Bias (‰) | RMSE (‰) | b (‰) | Bias (‰) | RMSE (‰) |
| –1 | 29 | 3.04 | 2.42 | |||
| 27 | 1.77 | 2.34 | 27 | 3.80 | 2.30 | |
| 25 | 0.50 | 2.27 | ||||
| –3 | 29 | 3.57 | 2.42 | |||
| 27 | 2.30 | 2.32 | 24 | 1.87 | 2.22 | |
| 25 | 1.03 | 2.24 | ||||
| –6 | 29 | 4.36 | 2.46 | |||
| 27 | 3.09 | 2.35 | 22 | 0.59 | 2.19 | |
| 15 | 1.82 | 2.25 | ||||
All other variables are as described in Model parameterization.
Discussion
Diurnal gm
Two diurnal gm trends were evident across the study. On 1 June, gm increased in the early morning period to relatively high values (∼2–3 μmol m−2 s−1 Pa−1) and then declined to lower values for the remainder of the day (∼1 μmol m−2 s−1 Pa−1), a pattern repeated on 20 June and the 23 August morning and mid-day periods. On 19 July, gm was highest during the earliest measurements (∼4 μmol m−2 s−1 Pa−1) and remained relatively high through the afternoon period (∼1.5–3 μmol m−2 s−1 Pa−1). These trends in diurnal gm probably represent a composite response to changes in plant microclimate and other regulators. Leaf water status and temperature are known to affect mesophyll conductance, with drought decreasing (Warren et al., 2004; Flexas et al., 2004) and higher temperature increasing gm (Bernacchi et al., 2002; but see Warren and Dreyer, 2006). The diurnal decline in gm observed in this study is consistent with previous work showing reduced gm under water-stressed conditions, though the range of pre-dawn Ψw seen during this study would be characterized as moderate water stress in juniper (Linton et al., 1998; McDowell et al., 2008b). Increases in TL across each day may have buffered any drought effect and prevented greater reduction of gm, but such complex interactions cannot be determined with the current data set. Finally, cooporins, the CO2-transporting protein channels, may have played a strong role in regulating diurnal shifts in gm, but their regulation and interactions are still not well understood (Uehlein et al., 2008; Heinen et al., 2009).
Significant relationships existed between gm and gsc, gm and TOD, and gm and PPFD. The gsc–gm data show that gm was higher than gsc, and thus was not substantially limiting CO2 transfer to the sites of carboxylation or, as discussed below, substantially affecting Δ. These findings agree with data in other species demonstrating that gm was higher than gsc (Loreto et al., 1992; Galmés et al., 2006), but differ from studies showing lower gm compared with gsc (Hanba et al., 2003). These comparisons could be confounded if point-based calculations consistently overestimated juniper gm, but estimates from this study are similar to point-based gm values observed in a previous study of juniper (Bickford et al., 2009). gsc–gm data in this study deviate from a 1:1 relationship, possibly due to different regulatory processes between stomatal and mesophyll conductance to CO2 (but see Mott, 2009). Consensus is lacking, as others have observed nearly 1:1 gsc–gm relationships (Lauteri et al., 1997), no significant relationship between gsc and gm (Bunce, 2009), and substantial variability in the gsc–gm relationship between species (Warren, 2008b). The diurnal decline in gm observed across all study dates did not consistently improve Δ predictions, but using TOD as a relatively simple method to capture recurrent diurnal environmental patterns (i.e. declining leaf water status and parabolic temperature shifts) that affect mesophyll conductance and other photosynthetic processes may be productive in other systems. The weak relationship between gm and PPFD shows that variation in light had little impact on juniper gm, a finding that generally agrees with a study showing no effect of light on gm in wheat (Tazoe et al., 2009) but contrasts with those showing stronger effects of light on gm (Loreto et al., 2009; Monti et al., 2009).
Δ, environmental, and physiological parameters
Diurnal patterns across the study were consistent with previous studies showing environmental regulation of Δobs. As previously observed in model and empirical studies, VPD and PPFD acted as environmental drivers of Δ (Baldocchi and Bowling, 2003; Chen and Chen, 2007; McDowell et al., 2008a; Bickford et al., 2009), probably through their strong influence on A and gsc. Leaf water status was also a likely co-regulator of discrimination. Δ was inversely related to Ψw, increasing when Ψw decreased from 1 June to 19 July, and decreasing when Ψw again increased in August. Δ was comparable with previous observations in juniper during the same months in 2006, but was lower on 23 August (Bickford et al., 2009), probably due to substantially more negative pre-dawn Ψw in August 2007 (−2.75 MPa) compared with August 2006 (−0.58 MPa; McDowell et al., 2008b). The non-significant relationship between Ψw and mean Δobs was probably due to low sample size (n=4).
Variation in the physiological parameters A and pi/pa, but not gsw, was correlated with variability in Δobs. Consistent with theory, Δobs was generally higher when A was low and pi/pa was high (Fig. 4). Conversely, Δobs tended to be lower when A was high and pi/pa was low. The diffuse pattern between Δobs and pi/pa seen at higher pi/pa (>0.7) is attributed to variation among measured trees (data not shown). A large range of Δobs was seen at low gsw, consistent with previous work showing relatively high Δ when gsw and A are low (Bickford et al., 2009), and probably contributed to the non-significant relationship between the two factors. This was unexpected because gsw regulates CO2 transport into the leaf, but the poor relationship may support an even stronger role for carboxylase activity in regulating Δ in juniper. Finally, the isotope effect associated with diffusion through airspaces and dissolution of CO2 to HCO3– to equilibrium is accounted for in Δcomp, but the diffusion or facilitated passage of CO2 or bicarbonate across the cell wall and organelle membranes is still being elucidated (Uehlein et al., 2008) and may create further fractionation events that influence the Δ that is measured, though these data do not demonstrate a strong gm effect on juniper Δ.
Model performance
Parameterizing gm based on its relationship to gs and TOD did not consistently improve model predictions over Δsimple, nor did the use of a mean gm in Δcomp. Incorporating gm via Δcomp did reduce model bias when set to low values, but had a negligible effect on the error term whether set to low or high values. Thus, much unexplained variance remains in predictions of juniper Δ in the field, as is evident in the large unresolved model bias between predicted and observed Δ inherent in all models tested across the four dates. From a whole-study perspective, the results demonstrate no improvement in model error when using Δcomp compared with Δsimple, supporting the use of the parsimonious simple model to predict juniper Δ over the diurnal periods and across the seasonal gradient in this study. It is possible, however, that utilizing the gm–TOD or gm–gsc relationship to parameterize Δcomp may result in significant reductions in model error in other plant systems. These findings contrast with previous work showing improved model fit when utilizing a mean gm in Δcomp across diurnal and seasonal time scales (Bickford et al., 2009), though Δsimple did outperform Δcomp on one date in that limited study. These results also contrast with a recent study showing improved model predictions of respired δ13C values when gm was linked to variation in gsw compared with using a static gm in model predictions (Cai et al., 2008). These discrepancies demonstrate the need for more studies in diverse systems. The substantial unexplained variance observed in the model bias, and subsequently in the error term, across all months warrants further examination. Model bias was relatively high on most days (Fig. 5), particularly 23 August, and in the pooled data (Table 2), showing that all models consistently overestimated Δobs. The most likely reason for this is model parameterization error (discussed below in the sensitivity analysis).
Sensitivity tests showed that variation in e and b improved model performance. Implementing an e value of −3‰ generally minimized error compared with values of −1‰ or −6‰, but did not show a similar reduction in model bias. Step-change reductions in b from the value used in this study (29‰), however, resulted in consistently lower model bias and error. Two factors could explain these findings: (i) that the fractionation associated with b was lower and/or more variable than that reported until recently; or (ii) that Rd was higher and/or more variable than estimated in this study. The simultaneous reduction in model bias and error observed in this study when reduced b values were implemented demonstrates the strong regulatory role of b in model performance, but without assays of PEP and Rubisco activity and Rubisco discrimination no conclusions about the isotope effect or variability in b over diurnal periods can be made. Importantly, this does not suggest that the result of the sensitivity tests demonstrates that b is lower than shown in theoretical (Tcherkez and Farquhar, 2005) or empirical studies (Roeske and O'Leary, 1984; McNevin et al., 2007). A lower b, however, could be explained by relatively high PEP carboxylation activity proportional to Rubisco activity (Farquhar and Richards, 1984; Lanigan et al., 2008), a lower intrinsic isotope effect of the carboxylases comprising b (Raven and Farquhar, 1990; Brugnoli and Farquhar, 2000), or temperature effects on carboxylase activity, as mean TL was >30 °C. PEP carboxylation is typically associated with C4 photosynthesis and results in low discrimination against 13C when hydration of CO2 to HCO3– by carbonic anhydrase is in equilibrium (approximately −5.7‰; Farquhar et al., 1989), but the extent of PEP carboxylase activity in C3 photosynthesis is not well understood.
Alternatively, the influence of respiratory activity may have been higher than was estimated in this study. Estimates were based on previous work showing a high dark respiration rate, which were used as a surrogate estimator of Rd, and a 2–3‰ dark respiration fractionation in juniper (Bickford et al., 2009). Error may have been introduced if Rd was subject to diurnal variation that was not accounted for, or if a substantial offset exists between e and the dark respiration fractionation. Recent evidence shows the day and dark respiratory biochemical pathways are not the same, and may result in different isotopic fractionation (Tcherkez et al., 2008); however, the magnitude of the difference at the leaf level is not yet understood.
Δsimple also showed sensitivity to variation in b, and sensitivity tests support greater variability in b among C3 plants than is currently assumed. Previous studies using Δsimple have shown b values <27‰ resulting in the best fit of observed Δ (Brugnoli and Farquhar, 2000), and this is usually attributed to the reduced b value accounting for omitted fractionation factors. Δcomp and Δsimple were tested with the same Δobs data set, however, and improvements were found in both models when lower b values were used. The results of the sensitivity tests are slightly confounded by the use of Δpred and Δef, of which e and b are components, in the calculations of gm. In this application, however, the impact on the sensitivity tests is minimal since the exercise was designed to illustrate the impact of varying b and e at given a constant gm. That said, the results would be strengthened by estimates of gm from an independent method such as chlorophyll fluorescence, which relies on assumptions different from those of the isotopic method (Pons et al., 2009). Previous work has shown similar gm estimates (Loreto et al., 1992) and small differences in gm estimates from the two methods (Vrábl et al., 2009), and chlorophyll fluorescence-based estimates may have provided useful data on the variability in gm observed in this study. Overall, the results of the model tests and sensitivity analysis show non-negligible model bias and error in predicting juniper leaf Δ which was not reconciled by incorporating variability in gm or other parameters.
Acknowledgments
We thank H. Powers, K. Brown, and C. Meyer for extensive technical support, and the Institute of Geophysics and Planetary Physics at Los Alamos National Laboratory (project 95566-001-05), the National Science Foundation (IOS-0719118), the UNM Biology Dept. Lynn A. Hertel Graduate Research Award, UNM Biology Dept. GRAC, and UNM SRAC for funding. We also thank E. Erhardt for statistical advice and P. Holland for assistance with R. W. Pockman, B. Helliker and two anonymous reviewers provided helpful comments on the manuscript.
References
- Baldocchi DD, Bowling DR. Modelling the discrimination of 13CO2 above and within a temperate broad-leaved forest canopy on hourly to seasonal time scales. Plant, Cell and Environment. 2003;26:231–244. [Google Scholar]
- Barbour MM, McDowell NG, Tcherkez G, Bickford CP, Hanson DT. A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant, Cell and Environment. 2007;30:469–482. doi: 10.1111/j.1365-3040.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology. 2002;130:1992–1998. doi: 10.1104/pp.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford CP, McDowell NG, Erhardt EB, Hanson DT. High frequency field measurements of carbon isotope discrimination and internal conductance in a semi-arid species, Juniperus monosperma. Plant, Cell and Environment. 2009;32:796–810. doi: 10.1111/j.1365-3040.2009.01959.x. [DOI] [PubMed] [Google Scholar]
- Bowling DR, Pataki DE, Randerson JT. Carbon isotope in terrestrial ecosystem pools and CO2 fluxes. New Phytologist. 2008;178:24–40. doi: 10.1111/j.1469-8137.2007.02342.x. [DOI] [PubMed] [Google Scholar]
- Breshears DD. Structure and function of woodland mosaics: consequences of patch-scale heterogeneity and connectivity along the grassland–forest continuum. In: Van Auken OW, editor. Western North American Juniperus woodland—a dynamic vegetation type. New York, NY: Springer Press; 2008. pp. 58–92. [Google Scholar]
- Brooks A, Farquhar GD. Effect of temperature on the CO2/O2specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- Brugnoli E, Farquhar GD. Photosynthetic fractionation of carbon isotopes. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: physiology and metabolism. Dordrecht, The Netherlands: Kluwer Academic; 2000. pp. 399–434. [Google Scholar]
- Bunce J. Use of the response of photosynthesis to oxygen to estimate mesophyll conductance to carbon dioxide in water-stressed soybean leaves. Plant, Cell and Environment. 2009;32:875–881. doi: 10.1111/j.1365-3040.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- Cai T, Flanagan LB, Jassal RS, Black TA. Modelling environmental controls on ecosystem photosynthesis and the carbon isotope composition of ecosystem-respired CO2 in a coastal Douglas-fir forest. Plant, Cell and Environment. 2008;31:435–453. doi: 10.1111/j.1365-3040.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- Chen B, Chen JM. Diurnal, seasonal and interannual variability of carbon isotope discrimination at the canopy level in response to environmental factors in a boreal forest ecosystem. Plant, Cell and Environment. 2007;30:1223–1239. doi: 10.1111/j.1365-3040.2007.01703.x. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA. Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant, Cell and Environment. 2006;29:2168–2184. doi: 10.1111/j.1365-3040.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Australian Journal of Plant Physiology. 1986;13:281–292. [Google Scholar]
- Evans JR, von Caemmerer S. Carbon dioxide diffusion inside leaves. Plant Physiology. 1996;110:339–346. doi: 10.1104/pp.110.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z. Effect of salinity and humidity on δ13C value of halophytes—Evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia. 1982a;52:121–124. doi: 10.1007/BF00349020. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology. 1982b;9:121–137. [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology. 2002;29:461–471. doi: 10.1071/PP01119. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology. 2004;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmes J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment. 2007;30:1284–1298. doi: 10.1111/j.1365-3040.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Galmes J, Medrano H, Flexas J. Acclimation of Rubisco specificity factor to drought in tobacco: discrepancies between in vitro and in vivo estimations. Journal of Experimental Botany. 2006;57:3659–3667. doi: 10.1093/jxb/erl113. [DOI] [PubMed] [Google Scholar]
- Galmes J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Gessler A, Tcherkez G, Peuke AD, Ghashghaie J, Farquhar GD. Experimental evidence for diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant, Cell and Environment. 2008;31:941–953. doi: 10.1111/j.1365-3040.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck FW, Lanigan G, Nogues S, Tcherkez G, Deleens E, Cornic G, Griffiths H. Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Reviews. 2003;2:145–161. [Google Scholar]
- Ghashghaie J, Duranceau M, Badeck F-W, Cornic G, Adeline M-T, Deleens E. δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant, Cell and Environment. 2001;24:505–515. [Google Scholar]
- Grassi G, Ripullone F, Borghetti M, Raddi S, Magnani F. Contribution of diffusional and non-diffusional limitations to midday depression of photosynthesis in Arbutus unedo L. Trees—Structure and Function. 2009;23:1149–1161. [Google Scholar]
- Hanba YT, Kogami H, Terashima I. The effect of internal CO2 conductance on leaf carbon isotope ratio. Isotopes in Environmental Health Studies. 2003;39:5–13. doi: 10.1080/1025601031000102233. [DOI] [PubMed] [Google Scholar]
- Heinen RB, Ye Q, Chaumont F. Role of aquaporins in leaf physiology. Journal of Experimental Botany. 2009;60:2971–2985. doi: 10.1093/jxb/erp171. [DOI] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Dependence on ribulosebisphosphate concentration, pH and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiology. 2008;148:2013–2020. doi: 10.1104/pp.108.130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauteri M, Scartazza A, Guido MC, Brugnoli E. Genetic variation in photosynthetic capacity, carbon isotope discrimination and mesophyll conductance in provenances of Castanea sativa adapted to different environments. Functional Ecology. 1997;11:675–683. [Google Scholar]
- Le Roux X, Bariac T, Sinoquet H, Genty B, Piel C, Mariotti A, Girardin C, Richard P. Spatial distribution of leaf water-use efficiency and carbon isotope discrimination within an isolated tree crown. Plant, Cell and Environment. 2001;24:1021–1032. [Google Scholar]
- Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Functional Ecology. 1998;12:906–911. [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology. 1992;98:1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Tsonev T, Centritto M. The impact of blue light on leaf mesophyll conductance. Journal of Experimental Botany. 2009;60:2283–2290. doi: 10.1093/jxb/erp112. [DOI] [PubMed] [Google Scholar]
- McDowell N, Baldocchi D, Barbour M, et al. Understanding the stable isotope composition of biosphere–atmosphere CO2 exchange. Eos. 2008a;89:94–95. [Google Scholar]
- McDowell NG, Pockman W, Allen C, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008b;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McNevin DB, Badger M, Whitney S, von Caemmerer S, Tcherkez G, Farquhar G. Differences in carbon isotope discrimination of three variants of d-ribulose-1,5-bisphosphate carboxylase/oxygenase reflect differences in their catalytic mechanisms. Journal of Biological Chemistry. 2007;282:36068–36076. doi: 10.1074/jbc.M706274200. [DOI] [PubMed] [Google Scholar]
- Monti A, Bezzi G, Venturi G. Internal conductance under different light conditions along the plant profile of Ethiopian mustard (Brassica carinata A. Brown) Journal of Experimental Botany. 2009;60:2341–2350. doi: 10.1093/jxb/erp032. [DOI] [PubMed] [Google Scholar]
- Mott K. Opinion: stomatal response to light and CO2 depend on the mesophyll. Plant, Cell and Environment. 2009;32:1479–1486. doi: 10.1111/j.1365-3040.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. Journal of Experimental Botany. 2009;60:2271–2282. doi: 10.1093/jxb/erp063. [DOI] [PubMed] [Google Scholar]
- Ogée J, Peylin P, Ciais P, Bariac T, Brunet Y, Berbigier P, Roche C, Richard P, Bardoux G, Bonnefond J-M. Partitioning net ecosystem carbon exchange into net assimilation and respiration using 13CO2 measurements: a cost-effective sampling strategy. Global Biogeochemical Cycles. 2003;17:1070–1070. [Google Scholar]
- Piel C, Frak E, Le Roux X, Genty B. Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut. Journal of Experimental Botany. 2002;53:2423–2430. doi: 10.1093/jxb/erf095. [DOI] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany. 2009;60:2217–2234. doi: 10.1093/jxb/erp081. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: Austria; 2009. ISBN 3-900051-07-0, URL http://www.R-project.org. Accessed 2 January 2010. [Google Scholar]
- Raven JA, Farquhar GD. The influence of N metabolism and organic acid synthesis on the natural abundance of isotopes of carbon in plants. New Phytologist. 1990;116:505–529. doi: 10.1111/j.1469-8137.1990.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Roeske CA, O'Leary MH. Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry. 1984;23:6275–6284. doi: 10.1021/bi00328a005. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Badger MR, Evans JR. Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. Journal of Experimental Botany. 2009;60:2291–2301. doi: 10.1093/jxb/erp035. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. How large is the carbon isotope fractionation of the photorespiratory enzyme glycine decarboxylase? Functional Plant Biology. 2006;33:911–920. doi: 10.1071/FP06098. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Bligny R, Gout E, Mahe A, Hodges M, Cornic G. Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proceedings of the National Academy of Sciences, USA. 2008;105:797–802. doi: 10.1073/pnas.0708947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J. In vivo respiratory metabolism of illuminated leaves. Plant Physiology. 2005;138:1596–1606. doi: 10.1104/pp.105.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Farquhar GD. Carbon isotope effect predictions for enzymes involved in the primary carbon metabolism of plant leaves. Functional Plant Biology. 2005;32:277–291. doi: 10.1071/FP04211. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Mahé A, Gauthier P, Mauve C, Gout E, Bligny R, Cornic G, Hodges M. In folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid ‘cycle’ in illuminated leaves. Plant Physiology. 2009;151:620–630. doi: 10.1104/pp.109.142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Nogues S, Bleton J, Cornic G, Badeck F, Ghashghaie J. Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiology. 2003;131:237–244. doi: 10.1104/pp.013078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. The Plant Cell. 2008;20:648–657. doi: 10.1105/tpc.107.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. Determination of the average partial pressure of CO2 in the chloroplasts from leaves of several C3 plants. Australian Journal of Plant Physiology. 1991;18:287–305. [Google Scholar]
- Vrábl D, Vašková M, Hronková M, Flexas J, Šantrůček J. Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. Journal of Experimental Botany. 2009;60:2315–2323. doi: 10.1093/jxb/erp115. [DOI] [PubMed] [Google Scholar]
- Warren CR. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany. 2008a;59:1475–1487. doi: 10.1093/jxb/erm245. [DOI] [PubMed] [Google Scholar]
- Warren CR. Soil water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. Journal of Experimental Botany. 2008b;59:327–334. doi: 10.1093/jxb/erm314. [DOI] [PubMed] [Google Scholar]
- Warren CR, Adams MA. Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant, Cell and Environment. 2006;29:192–201. doi: 10.1111/j.1365-3040.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- Warren CR, Dreyer E. Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. Journal of Experimental Botany. 2006;57:3057–3067. doi: 10.1093/jxb/erl067. [DOI] [PubMed] [Google Scholar]
- Warren CR, Livingston NJ, Turpin DH. Water stress decreases the transfer conductance of Douglas-fir (Pseudotsuga menziesii) seedlings. Tree Physiology. 2004;24:971–979. doi: 10.1093/treephys/24.9.971. [DOI] [PubMed] [Google Scholar]
- Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J. Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant, Cell and Environment. 2007;30:600–616. doi: 10.1111/j.1365-3040.2007.01647.x. [DOI] [PubMed] [Google Scholar]