Abstract
Aluminium (Al) toxicity and drought are the two major abiotic stress factors limiting common bean production in the tropics. Using hydroponics, the short-term effects of combined Al toxicity and drought stress on root growth and Al uptake into the root apex were investigated. In the presence of Al stress, PEG 6000 (polyethylene glycol)-induced osmotic (drought) stress led to the amelioration of Al-induced inhibition of root elongation in the Al-sensitive genotype VAX 1. PEG 6000 (>> PEG 1000) treatment greatly decreased Al accumulation in the 1 cm root apices even when the roots were physically separated from the PEG solution using dialysis membrane tubes. Upon removal of PEG from the treatment solution, the root tips recovered from osmotic stress and the Al accumulation capacity was quickly restored. The PEG-induced reduction of Al accumulation was not due to a lower phytotoxic Al concentration in the treatment solution, reduced negativity of the root apoplast, or to enhanced citrate exudation. Also cell-wall (CW) material isolated from PEG-treated roots showed a low Al-binding capacity which, however, was restored after destroying the physical structure of the CW. The comparison of the Al3+, La3+, Sr2+, and Rb+ binding capacity of the intact root tips and the isolated CW revealed the specificity of the PEG 6000 effect for Al. This could be due to the higher hydrated ionic radius of Al3+ compared with other cations (Al3+ >> La3+ > Sr2+ > Rb+). In conclusion, the results provide circumstantial evidence that the osmotic stress-inhibited Al accumulation in root apices and thus reduced Al-induced inhibition of root elongation in the Al-sensitive genotype VAX 1 is related to the alteration of CW porosity resulting from PEG 6000-induced dehydration of the root apoplast.
Keywords: Aluminium, apoplast, drought stress, intercellular space, organic acids, polyethylene glycol, root elongation
Introduction
Soil acidity (pH <5.5) is one of the important limitations to crop production worldwide. Acid soils make up approximately 30% of the world's total land area and more than 50% of the world's potentially arable lands, particularly in the tropics and subtropics (von Uexküll and Mutert, 1995; Kochian et al., 2004). When the pH drops below 5, aluminium (Al) is released into the soil solution and becomes the single most important factor limiting crop production on 67% of the total acid soil area (Eswaran et al., 1997).
Common bean (Phaseolus vulgaris L.) is the most important food legume for direct human consumption in the world, and it is a staple food crop for small farmers and the urban poor in many Latin American and African countries. It is also the second most important source of protein (65% of all protein consumed) and the third most important caloric source (32% of all calories consumed) after cassava (Manihot esculenta Crantz) and maize (Zea mays L.) (Rao, 2001; Broughton et al., 2003). Under field conditions, common bean often experiences different abiotic stresses including drought, toxicities of Al and manganese, low soil fertility, and high temperatures (Thung and Rao, 1999; Singh, 2001; Ishitani et al., 2004). Among these, Al toxicity and drought are the two major abiotic stresses for bean production in the tropics (Ishitani et al., 2004). About 40% of the common bean production areas in Latin America and 30–50% of central, eastern, and southern Africa are affected by Al phytotoxicity resulting in a yield reduction of 30–60% (CIAT, 1992).
The easily observable symptom of Al toxicity is a rapid (minutes to a few hours) inhibition of root growth (Horst et al., 1992; Delhaize and Ryan, 1995), resulting in a reduced and damaged root system that limits mineral nutrient and water uptake (Kochian et al., 2004). Ryan et al. (1993) found that the root apex is the most Al-sensitive root zone, and Sivaguru and Horst (1998) identified the distal transition zone (DTZ) as the specific site of Al injury in maize. However, in common bean, Rangel et al. (2007) showed that both the transition zone (TZ, 1–2 mm) and the elongation zone (EZ) are targets of Al injury. Aluminium resistance was related to a lower Al accumulation in the root tip (Shen et al., 2002; Rangel et al., 2007). Under short-term Al supply, Al accumulates primarily in the root apoplast (Taylor et al., 2000; Wang et al., 2004; Rangel et al., 2009), where Al3+ strongly binds to the negatively charged binding sites (Zhang and Taylor, 1989; Blamey et al., 1990; Horst et al., 2010) provided by unmethylated pectin in the cell wall (CW) (Schmohl et al., 2000; Eticha et al., 2005). Thus, a lower CW negativity reducing Al accumulation (Horst, 1995) and the detoxification of Al in the apoplast through root exudates play an important role in Al resistance. Lower Al accumulation in the root tips and thus Al resistance is mediated by citrate exudation in common bean (Mugai et al., 2000; Shen et al., 2002; Rangel et al., 2010).
Drought stress is another important limiting factor for common bean production in the developing world, since as much as 60% of the common bean production occurs under conditions of drought stress (Graham and Ranalli, 1997; Beebe et al., 2008). Particularly on many acid soils, dry spells during the main growing period of crops are a major yield-limiting factor (Welcker et al., 2005). Adaptation to drought involves complex multigenic components that interact holistically in plant systems (Cushman and Bohnert, 2000). In plants growing in dry soil, both shoot and root growth is hampered (Westgate and Boyer, 1985; Sharp et al., 1988). The maintenance of root growth during water deficit facilitates water uptake from the subsoil (Sponchiado et al., 1989; Serraj and Sinclair, 2002). However, the exploitation of the subsoil for water and thus the ability of the plants to withstand drought stress may be strongly impeded by Al toxicity in acid subsoils (Goldman et al., 1989). Thus, on acid soils that permit deep rooting, both Al and drought resistance are required for yield improvement particularly in common bean, a generally Al and drought-sensitive crop (Rao, 2001; Beebe et al., 2008). Therefore, studies on individual and combined stress factors of these two limitations are important to clarify the opportunities and constraints in breeding for adaptation to these abiotic stresses.
In light of the importance of root development under conditions of Al toxicity and drought, the short-term effects of combined Al toxicity and drought stress on root growth, with special emphasis on Al/drought interaction in the root apex, was investigated in the present study in hydroponics which allow a detailed study of Al toxicity. Drought stress was imposed through the application of polyethylene glycol (PEG). PEG 6000 is a high molecular weight solute, which cannot enter the apoplastic space (Carpita et al., 1979; Hohl and Schopfer, 1991). It is therefore being amply used as a non-absorbed osmoticum to induce osmotic stress and it allows the response of plants to drought stress in hydroponic studies (Jia et al., 2001; Fan and Neumann, 2004) to be mimicked.
Materials and methods
Plant materials and growing conditions
Seeds of the four common bean genotypes, Quimbaya, G 21212, BAT 477, and VAX 1 were germinated on filter paper sandwiched between sponges. After 3–4 d, uniform seedlings were transferred to a continuously aerated simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 (Rangel et al., 2007). Plants were cultured in a growth chamber under controlled environmental conditions of a 16/8 h light/dark cycle, 27/25 °C day/night temperature, 70% relative air humidity, and a photon flux density of 230 μmol m−2 s−1 of photosynthetically active radiation at plant height. The pH of the nutrient solution was gradually lowered to 4.5 within two days. Then the plants were transferred to treatment solutions containing a factorial combination of Al (0, 25 μM) and PEG 6000 (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) (0, 200 g l−1) for 24 h in the simplified nutrient solution, pH 4.5. At harvest, the culture solutions were collected and filtered immediately through 0.025 μm nitrocellulose membranes. Mononuclear Al (Almono) concentrations were measured colorimetrically using the pyrocatechol violet method (PCV) according to Kerven et al. (1989). The Almono concentration of the Al treatment solution was kept at 25 μM by adding Al stock solution when necessary to prevent a decrease of the Almono concentration in the solution owing to the Al absorption by the roots. There was no difference between the PEG treatments (data not shown), suggesting that PEG supply did not lead to precipitation or complexation of Al in the treatment solution.
If not otherwise mentioned PEG 6000 (PEG) was used. In some experiments different PEG 6000 concentrations were used. The corresponding osmotic potentials (OPs) of the 0, 50, 100, 150, 200, and 250 g l−1 PEG 6000 solutions were 0.00, –0.06, –0.24, –0.60, –1.20, and –2.10 MPa, respectively, measured with a cryoscopic osmometer (Osmomat 030, Gonotec GmbH, Berlin, Germany).
Dialysis membrane tubes (DMTs) (3500 Da MWCO, Spectra/Por, California, USA) were used to separate the roots from the PEG 6000 solution. After 2 d of acclimation, plants were transferred into DMTs, and then the DMTs were transferred into 200 g l−1 PEG treatment solution and kept in an upright position in solution for 8 h, then the DMTs were transferred to 100 μM Al treatment solution without or with 200 g l−1 PEG for 1 h. In parallel, experiments without DMT were conducted for comparison. The PEG and Al concentrations in the parallel experiments were 150 g l−1 and 25 μM, respectively. When treating the plants in the DMTs with 200 g l−1 PEG and 100 μM Al, inhibition of root elongation and Al contents were comparable to the treatment of the plants without DMTs at 150 g l−1 PEG and 25 μM Al, respectively (data not shown). Thus different concentrations of Al and PEG were used in the different growing systems.
Diffusion of low molecular weight (LMW) PEG through DMTs and the effect of LMW PEG on root growth and Al accumulation in the root apex
A 250 ml PEG 6000 (200 g l−1) solution in DMTs was incubated in 1.0, 1.5, and 2.0 l distilled water for 4 h. During this period, the external solution was stirred gently and subsamples were collected at 15 min interval. In these samples the OP was determined with a cryoscopic osmometer either directly or after concentrating ten times with a rotational-vacuum-concentrator RVC 2-25 (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode/Harz, Germany).
To compare the effect of different molecular weight PEG on root growth and Al accumulation in root apices, plants were pre-treated with PEG 1000, 3000, and 6000 (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) at different OPs (0, –0.06, –0.24, –0.60 MPa) for 8 h in simplified nutrient solution, pH 4.5. Then half of the plants were harvested for the determination of root elongation. The remaining plants were allowed to grow for 1 h in the same solutions in the presence of 25 μM Al, pH 4.5. After the Al treatment, 1 cm root tips were excised for Al analysis.
Measurement of root elongation rate
Two hours before the treatment was initiated tap roots were marked at 3 cm behind the root tip using a fine point permanent marker (Sharpie blue, Stanford) which did not affect root growth during the experimental period. Afterwards, the plants were transferred into a simplified nutrient solution (see above) without or with PEG in the absence or presence of 25 μM Al. Root elongation was measured after the treatment period using a mm scale.
Collection of root exudates and determination of organic acids in exudates and root apices
To collect root exudates from root apices, plants were pre-treated with 0 or 25 μM Al in the absence or presence of 150 g l−1 PEG for 3, 7, and 23 h, then ten plants were bundled in filter paper soaked with nutrient solution. Approximately 1 cm of the main root apex of each plant was immersed in 15 ml of a constantly aerated incubation solution containing 5 mM CaCl2, 1 mM KCl, 8 μM H3BO3, and 0 or 40 μM AlCl3, pH 4.5. During this treatment process, the basal part of the root system was constantly moistened with incubation solution (see above) to prevent dryness but avoiding dripping into the columns. After 2 h, the incubation solution containing the root exudates were immediately frozen at –20 °C. After thawing, the incubation solution was passed through 5 g of a cation-exchange resin (AG50W-X8 with a 75–150 μm mesh) in 20 ml poly-prep columns with a 200–400 μm mesh filter at the bottom of the column, at a flow rate of 1 ml min−1. The resulting solution containing the organic acids (OA) was concentrated to dryness in a rotary vacuum evaporator (RCT 10-22T, Jouan, Saint-Herblain, France). The residue from each sample was re-dissolved in 500 μl (10 mM) perchloric acid, sonicated for 15 min, filled into micro-filtration tubes with a membrane pore size of 0.45 μm (GHP Nanosep MF Centrifugal Device, Pall Life Sciences, Ann Arbor, USA), and filtered by centrifugation at 20 000 g for 25 s. The filtered samples were immediately used for measurement or frozen.
The OA content of root tips was determined by the modified method of de la Fuente et al. (1997). Plants were treated with 0 or 25 μM Al in the absence or presence of 150 g l−1 PEG for 4, 8, and 24 h, then the root tips (1 cm) were excised and frozen immediately in liquid nitrogen. Before thawing, 400 μl of cold 70% (v/v) ethanol was added to the samples which were then homogenized in a micro-homogenizer (MM200 Retsch, Haan, Germany) for 3 min at 20 cycles s−1. OAs were extracted at 75 °C for 1 h with intermittent shaking on a vortex every 15 min. Thereafter, the samples were centrifuged at 23 000 g for 10 min and the supernatant was transferred into a new Eppendorf tube. The supernatant was concentrated to dryness in a rotary vacuum evaporator. The concentrated residue from each sample was re-dissolved in 200 μl 10 mM perchloric acid, sonicated for 15 min, transferred to centrifugal micro-filtration tubes with a membrane pore size of 0.45 μm, and centrifuged at a speed of 20 000 g for 25 s. The samples were immediately used for measurement or frozen.
The concentrations of OAs in the root exudates as well as in the extracts of root tissue were measured by isocratic High Pressure Liquid Chromatography (HPLC, Kroma System 3000, Kontron Instruments, Munich, Germany). The OAs were detected through a 20 μl loop-injector (Auto-sampler 360) of the HPLC, separating different OAs on an Animex HPX-87H (300×7.8 mm) column (Bio-Rad, Laboratories, Richmond, California, USA), supplemented with a cation H+ micro-guard cartridge, using 10 mM perchloric acid as eluent at a flow rate of 0.5 ml min−1, at a constant temperature of 35 °C (Oven 480), and with a pressure of 7.4 kPa. Measurements were performed at λ=214 nm (UV Detector 320).
Freeze-fracture scanning electron microscopy
The effect of PEG on the structure of the root tips was studied at the Research Centre of Bayer CropSciences at Monheim, Rhein, Germany, in co-operation with P Baur and S Teitscheid. After treating the plant with PEG 6000 and PEG 1000 (–0.60 MPa OP) for 4 h, root tips were excised about 7 mm from the root apex and placed onto a custom-made specimen holder, partly embedded in Tissue-Tek OCT Compound (Sakura, Fine Technical, Tokyo, Japan), and then quickly frozen with liquid nitrogen. Frozen specimens were rapidly transferred to a pre-cooled (–150 °C) specimen stage in a freeze-etch unit and fractured with knife and tweezers at a distance of about 3 mm from the root apex. The samples were then etched for 15 min at 110 °C under 10−6 Torr to remove surface ice. The structure of root tip cross-sections was examined using a scanning electron microscope (SEM, JSM-5600 LV, Jeol, Tokyo, Japan) after gold sputtering with high vacuum in the SEI modus at 9 kV. The sample was kept at –95 °C by means of a Gatan Alto 2100 cryo system. Image recording was done with a Point Electronic, DISS5 scanning system.
Isolation of cell-wall material
After pre-treating with PEG (0–200 g l−1) for 24 h, 30 root tips of 1 cm length were excised and transferred to 1 ml of 96% ethanol (method A) or immediately frozen in liquid nitrogen and then ground to a fine powder with a mortar and pestle in liquid nitrogen before 1 ml of 96% ethanol was added (method B). Cell-wall material was prepared as an alcohol-insoluble residue after repeated washing with ethanol, modified after Schmohl and Horst (2000). Root samples were thoroughly homogenized in ethanol using a mixer mill at a 30 cycles s−1 for 2 min. The homogenization was repeated twice. Then the samples were centrifuged at 23 000 g for 15 min and the supernatant was discarded. One millilitre of 96% ethanol was added and the pellet was re-suspended. The washing procedure was repeated twice. The remaining CW material was dried using a centrifugal evaporator (RC10-22T, Jouan SA, France), weighed, and stored at 4 °C for further use.
Determination of pectin and its degree of methylation
The dried cell-wall material isolated from 1 cm root tips was weighed, hydrolysed according to Ahmed and Labavitch (1977) extending the incubation time to 10 min in concentrated H2SO4 and 2 h after each step of water addition. The uronic acid content was determined colorimetrically according to Blumenkrantz and Asboe-Hansen (1973) using a microplate spectrophotometer (μQuant™; Bio-Tek Instruments, Winooski, VT, USA). Galacturonic acid was used as a calibration standard; thus the root pectin content was expressed as galacturonic acid equivalent (GaE).
For the determination of the degree of methylation (DM), the cell-wall material from root apices was prepared in the same way as for pectin determination. Methanol was released from the cell-wall material by saponification according to Fry (1988), and modified after Wojciechowski and Fall (1996). After the addition of 2 U of alcohol oxidase (EC 1.1.3.13 from Pichia pastoris; Sigma, Deisenhofen, Germany) the complex of formaldehyde with Fluoral-P (15 mg ml−1) (Molecular Probes, Leiden, The Netherlands) was measured fluorometrically (excitation λ=405 nm, emission λ=503 nm). The degree of methylation (%) was calculated as the molar ratio of methanol/uronic acid×100.
Cell-wall binding-capacity and uptake of Al3+, La3+, Sr2+, Rb2+ in 1 cm root apices
The isolated cell-wall material from 30 root tips (approximately 3 mg) was incubated for 30 min in 1 ml of a solution (pH 4.3) containing 300 μM AlCl3 or 300 μM LaCl3, 450 μM SrCl2 or 900 μM RbCl without or with 150 g l−1 PEG. Then the suspension was centrifuged at 23 000 g for 10 min. The supernatant was discarded. The pellet was re-suspended in 1 ml of ultra-pure deionized water and centrifuged again. The procedure was repeated twice. Then the residues were prepared for Al, La, Sr, and Rb determination.
To study the effect of PEG on the accumulation of La3+, Sr2+, and Rb+ in the root apices, intact plants were pre-treated with the simplified nutrient solution and 0 or 50, 100, 150, or 200 g l−1 PEG (pH 4.5) for 8 h. Then the plants were treated with 25 μM AlCl3, 5 μM LaCl3, 2.5 mM SrCl2, or 0.5 mM RbCl minus or plus 150 g l−1 PEG in the same nutrient solution for 1 h, pH 4.5.
Determination of Al, La, Sr, and Rb
For the determination of Al, La, Sr, and Rb, 1 cm root tips or cell-wall material were digested in 500 μl ultra-pure HNO3 (65%, v/v) by overnight shaking on a rotary shaker. The digestion was completed by heating the samples in a water bath at 80 °C for 20 min. Then 1.5 ml ultra-pure deionized water was added after cooling the samples in an ice-water bath. Aluminium was measured with a Unicam 939 QZ graphite furnace atomic absorption spectrophotometer (GFAAS; Analytical Technologies Inc., Cambridge, UK) at a wavelength of 308.2 nm after appropriate dilution, and an injection volume of 20 μl. La, Sr, and Rb were measured by inductively coupled plasma mass spectroscopy (ICP-MS) (7500cx, Agilent Technology, Santa Clara, California, USA) after appropriate dilution.
Statistical analysis
A completely randomized design was used, with 4–12 replicates in each experiment. Statistical analysis was carried out using SAS 9.2. Means were compared using t or Tukey test depending on the number of treatments being compared. *, **, *** and ns denote significant differences at P <0.05, 0.01, 0.001, and not significant, respectively.
Results
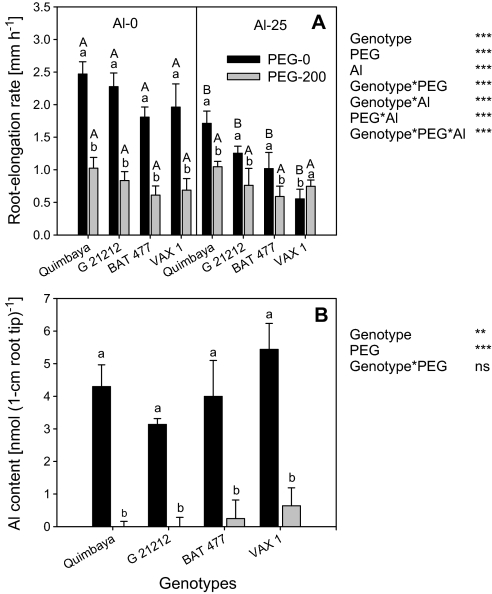
Four common bean genotypes differing in Al resistance were selected to investigate the relationship between Al toxicity and drought stress. The genotypes responded to Al treatment as previously reported, with Quimbaya as the most Al-resistant and VAX 1 as the most Al-sensitive (Fig. 1A; Rangel et al., 2005). PEG treatment led to severe osmotic stress and thus inhibition of root growth. Although the comparison of means did not show significant differences between genotypes in response to PEG, the ANOVA showed a highly significant genotype×Al interaction with genotype Quimbaya showing the highest and BAT 477 the lowest root growth in the presence of PEG. Combined Al and PEG stress did not lead to further inhibition of root growth. By contrast, PEG in addition to Al stress enhanced root growth compared with Al stress alone (highly significant PEG×Al interaction) particularly in genotype VAX 1 (highly significant genotype×PEG×Al interaction). The lack of Al-induced inhibition of root elongation and even the positive effect of PEG on root growth in the presence of Al can be explained by a strongly reduced Al accumulation in the root tips (Fig. 1B).
Fig. 1.
Root elongation rate (A) and Al content of 1 cm root tips (B) of four common bean genotypes under osmotic (200 g l−1 PEG) and Al stress (25 μM Al). Plants were pre-cultured in a simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 48 h for acclimation and pH adaptation, then treated without or with 25 μM Al in the absence or presence of 200 g l−1 PEG in the simplified nutrient solution for 24 h, pH 4.5. Bars represent means ±SD, n=12 for (A) and n=4 for (B). Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG treatments within Al supplies and comparison of Al treatments within PEG supplies, respectively. For the ANOVA, **, *** denote significant differences at P <0.01, P <0.001, respectively; ns, not significant.
Since among the genotypes tested the PEG-improved root growth in the presence of Al was most marked in VAX 1, the study was continued with this genotype only. The lower Al accumulation in the root apices of PEG-stressed plants could be due to an enhanced synthesis and exudation of organic acids because citrate exudation has been reported to be one of the most important mechanisms of Al resistance in common bean. Therefore, the contents and the exudation rates of organic acids were determined after 4, 8, and 24 h of PEG and Al treatment in order to take into account the adaptations to Al (Rangel et al., 2007) and PEG (data not shown) over the treatment period.
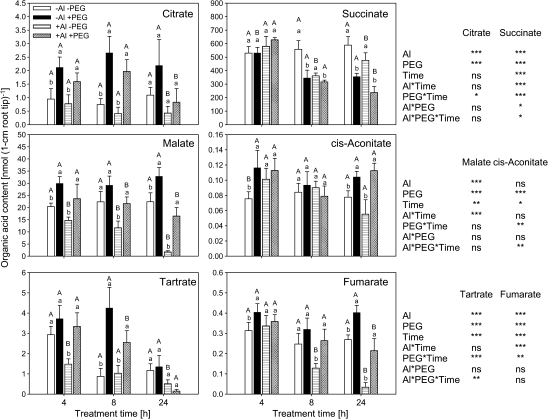
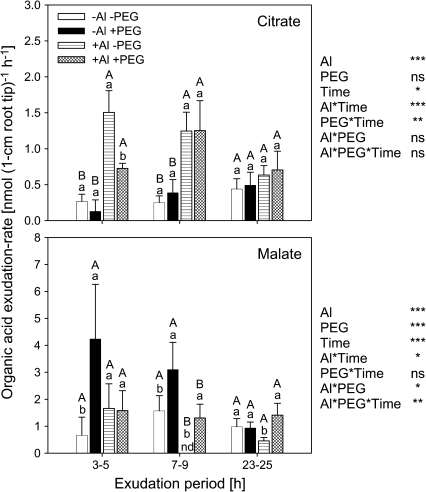
Whereas Al treatment decreased the contents of most organic acids with increasing treatment duration, PEG treatment/drought stress strongly enhanced OA contents in the root tissue, particularly of citrate and malate independent of the Al treatment (Fig. 2). Since organic acids could not be analysed in the presence of PEG and PEG could not be satisfactorily separated from the solution, organic acid anion exudation had to be determined during a 2 h period without PEG (but with Al) supply after the corresponding PEG pre-treatment. After the removal of PEG from the treatment solution, the amount of organic acid in 1 cm root apical tissues did not change during the subsequent 2 h exudate collection period and confirmed the organic acid contents (data not shown). Only malate, but not citrate exudation was affected by PEG treatment (Fig. 3). On the other hand, Al significantly enhanced citrate exudation independently of the PEG pre-treatments up to 9 h (Fig. 3).
Fig. 2.
Organic acid contents in 1 cm apical roots of common bean genotype VAX 1 (Al-sensitive) affected by osmotic stress and Al supply. Plants were pre-cultured in a simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 48 h for acclimation and pH adaptation, then treated without or with Al (25 μM) in the absence or presence of PEG (150 g l−1) in the simplified nutrient solution for 4, 8, and 24 h, pH 4.5. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG treatments within Al supplies and comparison of Al treatments within PEG supplies, respectively. For the ANOVA, *, **, *** denote significant differences at P <0.05, P <0.01, P <0.001, respectively; ns=not significant (F test).
Fig. 3.
Effect of PEG and Al treatment on organic acid exudation from 1 cm root apices of Al-sensitive common bean genotype (VAX 1). Plants were pre-cultured in a simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 48 h for acclimation and pH adaptation and then treated without or with Al (25 μM) in the absence or presence of PEG (150 g l−1) for 3, 7, and 23 h. Thereafter, the roots of 10 plants were bundled and the root tips (1 cm) were incubated in 15 ml of Al (0, 40 μM) treatment solution containing the above simplified nutrient solution without PEG for 2 h. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG treatments within Al supplies and comparison of Al treatments within PEG supplies, respectively. For the ANOVA, *, **, *** denote significant differences at P <0.05, P <0.01, P <0.001, respectively; ns=not significant (F test). nd=not detected.
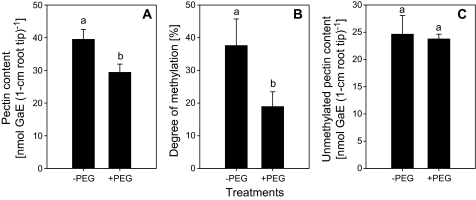
Another reason for the impeded Al accumulation in the root apices could be a lower negativity of the CWs formed in the presence of PEG. The cell-wall pectin-content and its degree of methylation determine the Al binding capacity of the root cell-wall (Schmohl and Horst, 2000). PEG treatment reduced total CW pectin content but also decreased the degree of methylation of pectin in 1 cm root tips. Thus the content of unmethylated pectin representing the negativity of the CWs remained unaffected by the PEG treatment (Fig. 4).
Fig. 4.
Total cell-wall pectin-content (A), its degree of methylation (B), and unmethylated pectin content (C) in 1 cm root tips of Al-sensitive common bean genotype (VAX 1). Plants were pre-treated without or with 150 g l−1 PEG in a simplified solution (pH 4.5) containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 24 h, then 30 root tips (1 cm) were harvested and cell-wall material was isolated according to Method A described in the Materials and methods for the determination of pectin content and degree of methylation. Bars represent means ±SD, n=4. Means with the same letters are not significantly different at P <0.05 (t test).
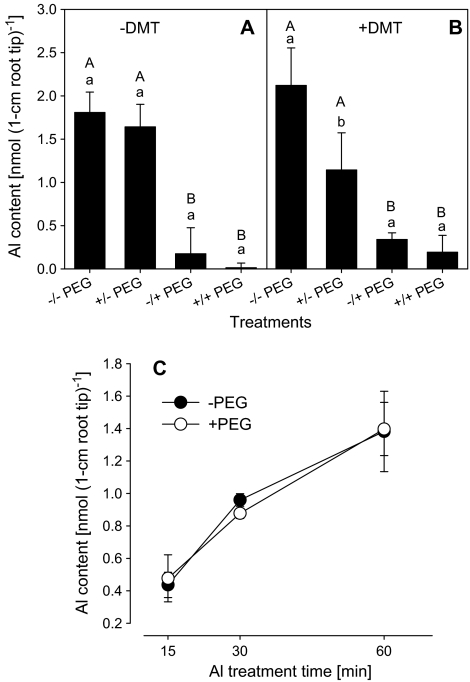
In order to differentiate between a direct effect of PEG accumulation on/in the root and of PEG-induced osmotic stress on Al accumulation in the roots, the roots were enclosed in a DMT, which has a molecular weight cut-off (MWCO) of 3500 Da and does not allow PEG 6000 to cross the membrane. Thus, the direct contact of PEG with the root was prevented while maintaining the osmotic stress. Higher PEG and Al concentrations were used with, rather than without, DMT according to preliminary experiments to compensate for impeded PEG and Al diffusion through the DMT (data not shown). As shown above, the presence of PEG during the Al treatment period of 1 h reduced the Al accumulation in the root tips to low levels even in plants not exposed to PEG during the 8 h pre-treatment period (–/+ PEG) (Fig. 5A). Discontinuing the PEG treatment during the 1 h Al treatment period after 8 h PEG pre-treatment (+/– PEG) completely restored the Al accumulation capacity of the root apices. This recovery is a very rapid process since, as early as 15 min after interrupting the PEG treatment, the difference in Al accumulation between PEG-treated and untreated plants disappeared (Fig. 5C). When the roots were protected against direct contact with PEG using DMT (Fig. 5B) Al accumulation by the roots was similarly reduced when osmotic stress was applied during the 1 h Al uptake period. However, when the osmotic stress was discontinued during the Al uptake period (+/– PEG), the Al uptake capacity was not fully restored to the level observed without DMT. This suggests a slower recovery from osmotic stress in the dialysis tubes.
Fig. 5.
Al content in 1 cm root tips of Al-sensitive common bean genotype (VAX 1). (A) Without dialysis membrane tubes (DMT); plants were pre-treated without or with 150 g l−1 PEG solution for 8 h, and then treated with 25 μM Al in the absence or presence of 150 g l−1 PEG solution for 1 h. (B) With DMT; plants were pre-treated without or with 200 g l−1 PEG for 8 h, then treated with 100 μM Al in the absence or presence of 200 g l−1 PEG solution for 1 h. (C) Without DMT; plants were pre-treated without or with 150 g l−1 PEG solution for 8 h, and then treated with 25 μM Al solution for 15, 30 and 60 min. The background solution of the above treatment solution was the simplified solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3, pH 4.5. –/– PEG: without PEG during pre-treatment and Al treatment; +/– PEG: with PEG during pre-treatment, without PEG during Al treatment; –/+ PEG: without PEG during pre-treatment, with PEG during Al treatment; +/+ PEG: with PEG during pre-treatment and Al treatment. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG pre-treatments within PEG re-treatments and comparison of PEG re-treatments within PEG pre-treatments, respectively.
Since the presence and thus penetration of the DMT by LMW PEG in PEG 6000 cannot be excluded, the OP was studied as an indirect measure of the presence of LMW PEG in the solution passing through the DMT in a model experiment in which the PEG 6000-filled DMT was incubated for 4 h. There was only a slight decrease of the OP which was only significant in the 10× concentrated incubation solution (see Supplementary Fig. S1 at JXB online). Even then the OP did not decrease beyond –0.06 MPa which did not affect root growth (Fig. 6A). This suggests that there is only a low amount of LMW PEG in the PEG 6000 product used for our experiments.
Fig. 6.
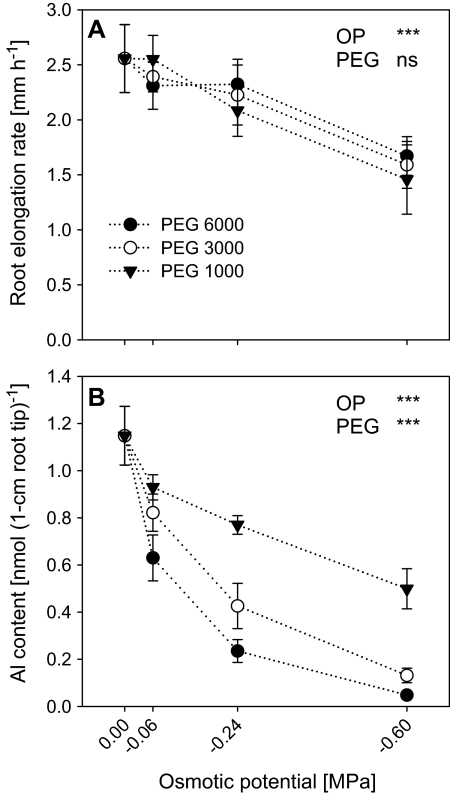
Effect of different molecular weight PEGs on root growth and Al accumulation in root tips of Al-sensitive common bean genotype (VAX 1). (A) Plants were pre-treated with different molecular weight PEGs at different osmotic potentials for 8 h. (B) Plants were pre-treated with different molecular weight PEGs at different osmotic potentials for 8 h, and then treated with 25 μM Al for 1 h in the presence of different molecular weight PEGs for 1 h. The background solution of the above treatment solution was the simplified solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3, pH 4.5. Bars represent means ±SD, n=4. For the ANOVA, *** denote significant differences at P <0.001; ns, not significant (F test).
To clarify how LMW PEG affects Al accumulation in the root apex, the effect of PEG 6000, PEG 3000, and PEG 1000 on Al contents in the root tips was compared at the same OPs corresponding to PEG 6000 concentrations of 0, 50, 100, 150 g l−1. The root elongation rate was decreased with decreasing OP independent of the molecular weight of the PEG (Fig. 6A). However, PEG 6000 reduced the Al contents of the root tips much more efficiently than PEG 3000 and particularly PEG 1000 (Fig. 6B).
The effect of different molecular weight PEG on the structure of the root tip has been studied using freeze-fracture electron microscopy. The resolution of the technique did not allow any conclusion to be drawn about the cell wall structure. However, the root cross-sections, shown in Supplementary Fig. S2 at JXB online, clearly showed that, in spite of comparable osmotic stress induced by the different molecular weight PEG (compare Fig. 6A), the effects on the root structure were different. In roots exposed to PEG 6000 (see Supplementary Fig. S2C, F at JXB online) the epidermis and the outer cortical cell layers were very closely packed and nearly all the intercellular spaces disappeared. By contrast, PEG 1000 (see Supplementary Fig. S2B, E at JXB online) hardly affected the intercellular space compared with the control (see Supplementary Fig. S2A, D at JXB online) indicating that in addition to osmotic stress, PEG 6000 dehydrates the root apoplast more than PEG 1000.
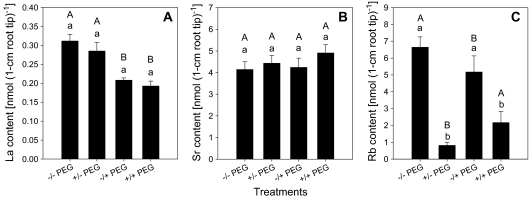
The specificity of the PEG 6000 effect on Al uptake into the root apex was evaluated using La, Sr, and Rb uptake for comparison (Fig. 7). PEG pre-treatment did not affect La uptake, while PEG applied together with La slightly but significantly decreased La accumulation (Fig. 7A). By contrast, neither PEG pre-treatment (+/–PEG) nor re-supply of PEG (–/+PEG) during the Sr uptake period affected Sr (as a tracer of Ca) accumulation in the root apices (Fig. 7B). However, Rb (as a tracer of K) accumulation was reduced by PEG pre-treatment (+/–PEG) and PEG application (–/+PEG) during the Rb exposure period (Fig. 7C), which might be explained by a significant increase of the K content in the root tips (from 212 to 342 nmol root tip−1, data not shown) caused by osmotic stress.
Fig. 7.
Effect of PEG pre-treatment/treatment on La (A), Sr (B), and Rb (C) accumulation of 1 cm root tips in Al-sensitive common bean genotype (VAX 1). Plants were pre-treated without (–PEG) or with 150 g l−1 PEG (+PEG) in a simplified solution (pH 4.5) containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 8 h. Then the plants were supplied with 5 μM LaCl3, 2.5 mM SrCl2 or 0.5 mM RbCl in absence (–/–, +/– PEG) or presence of 150 g l−1 PEG (–/+, +/+ PEG) in the same nutrient solution as described above for 1 h. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG pre-treatments within PEG re-treatments and comparison of PEG re-treatments within PEG pre-treatments, respectively.
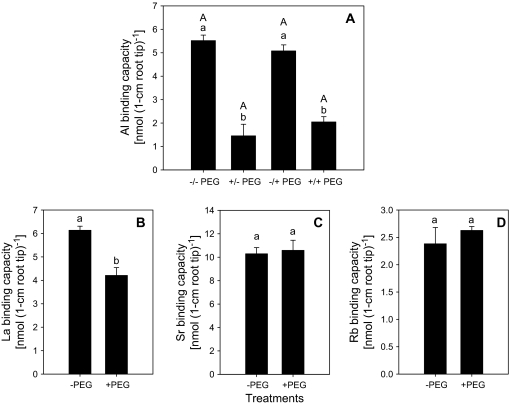
Cell-wall material isolated from 1 cm root apices of plants treated without or with PEG (150 g l−1) was exposed to Al, La, Sr, or Rb for 30 min in the absence or presence of PEG. PEG pre-treatment strongly reduced Al binding to the CWs (Fig. 8A). By contrast to Al, La accumulation was only slightly reduced (Fig. 8B), and Sr and Rb accumulation was not affected by PEG (Fig. 8C, D). Application of PEG during the Al loading period only did not affect the Al-binding properties of the isolated cell-wall material (Fig. 8A). Moreover, the different effects of osmotic stress on Rb accumulation in vivo (Fig. 7C) and in vitro (Fig. 8D) conditions suggest that the apoplast is not the main binding site of Rb, which may play an important role in the osmotic adjustment of the cytoplasm similar to K (Ogawa and Yamauchi, 2006).
Fig. 8.
Al3+ (A), La3+ (B), Sr2+ (C), and Rb+ (D) binding of cell-wall material isolated from of 1 cm root tips of Al-sensitive common bean genotype (VAX 1). Plants were pre-treated without or with 150 g l−1 PEG for 24 h in a simplified solution (pH 4.5) containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3. Then 30 root tips (1 cm) were harvested for each sample and cell-wall material isolated according to Method A described in the Materials and methods. Then the isolated cell-wall material was treated with 1 ml 300 μM Al minus or plus 150 g l−1 PEG, 300 μM LaCl3, 450 μM SrCl2, or 900 μM RbCl for 30 min, pH 4.3. –/– PEG: without PEG during pre-treatment and Al treatment; +/– PEG: with PEG during pre-treatment and without PEG during Al treatment; –/+ PEG: without PEG during pre-treatment and with PEG during Al treatment; +/+ PEG: with PEG during pre-treatment and Al treatment. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of PEG pre-treatments within PEG re-treatments and comparison of PEG re-treatments within PEG pre-treatments, respectively.
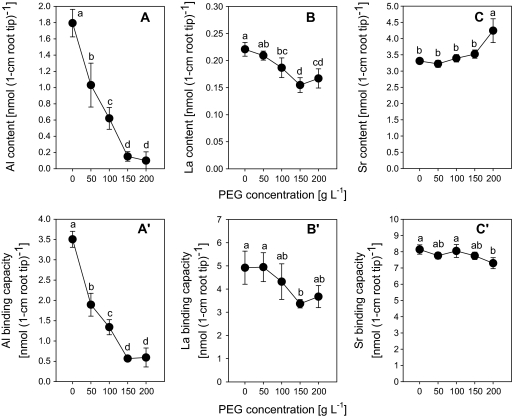
Al accumulation in 1 cm root apices of intact plants (Fig. 9A) and Al binding to the CWs of these root tips (Fig. 9A') decreased with increasing PEG concentration (0–150 g l−1) in the treatment solution. A similar decreasing tendency was also observed for La, although the relative change was much lower compared to Al (Fig. 9B, B'). Unlike that of Al and La, Sr uptake/binding was not reduced by PEG treatment (Fig. 9C, C'). A higher concentration of PEG (200 g l−1) did not further reduce Al and La uptake and its binding to the CW of root tips (Fig. 9). A PEG supply of 250 g l−1 was found to be lethal to the plants since it seriously damaged the root system (data not shown).
Fig. 9.
Effect of PEG treatment on Al, La, and Sr accumulation of 1 cm root tips (A, B, C) and binding of cell-wall material isolated from 1 cm root tips (A′, B′, C′) of Al-sensitive common bean genotype (VAX 1). (A, B, C) Plants were pre-treated with PEG (0–200 g l−1) for 8 h in a simplified solution (pH 4.5) containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3. Then the plants were supplied with 25 μM AlCl3, 5 μM LaCl3, or 2.5 mM SrCl2 in the presence of PEG (0–200 g l−1) in the same nutrient solution for 1 h as described above. (A′, B′, C′) Plants were pre-treated with PEG (0–200 g l−1) for 24 h in the simplified solution. Then 30 root tips (1 cm) were harvested for each sample and cell-wall material isolated according to Method A described in the Materials and methods. Then the isolated cell-wall material was treated with 1 ml 300 μM Al, 300 μM LaCl3, or 450 μM SrCl2 for 30 min, pH 4.3. Bars represent means ±SD (n=4). Means with the same letters are not significantly different at P <0.05 (Tukey test).
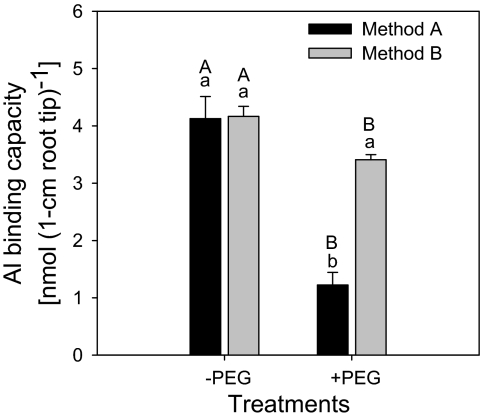
To elaborate the role of PEG-induced alteration of cell-wall structure on Al binding, a simple physical method (method B) was used to destroy the CW structure by vigorously grinding the root apices with a mortar and pestle in liquid nitrogen. PEG pre-treatment resulted in about a 70% reduction of Al binding when the CW structure was widely unaltered (method A; Fig. 10). However, by destroying the CW structure (method B) Al binding was restored in the PEG pre-treated samples. This indicates that PEG reduces CW porosity and restricts the access of Al ions to binding sites.
Fig. 10.
Al3+ binding of cell-wall material isolated from 1 cm root tips of Al-sensitive common bean genotype (VAX 1). Plants were pre-treated without or with 150 g l−1 PEG for 24 h in a simplified solution (pH 4.5) containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3. Then, 30 root tips (1 cm) were harvested and cell-wall material was isolated according to Method A or Method B, described in the Materials and methods. Then the isolated fine cell-wall powder was treated with 1 ml 300 μM Al for 30 min, pH 4.3. Bars represent means ±SD, n=4. Means with the same small letter and capital letter are not significantly different at P <0.05 (t test) for the comparison of the method of CW isolation within PEG pre-treatments and comparison of PEG pre-treatments within the method of CW isolation, respectively.
Discussion
Generally, there is a positive relationship between Al-induced short-term inhibition of root elongation and Al accumulation in the root tip apoplast of common bean (Rangel et al., 2009) indicating that Al resistance involves the exclusion of Al from the root tip apoplast (Horst et al., 2010). In the present study, PEG 6000-induced osmotic stress significantly inhibited Al accumulation in the root tips, almost reaching the level of the control (Fig. 1B). Consequently, there was no Al toxicity which is reflected by the lack of any additional Al effect on the root elongation of PEG 6000-stressed plants (Fig. 1A). The possibility that PEG or contaminants associated with PEG may decrease Al uptake into the root apex by complexing or precipitating Al in the treatment solution can be excluded because PEG application did not affect the mononuclear phytotoxic Al concentration of the treatment solution (data not shown).
Citrate exudation contributes to the Al resistance of common bean by excluding Al from the root apex. In the present study, Al stress significantly increased citrate exudation from the root apices during the early Al injury period (3–9 h), but the exudation was reduced with time (Fig. 3), which is typical for this Al-sensitive genotype VAX 1 (Rangel et al., 2010). The reduction of citrate exudation was related to the decreasing citrate content in the root apex (Fig. 2). These results confirm our previous studies that the Al resistance of common bean through citrate exudation requires the maintenance of the cytosolic citrate concentration through up-regulated synthesis and down-regulated degradation (Eticha et al., 2010; Rangel et al., 2010). Abscisic acid (ABA), known as a stress-inducible phytohormone, plays important regulatory roles in the adaptation of root growth to drought and salt stress (Sharp, 2002; Ren et al., 2010). As an early Al-stress signal it may also regulate citrate exudation since the exogenous application of ABA increased the activity of citrate synthase (CS) and citrate exudation, thus decreasing Al accumulation in the root apex of soybean (Shen et al., 2004). Therefore, it is speculated that drought stress-induced ABA synthesis may directly or indirectly enhance citrate exudation through stimulating citrate production in the root apex which detoxifies Al and contributes to improved root growth under Al stress condition. Under medium-term (4–24 h) Al stress, the citrate content in the root apex was enhanced by PEG (osmotic stress) treatment (Fig. 2). However, PEG pre-treatment did not affect citrate exudation from the root apex (Fig. 3), suggesting that osmotic stress did not induce the exclusion of Al from root apices by increasing citrate exudation. Since relieving of the osmotic stress by withdrawing PEG from the solution rapidly restored the Al accumulation capacity of the root apices (Fig. 5), the contribution of citrate exudation in reducing the Al binding capacity in the presence of PEG cannot be unequivocally ruled out.
The apoplast of the root apex has been proposed to be the primary site of Al toxicity (Horst, 1995; Horst et al., 2010). Many reports indicate that Al in the root primarily accumulates in the CW. Rangel et al. (2009) found that about 80% of the total Al in the root of common bean was bound in the CW. Similar findings were reported for soybean (Yang et al., 2009). The density of the negative charge carried by the CW is determined by the degree of methylation (DM) of pectin which thus determines the Al binding capacity of roots (Schmohl et al., 2000; Eticha et al., 2005; Yang et al, 2008). Therefore, reduced Al accumulation in PEG-stressed plants could be due to CW modification. However, in disagreement with salt (NaCl)-induced osmotic stress of our previous studies in maize, which led to an increased pectin content in root apices, enhanced Al accumulation, and thus higher Al sensitivity (Horst et al., 1999), our present results showed that PEG-induced osmotic stress did not affect the content of unmethylated pectin in the root apices of common bean (Fig. 4). Therefore, the results do not support the assumption that osmotic stress leads to low Al accumulation by decreasing the CW negativity.
The use of PEG in studies on osmotic stress relies on the assumption that this high molecular weight solute cannot enter the symplastic space of the root (see Introduction). However, there are several reports clearly showing that PEG may be accumulated in roots and even transported to the shoot (Lawlor, 1970; Janes, 1974; Yaniv and Werker, 1983; Jacomini et al., 1988). This may depend on the plant species, PEG source (contamination by LMW PEG) and concentration, time of exposure, and root damage. If PEG accumulates at the root surface or enters the root apoplast it may physically interfere with Al uptake and its binding to the CW. Therefore, in order to clarify the importance of apoplastic PEG or PEG-induced osmotic stress decreased Al accumulation in root tips, the roots were separated from the PEG in solution using DMT which has a molecular weight cut-off of 3500 Da. Aluminium accumulation in the root tips grown in DMTs was also strongly reduced by PEG treatment (Fig. 5) suggesting that it was not the physical presence of PEG 6000 but the PEG 6000-induced osmotic stress that was the cause of lower Al accumulation. A possible contribution of LMW PEG present in the PEG 6000 used in the experiments is unlikely because of two lines of evidence: (i) LMW PEG diffusing through the DMT reduced the OP of the equilibrium solution only to an OP value which hardly affected the Al binding of the roots (see Supplementary Fig. S1 at JXB online, Fig. 6B); (ii) PEG 6000 reduced the Al binding of the roots more than PEG 3000 and particularly PEG 1000 in spite of similar osmotic stress and inhibitory effects on root elongation rate (Fig. 6).
Compared with La, Sr, and Rb, the strong reduction of cation accumulation in the root apex by osmotic stress appears to be specific to Al. Osmotic stress had only a much smaller, yet, significant effect on La accumulation (Figs 7, 9). By contrast, neither PEG pre-treatment nor re-supply of PEG during the Sr uptake period affected Sr accumulation in the root apices (Fig. 7B). Rubidium accumulation was reduced by PEG pre-treatment and PEG application during the Rb exposure period (Fig. 7C). The reduction of Rb accumulation was only found under in vivo conditions. Binding of Rb to the isolated CW of root apices in vitro was not affected by PEG pre-treatment (Fig. 8D). This suggests that the apoplast is not the main binding site of Rb, which may play an important role in the osmotic adjustment of the cytoplasm similar to K (Premachandra et al., 1995; Ogawa and Yamauchi, 2006).
The specificity of cation accumulation might be related to the hydrated ionic radius of the cations: Al3+ (0.475 nm) > La3+ (0.452 nm) > Sr2+ (0.412 nm)=Ca2+ (0.412 nm) > K+ (0.331 nm) > Rb+ (0.329 nm) (Nightingale, 1959). Since the pore size of the CW plays an important role in the apoplastic transport of water, ions, metabolites, and proteins (Carpita et al., 1979; Brett and Waldron, 1996; Cosgrove, 2005), the differences between the ions in Al accumulation of the PEG-exposed root apices may suggest that PEG (osmotic stress) affects CW porosity. This assumption is supported by the fact that a similar reduction in accumulation specific for Al could also be observed in cell walls isolated from PEG-treated root tips (Fig. 8). Microscopic evaluation showed that the CW material was fairly intact (not shown) indicating that the CW porosity was not disrupted. After physically destroying the structure of the CW, Al binding to the CW was almost restored (Fig. 10).
The CW porosity is reported to be largely controlled by the pectin matrix (Baron-Epel et al., 1988). Schmohl and Horst (2000) suggested that the cross-linking of pectins by Al reduces the permeability of the CW for macromolecules such as proteins by reducing the CW porosity. McKenna et al. (2010) showed that Al and other metals reduced the hydraulic conductivity of bacterial cellulose–pectin composites, used as plant cell-wall analogues to about 30% of the initial flow rate. SEM revealed changes in the ultrastructure of the composites suggesting that metal binding decreased the hydraulic conductivity through changes in pectin porosity.
Pectin can form hydrated gels that push microfibrils apart, easing their sideway slippage during cell growth, while also locking them in place when growth ceases (Baron-Epel et al., 1988; Fleischer et al., 1999; Cosgrove, 2005). For example, Jarvis (1992) indicated that pectin may act as a hydrophilic filler to prevent aggregation and collapse of the cellulose network. Therefore, the reduction of pectin in the CW of root apices under osmotic stress (Fig. 4) may change the structure of the CW, consequently resulting in a rearrangement of wall polymers and affecting the porosity.
Generally, the pore diameter of the plant CW is in the range of 3.5–5.5 nm, which mainly depends on CW structure, hydrophobicity, CW chemical composition, and physical properties (Carpita et al., 1979; Chesson et al., 1997). Thus any change in these factors may result in the subsequent alteration of porosity. For example, Bauchot et al. (1999) reported that low temperature decreased the pore size of the CW of kiwifruit by modifying CW composition. The addition of boric acid to growing borate-deficient suspension-cultured Chenopodium album L. cells rapidly decreased the pore size of the CW by the formation of a borate ester cross-linked pectic network in the primary walls (Fleischer et al., 1999). However, although it is reported that plant cells interact with their environment through the porous network of the CW (Carpita et al., 1979), and water stress can induce changes in CW composition and CW properties of the roots (Iraki et al., 1989a, b; Wakabayashi et al., 1997; Leucci et al., 2008), to our knowledge, there is no report addressing the effect of drought stress on CW porosity.
Water is the most abundant component of the CW making up about two-thirds of the wall mass in growing tissues. This water is located mainly in the matrix (≈ 75–80% water), which suggests that the matrix has properties of a relatively dense hydro-gel (Cosgrove, 1997). This visco-elastic nature of the plant CW allows it to respond to stresses and limitations imposed upon it (Moore et al., 2008). Loss of water from the wall matrix can result in serious disruption to polymer organization. One obvious effect is that polymers usually well separated in the hydrated wall are brought into close proximity to each other, thus causing polymer adhesion or cross-linking under water stress. A model illustrating the effect of water loss on CW polymer organization was presented by Moore et al. (2008).
The extent of loss of water from the apoplast and, consequently, shrinkage of the root structure appeared to be dependent of the molecular size of the applied PEG: PEG 6000>PEG 3000>>PEG 1000 (see Supplementary Fig. S2 at JXB online). The difference between the PEG sources at the same OP of –0.60 MPa might be related to the penetration of the PEG molecules into the root apoplast: the higher the hydrodynamic radius the better the exclusion from the apoplast and, consequently, the dehydration of the apoplast. The estimated hydrodynamic radii of PEG 6000, 3000, and 1000 are 2.7, 1.6, and 0.89 nm, respectively (Kuga et al., 1981).
Also, the rapid recovery of Al accumulation in the living root apex after transfer of the roots into PEG-free solution (Fig. 5C) suggests that the water content of the apoplast is a decisive factor for PEG-induced alteration of CW porosity. However, the CW extension of living cells must involve biochemical (enzymatic) cleavage of load-bearing cross-linkages between wall polymers. Since the restoration of the Al accumulation capacity of the cell walls after the cessation of the PEG stress could only be observed in living root apices (Fig. 5), but not in ethanol-insoluble CW material isolated from root apices pre-treated with PEG (Fig. 8A), a role of enzymes mediating the inhibition of Al accumulation has to be postulated. Several CW proteins/enzymes are believed to play important roles in modifying the wall network and thus, possibly, the wall's ability to extend, such as expansin, xyloglucan endotransglycosylase (XET), and glucanase (Wu and Cosgrove, 2000). Therefore, it is speculated that some proteins related to the modification of the CW structure are involved in the PEG 6000 (osmotic stress)-induced alteration of CW porosity. This needs to be substantiated through further physiological and molecular studies.
In conclusion, the observed results provide circumstantial evidence that the osmotic stress-inhibited Al accumulation in root apices and thus reduced Al-induced inhibition of root elongation in the Al-sensitive common bean genotype VAX 1 is related to the alteration of CW porosity resulting from PEG 6000-induced dehydration of the root apoplast.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Diffusion of low molecular weight PEG through DMT.
Supplementary Fig. S2. Freeze-fracture scanning electron micrographs of root tip cross-sections of common bean genotypes VAX 1 in the presence of different molecular weight PEG.
Supplementary Material
Acknowledgments
This research was supported by a restricted core project from BMZ/GTZ (No. 05.7860.9-001.00) entitled ‘Fighting drought and aluminium toxicity: integrating functional genomics, phenotypic screening and participatory evaluation with women and small-scale farmers to develop stress-resistant common bean and Brachiaria for the tropics’, granted to the International Center for Tropical Agriculture (CIAT). We thank Dr Steve Beebe, Leader of the Bean Program of CIAT, for the supply of seed of the common bean genotypes, P Baur and S Treitscheid, Bayer CropScience, for supporting the freeze-fracture SEM analysis, and the China Scholarship Council for providing a scholarship to the first author.
References
- Ahmed AER, Labavitch JM. A simplified method for accurate determination of cell wall uronide content. Journal of Food Biochemistry. 1977;1:361–365. [Google Scholar]
- Baron-Epel O, Gharyal PK, Schindler M. Pectins as mediators of wall porosity in soybean cells. Planta. 1988;175:389–395. doi: 10.1007/BF00396345. [DOI] [PubMed] [Google Scholar]
- Bauchot AD, Hallett IC, Redgwell RJ, Lallu N. Cell wall properties of kiwifruit affected by low temperature breakdown. Postharvest Biology and Technology. 1999;16:245–255. [Google Scholar]
- Beebe S, Rao IM, Cajiao C, Grajales M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Science. 2008;48:582–592. [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. Role of root cation-exchange capacity in differential aluminium tolerance of Lotus species. Journal of Plant Nutrition. 1990;13:729–744. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Analytical Biochemistry. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brett C, Waldron K. Physiology and biochemistry of the plant cell wall. London: Chapman and Hall; 1996. [Google Scholar]
- Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J. Beans (Phaseolus spp.): model food legumes. Plant and Soil. 2003;252:55–128. [Google Scholar]
- Carpita N, Sabularse D, Montezinos D, Delmer DP. Determination of the pore size of cell walls of living plant cells. Science. 1979;205:1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Chesson A, Gardner PT, Wood TJ. Cell wall porosity and available surface area of wheat straw and wheat grain fractions. Journal of the Science of Food and Agriculture. 1997;75:289–295. [Google Scholar]
- CIAT. Constraints to and opportunities for improving bean production. 1992. A planning document 1993–98 and an achieving document 1987–92. Cali, Colombia, CIAT. [Google Scholar]
- Cosgrove DJ. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell and Developmental Biology. 1997;13:171–201. doi: 10.1146/annurev.cellbio.13.1.171. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ. Genomic approaches to plant stress tolerance. Current Opinion in Plant Biology. 2000;3:117–124. doi: 10.1016/s1369-5266(99)00052-7. [DOI] [PubMed] [Google Scholar]
- de la Fuente JM, Raminrez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiology. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran H, Reich P, Beinroth F. Global distribution of soils with acidity. In: Moniz AC, Furlani AMC, Schaffert RE, Fageria NK, Rosolem CA, Cantarella H, editors. Plant–soil interactions at low pH. Brazil: 1997. pp. 159–164. [Google Scholar]
- Eticha D, Stass A, Horst WJ. Cell-wall pectin and its degree of methylation in the maize root apex: significance for genotypic differences in aluminium resistance. Plant, Cell and Environment. 2005;28:1410–1420. [Google Scholar]
- Eticha D, Zahn M, Bremer M, Yang Z, Rangel AF, Rao IM, Horst WJ. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Annals of Botany. 2010 doi: 10.1093/aob/mcq049. doi: 10.1093/aob/mcq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Neumann PM. The spatially variable inhibition by water deficit of maize root growth correlates with altered profiles of proton flux and cell wall pH. Plant Physiology. 2004;135:2291–2300. doi: 10.1104/pp.104.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiology. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. The growing plant cell wall: chemical and metabolic analysis. New York, USA: Longman; 1988. [Google Scholar]
- Goldman IL, Carter TE, Jr, Patterson RP. A detrimental interaction of subsoil aluminum and drought stress on the leaf water status of soybean. Agronomy Journal. 1989;81:461–463. [Google Scholar]
- Graham PH, Ranalli P. Common bean (Phaseolus vulgaris L.) Field Crops Research. 1997;53:131–146. [Google Scholar]
- Hohl M, Schopfer P. Water relations of growing maize coleoptiles: comparison between mannitol and polyethylene glycol 6000 as external osmotica for adjusting turgor pressure. Plant Physiology. 1991;95:716–722. doi: 10.1104/pp.95.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Journal of Plant Nutrition and Soil Science. 1995;158:419–428. [Google Scholar]
- Horst WJ, Asher CJ, Cakmak J, Szulkiewicz P, Wissemeier AH. Short-term responses of soybean roots to aluminium. Journal of Plant Physiology. 1992;140:174–178. [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluska F, Sivaguru M. Does aluminium affect root growth of maize through interaction with the cell wall–plasma membrane–cytoskeleton continuum? Plant and Soil. 1999;215:163–174. [Google Scholar]
- Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany. 2010 doi: 10.1093/aob/mcq053. doi: 1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki NM, Bressan RA, Hasegawa PM, Carpita NC. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiology. 1989a;91:39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki NM, Singh N, Bressan RA, Carpita NC. Cell walls of tobacco cells and changes in composition associated with reduced growth upon adaptation to water and saline stress. Plant Physiology. 1989b;91:48–53. doi: 10.1104/pp.91.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Rao I, Wenzl P, Beebe S, Tohme J. Integration of genomics approach with traditional breeding towards improving abiotic stress adaptation: drought and aluminum toxicity as case studies. Field Crops Research. 2004;90:35–45. [Google Scholar]
- Jacomini E, Bertani A, Mapelli S. Accumulation of polyethylene glycol 6000 and its effects on water content and carbohydrate level in water-stressed tomato plant. Canadian Journal of Botany. 1988;66:970–973. [Google Scholar]
- Janes BE. The effect of molecular size concentration in nutrient solution and exposure time on the amount and distribution of polyethylene glycol. Plant Physiology. 1974;54:226–229. doi: 10.1104/pp.54.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MC. Control of thickness of collenchyma cell walls by pectins. Planta. 1992;187:218–220. doi: 10.1007/BF00201941. [DOI] [PubMed] [Google Scholar]
- Jia W, Zhang J, Liang J. Initiation and regulation of water deficit-induced abscisic acid accumulation in maize leaves and roots: cellular volume and water relations. Journal of Experimental Botany. 2001;52:295–300. [PubMed] [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kobot S. Aluminium determination in soil solution. II. Short-term colorimetric procedure for the measurement of inorganic monomeric aluminium in the presence of organic acid ligands. Australian Journal of Soil Research. 1989;27:91–102. [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kuga S. Pore size distribution analysis of gel substances by size exclusion chromatography. Journal of Chromatography. 1981;206:449–461. [Google Scholar]
- Lawlor DW. Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytologist. 1970;69:501–504. [Google Scholar]
- Leucci MR, Lenucci MS, Piro G, Dalessandro G. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. Journal of Plant Physiology. 2008;165:1168–1180. doi: 10.1016/j.jplph.2007.09.006. [DOI] [PubMed] [Google Scholar]
- McKenna BA, Kopittke PM, Wehr JB, Blamey FP, Menzies NW. Metal ion effects on hydraulic conductivity of bacterial cellulose-pectin composites used as plant cell wall analogs. Physiologia Plantarum. 2010;138:205–214. doi: 10.1111/j.1399-3054.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A. Adaptations of higher plant cell walls to water loss: drought versus desiccation. Physiologia Plantarum. 2008;134:237–245. doi: 10.1111/j.1399-3054.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Mugai EN, Agong SG, Matsumoto H. Aluminium tolerance mechanisms in Phaseolus vulgaris L.: citrate synthase activity and TTC reduction are well correlated with citrate secretion. Soil Science and Plant Nutrition. 2000;46:939–950. [Google Scholar]
- Nightingale ER., Jr Phenomenological theory of ion solvation. Effective radii of hydrated ions. Journal of Physical Chemistry. 1959;63:1381–1387. [Google Scholar]
- Ogawa A, Yamauchi A. Root osmotic adjustment under osmotic stress in maize seedlings. 2. Mode of accumulation of several solutes for osmotic adjustment in the root. Plant Production Science. 2006;9:39–46. [Google Scholar]
- Premachandra GS, Hahn DT, Rhodes D, Joly RJ. Leaf water relations and solute accumulation in two grain sorghum lines exhibiting contrasting drought tolerance. Journal of Experimental Botany. 1995;46:1833–1841. [Google Scholar]
- Rangel AF, Mobin M, Rao IM, Horst WJ. Proton toxicity interferes with the screening of common bean (Phaseolus vulgaris L.) genotypes for aluminium resistance in nutrient solution. Journal of Plant Nutrition and Soil Science. 2005;168:607–616. [Google Scholar]
- Rangel AF, Rao IM, Braun H-P, Horst WJ. Aluminium resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiologia Plantarum. 2010;138:176–190. doi: 10.1111/j.1399-3054.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. Journal of Experimental Botany. 2007;58:3895–3904. doi: 10.1093/jxb/erm241. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Intracellular distribution and binding state of aluminum in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiologia Plantarum. 2009;135:162–173. doi: 10.1111/j.1399-3054.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Rao IM. Role of physiology in improving crop adaptation to abiotic stresses in the tropics: the case of common bean and tropical forages. In: Pessarakli M, editor. Handbook of plant and crop physiology. New York: Marcel Dekker; 2001. pp. 583–613. [Google Scholar]
- Ren Z, Zheng Z, Chinnusamy V, Zhu J, Cui X, Lida K, Zhu J-K. RAS1, a quantitative trait locus for salt tolerance and ABA sensitivity in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2010;107:5669–5674. doi: 10.1073/pnas.0910798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany. 1993;44:437–446. [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cell grown in suspension culture. Plant, Cell and Environment. 2000;23:735–742. [Google Scholar]
- Schmohl N, Pilling J, Fisahn J, Horst WJ. Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiologia Plantarum. 2000;109:419–427. [Google Scholar]
- Serraj R, Sinclair TR. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant, Cell and Environment. 2002;25:333–341. doi: 10.1046/j.1365-3040.2002.00754.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE. Interaction with ethylene: changing views on the role of ABA in root and shoot growth responses to water stress. Plant, Cell and Environment. 2002;25:211–222. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiology. 1988;87:50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan XL, Matsumoto H. Effect of K-252a and abscisic acid on the efflux of citrate from soybean roots. Journal of Experimental Botany. 2004;55:663–671. doi: 10.1093/jxb/erh058. [DOI] [PubMed] [Google Scholar]
- Shen H, Yan X, Wang X, Zheng S. Exudation of citrate in common bean in response to aluminum stress. Journal of Plant Nutrition. 2002;25:1921–1932. [Google Scholar]
- Singh SP. Broadening the genetic base of common bean cultivars: a review. Crop Science. 2001;41:1659–1675. [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiology. 1998;116:155–163. [Google Scholar]
- Sponchiado BN, White JW, Castillo JA, Jones PG. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture. 1989;25:249–257. [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiology. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thung M, Rao IM. Integrated management of abiotic stresses. In: Singh SP, editor. Common bean improvement in the twenty-first century. Dordrecht: Kluwer Academic Publishers; 1999. pp. 331–370. [Google Scholar]
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant and Soil. 1995;171:1–15. [Google Scholar]
- Wakabayashi K, Hoson T, Kamisaka S. Changes in amounts and molecular mass distribution of cell wall polysaccharides of wheat (Triticum aestivum L.) coleoptiles under water stress. Journal of Plant Physiology. 1997;151:33–40. [Google Scholar]
- Wang Y, Stass A, Horst WJ. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiology. 2004;136:3762–3770. doi: 10.1104/pp.104.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker C, Théc C, Andréau B, et al. Heterosis and combining ability for maize adaptation to tropical acid soils: Implications for future breeding strategies. Crop Science. 2005;45:2405–2413. [Google Scholar]
- Westgate ME, Boyer JS. Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta. 1985;164:540–549. doi: 10.1007/BF00395973. [DOI] [PubMed] [Google Scholar]
- Wojciechowski CL, Fall F. A continuous fluorometric assay for pectin methylesterase. Analytical Biochemistry. 1996;137:103–108. doi: 10.1006/abio.1996.0206. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cosgrove DJ. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. Journal of Experimental Botany. 2000;51:1543–1553. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, You JF, Xu MY, Yang ZM. Interaction between aluminum toxicity and manganese toxicity in soybean (Glycine max) Plant and Soil. 2009;319:277–289. [Google Scholar]
- Yaniv Z, Werker E. Absorption and secretion of polyethylene glycol by Solanaceous plants. Journal of Experimental Botany. 1983;34:1577–1584. [Google Scholar]
- Zhang G, Taylor GJ. Kinetics of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars of Triticum aestivum L. Plant Physiology. 1989;91:1094–1099. doi: 10.1104/pp.91.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.