Abstract
Spectral reflectance indices can be used to estimate the water status of plants in a rapid, non-destructive manner. Water spectral indices were measured on wheat under a range of water-deficit conditions in field-based yield trials to establish their relationship with water relations parameters as well as available volumetric soil water (AVSW) to indicate soil water extraction patterns. Three types of wheat germplasm were studied which showed a range of drought adaptation; near-isomorphic sister lines from an elite/elite cross, advanced breeding lines, and lines derived from interspecific hybridization with wild relatives (synthetic derivative lines). Five water spectral indices (one water index and four normalized water indices) based on near infrared wavelengths were determined under field conditions between the booting and grain-filling stages of crop development. Among all water spectral indices, one in particular, which was denominated as NWI-3, showed the most consistent associations with water relations parameters and demonstrated the strongest associations in all three germplasm sets. NWI-3 showed a strong linear relationship (r2 >0.6–0.8) with leaf water potential (ψleaf) across a broad range of values (–2.0 to –4.0 MPa) that were determined by natural variation in the environment associated with intra- and inter-seasonal affects. Association observed between NWI-3 and canopy temperature (CT) was consistent with the idea that genotypes with a better hydration status have a larger water flux (increased stomatal conductance) during the day. NWI-3 was also related to soil water potential (ψsoil) and AVSW, indicating that drought-adapted lines could extract more water from deeper soil profiles to maintain favourable water relations. NWI-3 was sufficiently sensitive to detect genotypic differences (indicated by phenotypic and genetic correlations) in water status at the canopy and soil levels indicating its potential application in precision phenotyping.
Keywords: Canopy reflectance, canopy water content, leaf water potential, root growth, water index
Introduction
Crop water status is an important consideration in dryland agriculture and is influenced by many factors including environmental conditions, agronomic practices, soil properties, and crop growth (Hanks, 1988). Plant water status provides information that can be used to prevent crop water deficit through irrigation (Koksal, 2008), to select genotypes in breeding (Munjal and Dhanda, 2005), and to assess crop growth under drought conditions (Tucker, 1980; Peñuelas et al., 1993). Several methods are used to determine crop water content; leaf water potential (ψleaf) is the standard while leaf relative water content (RWC) is often used as a substitute (Slatyer, 1967). Other approaches for estimating water flow through plants include measuring leaf stomatal conductance in irrigated environments (Amani et al., 1996) and measuring canopy temperature (CT) as a relative measure of water flow associated with water extraction from the soil under water deficit (Reynolds et al., 2007).
Plant water status can also be assessed remotely by measuring canopy reflectance indices, since they change in response to crop water content (Peñuelas et al., 1997; Ustin et al., 1998; Stimson et al., 2005). As a technique, canopy spectral reflectance offers a number of advantages, such as easy and quick measurements, integration at the canopy level and the fact that additional parameters can be estimated simultaneously via a series of diverse spectral indices (i.e. photosynthetic capacity, leaf area index, intercepted radiation, and chlorophyll content) (Araus et al., 2001). Given its versatility, canopy reflectance is a valuable tool for high throughput phenotyping (Montes et al., 2007; Chapman, 2008).
Energy is strongly absorbed by water at specific wavelengths and different reflectance indices have been suggested for predicting crop water content (Peñuelas et al., 1993; Gao, 1996, 1997; Serrano et al., 2000; Stimson et al., 2005). Wavelengths in the near infrared (NIR; 700–1300 nm) and in the short infrared (SIR; 1300–2500 nm) regions have been employed for monitoring plant water status and several water bands have been proposed in the electromagnetic spectrum at 970, 1240, 1400, and 2700 nm (Tucker, 1980; Peñuelas et al., 1993; Gao, 1996; Zarco-Tejada and Ustin, 2001; Anderson et al., 2004; Stimson et al., 2005). Gao (1996) developed and proposed the normalized difference water index (NDWI; [R860–R1240]/[R860+R1240]) to sense vegetative water content using air-borne imagery with high image resolution. Anderson et al. (2004) utilized this index to determine canopy water content in soybean and corn by employing air-borne imagery (ASIRIS). Stimson et al. (2005) found that the NDWI and the normalized difference vegetation index (NDVI; [R900–R680]/[R900+R680]) showed significant correlation with leaf water content and water potential (r2=0.44–0.71) in two conifer species (Pinus edulis and Juniperus monosperma). Zarco-Tejada and Ustin (2001) and Zarco Tejeda et al. (2003) modelled the simple ratio water index (SRWI, R860/R1240) to estimate the vegetation water content in relation to leaf thickness, biomass, and leaf area index.
The water index (WI, R970/R900) proposed by Peñuelas et al. (1993) has been used to estimate water status in Phaseolus vulgaris, Capsicum annuum, and Gerbera jamesonii, and was associated with RWC under water-stressed conditions. In broccoli plants, the WI explained variations in plant water content as well as total biomass under diverse water treatments (El-Shikha et al., 2007). Babar et al. (2006) proposed two normalized water indices (NWI-–1=[R970–R900]/[R970+R900] and NWI-2=[R970–R850]/[R970+R850]) based on the water index proposed by Peñuelas et al. (1993) for screening grain yield in spring wheat genotypes under well-irrigated and water-deficient, stressed conditions. Two additional normalized water indices (NWI-3=[R970–R880]/[R970+R880] and NWI-4=[R970–R920]/[R970+R920]) have been proposed for use in screening the grain yield of advanced lines of winter wheat under rainfed conditions (Prasad et al., 2007). These five water indices (WI and four NWIs) have explained a large proportion of grain yield variability and are an alternative approach for selecting high yielding lines in wheat for diverse environments (Babar et al., 2006; Prasad et al., 2007). The water indices (WIs) are based on the hypothesis that the NIR wavelengths (970 nm) penetrate deeper into the canopy and therefore accurately estimate water content (Babar et al., 2006; Prasad et al., 2007; Gutierrez et al., 2010). The association between the WIs and grain yield indicates that canopy water content plays a vital role in determining yield of wheat genotypes under optimal as well as adverse growth conditions (Babar et al., 2006; Prasad et al., 2007).
Although a large number of indices at diverse wavelengths, based on theoretical perspectives, have been proposed, there is relatively little validation with field data (Serrano et al., 2000; Sims and Gamon, 2003). The objectives of the present study were (i) to establish which of a number of spectral reflectance indices showed the most reliable associations with the following plant and soil water status related parameters under a range of field conditions: ψleaf, RWC, CT, soil water potential (ψsoil), and available volumetric soil water (AVSW); (ii) assess the sensitivity of spectral water indices to detect genotype effects on plant water status and related traits using contrasting types of germplasm, and (iii) evaluate spectral water indices as a potential high throughput screening tool for water relations related traits in comparison to other methods.
Materials and methods
Experimental materials
Three types of wheat germplasm were used in this study which were evaluated and selected in previous breeding trials with a larger line number at the International Maize and Wheat Improvement Center (CIMMYT). The first germplasm set was composed of 16 advanced lines (ALN) previously selected and characterized as drought-resistant lines (high yielding) among other lines in earlier trials and used in our study during two growing seasons (2006 and 2007). The second germplasm was a subset of 14 bread wheat sister lines obtained from a larger population of random derived sister lines of the cross Seri-M82/Babax (elite/elite cross) plus the two parents previously selected and characterized as contrasting in drought resistance (Lopes and Reynolds, 2010). These sister lines and the two parents (SBS-I) were evaluated during 2006 and 2007. For the season 2008, the sister lines were reduced from 14 to six lines maintaining the two parents (SBS-II) based on the grain yield performance. The third germplasm set consisted of ten lines derived from inter-specific hybridization with wild relatives including the recurrent parents used to breed synthetic derived lines; [as described in Lage and Trethowan et al. (2008), Olivares-Villegas et al. (2007), and Reynolds et al. (2007), respectively]. The ten synthetic derivative lines (SYNDER), which were previously selected for high grain yield from a bigger yield trial (large line number), was also evaluated under water-stressed conditions during the 2008 season.
Growing conditions
The genotypes were grown during the winter season at CIMMYT's experimental station at Ciudad Obregon, Northwest Mexico (27.3° N, 109.9o W, 38 m above sea level). Weather conditions are mostly sunny and dry during the winter cropping cycle. The soil type is coarse sandy clay, mixed montmorillonitic type caliciorthid, low in organic matter, and slightly alkaline (pH 7.7) in nature (Sayre et al., 1997).
The seeding rate for each experiment was 78 kg ha−1. Nitrogen and phosphorus were applied to the plots at a rate of 150 kg ha−1 and 22 kg ha−1, respectively. Field plots consisted of two raised beds (28 cm apart) each 5 m long and 80 cm wide. An alpha lattice design with two repetitions was used for all experiments.
The planting dates were in November and plants reached booting and heading during February–March and were harvested in May. The crop growing seasons for all experiments are referred to as years: 2006 for the cycle 2005–2006, 2007 for the cycle 2006–2007, and 2008 for the cycle 2007–2008. The ALN, SBS-I, SBS-II, and SYNDER were planted under water stressed conditions in the 2006, 2007, and 2008 growing seasons.
Drought stressed conditions were achieved by applying one irrigation before seeding (which provided approximately 100 mm of available water) and then two irrigations (approximately 50–70 mm of available water each) were applied prior to the booting stage.
Folicur was applied at the booting, heading, and grain-filling stages at a rate of 0.5 l ha−1 to protect the experimental materials from leaf rust, caused by Puccinia triticina.
Spectral reflectance measurements
Canopy reflectance was measured in the 350–1100 nm range and collected at 1.5 nm intervals using a FieldSpec spectroradiometer (Analytical Spectral Devices, Boulder, CO). Data were collected during cloud-free days at midday (between 10.30 h and 14.00 h) after the machine was calibrated using a white plate of barium sulphate (BaSO4) which provides maximum reflectance (Labsphere Inc., North Sutton, USA). Four measurements in each plot were taken at a height of 0.5 m above the canopy and with a field of view of 25°. Readings were taken once during booting (SBS-II and SYNDER), anthesis (SBS-II, and SYNDER), and grain filling (all trials).
The water index proposed by Peñuelas et al. (1993) was calculated (WI=R970/R900) and four normalized water indices (NWIs) were also estimated according to Babar et al. (2006) and Prasad et al. (2007) (NWI-1=[R970–R900]/[R970+R900], NWI-2=[R970–R850]/[R970+R850], NWI–3=[R970–R880]/[R970+R880], and NWI-4=[R970–R920]/[R970+R920]).
Leaf and soil water potential and relative water content
Leaf water potential (ψleaf) was estimated on flag leaves during booting (SBS-II and SYNDER), anthesis (SBS-II, and SYNDER) and grain filling (all trials) one day before or one day after the spectral reflectance measurements. Four flag leaves in each plot were used to determine water potential using a pressurized pump (Scholander's pump) at midday (13.00–15.00 h). Water potential determined at night using flag leaves (22.00–24.30 h) was assumed to approximate soil water potential of the rhizosphere (ψsoil), as explained in the Discussion.
The relative water content (RWC) determined on flag leaves at grain filling was taken almost synchronously with the spectral measurements, and fresh samples of four flag leaves per plot (7–10 cm2) were collected and immediately weighed (fresh weight, FW). Intact leaves were transferred to sealed tubes, rehydrated in de-ionised water (around 8–12 h, until fully turgid at 25 °C), and weighed again (turgid weight, TW). Finally, the leaf samples were oven-dried at 78 °C for 24 h and then weighed (dry weight, DW). The RWC was calculated using the following formula:
Canopy temperature
A hand-held infrared thermometer (Mikron M90 Series, Mikron Infrared Instrument Co. Inc., Oakland, NJ) was used in all experiments to measure canopy temperature (CT) during booting and grain filling. The mean of four readings was obtained from the same side of each plot at an angle of approximately 30° with respect to the horizontal angle to integrate as many leaves as possible without capturing the soil in the measurement. The measurements were taken in the afternoon (13.00–14.00 h) when the crop experienced maximum transpiration rates. The WIs determined at booting, anthesis, and grain filling were related to the CT readings of booting and grain filling (same for anthesis and grain filling of NWI-3).
Available volumetric soil water
To estimate the available volumetric soil water (AVSW), a hydraulic probe (tube 6.54 cm in diameter and 2 m in length) connected to a tractor was used for collecting soil samples at different depths (30–60, 60–90, and 90–120 cm deep) during booting, anthesis, and grain filling in the SBS-II and SYNDER experiments. For the SBS-I and ALN, the AVSW was determined only after physiological maturity (2006 and 2007). During 2008, AVSW determined at booting, anthesis and grain filling was compared to WIs and CT values determined at the same respective growth stages.
Grain yield and biomass
In all experiments, grain yield was determined after physiological maturity by harvesting and threshing the entire plot, excluding a 0.5 m border at each end. Prior to grain harvest, a random subsample of 100 spike-bearing culms was removed from the plots. The subsample was oven-dried, weighed, and threshed. The grain weight was recorded and individual kernel weight estimated using a subsample of 200 kernels.
For biomass harvesting, all plants in a 0.5 m long area were cut at soil level in one of the two beds of each plot. The area harvested for biomass was 0.4 m2 (0.5 m by 0.8 m). The canopy reflectance measurements were taken randomly before biomass harvesting. After biomass harvesting, the total fresh weight was taken and oven-dried at 78 °C for 48 h. The dry weight of the biomass was recorded for estimating biomass by area (g m−2). The biomass was sampled at booting, anthesis, and maturity in the SBS-II and SYNDER experiments for the year 2008. During the previous years (2006 and 2007), biomass was not measured.
Estimation of genetic correlations
Genetic correlations between the NWI-3 and water relations parameters were estimated using the SAS software with Proc Mixed, following the method described by Singh and Chaudhary (1977) (SAS Institute, 2001). The formula used to estimate genetic correlation was:
where Var and Cov, respectively, refer to the components of variance and covariance.
The genetic correlations were estimated by combining years in ALN and SBS-I (2006 and 2007) for the grain-filling stage, while for SBS-II and SYNDER were estimated by combining growth stages (booting, heading, and grain filling) during 2008.
Statistical analyses
All experimental data were analysed according to the alpha lattice design by using Proc Mixed in the SAS program for each growth stage and year (SAS Institute, 2001). Pearson correlation coefficients were estimated using adjusted means to estimate the phenotypic relationship of the water indices to ψleaf, ψsoil, RWC, AVSW, grain yield, and biomass.
Results
The unselected recombinant inbred lines, elite advanced lines, and synthetic derivative genotypes showed differences in drought resistance under high levels of water stress measured as ψleaf (ranging from –2.49 to –3.59 MPa) across growth stages and across growing seasons (Table 1). Furthermore, genotypic differences were maintained as the magnitude of water stress increased across growth stages (–1.2 MPa for booting, –2.2 MPa for anthesis, and –3.3 MP for grain filling) where drought-resistant genotypes always had higher ψleaf (Fig. 1). The five canopy spectral water indices (WI and four normalized WIs) tested in the present study demonstrated strong associations with the plant and some soil water parameters under field water-stressed conditions, with NWI-3 demonstrating the strongest associations (Table 2). The four normalized WIs (NWI-1, NWI-2, NWI-3, and NWI-4) sometimes demonstrated similar results without significant differences among them, but NWI-3 generally showed stronger relationships (ranging from 1–6% stronger compared with the other WIs) in all germplasm sets evaluated during three growing seasons (Table 2). Therefore, data for NWI-3 was mostly presented in the current study to illustrate the relationship of WI with water status parameters.
Table 1.
Mean and least significant difference (LSD) for the normalized water index 3 (NWI-3), leaf and soil water potential, canopy temperature, grain yield, and biomass in a subset of sister lines (SBS-I and SBS-II), advanced lines (ALN), and synthetic derivatives (SYNDER) grown under water-stressed conditions
| Trial | Year | Mean | LSD | Signif.a |
| NWI–3 | ||||
| SBS-I | 2006–2007 | –0.018 | 0.021 | * |
| ALN | 2006–2007 | –0.013 | 0.018 | * |
| SBS-II | 2008 | –0.038 | 0.007 | ** |
| SYNDER | 2008 | –0.036 | 0.010 | ** |
| Leaf water potential (MPa) | ||||
| SBS-I | 2006–2007 | –2.66 | 0.65 | ** |
| ALN | 2006–2007 | –2.49 | 1.44 | * |
| SBS-II | 2008 | –3.04 | 0.35 | * |
| SYNDER | 2008 | –3.59 | 0.20 | ** |
| Soil water potential (MPa) | ||||
| SBS-I | 2008 | –1.39 | 0.25 | ** |
| ALN | 2008 | –1.25 | 0.21 | ** |
| Canopy temperature (°C) | ||||
| SBS-I | 2006–2007 | 27.7 | 1.02 | * |
| ALN | 2006–2007 | 32.3 | 1.83 | * |
| SBS-II | 2008 | 29.6 | 0.23 | ** |
| SYNDER | 2008 | 29.0 | 1.23 | ** |
| Grain yield (kg ha−1) | ||||
| SBS-II | 2008 | 1.01 | 0.16 | ** |
| SYNDER | 2008 | 1.29 | 0.18 | ** |
| SBS-II | 2008 | 3.25 | 0.71 | ** |
| SYNDER | 2008 | 2.98 | 1.88 | * |
| Biomass (kg ha−1) | ||||
| SBS-I | 2006–2007 | 6.34 | 0.57 | ** |
| ALN | 2006–2007 | 4.78 | 0.57 | * |
*,** Significant at the 0.05 and 0.01 probability levels, respectively.
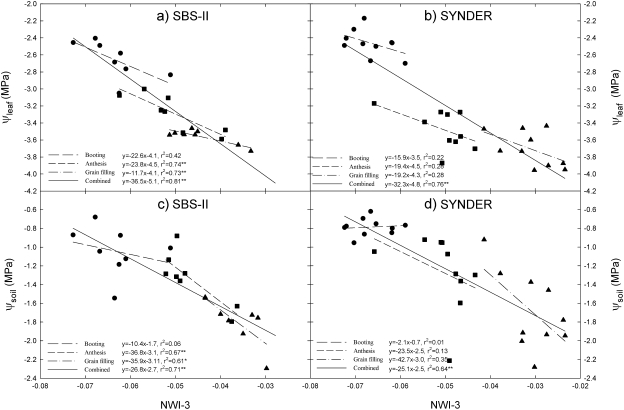
Fig. 1.
Relationship of the normalized water index 3 (NWI-3) with leaf water potential (ψleaf) and soil water potential (ψsoil) in a subset of sister lines (SBS-II) and synthetic derivatives lines (SYNDER) grown under water-stressed conditions during 2008.
Table 2.
Correlation coefficients at grain filling of five water indices with leaf water potential (ψleaf), relative water content (RWC), canopy temperature (CT), and available volumetric soil water (AVSW) content in a subset of sister lines (SBS-I) and advanced lines (ALN) grown under water-stressed conditions during 2006 and 2007
| 2006–2007b | Water statusa |
Available volumetric soil watera |
||
| ψleaf | RWC | CT | 30–90 cm | |
| SBS-I | ||||
| WI | –0.47* | –0.23 | 0.46 | –0.43 |
| NWI-1 | –0.47* | –0.23 | 0.47* | –0.43 |
| NWI-2 | –0.46 | –0.23 | 0.47* | –0.42 |
| NWI-3 | –0.49* | –0.24 | 0.49* | –0.44 |
| NWI-4 | –0.48* | –0.24 | 0.46 | –0.42 |
| ALN | ||||
| WI | –0.58* | –0.14 | 0.51* | –0.57* |
| NWI-1 | –0.58* | –0.14 | 0.51* | –0.57* |
| NWI-2 | –0.56* | –0.07 | 0.39 | –0.56* |
| NWI-3 | –0.58* | –0.14 | 0.53* | –0.58* |
| NWI-4 | -0.55* | –0.09 | 0.53* | –0.55* |
* Significant at the 0.05 probability level.
WI, water index; NWI, normalized water index 1, 2, 3, and 4.
Association of the normalized water indices with water status-related parameters
NWI-3 showed negative relationships with ψleaf at individual growth stages when advanced lines (ALN), recombinant inbred lines (SBS-I and SBS-II), and synthetic lines (SYNDER) were compared under diverse water stress levels during the three growing seasons (2006–2007 and 2008) (Table 2; Fig. 1a, b). For the ALN and SBS-I, both parameters were only determined at grain filling, while for SBS-II the same relationship was significant at anthesis and grain filling. The association of NWI-3 with ψleaf was stronger when booting, anthesis, and grain filling were combined in SBS-II and SYNDER during 2008 (Fig. 1a, b). Similarly, NWI-3 showed a negative association with ψsoil at booting, anthesis, and grain filling in SBS-II, but again the relationship was stronger when the three growth stages were combined (Fig. 1c, d). Variation in NWI-3 across the growth stages (booting, anthesis, and grain filling) followed ψleaf and ψsoil changes in SBS-II and SYNDER (significant associations at P ≤0.05 and 0.01) (Fig. 1).
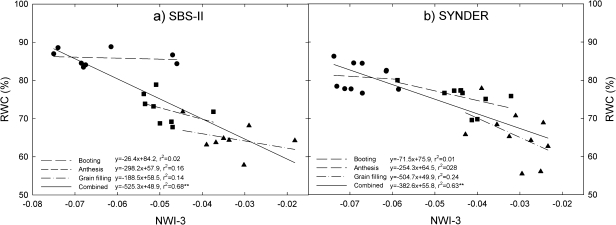
The negative correlation between NWI-3 and RWC were non-significant at individual growth stages in the three germplasm sets across growing seasons (ALN, SBS, SYNDER) (Table 2; Fig. 2), but were significant when combining data for booting, anthesis, and grain filling stages for the two trials evaluated during 2008 (Fig. 2).
Fig. 2.
Relationship between the normalized water index 3 (NWI-3) and relative water content (RWC) in a subset of sister lines (SBS-II) and synthetic derivatives lines (SYNDER) grown under water-stressed conditions during 2008.
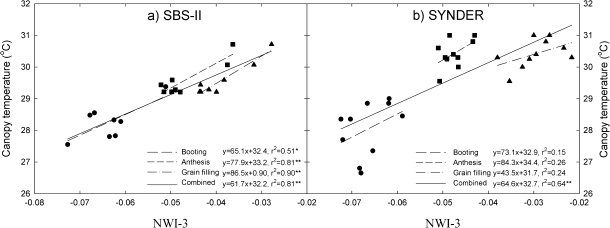
Correlations between NWI-3 and CT showed a positive trend in the three growing seasons for SBS-I and SYNDER and were generally consistent at individual growth stages for ALN and SBS-I during 2006–2007, and SBS-II during 2008 (Table 2; Fig. 3). However, when the growth stages were combined during 2008, CT showed a stronger relationship with NWI-3 in SBS-II (r2=0.81) and SYNDER (r2=0.64) (Fig. 3). In addition, CT showed a negative association with ψleaf, but only one relationship was significant at anthesis for SBS-II (Fig. 4). Combining the three growth stages, the negative relationship was significant for SBS-II (r2=0.61) and SYNDER (r2=0.73).
Fig. 3.
Relationship between the normalized water index 3 (NWI-3) and canopy temperature in a subset of sister lines (SBS-II) and synthetic derivatives lines (SYNDER) grown under water-stressed conditions during 2008.
Fig. 4.
Relationship between canopy temperature and leaf water potential (ψleaf) in a subset of sister lines (SBS-II) and synthetic derivatives lines (SYNDER) grown under water-stressed conditions during 2008.
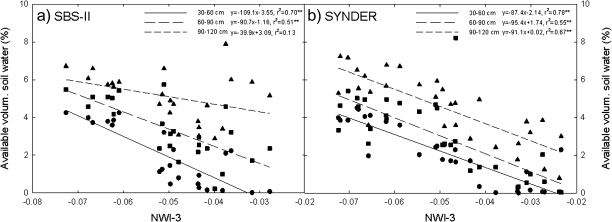
NWI-3 showed negative significant relationships with AVSW at grain filling in SBS-I and by combining growth stages (booting, heading, and grain filling) in SBS-II (Table 2; Fig. 5). The relationship between NWI-3 and AVSW was significant at superficial and deeper soil layers for SBS-II (r2 ranged from 0.40 to 0.72) and SYNDER (r2 ranged from 0.58 to 0.67) during 2008 (Fig. 5).
Fig. 5.
Relationship between the normalized water index 3 (NWI-3) and available volumetric soil water at three soil depths in a subset of sister lines (SBS-II) and synthetic derivatives lines (SYNDER) grown under water-stressed conditions during 2008.
NWI-3 also showed a consistent negative relationship with grain yield and biomass across growing seasons in all germplasm sets; being stronger when growth stages were combined for SBS-II and SYNDER during 2008 (Table 3). Similarly, CT showed strong negative relationships with grain yield (ALN, SBS-I, SBS-II, and SYNDER) and biomass (SBS-II and SYNDER) across seasons and by combining growth stages.
Table 3.
Correlation coefficients of grain yield and biomass with the normalized water index three (NWI-3) and canopy temperature (CT) in a subset of sister lines (SBS-I and SBS-II), advanced lines (ALN), and synthetic derivatives lines (SYNDER) grown under water-stressed conditions
| Parameter | Triala | Triala | ||
| SBS–I | ALN | |||
| 2006–2007 | Grain yield | Grain yield | ||
| NWI-3 | –0.49* | –0.56* | ||
| CT | –0.58* | –0.64** | ||
| SBS–II | SYNDER | |||
| 2008 | Grain yield | Biomass | Grain yield | Biomass |
| NWI-3 | –0.95** | –0.96** | –0.68* | –0.64* |
| CT | –0.95** | –0.94** | –0.68* | –0.76** |
*,**Significant at the 0.05 and 0.01 probability levels, respectively.
Genotypic differences explained by the normalized water indices
There were genotypic differences (P ≤0.01 and 0.05) for NWI-3, ψleaf, ψsoil, CT, grain yield, and biomass for the advanced lines (ALN), recombinant inbred lines (SBS-I and SBS-II), and synthetic derivatives lines (SYNDER) across growing seasons (Table 1). AVSW also showed genotypic differences at different soil depths (30–60 cm, 60–90 cm, and 90–120 cm) and across growth stages in the three growing seasons.
The relationship of NWI-3 with ψleaf and CT across growth stages demonstrated that NWI-3 was sensitive to genotypic differences in drought resistance at the canopy level in each germplam set at different water stress levels across years (Table 2; Figs 1a, b, 3). At the soil level, the changes in AVSW that were associated with NWI-3 in SBS-II and SYNDER also demonstrated that the genotypic differences at superficial and deeper soil layers could be related to differential capacity of root systems to explore water at low ψsoil (Figs 1c–d, 5).
The NWI-3 gave significant genetic correlations with the water relations parameters across growing seasons (ALN and SBS-II) and across growth stages (SBS-II and SYNDER) (Table 4). The genetic correlation between NWI-3 and canopy and soil water parameters was generally similar or higher to the phenotypic correlation values (Tables 2, 4; Figs 1–5). Also, the genetic correlation showed the same trends for each germplasm set when individual growth stages were tested (data not shown).
Table 4.
Genetic correlations between the normalized water index 3 (NWI-3) and water relations parameters for a subset of sister lines (SBS-I and SBS-II), advanced lines (ALN), and synthetic derivatives (SYNDER) grown under water-stressed conditions
| Trial | Season | ψleafa | ψsoila | RWC | CTa | Available volumetric soil watera |
| 30–90 cm | ||||||
| ALNb | 2006–2007 | –0.73** | –0.40 | 0.75** | –0.51* | |
| SBS-Ib | 2006–2007 | –0.53* | –0.29 | 0.55* | –0.63** | |
| SBS-IIc | 2008 | –0.88* | –0.70* | –0.27 | 0.78* | –0.82** |
| SYNDERc | 2008 | –0.65* | –0.52 | –0.40 | 0.68* | –0.83** |
*,**Significant at the 0.05 and 0.01 probability levels, respectively.
Grain filling combined across years (2006-2007).
Booting, heading, and grain filling combined for 2008.
Water stress levels and water relation parameters using a combined analysis
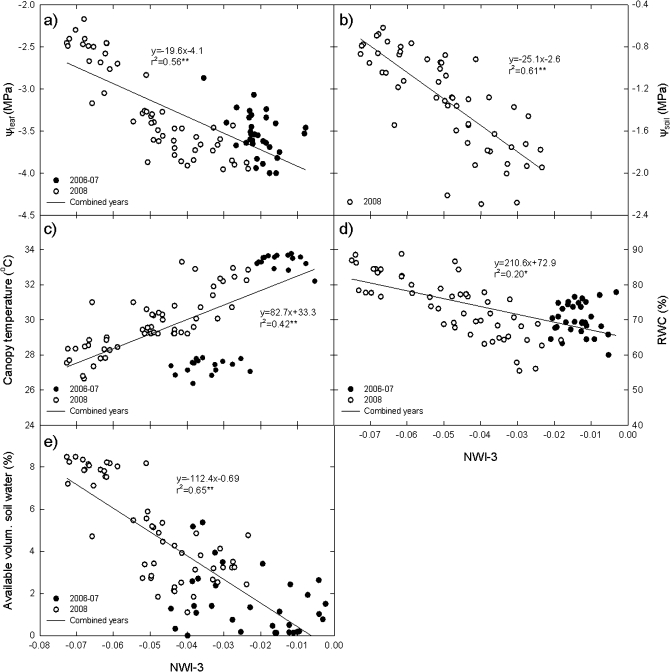
Since the different germplasm sets showed similar associations of WIs with water relations parameters, a combined analysis of all germplasm sets across growing seasons was performed (Fig. 6). The relationship of NWI-3 with all water parameters was strengthened at both the canopy level (ψleaf, RWC, and CT) and at the root level (ψsoil and AVSW).
Fig. 6.
Relationships of the normalized water index 3 (NWI-3) with leaf water potential (ψleaf), soil water potential (ψsoil), leaf relative water content (RWC), canopy temperature (CT), and available volumetric soil water (AVSW) by combining determinations across environments for a subset of sister lines (SBS-I and SBS-II), advanced lines (ALN), and synthetic lines (SYNDER).
When the relationship of NWI-3 with ψleaf was considered for all germplasm sets across growing seasons the association was clearly strong across a wide range of environments (Fig. 6a). Similarly, CT showed a strong relationship with NWI-3 (Fig. 6c) by combining all germplasm sets, but RWC showed a relatively low relationship with NWI-3 across growing seasons (Fig. 6d).
At the soil level, ψsoil and AVSW were highly associated with NWI-3 at diverse water stress levels when all germplasm sets were combined across years and stages (Fig. 6b, e).
Discussion
The potential of water indices to screen canopy water content
The value of spectral reflectance indices to sense plant water status, based on strong absorption by water at specific wavelengths such as 970 nm, has been reported in different crops, conifers, shrubs, and other plant species under water stressed conditions (Table 5). Other spectral reflectance indices based on visible, near, and far infrared regions have also been associated with plant water status parameters (i.e. ψleaf) under different levels of water stress (Table 5). Some of these indices were based on ground-based canopy reflectance to estimate crop water content, while others utilized satellite and aircraft imagery in forest species and farm fields to estimate vegetative water content. The WI (source for the four normalized water indices used in the current study) proposed by Peñuelas et al. (1993) has been widely associated with diverse water relations parameters in a variety of crops (Table 5). WI showed a good association with RWC and ψleaf (r=0.60–0.80) by inducing artificial leaf dehydration of gerbera and pepper plants growing under greenhouse and growth chamber conditions, but WI had weaker associations at moderate water stress (RWC <85% and ψleaf–1.55 MPa) (Peñuelas et al., 1993). In another study, the plant water content of the seedlings of several shrubs and tree species was highly correlated with WI (r=0.61–0.75) when plants were grown in plastic tubes; however, under natural conditions, the association was reduced (r=0.05–0.56) (Peñuelas et al., 1997). Sim and Gamon (2003) studied several plant species (annual and perennial species) grown in natural conditions and found that WI gave a better association with plant water content than did other indices (NDVI, NDWI, and SR). Eitel et al. (2006) also used WI, but it showed a weak relationship with RWC and ψleaf at the leaf and canopy level, compared to NDWI and the maximum difference water index (MDVI) in Populus tree species grown in greenhouse conditions. MDWI [Rmax(1500–1750)–Rmin(1500–1750)]/[Rmax(1500–1750)+Rmin(1500–1750)] was a newly proposed water index that incorporated short-wave infrared wavelengths and was more efficient at detecting changes in plant water status compared with the indices that employ near infrared wavelengths (NDWI and WI) (Eitel et al., 2006).
Table 5.
Spectral water indices and their relationship with water relation parameters in diverse plant species and growth conditions using ground based, aircraft, and satellite spectrometers
| Water index | Parameter related | Growth conditions | Plant species | Comments | Reference |
| WI | RWC and ψleaf | Greenhouse and growth chambers | Gerbera and pepper | Ground-based spectrometer. Artificial leaf dehydration and weaker association at lower RWC <(85%) and ψleaf (–1.55 MPa) (r=0.60–0.80) | Peñuelas et al. (1993) |
| NDWI | Vegetation water content | Field and laboratory | Natural vegetation and irrigated fields | Airborne imaging spectrometer (AVIRIS). NDWI was highly related to the vegetation water content. | Gao (1996) |
| WI | Plant and seedling water content | Plastic tunnels and natural conditions | Shrubs and tree species | Ground-based spectrometer. Weaker association when plants are growing in natural conditions (r=0.05–0.75) | Peñuelas et al. (1997) |
| NDWI, SRWI, and PWI | Plant water status | Natural vegetation and farm fields | Forest and wheat | Satellite spectrometer (MODIS). Simulated models for estimating vegetation water content in relation to leaf thickness, biomass, and leaf are index | Zarco-Tejada and Ustin (2001); Zarco-Tejada et al. (2003) |
| 975, 1200, and 1750 nm for diverse ratios | RWC | Laboratory (leaves collected from trees of urban areas) | Quercus species | Ground-based spectrometer. High relationship between diverse ratios using 975, 1200, and 1750 nm wavelengths | Pu et al., 2003 |
| NDVI, SR, NDVI, and WI | Tissue water content of leaves, fruits, stems, and flowers | Natural vegetation | Annual species and perennial species (vines, shrubs, and tree species)s | Ground-based spectrometer. WI gave better results for estimating tissue water content (r2=0.51) | Sims and Gamon (2003) |
| NDWI and NDVI | Leaf and stem water content | Farm fields | Soybean and corn | Airborne imagery. Vegetation water content according to leaf area index | Anderson et al. (2004) |
| NDWI and NDVI | Leaf water content and ψleaf | Farm field | Corn and soybean | Imagery (Landsat satellite). NDWI resulted better to mapping vegetation water content (r2=0.44–0.68) | Jackson et al. (2004) |
| NDWI, NDVI, WI, and 680–780 red edge band | Plant water content | Experimental field plots | Winter wheat varieties | Ground-based spectrometer. Plant water content was better estimated using a red edge wavelengths (680–780 nm) and ψleaf were better estimated using 970 nm and NDWI (r=0.34–0.75) | Liu et al. (2004) |
| 965–1085 nm, 1192–1282 nm, and others | Leaf water content | Experimental field plots | Wheat | Ground-based spectrometer. 965–1085 nm and 1192–1282 nm gave stronger association with leaf water content | Zhao et al. (2004) |
| NDWI, NDVI, 970, and 1200 nm | Leaf water content and ψleaf | Natural vegetation | Two conifers (Pinus edulis and Juniperus monosperma) | Ground-based spectrometer. Leaf water content and ψleaf were better estimated using 970 nm and MDWI (r2=0.44–0.68) | Stimson et al. (2005) |
| NDWI and WI | RWC and ψleaf | Growth chambers | Populus spp. | Ground-based spectrometer. Excluding ψleaf of –1.6 MPa, high relationship at the leaf level using NDWI | Eitel et al. (2006) |
| WDI | Experimental field plots | Broccoli plants | Ground-based spectrometer. WDI detected differences in canopy water content | El-Shikha et al. (2007) |
NDVI, normalized difference vegetation index; NDWI, normalized difference Water index; MDWI, maximum difference water index; PWI, plant water index; SR, simple ratio; SRWI, simple ratio water index; WI, water index; WDI, water differential index.
In the current study, the 970 nm wavelength, used in the five WIs tested, was clearly sensitive to the water content differences among genotypes over a range of water deficits, showing strong linear relationships with most of the water relations parameters measured (Table 2; Fig. 6). These indices compare the energy absorbed by water at 970 nm with different reference wavelengths (850, 880, 900, and 920 nm), which do not show energy absorption by water (Peñuelas et al., 1997; Babar et al., 2006; Prasad et al., 2007). Overall, the reference wavelength at 880 nm (employed for the NWI-3) resulted a little better than the others (Table 2). The current study is the first to report genetic effects within a species (as far as the authors are aware), suggesting the potential for application of WI in high-throughput screening of genotypes for water relations parameters; this will be discussed subsequently.
Environmental range in which NWI-3 is associated with water relations parameters
The fact that NWI-3 was measured across distinct crop stages and crop cycles, in addition to in different genetic materials, afforded the opportunity to assess the association of NWI-3 with water relations parameters across a broad range of expression. In general, water stress intensified over the course of the crop cycle and there was also significant seasonal variation when comparing crop cycles. This environmental variation in water stress was manifest in the wide range of expression of water relations parameters, and NWI-3 showed relatively strong linear associations with most water relations parameters (Figs 1–6). For example, ψleaf and ψsoil were expressed in the range –2.0 to –4.0 MPa and –0.5 to –2.0 MPa, respectively, and showed linear associations with NWI-3 across this range explaining 60–80% of the variation between the traits, depending on germplasm set. The data presented in this study give a reasonably comprehensive idea of the range of water relations traits and their levels of expression for which NWI-3 can be expected to serve as a proxy in situations where inexpensive or rapid estimation of water status of plants is useful.
NWI-3 and water status parameters
NWI-3 showed strong association with ψleaf which was generally the water relation parameter most consistently associated with NWI-3 (Table 2; Fig. 1). ψleaf determined at night was assumed to approximate the ψsoil of the active root environment for each genotype as plants tend to equilibrate with the soil when demand for water from the atmosphere is negligible (Nobel, 1983). The expression of genetic effects for ψsoil indicates that different genotypes explore distinct soil water profiles. Furthermore, its association with expression of ψleaf (r2=0.52–0.76) and NWI-3 (r2=0.59–0.64) indicates that hydration status during the day could be related to the ability of roots to explore wetter soil profiles (Fig. 5), as opposed to adopting a more conservative water relations strategy. Our results suggest that drought-resistant genotypes maintain a better canopy water content compared with susceptible genotypes across a range of developmental stages (Table 2; Figs 1–6).
RWC, a useful indicator of plant hydration status under water stress (Slatyer, 1967; Chaves et al., 2002), showed low relationship at individual growth stages (Table 2; Fig. 2), but combining growth stages RWC showed significant association with NWI-3 during 2008 and across seasons (Figs 2, 6). RWC has also been associated with other spectral water indices (i.e. WI and NDWI) and specific wavelengths in crops evaluated under water stress (Table 5) (Peñuelas et al., 1993; Pu et al., 2003; Eitel et al., 2006). Boyer et al. (2008) found that barley and wheat leaves absorb excess water (10–15%) as a result of osmotic adjustment during water incubation to obtain full leaf turgidity, thereby overestimating RWC, a source of error that may have affected results in the current study.
The strong relationship between CT and NWI-3 (Fig. 3) and between CT and ψleaf (Fig. 4) of genotypes corroborate the argument that better performance associated with cooler canopies is a function of improved hydration status (Olivares-Villegas et al., 2007) which is associated with roots that access deeper soil profiles (Lopes and Reynolds, 2010). The association between NWI-3 and AVSW at different soil profiles in the current study further supports this (Table 2; Fig. 5). Under soil water deficits, plants close stomata to reduce water loss (Medrano et al., 2002) while resistant plants may develop deeper roots to maintain better gas exchange (transpiration and photosynthesis) and higher growth rates (Passioura, 1983). The association reported here between NWI-3 and AVSW (Fig. 5) suggest its potential application in screening for genotypes that more effectively access water at soil depth (Table 2; Fig. 5).
Genotypic differences in canopy water content
NWI-3 showed a strong relationship with ψleaf at individual growth stages in all three sets of germplasm ALN, SBS-I, and SBS-II (Table 2; Fig. 1). Genetic correlations made using data combined across growth stages in the SBS-II and SYNDER and across growing seasons in ALN and SBS-I, demonstrated that NWI-3 is able to detect genetic differences among the lines evaluated in the present study (Table 4). The significant genetic correlations between NWI-3 and the majority of water relations parameters (as indicated by ψleaf, cooler canopies and the ability more effectively to dry the soil) indicate strong genetic effects (Table 4).
While, in general, NWI-3 was significantly associated with water relations parameters at all growth stages measured, a possible reason for low association at some individual growth stages was the environmental field variation associated with the high water-stress levels. The idea that associations may have been weakened by environmental variance is supported by the fact that when growth stages in SBS-II and SYNDER were combined, the relationships were strengthened. A cross correlation analysis between NWI-3 and ψleaf during two seasons (2006 and 2007) for ALN and SBS-I and during three growth stages (booting, anthesis, and grain filling) for SBS-II and SYNDER also showed strong associations (r ranged from –0.45 to –0.86 for ψleaf, ψsoil, RWC, and AVSW and from 0.41 to 0.87 for CT). The use of NWI-3, or other spectral water indices, to explain genotypic differences for drought resistance in relation to the canopy water content (ψleaf and CT) and root capacity (ψsoil and AVSW) under water-stressed conditions has not previously been reported. Liu et al. (2004) compared three wheat cultivars and combined four irrigation regimes and four nitrogen treatments using a red edge band (740–930 nm) to explain plant water content. Measuring canopy reflectance at six different growth stages (tillering to milking stage), the red edge band showed significant association with plant water content. Under both drought and hot-irrigated environments, deeper root growth permits better access to soil water to maintain better plant water content (Reynolds et al., 2007). McKenzie et al. (2009) found that the root mass of several barley genotypes was associated with subsoil water extraction and similar results have been shown in bread wheat (Lopes and Reynolds, 2010). In our study, WIs indicate improved hydration status in resistant genotypes and their relationship with AVSW at different soil depths (Fig. 5) could indicate an association with root capacity.
The argument that NWI-3 is sensitive to genetic differences in water relations parameters is supported by the results of this study, including phenotypic and genetic correlations within different classes of breeding material (Tables 2, 4; Figs 1–5). Not surprisingly there is also an association of NWI-3 with grain yield in genotypes that maintained better water status (Table 3).
Water indices and other water relations methods
ψleaf is considered the most reliable indicator of plant water status and has been used to evaluate drought resistance among wheat genotypes (Munjal and Dhanda, 2005). Given the strong association between NWI-3 and ψleaf in the present study, the advantages of using NWI-3 to estimate plant water status, instead of the time-consuming method of measuring water potential with Scholander's pressure pump, are self evident (20–30 samples h−1 for ψleaf and 150–200 readings h−1 for WIs). The spectral reflectance method is much more rapid at integrating several leaves on the canopy avoiding cutting leaf samples from plants. Other methods, such as measurement of RWC, are also time-consuming and less integrative when compared with using WIs to estimate water status. NWI-3 integrates dozens of leaves on the canopy and additional parameters can be estimated simultaneously through other spectral indices, such as photosynthetic capacity (NDVI), leaf area index (GNDVI), intercepted radiation (PRI), chlorophyll a/b (RARSa and RARSb), etc (Araus et al., 2001).
The association between CT and NWI-3 (Fig. 3) confirms that CT is also a good indicator of hydration status. In this study, NWI-3 showed a better association with ψleaf and AVSW (r2=0.56–0.81) (Figs. 1, 5) than CT (r2=0.13–0.72). The robustness of the WIs as an indicator of ψleaf at different growth stages (Table 2; Fig. 1) could also indicate its value in irrigation decisions, to avoid water stress at critical growth stages during the cropping season (Koksal, 2008). Irrigation scheduling is an important goal in remote sensing; crop water status information and several indices (NDVI and NDWI) have been proposed for improving this (Jackson, 1986; Jackson et al., 2004). Data presented in this study suggest that NWI-3 may be a more reliable index for application in irrigation scheduling, though confirmation would have to come ultimately from calibration studies to establish threshold NWI-3 values that correspond to the water relations parameters associated with standard irrigation intervals.
Conclusions
The relationship between NWI-3 and ψleaf was generally consistent across years, and across growth stages in unselected recombinant inbred lines, elite advanced lines, and synthetic derivative genotypes under a wide range of water-stressed conditions. Results show a link between hydration status, transpiration rates (cooler canopies), water extraction capacity, and improved yield in drought-resistant genotypes under diverse water stress levels. The argument that genotypes with better canopy water content can access deeper soil layers for water uptake was supported by the association of the NWI-3 with AVSW and ψsoil. In addition, the genetic correlations between NWI-3 and water relations parameters support the idea that NWI-3 is able to distinguish genotypic differences in drought resistance at the canopy and soil level during the crop cycle. NWI-3 offers significant advantages for screening water relation traits since it integrates at the canopy level and can evaluate a large number of genotypes quickly and cheaply, compared with other methods that have been described in the literature.
Acknowledgments
This research was partially funded by The Oklahoma Wheat Commission, International Maize and Wheat Improvement Center (CIMMYT), the National Council of Science and Technology of Mexico (CONACYT), and the Australian Grains Research Development Corporation (GRDC). We are also thankful to Jose Luis Barrios Gonzalez and Vania Tellez Arce for assisting with water potential measurements; and to Marta Lopes and Dan Mullan for reviewing an earlier version of this manuscript.
Glossary
Abbreviations
- ALN
advanced lines
- CIMMYT
International Maize and Wheat Improvement Center
- CT
canopy temperature
- NDVI
normalized difference vegetation index
- NDWI
normalized difference water index
- NWI-1
normalized water index-1
- NWI-2
normalized water index-2
- NWI-3
normalized water index-3
- NWI-4
normalized water index-4
- NWIs
normalized water indices
- RWC
relative water content
- SBS-I
subset of advanced sister lines in the years 2006 and 2007
- SBS-II
subset of sister lines in the year 2008
- SRWI
simple ratio water index
- SYNDER
synthetic derivative lines
- WI
water index
- AVSW
available volumetric soil water
- ψleaf
leaf water potential
- ψsoil
soil water potential
References
- Amani I, Fischer RA, Reynolds MP. Canopy temperature depression association with yield of irrigated spring wheat cultivars in hot climate. Journal of Agronnomy and Crop Science. 1996;176:119–129. [Google Scholar]
- Anderson MC, Neale CMU, Li F, Norman JM, Kustas WP, Jayanthi H, Chavez J. Upscaling ground observations of vegetation water content, canopy height, and leaf area index during SMEX02 using aircraft and Landsat imagery. Remote Sensing of Environment. 2004;92:447–464. [Google Scholar]
- Araus JL, Casadesus J, Bort J. Recent tools for the screening of physiological traits determining yield. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. Mexico, DF: CIMMYT; 2001. pp. 59–77. [Google Scholar]
- Babar MA, Reynolds MP, van Ginkel M, Klatt AR, Raun WR, Stone ML. Spectral reflectance indices as a potential indirect selection criteria for wheat yield under irrigation. Crop Science. 2006;46:578–588. [Google Scholar]
- Boyer JS, James RA, Munns R, Condon T, Passioura JB. Osmotic adjustment leads to anomalously low estimates of relative water content in wheat and barley. Functional Plant Biology. 2008;35:1172–1182. doi: 10.1071/FP08157. [DOI] [PubMed] [Google Scholar]
- Chapman SC. Use of crop models to understand genotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica. 2008;161:195–208. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. How plants cope with drought in the field? Photosynthesis and growth. Annals of Botany. 2002;89:907–916. doi: 10.1093/aob/mcf105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel JUH, Gessler PE, Smith AMS, Robberecht R. Suitability of existing and novel spectral indices to remotely detect water stress in Populus spp. Forest Ecology and Management. 2006;229:170–182. [Google Scholar]
- El-Shikha DM, Waller P, Hunsaker D, Clarke T, Barnes E. Ground-based remote sensing for assessing water and nitrogen status of broccoli. Agricultural Water Management. 2007;92:183–193. [Google Scholar]
- Gao B. NDWI a normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sensing of Environment. 1996;58:257–266. [Google Scholar]
- Gutierrez M, Reynolds MP, Raun WR, Stone ML, Klatt AR. Spectral water indices for assessing yield in elite bread wheat genotypes in well irrigated, water stressed, and high temperature conditions. Crop Science. 2010;50:197–214. [Google Scholar]
- Hanks RJ. Measurement of soil and plant water status. Irrigation Science. 1988;9:253. [Google Scholar]
- Jackson RD. Remote sensing of biotic and abiotic plant stress. Annual Review of Phytopathology. 1986;4:289–297. [Google Scholar]
- Jackson TJ, Chen D, Cosh M, Li F, Anderson M, Walthall C, Doriaswamy P, Hunt ER. Vegetation water content mapping using Landsat data derived normalized difference water index for corn and soybeans. Remote Sensing of Environment. 2004;92:475–482. [Google Scholar]
- Koksal E. Irrigation water management with water deficit index calculated based on oblique viewed surface temperature. Irrigation Science. 2008;27:41–56. [Google Scholar]
- Lage J, Trethowan RM. CIMMYT's use of synthetic hexaploid wheat in breeding for adaptation to rainfed environments globally. Australian Journal of Agricultural Research. 2008;59:461–469. [Google Scholar]
- Liu L, Wang J, Huang W, Zhao C, Zhang B, Tong Q. Estimating winter wheat plant water content using red edge parameters. International Journal of Remote Sensing. 2004;25:3331–3342. [Google Scholar]
- Lopes M, Reynolds MP. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology. 2010;37:147–156. [Google Scholar]
- McKenzie BM, Bengough AG, Hallett PD, Thomas WTB, Forster B, McNicol JW. Deep rooting and drought screening of cereal crops: a novel field-based method and its application. Field Crops Research. 2009;112:165–171. [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulias J, Flexas J. Regulation of photosynthesis of C-3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes JM, Melchinger AE, Reif JC. Novel throughput phenotyping platforms in plant genetic studies. Trends in Plant Science. 2007;12:433–436. doi: 10.1016/j.tplants.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Munjal R, Dhanda SS. Physiological evaluation of wheat (Triticum aestivum L.) genotypes for drought resistance. Indian Journal of Genetics and Plant Breeding. 2005;65:307–308. [Google Scholar]
- Nobel PS. Biophysical plant physiology and ecology. San Francisco, CA: WH Freeman and Company; 1983. [Google Scholar]
- Olivares-Villegas JJ, Reynolds MP, McDonald GK. Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Functional Plant Biology. 2007;34:189–203. doi: 10.1071/FP06148. [DOI] [PubMed] [Google Scholar]
- Passioura JB. Roots and drought resistance. Agricultural Water Management. 1983;7:265–280. [Google Scholar]
- Peñuelas J, Filella I, Biel C, Serrano L, Save R. The reflectance at the 950–970 mm region as an indicator of plant water status. International Journal of Remote Sensing. 1993;14:1887–1905. [Google Scholar]
- Peñuelas J, Isla R, Filella I, Araus JL. Visible and nearinfrared reflectance assessment of salinity effects on barley. Crop Science. 1997;37:198–202. [Google Scholar]
- Prasad B, Carver BF, Stone ML, Babar MA, Raun WR, Klatt AR. Genetic analysis of indirect selection for winter wheat grain yield using spectral reflectance indices. Crop Science. 2007;47:1416–1425. [Google Scholar]
- Pu R, Ge S, Kelly NM, Gong P. Spectral absorption features as indicators of water status in coast live oak (Quercus agrifolia) leaves. International Journal of Remote Sensing. 2003;24:1799–1810. [Google Scholar]
- Reynolds MP, Dreccer F, Trethowan R. Drought adaptive traits derived from wheat wild relatives and landraces. Journal of Experimental Botany. 2007;58:177–186. doi: 10.1093/jxb/erl250. [DOI] [PubMed] [Google Scholar]
- Singh RK, Chaudhary BB. Biometrical method in quantitative genetic analysis. New Delhi: Kalyani Publishers; 1977. [Google Scholar]
- SAS Institute. The SAS system for windows, version 8.2. Cary, NC: SAS Institute; 2001. [Google Scholar]
- Sayre KD, Rajaram S, Fischer RA. Yield potential progress in short bread wheat in Northern Mexico. Crop Science. 1997;37:36–42. [Google Scholar]
- Serrano L, Filella I, Peñuelas J. Remote sensing of biomass and yield of winter wheat under different nitrogen supplies. Crop Science. 2000;40:723–731. [Google Scholar]
- Sims DA, Gamon JA. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: a comparison of indices based on liquid water and chlorophyll absorption features. Remote Sensing of Environment. 2003;84:526–537. [Google Scholar]
- Slatyer RO. Plant–water relationships. New York: Academic Press; 1967. [Google Scholar]
- Stimson HC, Breshears TDD, Ustin SL, Kefauver SC. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote Sensing of Environment. 2005;96:108–118. [Google Scholar]
- Tucker CJ. Remote sensing of leaf water content in the near infrared. Remote Sensing of Environment. 1980;10:23–32. [Google Scholar]
- Ustin SL, Roberts DA, Pinzon J, Jacquemoud S, Gardner M, Scheer G, Castaneda CM, Palacios-Orueta A. Estimating canopy water content of chaparral shrubs using optical methods. Remote Sensing of Environment. 1998;65:280–291. [Google Scholar]
- Zarco-Tejada PJ, Ustin SL. Modeling canopy water content for carbon estimates from MODIS data at land EOS validation sites. Proceeding of the 2001 international geoscience and remote sensing symposium. 2001;Vol. 1 July 9–13. Sydney, Australia, 342–344. [Google Scholar]
- Zarco-Tejada PJ, Rueda CA, Ustin SL. Water content estimation in vegetation with MODIS reflectance data and model inversion methods. Remote Sensing of Environment. 2003;85:109–124. [Google Scholar]
- Zhao CJ, Zhou Q, Wang J, Huang WJ. Band selection for analysing wheat water status under field conditions using relative depth indices (RDI) International Journal of Remote Sensing. 2004;25:2575–2584. [Google Scholar]