Abstract
Non-syndromic thoracic aortic aneurysms and dissections (TAADs) are inherited in an autosomal dominant manner in ~20% of cases. Familial TAAD is genetically heterogeneous and four loci have been mapped for this disease to date, including a locus at 16p for TAAD associated with patent ductus arteriosus (PDA). The defective gene at the 16p locus has recently been identified as the smooth muscle cell (SMC)-specific myosin heavy chain gene (MYH11). On sequencing MYH11 in 93 families with TAAD alone and three families with TAAD/PDA, we identified novel mutations in two families with TAAD/PDA, but none in families with TAAD alone. Histopathological analysis of aortic sections from two individuals with MYH11 mutations revealed SMC disarray and focal hyperplasia of SMCs in the aortic media. SMC hyperplasia leading to significant lumen narrowing in some of the vessels of the adventitia was also observed. Insulin-like growth factor-1 (IGF-1) was upregulated in mutant aortas as well as explanted SMCs, but no increase in transforming growth factor-β expression or downstream targets was observed. Enhanced expression of angiotensin-converting enzyme and markers of Angiotensin II (Ang II) vascular inflammation (macrophage inflammatory protein-1α and β) were also found. These data suggest that MYH11 mutations are likely to be specific to the phenotype of TAAD/PDA and result in a distinct aortic and occlusive vascular pathology potentially driven by IGF-1 and Ang II.
INTRODUCTION
Aortic aneurysms and dissections are the major diseases affecting the aorta and a leading cause of morbidity and mortality in the USA (1,2). Both aneurysms and dissections are classified on the basis of their anatomic location. Thoracic aortic aneurysms and aortic dissections (TAADs) affect the ascending aorta and are inherited in an autosomal dominant manner in up to 20% of patients (3,4). A majority of patients with a family history of TAAD do not have a known syndrome associated with aneurysm formation, such as Marfan syndrome or Loeys–Dietz syndrome (3,5). Additionally, familial TAAD is genetically heterogeneous and four loci have been mapped for non-syndromic familial TAAD to date: TAAD1, FAA1, TAAD2 and a locus at 16p for TAAD associated with patent ductus arteriosus (PDA) (6–9). The gene at the TAAD2 locus on 3p24–25 has been identified as transforming growth factor beta-type II receptor (TGFBR2), implicating the TGF-β pathway in aortic disease (10). More recently, the defective gene at the 16p locus has been identified as smooth muscle cell (SMC)-specific myosin heavy chain (MYH11) through the detection of mutations in two families with TAAD and PDA, suggesting that a viable contractile machinery in SMCs is also essential to prevent aortic disease (11).
This study sought to define the frequency and spectrum of familial thoracic aortic disease resulting from MYH11 mutations and investigate the pathological changes and disease pathways associated with MYH11 mutations. Unlike the two previously reported mutations resulting in in-frame deletions in the coiled–coil region of MYH11, we found mis-sense point mutations in the gene in two families with TAAD/PDA. In addition to regions of cell loss and proteoglycan accumulation typical of medial degeneration, we observed SMC disarray and focal regions of hyperplasia of the SMCs in the aortic media in aortas from patients carrying MYH11 mutations. Increased insulin-like growth factor-1 (IGF-1), angiotensin-converting enzyme (ACE) and macrophage inflammatory protein-1α and β (MIP-1-α and MIP-1-β) expression were found in the aorta and explanted aortic SMCs of a patient with an MYH11 mutation implicating IGF-1 and Ang II in the disease pathology, although no evidence of increased TGF-β expression or signaling was found. Our data further delineate the clinical phenotype associated with MYH11 and provide evidence that a subset of aortic disease is driven by increased IGF-1 and Ang II rather than increased TGF-β signaling.
RESULTS
Genetic analysis
Probands from three TAAD families with one or more members with PDA were screened for MYH11 mutations, along with probands from 93 unrelated TAAD families without PDA (Fig. 1A). Sequencing revealed MYH11 alterations in the probands from two of the three TAAD/PDA families: two closely linked missense alterations, L1264P (3791T > C) and R1275L (3824G > T), in the coiled–coiled domain in family TAA027 and the missense alteration R712Q (2153C > T), in the MYH11 ATPase head region in TAA069 (based on Genbank accession #s NM_002474, NM_022844) (Fig. 1A). These alterations segregated with the TAAD and/or PDA phenotype in the families and were not present in 360 control chromosomes. Co-segregation of the two alterations in family TAA027 suggests that the mutations are in linkage disequilibrium. No disease causing MYH11 variants were identified in the other 94 families analyzed.
Figure 1.
TAAD/PDA pedigrees with MYH11 mutations and clinical imaging. (A) Affected members of the TAA027 family carry two closely linked missense alterations, L1264P (3791T > C) and R1275L (3824G > T), in the coiled–coil domain, and those of the TAA069 family carry the missense alteration, R712Q (2153C > T), in the MYH11 ATPase head region. (B) MRA three-dimensional reconstruction with gadolinium contrast-enhanced angiogram of thoracic aorta of TAA027:III:2 obtained with a SENSE factor of 4. Arrows indicate location of the aneurysm involving the ascending aorta and sparing the sinuses of Valsalva. (C) Presence of greatly increased vasa vasorum noted at surgery.
The proband in family TAA027, individual TAA027:II:3, a 37-year-old Caucasian woman, presented with a chronic descending aortic dissection and PDA. She underwent a thora-coabdominal aortic aneurysm repair, extent I, and died suddenly 6 months later because of a type A dissection (Fig. 1A). Her father had a history of PDA repair at the age of 30 and died suddenly at 34 years after experiencing chest pain. The paternal grandfather also died suddenly at the age of 30 years of unknown causes. The proband’s sister’s ascending aortic dilatation was being monitored without beta-blocker therapy, when she experienced a type A dissection at 48 years; her aortic root was 4.4 cm at the time of dissection. On detailed examination, she had livedo reticularis (LR) on her legs. Her 22-year-old son’s (individual TAA027:III:2 in Fig. 1A) ascending aorta distal to the sinuses of Valsalva enlarged from 4.1 to 4.7 cm in 6 months, prompting surgical repair (Fig. 1B). The aneurysm was noted to be highly vascularized at the time of surgery (Fig. 1C). There was no history of hypertension in the affected family members.
The proband in family TAA069, individual TAA069:II:1 (Fig. 1A), had his ascending aorta emergently repaired after an acute type A dissection at the age of 39 years. Owing to complications from his chronic dissection, he also required an ascending aorto-to-abdominal aortic bypass graft. His father presented initially with bilateral narrowing of the superficial femoral arteries at the age of 36 years and underwent bilateral femoral popliteal bypass. He subsequently had an acute myocardial infarct (MI) at the age of 47 years and underwent coronary artery bypass surgery. At the age of 50 years, he underwent aortobifemoral bypass grafting for continued arterial occlusive disease. At the age of 56 years, he had a type B dissection of his aorta and was treated medically. He died of congestive heart failure at the age of 64 years. He had diabetes and a history of smoking. The proband’s brother had a PDA repaired at the age of 1 month and he has a normal sized aorta by echocardiogram at 40 years. Second brother died at the age of 43 years because of acute MI. The proband’s daughter is 3 years of age and has a PDA without aortic enlargement. Individuals carrying MYH11 mutations and presenting with PDA were not born prematurely and had no facial features suggestive of Char syndrome (MIM no. 169100).
Molecular basis of dysfunction caused by the MHY11 mutations
Cyclic interaction of the myosin motor with actin filaments, fueled by ATP hydrolysis, leads to SMC shortening and contractile force generation (12,13). Smooth muscle myosin consists of two heavy chains and four light chains. The long C-terminal tails of heavy chains dimerize to yield an α-helical coiled–coil. The N-terminus harbors the motor domain (MD), containing both ATP-and actin-binding sites, which together with the light chains form two globular heads (cross-bridges). The tail portion functions as a lever arm that translates conformational changes within the MD into ‘rowing’ motions by which myosin moves actin (14). The SH1 α-helix, which functions as a joint between MD and the converter/lever arm unit, is key to force development (Fig. 2A–C). Both biochemical and structural studies illustrate that the SH1 helix undergoes conformational changes during ATP hydrolysis (15). Arg-712, invariant in all type II myosins, is part of this α-helix and its guanidinum side chain entertains two key hydrogen bonds with the backbone carbonyl groups of Ala-91 and Leu-93. The R712Q mutation will introduce a shorter carboxamide side chain that is incapable of mediating these interactions, thus destabilizing the SH1 helix and disabling communication between MD and the converter/lever arm module. Curiously, Arg-712 of MYH11 is paralogous to Arg-705 in MYH9 and an R705H mutation in the latter leads to non-syndromic hereditary deafness (DFNA17) (16). More interestingly, introducing this substitution within the SH1 helix of the homologous Dictyostelium myosin II perturbs cross-bridge elasticity, but not the ATPase activity (17). Analogously, an Arg mutation in the converter domain causes familial hypertrophic cardiomyopathy (HCM) characterized by increased fiber stiffness (18). Taken together, our results indicate that the aortic phenotype observed in patients with the MYH11 R712Q mutation is most likely a consequence of diminished myosin motor elasticity. CLUSTALW analysis confirms conservation of this residue in all available normal orthologs (Fig. 2D).
Figure 2.
Structural analysis of the MYH11 mutations identified in this study. (A) Three-dimensional structure of vertebrate smooth muscle myosin MD bound to Mg-ADP- (PDB ID: 1BR2). SH1 and SH2 helices are shown in red and pink, respectively. The converter domain attached to the C-terminus of the SH1 helix is presented in green; the remainder of the structure is shown in gray. (B) A close-up view shows detail of the structural elements highlighted. Arg-712 (red) is located near the end of SH1 helix and its hydrogen-bonding interactions with the neighboring backbone carbonyl groups are shown using dashed lines. The values adjacent to these lines indicate H-bond distances (in Å). (C) Unwinding of the SH1 helix in the scallop myosin S1 structure complexed with Mg-ADP. Note that the converter domain occupies a completely different orientation when compared with the original structure depicted in the middle panel. In addition, the distance of separation between the two reactive thiol groups (C701 and C711, marked by blue asterisks) is reduced to ~7 from ~18 Å in the configuration shown in (B). (D) CLUSTALW alignment of MYH11 orthologs showing conservation of Arg-712 across species. This residue is evolutionally conserved among all species examined, and alteration of the equivalent residue in MYH9 causes hereditary deafness (DFNA17). (E) COILS output showing significantly decreased probability of coiled–coil formation when the L1264P mutation is introduced in the normal protein sequence.
Although similar structural details are unavailable for the region encompassing Leu-1264, analysis of the 30 amino this region using the COILS program supports the conclusion that the introduction of a helix breaking residue (proline) at this position disrupts coiled–coil formation, whereas R1275L does not impact the probability of coiled–coil formation (Fig. 2E and F). As L1264P is located in the regulatory region of MYH11, it is unlikely to perturb the catalytic activity of the myosin motor. Instead, an unstable coiled–coil region is likely to interfere with its function in scenarios like protein–protein interactions.
Pathology associated with aneurysms
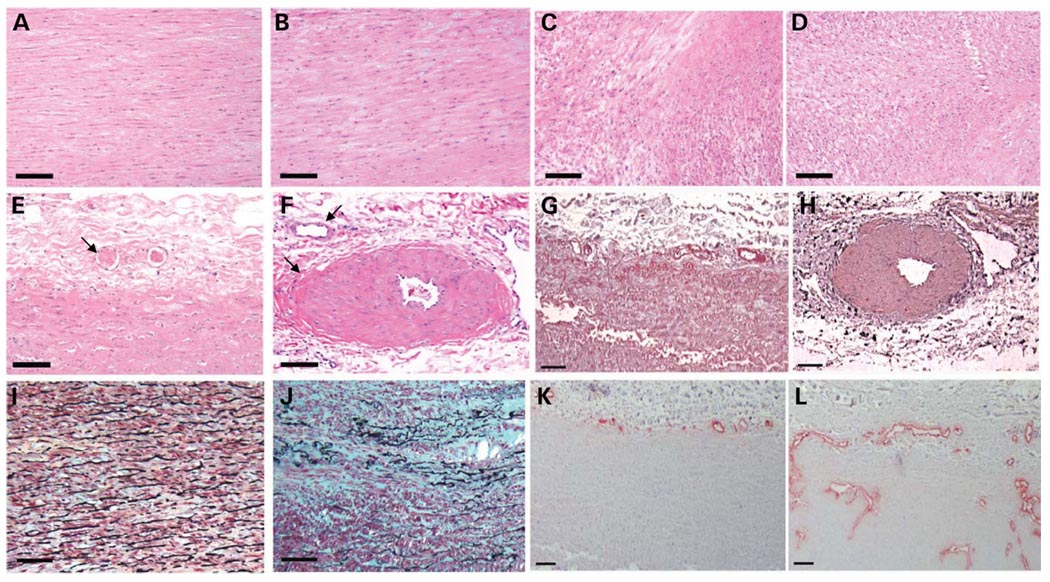
Haemotoxylin and eosin (H&E) staining of aortic tissue from two patients revealed areas of focal medial degeneration with increased proteoglycans and decreased elastic fibers and SMCs, along with areas of increased SMCs. These areas of hyperplasia of the medial SMCs in both patients were remarkable for a lack of structured orientation parallel to the lumen of the aorta, as typically seen in the aorta from controls and Marfan syndrome patients (Fig. 3A–D). Instead, the SMCs were randomly oriented with respect to one another and did not appear to be enlarged. The pathology of the aorta from TAA027:III:2 was also remarkable for focal fibromuscular dysplasia of the arteries in the vasa vasorum when compared with controls; no adventitia was available on the two H&E slides from TAA069:II:1 (Fig. 3E and F). Smooth muscle α-actin staining confirmed that the fibromuscular dysplasia was due to hyperplasia of the SMCs (Fig. 3G and H). Movat pentachrome staining of the aorta from TAA027:III:2 confirmed increased proteoglycans characteristic of medial degeneration but no evidence of increased collagen in the patient’s aorta (Fig. 3I and J). In addition, staining endothelial cells with an anti-von Willebrand factor antibody demonstrated substantially increased vascularity in the adventita and also showed that the vasa vasorum penetrated into the media of the patient’s aorta, but not in the control aorta (Fig. 3K and L).
Figure 3.
Aortic pathology associated with MYH11 mutations. H&E staining of aortic media from (A) control, (B) Marfan patient and two MYH11 patients [(C) TAA069:II:1 and (D) TAA027:III:2] shows regions of SMC disarray and hyperplasia in the patients with MYH11 mutations. H&E staining of the vasa vasorum showed normal vessels in the control (E, arrow), whereas some vessels in the patient vasa vasorum exhibit fibromuscular dysplasia due to SMC hyperplasia in the vessels and others appear normal (F, arrows). α-actin staining of the vasa vasorum confirms that the fibromuscular dysplasia in patient TAA027:III:2 (H) is due to SMC hyperplasia and is absent in the control (G). Movat staining of aortic media from a control (I) and TAA027:III:2 (J) shows medial degeneration characterized by proteoglycan accumulation (stained blue), loss and fragmentation of elastic fibers (stained black), no collagen accumulation (stained yellow) and areas of SMC (stained red) loss in patient when compared with control aorta. von Willebrand factor staining of endothelial cells shows increased vasa vasorum penetrating deeper into the medial layer in TAA027:III:2 (L) compared with control (K). Scale bar = 100 µm.
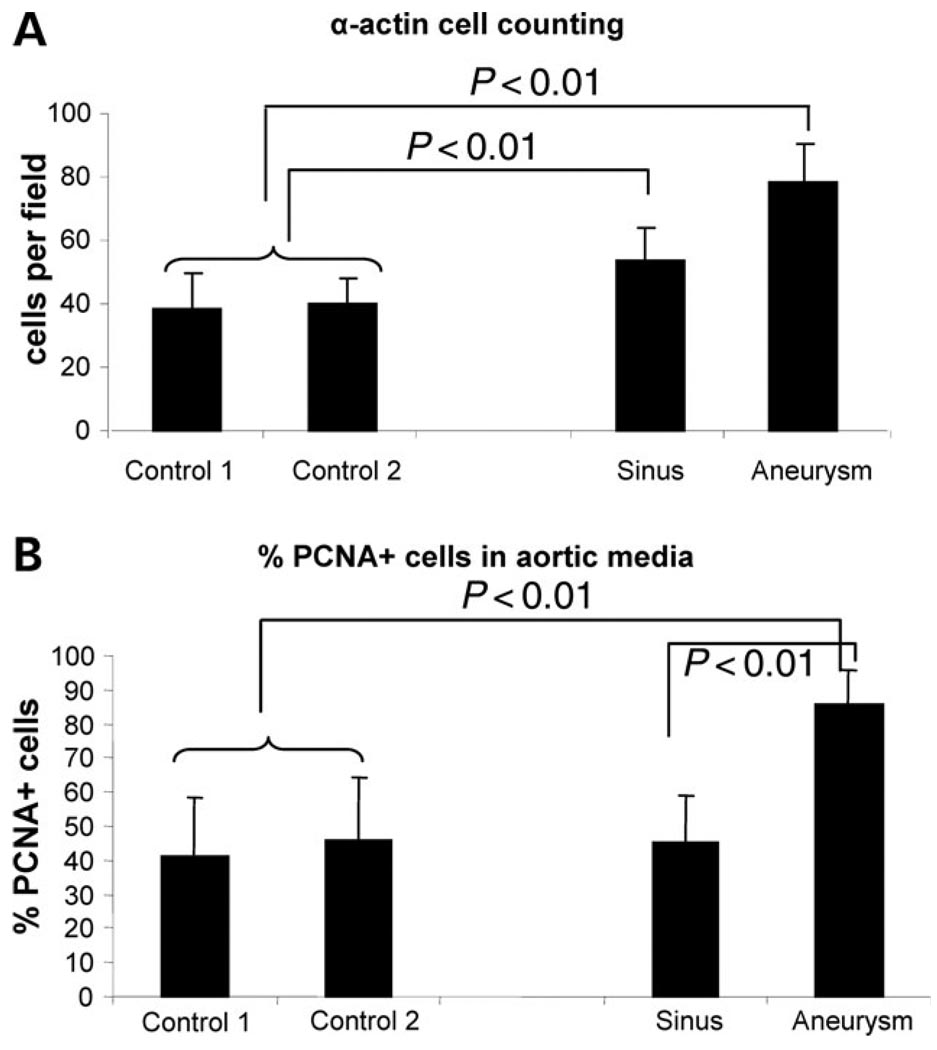
Despite some regions of SMC loss typical of medial degeneration, hyperplasia of the SMCs in the aortic media from patient TAA027:III:2 was demonstrated by counting α-actin positive cells in the aneurysms and the sinuses of Valsalva and comparing these results with α-actin positive cells in two control aortas (Fig. 4A) (P < 0.01). In addition, the percentage of proliferating cell nuclear antigen (PCNA)+ cells in the aneurysm was significantly higher in the aneurysm than in the controls (P < 0.01), indicating that SMCs in the patient’s aorta were more proliferative more rapidly than those in the control aorta (Fig. 4B).
Figure 4.
Hyperplasia and increased cell proliferation in MYH11 mutant aneurysms. (A) The number of α-actin positive cells per field is significantly higher (P < 0.001) in the aortic aneurysm and the sinuses of Valsalva when compared with two control aortas. (B) PCNA staining shows increased proliferation in the aneurysm media. The percentage of proliferating (PCNA positive) cells in the medial layer from the aortic aneurysm is significantly higher (P < 0.001) than that observed in two control aortas or the sinuses of Valsalva from the aneurysm patient.
Analysis of growth factors
The observed SMC hyperplasia led to an investigation of known SMC mitogen production by these cells, specifically IGF-1, PDGF-B and genes comprising the renin–angiotensin system (RAS). The TGF-β pathway was also investigated because increased TGF-β signaling has previously been implicated in aortic disease (5,19). Quantitative RT-PCR (qPCR) analysis of explanted SMCs from an MYH11 patient (TAA027:III:2) and two age-matched control SMCs showed that IGF-1 expression was significantly increased in the explanted patient SMCs when compared with two controls (P < 0.02). The mRNA expression of PDGF-B and TGF-β1 was similar to control SMCs (Fig. 5A). Of the genes in the RAS pathway [renin, angiotensinogen, ACE and the type I receptor (AT1R)] examined, only ACE was significantly upregulated in patient SMCs (Fig. 5B). Aortic tissue derived from the patient demonstrated increased staining for IGF-1 in the media and the vasa vasorum when compared with control aortas (Fig. 5C–F), whereas there was no increase in expression or staining of connective tissue growth factor (CTGF) or plasminogen activator inhibitor 1 (PAI-1) and markers of increased TGF-β signaling (data not shown) (20).
Figure 5.
Increased IGF-1 expression is present in cells and tissues from patients with MYH11 mutations. (A) Quantitative real-time PCR analysis shows a significant increase in IGF-1 expression in SMCs from TAA:027:III:2, compared with two controls, with no significant change in the patient SMC expression of PDGF-B and TGF-β1. (B) Quantitative realtime PCR analysis of the RAS components renin (REN), angiotensinogen (AGT), angiotensin-converting enzyme (ACE) and the type I angiotensin II receptor (AT1R) shows a significant increase in ACE in the patient SMCs. IGF-1 immunohistochemistry shows increased IGF-1 staining in the aortic media (D) as well as vessels of the vasa vasorum (F) of TAA027:III:2 when compared with control media (C) and vasa vasorum (E). Scale bar = 100 µm.
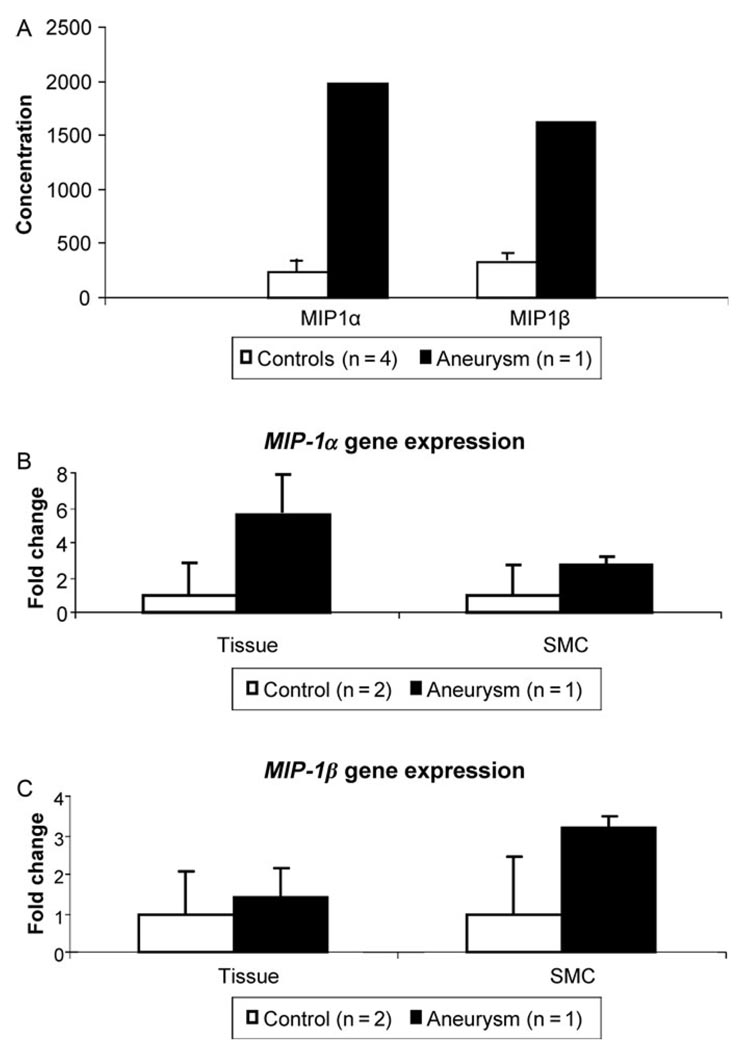
Other cytokines that can drive angiogenesis and hyperplasia were surveyed using organ culture and the Luminex cytokine platform. When the patient aorta was compared with four control aortas, the most striking changes occurred in the macrophage inflammatory proteins α and β (MIP-1α and -β), with 8.3-fold increase in MIP-1α expression and 4.9-fold increase in MIP-1β observed in the patient aorta (Fig. 6A). The increase in MIP-1α and β was confirmed by qPCR using aortic RNA (Fig. 6B and C). Evaluation of the number of macrophages (CD68+) and T-lymphocytes (CD3+) through immunostaining did not demonstrate any significant increase in these cells in patient aorta when compared with control aorta (data not shown), suggesting that cells other than macrophages and T-lymphocytes were producing the increased MIP-1α and β in the diseased aorta. Subsequent assessment of the expression of MIP-1α and β in explanted SMCs from the patient showed increased expression levels of these two genes when compared with two control SMCs (Fig. 6B and C).
Figure 6.
Increased MIP-1α and MIP-1β expression in MYH11 mutant aorta and SMCs. (A) Analysis of cytokines by organ culture analysis shows a significant increase in the secretion of chemokines MIP-1α and MIP-1β in the MYH11 patient aorta when compared with four control aortas. (B and C) Quantitative real-time PCR analysis shows a similar trend for increased MIP-1α and MIP-1β expression in aortic tissue as well as SMCs from the patient, compared with controls.
DISCUSSION
This study is the first to demonstrate that MYH11 mutations are a rare cause of familial TAAD. At the same time, we confirm that MYH11 are a common cause of familial TAAD associated with PDA. Two of three families with TAAD in conjunction with PDA were found to carry novel missense point mutations in MYH11, whereas none of the 93 families with familial TAAD alone was found to have mutations in this gene. By structural analysis, the mutations found in the TAAD/PDA families are predicted to be deleterious to protein function. Similar to two previously reported families with MYH11 mutations, we observed variable expressivity of the mutant gene in these families, with some family member presenting with TAAD in conjunction with PDA, some with PDA or some with TAAD alone. In addition, similar to our observations with TGFBR2 mutations (10), decreased penetrance of the mutation was observed in some adults, who had no known cardiovascular disease (Fig. 1A). Finally, the aneurysm in patients with MYH11 mutations involved the ascending aorta, a location where a majority of force is placed on the aortic wall with each cardiac contraction and spared the sinuses of Valsalva, which is the location of aneurysms in Marfan patients and TAAD patients with TGFBR2 mutations (Fig. 1B) (21).
Interestingly, two individuals with MYH11 mutations from TAA069 presented with premature occlusive arterial disease, resulting in coronary artery disease and peripheral vascular disease. Additionally, one individual from TAA027 who carried an MYH11 mutation was determined to have persistent and severe LR, occlusive disease. In addition to this evidence of occlusive disease in TAA027 and TAA069, death due to strokes occurred in three individuals who were carriers of MYH11 mutations in a previously reported family (11), raising the intriguing possibility that occlusive vascular disease is associated with MYH11 mutations.
It is notable that mutations in the paralogous cardiac-specific myosin heavy chain gene (MYH7) are responsible for ~30–40% of HCM, an autosomal dominant disease characterized pathologically by the presence of myocyte disarray, myocyte hypertrophy and interstitial fibrosis (22,23) Indeed, myocyte disarray, i.e. the presence of abnormally shaped cells that are disoriented with respect to one another, is considered a pathological hallmark of HCM (24,25). These pathological findings in HCM are thought to occur secondary to the activation of specific trophic and mitotic factors in the heart, and studies demonstrating increased IGF-1 and TGF-β1 levels in HCM implicate these growth factors in disease progression (26,27). Our data suggest that MYH11 mutations result in a similar pathology in SMCs forming the medial layer of blood vessels.
Our data demonstrate a specific aortic pathology associated with MYH11 mutations, which is distinct from pathology previously described for thoracic aneurysms. The pathological hallmark of HCM, myocyte disarray, is similar to the SMC disarray observed in the aortic media in two unrelated patients with MYH11 mutations. In contrast to HCM, there was no evidence of hypertrophy of aortic SMCs, but rather focal hyperplasia of SMCs was observed, both in the aortic media and vasa vasorum of patients with MYH11 mutations. In addition, there were areas of SMC loss and typical medial degeneration, which might be postulated to represent a later stage. Given that the aorta we were able to assess in detail had been prophylactically rather than emergently repaired in a young individual, overall greater numbers of SMCs were observed. Although terminally differentiated cardiac myocytes lack replicative capacity and respond to stress either by an increase in size leading to hypertrophy or by premature death leading to fibrosis, SMCs can switch between differentiated and prolif-erative phenotypes in response to intracellular or extracellular cues. Our data suggest that SMCs proliferate rather than hypertrophy as a result of MYH11 mutations, implying that although paralogous genes are altered between TAAD and HCM, there is a cell type-specific response to these alterations. Therefore, although some pathological alterations are similar, e.g. cellular disarray, there are differences observed between cardiomyocytes and SMCs, resulting from programmed responses in these cells.
Further similarities between HCM due to MYH7 mutations and aortic disease due to MYH11 mutations may be drawn when the stress induced pathways leading to disease are examined in the MYH11 patient. Previous data indicate that expression of mutant sacromeric proteins in cardiomyocytes in HCM impairs their mechanical performance, leading to compensatory hypertrophy (28). Growth factors, including IGF-1 and TGF-β1, are upregulated in the myocardium of HCM patients and may contribute to the hypertrophy and interstitial fibrosis (27). Ang II produced by a local RAS has been implicated in both promoting myocyte hypertrophy and increasing cardiac fibrosis, and its antagonist, losartan, inhibits load-induced cardiac hypertrophy and reverses cardiac interstitial fibrosis (29).
Increased IGF-1 expression and production were found in the aortic media, vasa vasorum and explanted SMCs from a patient with an MYH11 mutation. IGF-1 expression is known to increase in response to cyclic stretch, possibly as an adaptive response to maintain vessel tone and function in response to impaired contraction (30). Increased IGF-1 intiates two signaling pathways in SMCs—the phosphoinositide-3 kinase (PI3K) pathway leading to increased production of contractile proteins and the extracellular signal-regulated kinase (ERK) pathway, leading to increased proliferation. (31). The focal nature of the SMC hyperplasia suggests that the presence of the mutant MYH11 protein alone is not sufficient to induce hyperplasia and a second, as yet unknown, event is needed to elicit this response.
The increased ACE expression by explanted MYH11 mutant SMCs suggests that increased tissue production of Ang II may occur as part of disease process, augmented by the increased blood flow associated with the increased vascularity observed. Cytokine profiling and expression analysis of MYH11 mutant aortic tissue and SMCs indicated significant upregulation of MIP-1α and β. MIP-1α has also been shown to be upregulated in aortic explants from a mouse model of Ang II infusion, suggesting that MIP-1α is downstream of Ang II signaling (32). Thus, the ACE upregulation seen in mutant aortic SMCs appears to functionally result in enhanced tissue response to Ang II. There was no evidence of increased TGF-β1 production or signaling in the aorta carrying the MYH11 mutation, as assessed by the expression of the TGF-β responsive genes PAI-1 and CTGF. However, substantial crosstalk between the Ang II and TGF-β signaling pathways makes it difficult to assess the individual impact of these candidate pathways in this study.
Our findings further confirm the association of PDA with MYH11 mutations. Current data suggest that closure of the ductus arteriosus requires several events, including a postnatal constriction of the ductus caused by SMC contraction, partly in response to declining prostaglandin levels, followed by formation of the intimal cushions by SMC migration and endothelial/SMC proliferation (33). This remodeling results in permanent ductus closure. The MYH11 mutations identified are predicted to decrease SMC contractility, thereby disrupting proper ductal closure. In addition, the aortic medial SMC disarray associated with MYH11 mutations suggests compromised SMC alignment and migration in response to cellular signals. Additional studies are required to determine the frequency of MYH11 alterations in children with isolated PDA and whether these mutations predict adult-onset vascular disease in these children.
The paucity of aortic samples available from patients with MYH11 mutations for pathological and functional analyses is an unavoidable limitation of this study. However, our observations of SMC disarray are based on findings in the ascending aorta of two unrelated patients, and the pathological parallels with HCM aid in validating these findings. The observation of focal fibromuscular dysplasia associated with these mutations is not only supported by the increased thickness of arteries and the presence of premature occlusive vessel diseases including CAD and LR in patients with MYH11 mutations in this study, but also by the increased occurrence of strokes in previously reported families with MYH11 mutations. Further studies are required to verify and fully understand the molecular consequences of MYH11 mutations leading to aortic and potentially other vascular diseases including strokes and CAD.
MATERIALS AND METHODS
Patient material
This study was approved by the Institutional Review Board at the University of Texas Health Science Center at Houston. Families with multiple members with ascending thoracic aortic aneurysms and/or aortic dissections were recruited for research. Clinical information and family histories were collected from medical records and interviews conducted by a research nurse, genetic counselor or geneticist. Blood or buccal cell samples and medical records pertaining to cardiovascular disease were collected after consent was obtained. Family members underwent imaging (echocardiograms, computed tomography (CT) or magnetic resonance angiogram (MRA)) to assess heart structure and function and aortic root dimensions. Aortic diameters at the sinuses of Valsalva, the supra-aortic ridge and the ascending aorta were measured from cross-sectional echocardiography images in the parasternal long-axis orientation and plotted against nomograms derived from normal individuals’ measurements. Individuals were considered affected if they had a true aneurysm or dissection of the thoracic aorta or pre-aneurysmal dilation of the ascending aorta. DNA was obtained from 100 individuals without known aortic disease for use as controls.
Aortic tissue
H&E-stained slides were available from one MYH11 patient (TAA069:II:1). Full-thickness aortic wall specimens were collected at the sinuses of Valsalva and the aneurysm at maximal dilatation from another patient heterozygous for an MYH11 mutation undergoing prophylactic aneurysm repair (TAA027:III:2). Control ascending aortas were obtained from two individuals who died because of unrelated causes. Specimens were immediately transferred to the laboratory in cold Waymouth MB 752/1 medium (Invitrogen Corporation, CA, USA). Protein and RNA were extracted from frozen aortic tissue. Representative sections of the aortic aneurysm and sinuses of Valsalva were paraffin embedded and slides were prepared for immunostaining and histopathology. SMCs were explanted as described previously (34).
Mutational analysis
Genomic DNA was extracted from samples (peripheral blood or buccal cells) according to the manufacturer’s protocol using the PureGene genomic DNA isolation kit (Gentra Systems, MN, USA). Mutational analysis of the MYH11 gene was performed by bidirectional direct sequencing of amplified genomic DNA fragments using intron-based exon specific primers (primer sequences and amplification conditions are available on request). Sequencing was performed using Big Dye chemistry and products were analyzed on the ABI 3130 Genetic Analyzer (Applied Biosystems, CA, USA). Sequence was inspected using software from the Staden package and also visually curated. BLAST alignment was used to identify sequence alterations. All alterations were verified by a second-independent amplification and sequencing reaction. DNA from family members of probands with mutations was sequenced to determine whether the mutation was present.
Secondary structure analysis
The atomic coordinates of myosin crystal structures were obtained from the Protein Data Bank. Structure superposition and visualization were performed with the program O (35). Non-bonded and hydrogen-bonded contacts were determined using HBPLUS (36). Figures were generated using PyMOL (www.pymol.org) (37). The online program COILS was used for the prediction of coiled–coil regions in normal and mutant MYH11 protein (38).
Immunostaining
The antibodies used in this study are listed in Table 1. Biotin-conjugated antibodies (Vector Laboratories, Inc., CA, USA) were used as secondary antibody. An avidin-alkaline phosphatase–fast red reagent or the peroxidase-3,3′-diaminobenzidme tetrahydrochloride (DAB) system was used to visualize the antibody stains. The number of SMCs in the vessel wall was evaluated by immunohistochemistry for α-actin. Infiltrating inflammatory cells and α-actin positive SMCs were counted in 10 contiguous high-power (400×) fields under a fluorescent microscope by two independent observers. To obtain an index of cell proliferation, the expression of PCNA was measured by immunocytochemistry using a kit (Zymed, CA, USA) and the manufacturer’s protocol. PCNA+ cell nuclei were counted in 10 high-power fields (400×). A ratio of PCNA+ nuclei to total nuclei in each field was calculated and expressed as a percentage.
Table 1.
Antibodies used for immunohistochemistry analyses
| Antibody | Cell type | Source | Manufacturer | Dilution |
|---|---|---|---|---|
| SMC α-actin | SMC | Mouse monoclonal | Sigma | 1:200 |
| IGF-1 | Various | Rabbit polyclonal | Santa Cruz Biotechnology | 1:25 |
| PCNA | Proliferating cells | Mouse monoclonal | Zymed | Pre-diluted |
| CD3 | Pan T cell | Mouse monoclonal | DAKO | 1:200 |
| CD68 | Macrophage | Rabbit polyclonal | Santa Cruz Biotechnology | 1:50 |
| vWF | Endothelial | Rabbit polyclonal | DAKO | 1:200 |
Detection and quantification of cytokine levels
In vitro analysis of cytokine expression was performed using strips of ascending thoracic aorta, as previously described (32). It has been confirmed that the cytokines measured in aortic explants by this method are due to local production and not a vascular leak. Results are expressed in pg/mL.
Quantitative RT-PCR (qPCR) analysis
qPCR analysis was carried out using pre-designed TaqMan assays from Applied Biosystems using manufacturer’s protocols and reagents and run on an ABI Prism 7700 Sequence Detection System (Applied Biosystems).
ACKNOWLEDGEMENTS
We would like to thank the patients and their families for participating in these studies. We are also indebted Dr Sudha Veeraraghavan for help with generating the structure panels and to Dr Jeunemaitre and Dr Zhu at the Assistance Publique Hopitaux de Paris for sharing information about polymorphisms in MYH11. S.R. is a few scholar. D.M.M. is a Doris Duke Distinguished Clinical Scientist.
FUNDING
National Institutes of Health (RO1 HL62594 to D.M.M.), P50HL08379-01, MO1RR02558 (General Clinical Research Center); S10 RR19186; the Texgen Research Foundation.
Footnotes
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lilienfeld DE, Gunderson PD, Sprafka JM, Vargas C. Epidemiology of aortic aneurysms: I. Mortality trends in the United States, 1951 to 1981. Arteriosclerosis. 1987;7:637–643. doi: 10.1161/01.atv.7.6.637. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay J., Jr Diagnosis and treatment of diseases of the aorta. Curr. Probl. Cardiol. 1997;22:485–542. doi: 10.1016/s0146-2806(97)80004-7. [DOI] [PubMed] [Google Scholar]
- 3.Biddinger A, Rocklin M, Coselli J, Milewicz DM. Familial thoracic aortic dilatations and dissections: a case control study. J. Vasc. Surg. 1997;25:506–511. doi: 10.1016/s0741-5214(97)70261-1. [DOI] [PubMed] [Google Scholar]
- 4.Coady MA, Davies RR, Roberts M, Goldstein LJ, Rogalski MJ, Rizzo JA, Hammond GL, Kopf GS, Elefteriades JA. Familial patterns of thoracic aortic aneurysms. Arch. Surg. 1999;134:361–367. doi: 10.1001/archsurg.134.4.361. [DOI] [PubMed] [Google Scholar]
- 5.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 6.Guo D, Hasham S, Kuang SQ, Vaughan CJ, Boerwinkle E, Chen H, Abuelo D, Dietz HC, Basson CT, Shete SS, Milewicz DM. Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13–14. Circulation. 2001;103:2461–2468. doi: 10.1161/01.cir.103.20.2461. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan CJ, Casey M, He J, Veugelers M, Henderson K, Guo D, Campagna R, Roman MJ, Milewicz DM, Devereux RB, Basson CT. Identification of a chromosome 11q23.2–q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation. 2001;103:2469–2475. doi: 10.1161/01.cir.103.20.2469. [DOI] [PubMed] [Google Scholar]
- 8.Hasham SN, Willing MC, Guo DC, Muilenburg A, He R, Tran VT, Scherer SE, Shete SS, Milewicz DM. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24–25. Circulation. 2003;107:3184–3190. doi: 10.1161/01.CIR.0000078634.33124.95. [DOI] [PubMed] [Google Scholar]
- 9.Van Kien PK, Mathieu F, Zhu L, Lalande A, Betard C, Lathrop M, Brunotte F, Wolf JE, Jeunemaitre X. Mapping of familial thoracic aortic aneurysm/dissection with patent ductus arteriosus to 16p12.2–p13.13. Circulation. 2005;112:200–206. doi: 10.1161/CIRCULATIONAHA.104.506345. [DOI] [PubMed] [Google Scholar]
- 10.Pannu H, Fadulu V, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Vranckx R, Van Kien PK, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 12.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- 13.Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 14.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 15.Huston EE, Grammer JC, Yount RG. Flexibility of the myosin heavy chain: direct evidence that the region containing SH1 and SH2 can move 10 A under the influence of nucleotide binding. Biochemistry. 1988;27:8945–8952. doi: 10.1021/bi00425a011. [DOI] [PubMed] [Google Scholar]
- 16.Lalwani AK, Goldstein JA, Kelley MJ, Luxford W, Castelein CM, Mhatre AN. Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am. J. Hum. Genet. 2000;67:1121–1128. doi: 10.1016/s0002-9297(07)62942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwai S, Hanamoto D, Chaen S. A point mutation in the SH1 helix alters elasticity and thermal stability of myosin II. J. Biol. Chem. 2006;281:30736–30744. doi: 10.1074/jbc.M605365200. [DOI] [PubMed] [Google Scholar]
- 18.Kohler J, Winkler G, Schulte I, Scholz T, McKenna W, Brenner B, Kraft T. Mutation of the myosin converter domain alters cross-bridge elasticity. Proc. Natl Acad. Sci. USA. 2002;99:3557–3562. doi: 10.1073/pnas.062415899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am. J. Pathol. 2003;162:533–546. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111:e150–e157. doi: 10.1161/01.CIR.0000155243.70456.F4. [DOI] [PubMed] [Google Scholar]
- 22.Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 23.Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113:e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu. Rev. Genomics Hum. Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 25.Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. doi: 10.1111/j.1365-2559.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- 26.Broglio F, Fubini A, Morello M, Arvat E, Aimaretti G, Gianotti L, Boghen MF, Deghenghi R, Mangiardi L, Ghigo E. Activity of GH/IGF-I axis in patients with dilated cardiomyopathy. Clin. Endocrinol. (Oxf) 1999;50:417–430. doi: 10.1046/j.1365-2265.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Borger MA, Williams WG, Weisel RD, Mickle DA, Wigle ED, Li RK. Regional overexpression of insulin-like growth factor-I and transforming growth factor-beta1 in the myocardium of patients with hypertrophic obstructive cardiomyopathy. J Thorac. Cardiovasc. Surg. 2002;123:89–95. doi: 10.1067/mtc.2002.118275. [DOI] [PubMed] [Google Scholar]
- 28.Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2001;33:655–670. doi: 10.1006/jmcc.2001.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standley PR, Obards TJ, Martina CL. Cyclic stretch regulates autocrine IGF-I in vascular smooth muscle cells: implications in vascular hyperplasia. Am. J. Physiol. 1999;276:E697–E705. doi: 10.1152/ajpendo.1999.276.4.E697. [DOI] [PubMed] [Google Scholar]
- 31.Allen TR, Krueger KD, Hunter WJ, III, Agrawal DK. Evidence that insulin-like growth factor-1 requires protein kinase C-epsilon, PI3-kinase and mitogen-activated protein kinase pathways to protect human vascular smooth muscle cells from apoptosis. Immunol. Cell Biol. 2005;83:651–667. doi: 10.1111/j.1440-1711.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 32.Recinos AIII, Lejeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.10.013. doi: 10.1016/j.atherosclerosis.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clyman RI. Mechanisms regulating the ductus arteriosus. Biol. Neonate. 2006;89:330–335. doi: 10.1159/000092870. [DOI] [PubMed] [Google Scholar]
- 34.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 36.McDonald IK, Thornton JM. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 37.Delano WL. The PyMOL User’s Manual. San Carlos, CA, USA: DeLano Scientific; 2002. [Google Scholar]
- 38.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]