Introduction
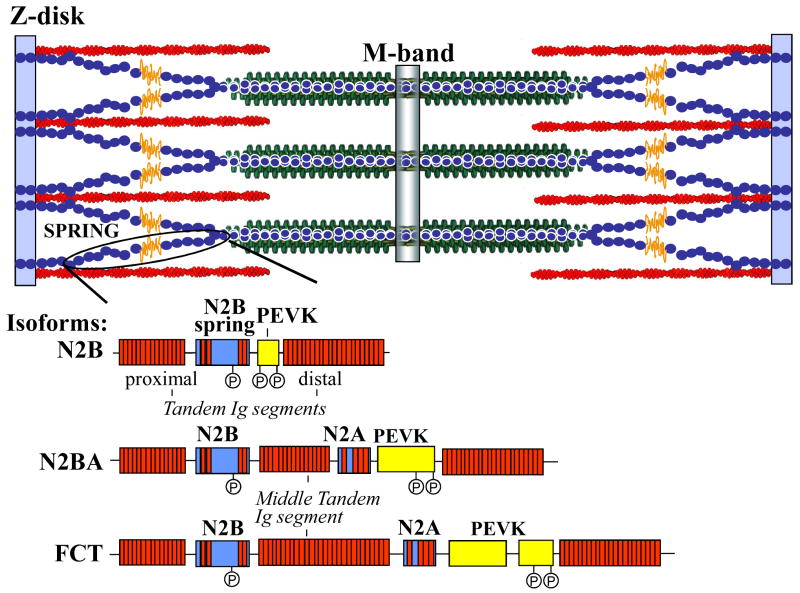
Titin constitutes the third myofilament of cardiac muscle, with a single giant polypeptide spanning from Z-disk to the M-band region of the sarcomere1 (Fig. 1). The ∼1.0 MDa region in the I-band is extensible and consists of tandemly arranged immunoglobulin (Ig)-like domains that make up proximal (near Z-disk) and distal (near A-I junction) segments, interspersed by the PEVK sequence (rich in proline, glutamate, valine, and lysine residues) and the N2B element2. Each functions as a distinct spring element3. The C-terminal ∼2 MDa of titin is located in the A-band and is inextensible. It is composed of regular arrays of Ig and fibronectin type 3 (Fn3) modules forming so-called super-repeats2. A-band titin may function as a molecular ruler, regulating assembly of the thick filament2,4,5. Titin's ∼250-kDa COOH-terminal region is an integral part of the M-band and contains a kinase domain6,7. As in the Z-disk, where titin filaments from opposite sarcomeres overlap, titin filaments from opposite half-sarcomeres overlap within the M-band, where they are interconnected by M-band proteins8. Thus, titin filaments with opposite polarity overlap in both Z-disk and M-band, forming a contiguous filament along the myofibril. In this review, we discuss titin's functions in the heart, with an emphasis on its role in diastolic function and the various mechanisms whereby passive stiffness can be tuned. Due to space constraints, it has not been possible to provide inclusive references to all original articles in the field.
Figure 1.
Schematic of titin in sarcomere.
Differential splicing
Titin is encoded by a single gene containing 368 exons. Multiple splice pathways in the I-band encoding region (∼230 exons) give rise to isoforms with different spring composition9. The three cardiac isoform classes are shown in Fig. 1. The relatively small ∼3.0 MDa isoform is known as N2B titin (it contains the N2B element)9. A second class also contains the N2A element, and is termed N2BA titin. N2BA titins have a longer PEVK segment and a variable number of additional Ig domains resulting in a ∼3.3-3.5 MDa size9. The third class includes isoforms that predominate in fetal-neonatal life which contain additional spring elements in both tandem Ig and PEVK regions, resulting in a ∼3.6-3.8 MDa size protein10-12. These isoforms gradually disappear during postnatal development. Regulation of the spring composition of titin in fetal-neonatal myocardium allows adjustment of diastolic filling properties during development.
Titin and muscle mechanics
Passive force
The best understood mechanical role of titin is its contribution to passive cardiomyocyte tension3,13-16. Passive tension results from extension of titin's I-band region, which elongates in a complex fashion as sarcomere length (SL) increases. The importance of titin is demonstrated by the fact that virtually no tension develops over the physiologic SL range after titin's I-band region is proteolyzed or detached from the thick filament2,3,13.
As discussed above, the spring portion of cardiac titin is composed of tandem Ig, PEVK and N2B segments9,17. Single molecule studies employing laser-tweezers and atomic force microscopy18-22 demonstrate that spring elements behave according to the wormlike chain entropic model applicable to flexible polymers. In the unstressed state spring segments have an end-to-end length close to zero. External force increases end-to-end length in association with reduced bending movements. The latter results in decreased entropy, manifested as increased passive force generation. This model is consistent with the non-linear relation between SL and cardiomyocyte passive tension that has been appreciated for many years, and explains elongation of the various segments of I-band titin as external force is applied18. As delineated in rodent left ventricular (LV) myocardium using immunolabeling of selected epitopes14,15,23, tandem Ig segments are extended first, followed by the PEVK segment, with the N2B segment elongating last.
N2BA titin has a longer extensible I-band region and is more compliant than N2B titin23. Both isoforms are co-expressed within the sarcomere; their ratio determines passive stiffness24. In adult rodents, N2B titin predominates in the LV and passive stiffness is therefore high16. In larger mammals, the proportion of N2BA titin increases, roughly paralleling body size. In human LV, the N2BA/N2B ratio is ∼0.6. The atria contain largely N2BA titin. Reflecting their isoform composition, rodent LV cardiomyocytes are much stiffer than cardiomyocytes from larger mammals16.
Myocardial passive tension includes contributions from cardiomyocytes (i.e., titin-dependent force) and collagen25. Titin's contribution is larger than collagen's at shorter SLs. At longer SLs collagen fibrils straighten and their stiffness increases. However, even at long SLs titin-dependent tension remains a substantial portion of total tension. Both titin- and collagen- dependent passive tension are higher in rodents than in larger mammals25. In consequence, passive myocardial stiffness and diastolic LV chamber stiffness are also greater in rodents. Recently, we generated two mouse knockouts (KOs) in which N2B or PEVK elements were excised26,27. The remaining spring elements (the tandem Ig and PEVK segments in the N2B KO; the tandem Ig and N2B segments in the PEVK KO) extend to a greater degree, explaining the increased titin-based passive tension of KO myocytes. Furthermore, in vivo pressure-volume loops revealed increased chamber stiffness, further establishing the importance of titin for diastolic function. Interestingly, although both models had elevated passive tension accompanied by diastolic dysfunction, the N2B KO model displayed cardiac atrophy and the PEVK KO hypertrophy (see also below).
Modulation of Titin-Dependent Passive Force
Titin-dependent passive tension can be modulated by post-translational modification, primarily phosphorylation. Yamasaki et al28 discovered that β-adrenergic stimulation of intact rat cardiac myocytes results in protein kinase A (PKA) phosphorylation of the cardiac-specific N2B sequence, which reduces passive stiffness. This occurs in many species, including canines and humans, and is more pronounced in N2B than N2BA titin28-30.
Krueger et al.31 showed that cGMP-dependent protein kinase (PKG) phosphorylates titin in canines and human. cGMP is a second messenger of NO and natriuretic peptides. The cGMP/PKG signaling cascade phosphorylates many sarcomeric and cytosolic proteins, with effects that include improvement in diastolic function (reviewed in32). Like PKA, PKG phosphorylates the N2B element; in human titin, this takes place on Serine 46931. Interestingly, sequence analysis indicates that S469 is not conserved in other species (Hidalgo and Granzier, unpublished data). Similar to PKA, PKG phosphorylation reduces passive stiffness30. Thus, the N2B element is a cardiac-specific sequence that can be phosphorylated by both PKA and PKG, with resulting decreased stiffness.
Hidalgo et al33 recently demonstrated that titin is also phosphorylated by protein kinase C (PKC). PKC regulates cardiac contractility by phosphorylating multiple thin- and thick-filament proteins. Titin phosphorylation was observed in skinned myocardium following incubation with PKCα. In vitro phosphorylation of recombinant protein representing titin's spring elements showed that PKCα targets the PEVK element at two highly conserved residues (S11878 and S12022). Mechanical experiments in both mouse and pig myocardium revealed that PKCα increases titin-based passive tension (increased tension is due to a reduced persistence length of the PEVK, and is borne by increased fractional extension of both N2B and tandem Ig segments). Thus, PKCα phosphorylation of titin links myocardial signaling and stiffness33. It is noteworthy that PKCα phosphorylation increases passive tension, whereas PKA/PKG produce the opposite effect. This is analogous to kinase effects on thin filament regulatory proteins where, for example, phosphorylation of TnI by PKA reduces and phosphorylation by PKC increases calcium sensitivity. The role of this novel PKC pathway for altering passive stiffness under physiological and pathological conditions remains to be established.
It will be important in the future to study the phosphorylation state of titin's PEVK segment in various disease states including heart failure, where PKC protein levels and activity are increased. Inhibiting PKCα has been proposed as a therapeutic strategy for treating heart failure. Our recent findings suggest that improving diastolic function via lowering titin phosphorylation could be one of its benefits.
In addition to isoform and phosphorylation effects, it was recently suggested that disulfide bridge formation in the N2B element can increase passive stiffness34. Because disulfide bridges require oxidizing conditions that are unlikely to exist in the sarcoplasm of healthy cells, this mechanism is unlikely to be relevant in normal physiology; the relevance in pathological states needs to be established. Calcium binding to titin may also alter passive tension35-37. This is related in part to binding to E-rich motifs in the PEVK segment36. Additionally, the PEVK domain in the extensible region of the N2B isoform interacts with actin in a [Ca2+] dependent fashion38,39, which may retard sliding of the thin filament on titin and increase passive stiffness.
The physiologic significance and interplay between the various mechanisms whereby titin-dependent passive stiffness is tuned remain to be established. Some mechanisms such as PKA and PKG are expected to be highly interactive since they appear to phosphorylate the same site in the N2B element. A full understanding of these interactions should be a major goal of future work.
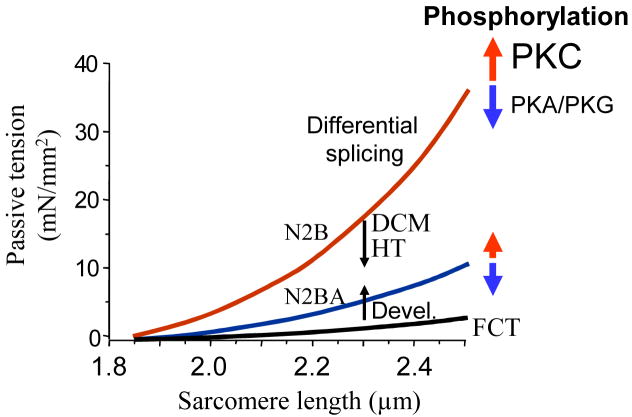
The passive tension –SL relations of the three classes of cardiac isoforms and the effects of phosphorylation are shown in Fig 2. Differential splicing is highly effective in altering titin-based passive stiffness, but is a slow process. Changes in passive tension resulting from PKA, PKG and PKC phosphorylation allow for rapid modulation of passive stiffness. PKA effects on passive stiffness are most prominent at shorter SLs28, whereas PKC effects are more prominent at longer SLs33.
Figure 2.
Titin-based passive stiffness tuning-mechanisms. Differential splicing gives rise to isoforms of varying stiffness. During postnatal development (Devel) passive stiffness increases due to switching of fetal cardiac titin (FCT) to adult N2B and N2BA isoforms; hypothyroidism (HT) and dilated cardiomyopathy (DCM) alter splicing in the opposite direction. PKA and PKG phosphorylation reduce and PKC phosphorylation increases passive stiffness.
Restoring Force
Cardiomyocytes recoil after contracting because they develop a restoring force (RF) at systolic SLs below the slack value of ∼ 1.9 μm. We estimated that titin accounts for at least 50% of RF40,41. The mechanism of titin-based RF is thought to be reverse extension at short SLs during contraction, i.e., movement of the thick filament during shortening extends the spring segments of titin in the opposite direction from when they are passively lengthened. With relaxation, the stretched springs recoil. The magnitude of the RF and the velocity of recoil are proportional to the stiffness of titin.
The titin RF may contribute to suction42, an important mechanism of early diastolic filling. Other mechanisms of suction likely include three dimensional systolic deformations and stretching of functional springs within the extracellular matix43. Suction is more pronounced at smaller end-systolic volumes, in parallel with the increased titin-dependent RF at shorter SLs. The direct relation between stiffness of titin and the magnitude of its RF implies that changes in stiffness may have divergent effects on diastolic function in the intact ventricle. Stiffer titin results in higher passive myocardial and ventricular end-diastolic chamber stiffness, while an increased RF may facilitate early diastolic filling. Rodents with rapid heart rates may benefit from augmented recoil that facilitates early filling during short cycles and a stiffer LV chamber later in diastole, which combine to rapidly set end-diastolic volume. Moreover, operating SLs of rodents are shorter than those of large mammals44. The latter further augments the titin-dependent RF while higher chamber stiffness can be tolerated because shorter SLs prevent excessive diastolic pressures.
Length-Dependent Activation
Increases in SL within the physiologic range result in increased myofilament calcium sensitivity, i.e., length-dependent activation (LDA). LDA is an important mechanism of the Frank-Starling relation and involves length-dependent thin filament activation45. A full discussion of LDA is beyond the scope of this review. However, titin appears to play a role because LDA varies with the level of passive tension at a given SL46-51. This has been explained by a reciprocal relationship between titin-dependent passive tension and inter-filament lattice spacing47. Another possibility is that longitudinal strain exerted by titin on the thick filament increases actin-myosin interaction52.
Titin-Binding Proteins
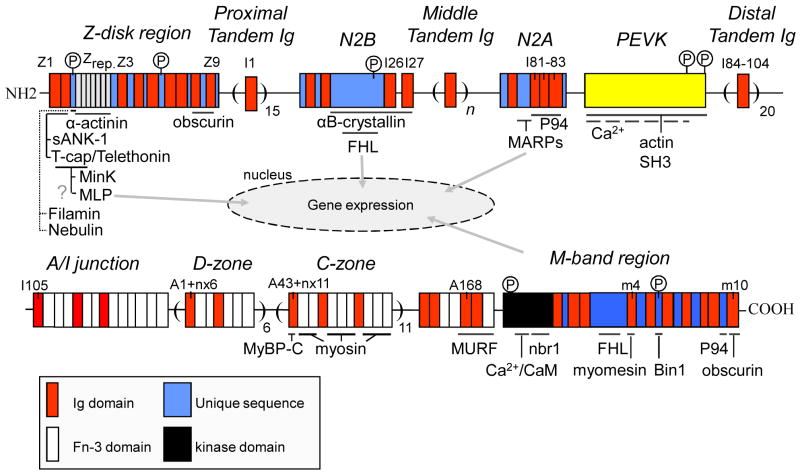
A variety of titin-binding proteins have been discovered (Fig. 3). Titin's two most N-terminal domains (Z1 and Z2) bind to small ankyrin-1 (sANK-1), a 17-kDa sarcoplasmic reticulum (SR) transmembrane protein53. This interaction is thought to play a role in organizing the SR around the contractile apparatus at the Z-disk. Furthermore, Z1 and Z2 interact with Tcap (telethonin), which assembles titin filaments into a tightly packed anti-parallel sandwich structure that is resistant to stretch54. Additional Z-disk strength is provided by titin's Z-repeats, 45-amino-acid repeats that bind α-actinin55,56. Tcap also interacts with the potassium-channel subunit MinK found in T-tubules57, which may modulate stretch-sensitive channel function. Furthermore, it has been suggested that Tcap is part of a mechanosensor by virtue of its interaction with muscle specific LIM protein (MLP)58. Polyclonal antibody studies have placed MLP in the Z-disk and the nucleus, where it may interact with the muscle transcriptional regulators, MyoD, MRF4, and myogenin. However, more recent work with a monoclonal MLP antibody59 shows that it is mainly cytoplasmic, with little preference for sarcomeric structures. That MLP is part of a stretch responsive signalling pathway is supported by mutations that cause dilated cardiomyopathy (DCM ) or hypertrophic cardiomyopathy (HCM)58 and by a MLP KO mouse which shows cardiac hypertrophy, myofibrillar disarray, and reduced myocardial stiffness58. Whether this involves a direct interaction between Tcap and MLP requires further study.
Figure 3.
Proteins that bind to titin.
The central I-band region of titin is a second hotspot for protein interactions. The N2B element has two established binding partners. One is αB-crystallin, a member of the small heat shock protein family that functions as a molecular chaperone 60. Upregulation of αB-crystallin occurs in several cardiac disorders. Overexpression protects the cardiomyocyte from ischemia-reperfusion injury (for review see 61). Using single molecule force spectroscopy we studied how N2B element extensibility is affected by wild-type and mutant αB-crystallin harboring the DCM missense mutation, R157H, or the desmin-related myopathy mutation, R120G62. Wild-type αB-crystallin lowers the compliance of the N2B element and increases the unfolding force of the flanking Ig domains. These effects are attenuated in R157H and abolished in the R120G mutant. Thus, αB-crystallin may normally protect titin from damage, an effect that is either attenuated or lost in disease-causing mutations.
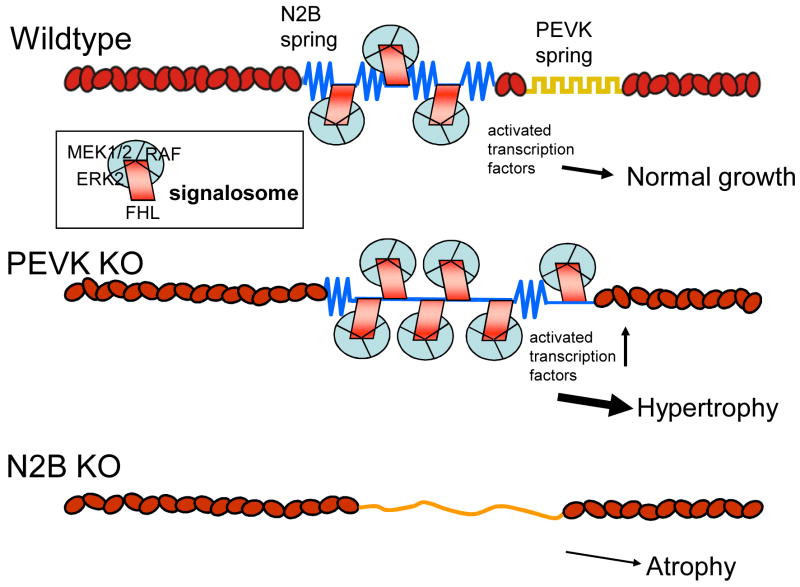
Titin also interacts with members of the four-and-a-half LIM (FHL) protein family, a newly identified group of LIM proteins characterized by 4 complete LIM domains and an N-terminal half LIM domain. FHL-1 is found in cardiac and skeletal muscle and FHL-2 mainly in myocardium. FHL-1 and -2 bind to the extensible region of the N2B element26,63. FHL proteins have varied biological functions64. Lange et al65 showed that FHL-2 couples metabolic enzymes. Sheikh et al63 showed that FHL-1 deficiency protects from pathological hypertrophy. Interestingly, we recently found that in PEVK KOs, where N2B element strain is enhanced (see above), FHL-1 and FHL-2 are upregulated and hypertrophy occurs26. Furthermore, the N2B KO, in which the N2B element is absent, has cardiac atrophy and decreased FHL levels27. Additionally, FHL-1 interacts with members of the MAPK signaling pathways (Raf1, MEK1/2, and ERK2) that co-localize with N2B in the sarcomere63. Together, these findings suggest that N2B facilitates assembly of a signaling complex that triggers hypertrophy in response to non-physiological N2B strain (as in pressure overload63 or the PEVK KO26). The blunted hypertrophy obtained when Gq overexpressing mice are crossed with FHL1 KO mice, a finding reported by Sheik at al63, suggests that the N2B-FHL-based signalosome receives input from G-protein receptors. The model shown in Fig. 4 emphasizes our view that the FHL-based signalosome is a strain sensor that triggers hypertrophy in response to excessive titin strain.
Figure 4.
Schematic of N2B-based signalosome. Adaptor molecules belonging to the FHL family bind to N2B element and sequester kinases of the MAPK signaling pathway. Bottom: increased N2B strain (PEVK KO) results in additional signalosomes, shifting the balance towards hypertrophy; absence of the N2B element (N2B KO) results in hypertrophy.
Additionally, the N2A element binds to three homologous muscle ankyrin repeat proteins (MARPs): CARP, ankrd2, and DARP66. MARPs participate in stress-activated pathways and are upregulated after mechanical or metabolic challenge67. Cyclic stretching of cultured cardiomyocytes induces expression of MARPs in the nucleus and the sarcomeric I-bands66. We showed that expression of MARPs is increased in end-stage DCM68. Analogous to the regulatory mechanism for MLP, dual localization of MARPs (titin's I-band region and the nucleus) may link stretch to gene expression. The N2A element also interacts with the Ca2+-dependent muscle protease calpain3/P94; this interaction may modulate P94 function in protein degradation69. P94 appears to be expressed in the heart only during early embryonic development70.
In the A-band, the first Ig domain of each 11-domain super-repeat interacts with myosin binding protein C (MyBP-C)71, whereas the FN3 domains bind to myosin72. Because A-band titin provides regularly spaced binding sites for myosin and MyBP-C, it may function as a molecular ruler which controls assembly and length of the thick filament. The M-line region of titin contains a serine/threonine kinase domain73. Little is known about its substrates and function. In vitro studies with a mutant kinase domain indicate that T-cap is a substrate in embryonic muscle74. Furthermore, titin kinase may play a role in embryonic sarcomere development, specifically, integration of titin in the A-band75 and sarcomere structure maintenance7. It has also been proposed that titin kinase is a mechano-sensor that regulates muscle protein expression in a strain-dependent fashion6. Lange et al6 proposed that titin kinase assembles an nbr1-based signalosome that communicates with the nucleus and modulates, in a stretch-dependent manner, protein expression and turnover. Finally, recent studies from our laboratory suggest that titin kinase affects cardiac contractility due to decreased SR calcium uptake76.
Near the edge of titin's M-band region (A168-170) is a binding site for muscle specific ring finger protein (MURF)77-79. MURF-1 is a sarcomere-associated protein that is an E3 ubiquitin ligase that conjugates ubiquitin to proteins destined for proteolysis. The middle of M-line titin contains a binding site for FHL-265. Closer to the C-terminus is a binding site for P9480. The most C-terminal domain of titin (m10) contains a binding sites for obscurin81,82, which is important for M-band stability.
In summary, titin-binding proteins have diverse roles in sarcomeric structure, protein turnover, biomechanical sensing and signaling. This suggests that titin has complex and important integrative functions. These functions are not expected to be equally represented in the different isoforms. N2BA and fetal cardiac titins but not N2B titin are expected to be involved in functions that require P94 and/or MARPs (which bind to the N2A element). Because the N2B isoform develops the highest force, functions that respond to stress (Z-disk, N2B element and M-band signalling) are expected to be accentuated in this isoform. Hence, as isoform shifts occur in disease (see below) changes in titin-based signalling are likely to occur.
Human Heart Disease
Due to its large size titin is expected to be a frequent target for mutations, but a total of only 20 mutations have been identified to date (for a complete list, see83), 1/10 of the number of mutations in β-MHC (which is <1/10 the size of titin). This low number of known titin mutations is likely, at least in part, due to the large message size which makes sequencing extremely demanding. As sequencing time and expense decrease many additional mutations will likely be discovered. Interestingly, some of the known mutations are in part of the gene that is expressed in cardiac as well as skeletal muscles, but for unknown reasons patients have a detectable phenotype in only one of the two muscle types. Exceptions to the rule are two recently discovered M-band mutations, both upstream of the kinase84. The patients have a similar clinical phenotype, with skeletal myopathy and fatal DCM. It is also noteworthy that ∼90% of the cardiac-specific mutations have a DCM phenotype with the remaining ∼10% having a HCM phenotype83. More work is required to understand the mechanism(s) by which titin mutations lead to either DCM or HCM. The recently introduced method85 of making a knock-in mouse model that contains a titin mutation similar to found in humans and then inducing a phenotype by stressing the heart may be valuable for this purpose.
Titin isoform shifts have also been reported in several diseases. Modest shifts can have significant effects because of the marked stiffness difference between N2B and N2BA titin. We were the first to report a disease-related shift in a large mammal, using the pacing tachycardia canine model 42,86, findings that were recently confirmed87. Here, the N2BA/N2B ratio was decreased in association with increased titin-dependent myocardial stiffness. Subsequently, we and others reported opposite results in explanted hearts from patients with end-stage DCM, i.e., increased N2BA/ N2B ratio and decreased titin-dependent tension68,88,89. In one study 68, levels of several N2A binding proteins were increased, suggesting a link between isoform shifts and signaling. Our results in pacing tachycardia suggest that with respect to titin this model does not mimic DCM patients. van Heerebeek et al90 measured isoform ratios in patients with non-ischemic DCM and HF with normal EF [diastolic HF(DHF)]. In contrast to earlier studies 68,88,89, DCM tissue was not from explanted hearts. They reported an N2BA/N2B ratio of 17/83 in DHF, lower than the DCM value of 35/65. The ratio in DCM was lower than reported previously in both explanted DCM hearts and their controls68,88,89. Thus, it is possible that DCM patients with earlier stage disease more closely resemble the tachycardia model. (This may be consistent with the finding of upregulated N2B titin in an earlier report in a single DCM patient91). In contrast to explanted heart studies68,88,89, in many patients in the more recent reports90,92 tissue was obtained via LV endomyocardial biopsy. It is possible that regional variation along with other as yet unspecified factors and associated conditions could contribute to varying isoform ratios.
There are two reports of isoform shifts in aortic stenosis (AS). We reported decreased N2BA/N2B in AS compared with transplant donor hearts93. In contrast, Borbely et al 92 reported increased N2BA/N2B in endomyocardial biopsies compared with endomyocardial tissue from several groups of control patients. The reason for this apparent discrepancy is not clear.
Recent studies indicate that alterations in titin phosphorylation may also occur in acquired disease. Paulus and colleagues have made major contributions to this emerging area92,94. In a 2005 report94 they studied skinned cardiomyocytes (endomyocardial biopsies) from patients with DHF and controls. Cardiomyocyte resting tension was markedly increased in DHF; this was reversed by PKA treatment. These results suggest that PKA phosphorylation of either titin or troponin I is reduced in DHF, both of which could raise resting tension. However, many DHF and control patients in this study were transplant recipients, which could have influenced myocardial and cardiomyocyte function. Moreover, titin isoforms were not reported.
Most recently, patients with HF (DCM and DHF), AS and controls were studied92. Cardiomyocyte resting tension was higher in both HF groups compared with AS and controls. N2BA/N2B ratios were increased in both HF groups (which by itself decreases tension). Treatment with gelsolin, which removes the thin filament, and BDM, which abolishes crossbridge cycling, did not alter passive force. This argues against a contribution of the thin filament and/or diastolic crossbridge cycling to increased passive force and implicates a titin-based mechanism. Both PKA and PKG treatment restored passive force toward normal. Overall titin phosphorylation was not different between HF and AS. However, in HF phosphorylation of the N2B isoform was reduced relative to N2BA titin, possibly accounting for higher passive tension in HF since hypophosporylated N2B titin generates higher passive tension than hypophosphorylated N2BA titin.
The phosphorylation state of titin's PEVK region was not investigated in the above studies (this pathway was discovered only recently). Thus, it is possible that phosphorylation of this region is increased in HF, resulting in higher passive tension. This is consistent with the finding that following PKA phosphorylation, HF cardiomyocytes still develop higher tension than AS cardiomyocytes92 despite the increased N2BA/N2B ratio.
A possible connection between titin and diabetic myocardial disease was suggested in another study by van Heerebeek et al95. Diastolic dysfunction is common in diabetes mellitus (DM)96. Van Heerebeek et al95 estimated diastolic stiffness in patients with HF (DCM and DHF) with and without DM. Here again, there were no non-failing controls. Cardiomyocyte resting tension was significantly higher in DM patients with normal EF compared with the other groups.
Last, we recently reported increased N2BA titin in rats with hypothyroidism97. Cardiomyocytes and skinned muscle strips demonstrated the expected decreases in titin-dependent passive tension and RF. Since diastolic dysfunction was present in hypothyroid animals, it was ascribed to increased collagen-dependent tension. Evidence of a role for thyroid hormone in isoform switching was also obtained in a recent cell culture study98. However, severely reducing thyroid hormone levels in utero and during early neonatal development had no detectable effect on isoform expression in either skeletal or cardiac muscle99. Clearly, further work is needed to delineate the role of thyroid hormone in titin isoform switching. Whether titin plays a role in myocardial abnormalities in patients with hypo- or hyperthyroidism also merits further study.
Summary
Titin is responsible for the passive and restoring force of the cardiac sarcomere and makes a major contribution to the diastolic wall stress of the LV, the level of which can be tuned through differential splicing and phosphorylation. PKA and PKG phosphorylation lower stress and PKC increases it. Changes in titin phosphorylation and titin splicing occur in cardiac disease, in addition to mutations in the titin gene. A host of titin-binding proteins has been discovered that implicate titin as a key player in the organization and development of the sarcomere, in protein turnover, and in sensing mechanical stress. Several stress sensing signalosomes along the molecule have been discovered, of which only the FHL-based signalosome binds to a spring element (N2B). This N2B-FHL signalosome is ideally situated to sense sarcomere strain and link diastolic dysfunction to hypertrophy signaling.
Acknowledgments
Funding Sources: Supported by NIH grants HL61497 and HL062881.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106:1563–72. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–6. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 3.Helmes M, Trombitas K, Centner T, Kellermayer M, Labeit S, Linke WA, Granzier H. Mechanically driven contour-length adjustment in rat cardiac titin's unique N2B sequence: titin is an adjustable spring. Circ Res. 1999;84:1339–52. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 4.Wang K. Titin/connectin and nebulin: giant protein rulers of muscle structure and function. Adv Biophys. 1996;33:123–34. [PubMed] [Google Scholar]
- 5.Trinick J. Titin and nebulin: protein rulers in muscle? Trends Biochem Sci. 1994;19:405–9. doi: 10.1016/0968-0004(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 7.Gotthardt M, Hammer RE, Hubner N, Monti J, Witt CC, McNabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem. 2003;278:6059–65. doi: 10.1074/jbc.M211723200. [DOI] [PubMed] [Google Scholar]
- 8.Obermann WM, Gautel M, Steiner F, van der Ven PF, Weber K, Furst DO. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol. 1996;134:1441–53. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89:1065–72. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 10.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–13. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 11.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res. 2004;94:967–75. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- 12.Greaser ML, Krzesinski PR, Warren CM, Kirkpatrick B, Campbell KS, Moss RL. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. J Muscle Res Cell Motil. 2005;26:325–32. doi: 10.1007/s10974-005-9039-0. [DOI] [PubMed] [Google Scholar]
- 13.Trombitas K, Jin JP, Granzier H. The mechanically active domain of titin in cardiac muscle. Circ Res. 1995;77:856–61. doi: 10.1161/01.res.77.4.856. [DOI] [PubMed] [Google Scholar]
- 14.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin's extensibility. Biophys J. 1999;77:3189–96. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, Gregorio CC. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol. 1999;146:631–44. doi: 10.1083/jcb.146.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86:1114–21. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, Granzier H. Molecular mechanics of cardiac titin's PEVK and N2B spring elements. J Biol Chem. 2002;277:11549–58. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Muhle-Goll C, Kellermayer MS, Labeit S, Granzier H. Different molecular mechanics displayed by titin's constitutively and differentially expressed tandem Ig segments. J Struct Biol. 2002;137:248–58. doi: 10.1006/jsbi.2002.4458. [DOI] [PubMed] [Google Scholar]
- 20.Kellermayer MS, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–6. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Linke WA, Oberhauser AF, Carrion-Vazquez M, Kerkvliet JG, Lu H, Marszalek PE, Fernandez JM. Reverse engineering of the giant muscle protein titin. Nature. 2002;418:998–1002. doi: 10.1038/nature00938. [DOI] [PubMed] [Google Scholar]
- 22.Linke WA, Granzier H. A spring tale: new facts on titin elasticity. Biophys J. 1998;75:2613–4. doi: 10.1016/S0006-3495(98)77706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79:3226–34. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trombitas K, Wu Y, Labeit D, Labeit S, Granzier H. Cardiac titin isoforms are coexpressed in the half-sarcomere and extend independently. Am J Physiol Heart Circ Physiol. 2001;281:H1793–9. doi: 10.1152/ajpheart.2001.281.4.H1793. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32:2151–62. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 26.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, King NM, Yu Q, Tschope C, McNabb M, Larson DF, Labeit S, Gotthardt M. Truncation of titin's elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105:557–64. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104:3444–9. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–8. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–71. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–44. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 31.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 32.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;152:855–69. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–8. doi: 10.1161/CIRCRESAHA.109.198465. 17 p following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grutzner A, Garcia-Manyes S, Kotter S, Badilla CL, Fernandez JM, Linke WA. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J. 2009;97:825–34. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellermayer MS, Granzier HL. Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett. 1996;380:281–6. doi: 10.1016/0014-5793(96)00055-5. [DOI] [PubMed] [Google Scholar]
- 36.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci U S A. 2003;100:13716–21. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL. Titin isoform-dependent effect of calcium on passive myocardial tension. Am J Physiol Heart Circ Physiol. 2004;287:H2528–34. doi: 10.1152/ajpheart.00553.2004. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J. 2001;81:2297–313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res. 2001;89:874–81. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 40.Helmes M, Trombitas K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res. 1996;79:619–26. doi: 10.1161/01.res.79.3.619. [DOI] [PubMed] [Google Scholar]
- 41.Preetha N, Yiming W, Helmes M, Norio F, Siegfried L, Granzier H. Restoring force development by titin/connectin and assessment of Ig domain unfolding. J Muscle Res Cell Motil. 2005;26:307–17. doi: 10.1007/s10974-005-9037-2. [DOI] [PubMed] [Google Scholar]
- 42.Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res. 2000;87:235–40. doi: 10.1161/01.res.87.3.235. [DOI] [PubMed] [Google Scholar]
- 43.Bell SP, Fabian J, LeWinter MM. Effects of dobutamine on left ventricular restoring forces. Am J Physiol. 1998;275:H190–4. doi: 10.1152/ajpheart.1998.275.1.H190. [DOI] [PubMed] [Google Scholar]
- 44.Toh R, Shinohara M, Takaya T, Yamashita T, Masuda S, Kawashima S, Yokoyama M, Yagi N. An X-Ray diffraction study on mouse cardiac cross-bridge function in vivo: effects of adrenergic {beta}-stimulation. Biophys J. 2006;90:1723–8. doi: 10.1529/biophysj.105.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ Res. 2007;101:1081–3. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- 46.Cazorla O, Vassort G, Garnier D, Le Guennec JY. Length modulation of active force in rat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol. 1999;31:1215–27. doi: 10.1006/jmcc.1999.0954. [DOI] [PubMed] [Google Scholar]
- 47.Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–35. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001;104:1639–45. doi: 10.1161/hc3901.095898. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol. 2003;553:147–54. doi: 10.1113/jphysiol.2003.049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch. 2005;449:449–57. doi: 10.1007/s00424-004-1354-6. [DOI] [PubMed] [Google Scholar]
- 51.Terui T, Sodnomtseren M, Matsuba D, Udaka J, Ishiwata S, Ohtsuki I, Kurihara S, Fukuda N. Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle. J Gen Physiol. 2008;131:275–83. doi: 10.1085/jgp.200709895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci. 2008;58:151–9. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- 53.Kontrogianni-Konstantopoulos A, Bloch RJ. The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin. J Biol Chem. 2003;278:3985–91. doi: 10.1074/jbc.M209012200. [DOI] [PubMed] [Google Scholar]
- 54.Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006;439:229–33. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]
- 55.Sorimachi H, Freiburg A, Kolmerer B, Ishiura S, Stier G, Gregorio CC, Labeit D, Linke WA, Suzuki K, Labeit S. Tissue-specific expression and alpha-actinin binding properties of the Z-disc titin: implications for the nature of vertebrate Z-discs. J Mol Biol. 1997;270:688–95. doi: 10.1006/jmbi.1997.1145. [DOI] [PubMed] [Google Scholar]
- 56.Gautel M, Goulding D, Bullard B, Weber K, Furst DO. The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J Cell Sci. 1996;109(Pt 11):2747–54. doi: 10.1242/jcs.109.11.2747. [DOI] [PubMed] [Google Scholar]
- 57.Furukawa T, Ono Y, Tsuchiya H, Katayama Y, Bang ML, Labeit D, Labeit S, Inagaki N, Gregorio CC. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001;313:775–84. doi: 10.1006/jmbi.2001.5053. [DOI] [PubMed] [Google Scholar]
- 58.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–55. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 59.Gehmlich K, Geier C, Milting H, Furst D, Ehler E. Back to square one: what do we know about the functions of Muscle LIM Protein in the heart? J Muscle Res Cell Motil. 2008;29:155–8. doi: 10.1007/s10974-008-9159-4. [DOI] [PubMed] [Google Scholar]
- 60.Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, Ding L, Labeit S, Horwitz J, Leonard KR, Linke WA. Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem. 2004;279:7917–24. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Osinska H, Gerdes AM, Robbins J. Desmin filaments and cardiac disease: establishing causality. J Card Fail. 2002;8:S287–92. doi: 10.1054/jcaf.2002.129279. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Y, Bogomolovas J, Labeit S, Granzier H. Single molecule force spectroscopy of the cardiac titin N2B element: effects of the molecular chaperone alphaB-crystallin with disease-causing mutations. J Biol Chem. 2009;284:13914–23. doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, 2nd, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–80. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev. 2000;95:259–65. doi: 10.1016/s0925-4773(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 65.Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–36. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- 66.Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–64. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Witt CC, Ono Y, Puschmann E, McNabb M, Wu Y, Gotthardt M, Witt SH, Haak M, Labeit D, Gregorio CC, Sorimachi H, Granzier H, Labeit S. Induction and myofibrillar targeting of CARP, and suppression of the Nkx2.5 pathway in the MDM mouse with impaired titin-based signaling. J Mol Biol. 2004;336:145–54. doi: 10.1016/j.jmb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 69.Ono Y, Torii F, Ojima K, Doi N, Yoshioka K, Kawabata Y, Labeit D, Labeit S, Suzuki K, Abe K, Maeda T, Sorimachi H. Suppressed disassembly of autolyzing p94/CAPN3 by N2A connectin/titin in a genetic reporter system. J Biol Chem. 2006;281:18519–31. doi: 10.1074/jbc.M601029200. [DOI] [PubMed] [Google Scholar]
- 70.Fougerousse F, Durand M, Suel L, Pourquie O, Delezoide AL, Romero NB, Abitbol M, Beckmann JS. Expression of genes (CAPN3, SGCA, SGCB, and TTN) involved in progressive muscular dystrophies during early human development. Genomics. 1998;48:145–56. doi: 10.1006/geno.1997.5160. [DOI] [PubMed] [Google Scholar]
- 71.Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem. 1996;235:317–23. doi: 10.1111/j.1432-1033.1996.00317.x. [DOI] [PubMed] [Google Scholar]
- 72.Muhle-Goll C, Habeck M, Cazorla O, Nilges M, Labeit S, Granzier H. Structural and functional studies of titin's fn3 modules reveal conserved surface patterns and binding to myosin S1--a possible role in the Frank-Starling mechanism of the heart. J Mol Biol. 2001;313:431–47. doi: 10.1006/jmbi.2001.5017. [DOI] [PubMed] [Google Scholar]
- 73.Labeit S, Gautel M, Lakey A, Trinick J. Towards a molecular understanding of titin. Embo J. 1992;11:1711–6. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–9. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 75.Weinert S, Bergmann N, Luo X, Erdmann B, Gotthardt M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. J Cell Biol. 2006;173:559–70. doi: 10.1083/jcb.200601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng J, Raddatz K, Molkentin JD, Wu Y, Labeit S, Granzier H, Gotthardt M. Cardiac hypertrophy and reduced contractility in hearts deficient in the titin kinase region. Circulation. 2007;115:743–51. doi: 10.1161/CIRCULATIONAHA.106.645499. [DOI] [PubMed] [Google Scholar]
- 77.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–22. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 78.McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC. Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci. 2004;117:3175–88. doi: 10.1242/jcs.01158. [DOI] [PubMed] [Google Scholar]
- 79.Hirner S, Krohne C, Schuster A, Hoffmann S, Witt S, Erber R, Sticht C, Gasch A, Labeit S, Labeit D. MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J Mol Biol. 2008;379:666–77. doi: 10.1016/j.jmb.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 80.Kinbara K, Sorimachi H, Ishiura S, Suzuki K. Muscle-specific calpain, p94, interacts with the extreme C-terminal region of connectin, a unique region flanked by two immunoglobulin C2 motifs. Arch Biochem Biophys. 1997;342:99–107. doi: 10.1006/abbi.1997.0108. [DOI] [PubMed] [Google Scholar]
- 81.Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–36. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci. 2008;121:1841–51. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- 83.Greaser ML. Stressing the giant: a new approach to understanding dilated cardiomyopathy. J Mol Cell Cardiol. 2009;47:347–9. doi: 10.1016/j.yjmcc.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–51. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 85.Gramlich M, Michely B, Krohne C, Heuser A, Erdmann B, Klaassen S, Hudson B, Magarin M, Kirchner F, Todiras M, Granzier H, Labeit S, Thierfelder L, Gerull B. Stress-induced dilated cardiomyopathy in a knock-in mouse model mimicking human titin-based disease. J Mol Cell Cardiol. 2009;47:352–8. doi: 10.1016/j.yjmcc.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106:1384–9. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 87.Jaber WA, Maniu C, Krysiak J, Shapiro BP, Meyer DM, Linke WA, Redfield MM. Titin isoforms, extracellular matrix, and global chamber remodeling in experimental dilated cardiomyopathy: functional implications and mechanistic insight. Circ Heart Fail. 2008;1:192–9. doi: 10.1161/CIRCHEARTFAILURE.108.768465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–41. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 89.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–16. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 90.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y, Labeit S, Lewinter MM, Granzier H. Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM. J Card Fail. 2002;8:S276–86. doi: 10.1054/jcaf.2002.129278. [DOI] [PubMed] [Google Scholar]
- 92.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 93.Williams L, Howell N, Pagano D, Andreka P, Vertesaljai M, Pecor T, Frenneaux M, Granzier H. Titin isoform expression in aortic stenosis. Clin Sci (Lond) 2009 doi: 10.1042/CS20080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 95.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 96.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548–51. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 97.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. J Mol Cell Cardiol. 2007;42:186–95. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 98.Kruger M, Sachse C, Zimmermann WH, Eschenhagen T, Klede S, Linke WA. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/ AKT pathway. Circ Res. 2008;102:439–47. doi: 10.1161/CIRCRESAHA.107.162719. [DOI] [PubMed] [Google Scholar]
- 99.Ottenheijm CA, Knottnerus A, Buck D, Luo X, Greer K, Hoying A, Labeit S, Granzier H. Tuning Passive Mechanics through Differential Splicing of Titin during Skeletal Muscle Development. Biophys J. 2009;97:2277–2286. doi: 10.1016/j.bpj.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]