Abstract
Preferential activation of regulatory T (Treg) cells limits autoimmune tissue damage during chronic immune responses but can also facilitate tumor growth. Here, we show that tissue-produced inflammatory mediators prime maturing dendritic cells (DC) for the differential ability of attracting anti-inflammatory Treg cells. Our data show that prostaglandin E2 (PGE2), a factor overproduced in chronic inflammation and cancer, induces stable Treg-attracting properties in maturing DC, mediated by CCL22. The elevated production of CCL22 by PGE2-matured DC persists after the removal of PGE2 and is further elevated after secondary stimulation of DC in a neutral environment. This PGE2-induced overproduction of CCL22 and the resulting attraction of FOXP3+ Tregs are counteracted by IFNα, a mediator of acute inflammation, which also restores the ability of the PGE2-exposed DC to secrete the Th1-attracting chemokines: CXCL9, CXCL10, CXCL11, and CCL5. In accordance with these observations, different DCs clinically used as cancer vaccines show different Treg-recruiting abilities, with PGE2-matured DC, but not type 1–polarized DC, generated in the presence of type I and type II IFNs, showing high Treg-attracting activity. The current data, showing that the ability of mature DC to interact with Treg cells is predetermined at the stage of DC maturation, pave the way to preferentially target the regulatory versus proinflammatory T cells in autoimmunity and transplantation, as opposed to intracellular infections and cancer.
Introduction
Type 1 immune responses, dominated by the activation of type 1 effector T cells (Teff; CTLs and Th1) and natural killer (NK) cells, are critical for the elimination of intracellular pathogens and tumor cells but can lead to autoimmune tissue damage. The magnitude, duration, and eventual outcome of type 1 responses are decided by the balance between immune effector cells and regulatory T (Treg) cells, characterized by the CD4+CD25high surface phenotype and the intracellular expression of FOXP3+ (1, 2). In accordance with the observations of immune dysfunction associated with advanced cancer, the numbers of FOXP3+ T cells are elevated in blood and affected tissues of cancer patients. Two mechanisms have been proposed to be responsible for this phenomenon: the preferential recruitment of Treg cells to tumor sites, mediated by the chemokines CCL22 (MDC/STCP-1) and CXCL12 (SDF-1), the respective ligands for the Treg-expressed chemokine receptors CCR4 and CXCR4 (3–8), and enhanced Treg induction (9–11).
Dendritic cells (DC) are the major type of antigen-presenting cells involved in the induction of immune responses and the regulation of its character (12–14). The high potency of mature DC in activating and reverting anergy of T cells has led to the application of ex vivo–generated DC as cancer vaccines (12, 13). The current cancer vaccines involving DC matured in the presence of the prostaglandin E2 (PGE2)-containing cytokine cocktail or macrophage-conditioned medium have proved to be highly effective in inducing circulating tumor-specific T cells in the blood of cancer patients (15–18), but their effectiveness in inducing clinical responses is still below expectations (19). Although this discrepancy is likely to result from multiple factors, the recently reported expansion of FOXP3+ Treg cells in the patients receiving such vaccines (20) represents one undesirable aspect of vaccination.
To develop a means of limiting the ability of DC-based vaccines to interact with Treg cells and to test if the ability of DC to interact with the different T-cell subsets can be imprinted by the conditions of DC maturation, we evaluated the mechanism of Treg attraction to human monocyte-derived DCs and analyzed the effect of different inflammatory mediators on the subsequent ability of mature DC to attract FOXP3+ Treg cells. We compared the effect of PGE2, a factor prevalent in chronic inflammation and cancer (21) and previously implicated in the CCL22-mediated attraction of Th2 cells (22–24), and IFNα, a mediator of acute inflammation (25) involved in the induction of Th1-attracting CXCR3 ligands (26–28).
Our data show that CCL22, but not other chemokines similarly implicated in Treg attraction to different tissues, constitutes the key DC-produced Treg-attracting chemokine. We report that the elevated Treg-promoting activity of DCs is a stable feature of the DCs that have matured in the presence of PGE2, but it can be antagonized by the presence of IFNα during DC maturation. The current observations that distinct chemokine profiles are imprinted in DCs during their maturation and are preserved in mature DCs after their removal from different inflammatory environments help to design vaccines that preferentially target the functionally different T-cell subsets.
Materials and Methods
Media and reagents
Serum-free CellGenix DC medium (CellGenix, Inc.) was used to generate DC. The following factors were used to generate mature DC: recombinant human (rhu) granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 (gifts from Schering-Plough); IFNα (Intron A, IFNα-2b, Schering-Plough); rhuTNFα, rhuIL-1β, and rhuIFNγ (Strathmann Biotech); rhuIL-6 (Genzyme); and PGE2 and polyinosinic acid:poly-CMP (polyI:C; both from Sigma). CD40L-transfected J558 cells (gift from Dr. P. Lane, University of Birmingham, Birmingham, United Kingdom) were used as an equivalent to activated CD4+ T cells (29). All recombinant chemokines used in this study and all chemokine-specific primary and biotinylated secondary antibodies used in ELISA assays were purchased from PeproTech.
DC culture
Peripheral blood mononuclear cells obtained from healthy donors were isolated with lymphocyte separation medium (Cellgro, Mediatech). Monocytes were isolated on density gradients using Percoll (Sigma) or Isolate (Irving Scientific) followed by plastic adherence, resulting in purity of >90% (see refs. 36, 39). Monocytes were cultured for 6 d in 24-well plates (Falcon, Becton Dickinson Labware) at 5 × 105 cells per well in rhuGM-CSF and IL-4 (both 1,000 IU/mL). On day 6, DC maturation (48 h) was induced using the indicated combinations of the following factors: IL-1β (25 ng/mL), TNFα (50 ng/mL), IFNγ (1,000 units/mL), IL-6 (1,000 units/mL), PGE2 (10−6 mol/L), polyI:C (20 μg/mL), and IFNα (3,000 units/mL). When indicated, DCs were harvested and seeded at density of 5 × 104 in 500 μL with or without J558 cells in CellGenix medium without any additional factors for the following 24h. The supernatants were filtered (0.22 μm) before their use in migration experiments.
Isolation of CD4+ T cells
Mononuclear cells from peripheral blood of healthy donors were isolated by density gradient separation using Lymphocyte Separation Medium (Cellgro, Mediatech). CD4+ T cells were isolated by negative magnetic selection using the EasySep Human CD4 T Cell Enrichment kit (StemCell Technologies, Inc.) according to the manufacturer's protocol.
Analysis of chemokine production by Taqman and ELISA
Based on preliminary kinetic experiments, we determined 6 h to be the optimum time to measure mRNA expression for IFNα-inducible chemokines and 48 h to be the optimum for PGE2-inducible chemokines. ELISA analysis of protein secretion by DC for all the chemokines was performed at 48 h, unless specifically indicated.
For the Taqman analysis, total RNA was extracted using Qiagen RNeasy kit according to the manufacturer's protocol. cDNA synthesis was performed on 2 μg of extracted RNA in 20 μL reaction volume using Retroscript kit (Ambion) according to the manufacturer's protocol. Real-time analysis was performed on 25 ng of sample cDNA using premade Taqman primers and probes (see Supplementary Table S1), either from GenScript or Applied Biosystems, in 25 μL reaction volume using Universal Taqman kit with UNG (Applied Biosystems) following the manufacturer's protocol. Samples were analyzed with an ABI Prism 7700 sequence analyzer (Applied Biosystems). The expression of each gene was normalized to HPRT1 and expressed as fold increase (2−ΔCT), where ΔCT= [CT (Target gene)] − [CT (HPRT1)].
Supernatants of DC cultures were analyzed for CCL5, CCL22, CXCL9, and CXCL10 proteins by indirect sandwich ELISA. Briefly, ELISA plates (Corning, Inc.) were coated overnight at room temperature with 100 μL of primary antibody at 10 μg/mL followed by washing and blocking with PBS + 4% bovine serum albumin (BSA) for 1 h. Samples (50 μL) were added to the wells and incubated for 1 h and subsequently washed and incubated with 50 μL of biotinylated secondary antibodies at 2.5 μg/mL for 1 h. The plates were washed and incubated for 30 min with streptavidin-horseradish peroxidase conjugate (Pierce Biotechnology, Inc.) and diluted 1:8,000 in wash buffer (50 mmol/L Tris, 0.2% Tween). The plates were washed and detected with 50 μL of 3,3′,5,5′-tetramethylbenzidine substrate (Pierce Biotechnology). Reactions were stopped with 2% H2SO4 and absorbance at 450 nm was measured.
Chemotaxis
Chemotaxis assays were performed in 96-(Trans)well plates with a 3-μm-pore-size polycarbonate filter (Corning). The lower chamber was filled with 200 μL of rhuCCL22 (100 ng/mL) or CXCL10 (1,000 ng/mL) and CXCL11 (1,000 ng/mL) in RPMI 1640 with 0.5% BSA (chemotaxis medium) or DC culture supernatant and 50 μL (2.0 × 105 cells) of purified CD4+ T cells were added to the upper chamber. When indicated, CD4+ T cells were treated for 30 min with anti-CCR4 antibody (1G1, 20 μg/mL; BD Biosciences) before chemotaxis to block the CCR4-dependent chemotaxis. Alternatively, CCL22 (200 ng/mL) was added to the upper chamber of the migration system to abrogate the CCL22 gradient and desensitize the CCR4 responsiveness. Migration chambers were incubated for 3 h at 37°C. Subsequently, cells from multiple wells were pooled and total RNA was extracted. The presence of Treg cells within the migrated cell population was analyzed by Taqman for the expression of FOXP3 or CTLA4 mRNA using the FOXP3-specific (Hs00203958_m1) and CTLA4-specific (Hs00175480_m1) expression Assay-on-Demand (Applied Biosystems) and expression was normalized to HPRT1. The FOXP3 protein expression was confirmed by flow cytometry using the FOXP3 staining kit (eBioscience). Photomicrographs of total (CD3+) T cells migrated in response to medium, αDC1, or “standard” DC (sDC) supernatants were obtained using a Zeiss Axiovert200 inverted microscope with ZeissCAM.
Statistical analysis
All data were evaluated using Student's t test (two tailed), with P < 0.05 considered as significant.
Results
IFNα and PGE2 cross-regulate the chemokine production profiles of maturing DC
To develop a means to regulate the ability of DC to interact with Tregs (as opposed to Teff cells), we analyzed the production of the chemokines previously implicated in the attraction of Treg cells, CCL22 (3, 5) and CCL17 (as an alternative CCR4 ligand), CXCL12 (8), and CCL20 (4, 30), by the human monocyte-derived DC matured in different inflammatory conditions. As representatives of Teff-attracting chemokines, we analyzed the production of the known CXCR3 ligands (CXCL9, CXCL10, and CXCL11) and the production of CCL5, implicated in the attraction of type 1 immune cells (31–34). Because the CCL22-dependent attraction of Th2 cells can be enhanced by PGE2 (22–24), we tested the effect of PGE2 on production of the above Treg-attracting chemokines, contrasting it with IFNα, as a representative mediator of acute inflammation.
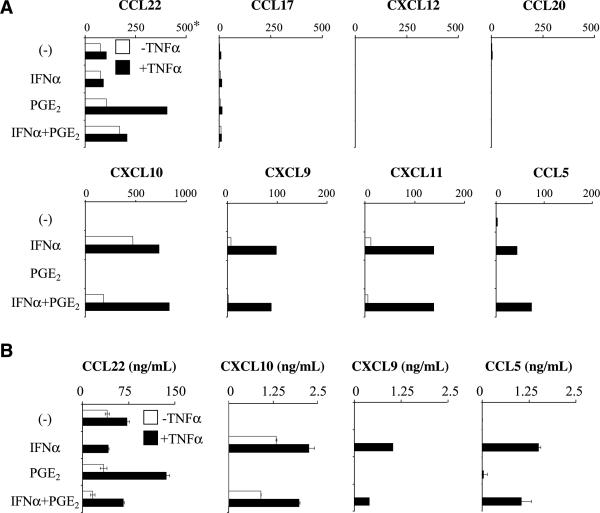
Immature DC or DC matured in the presence of TNFα expressed low levels of CCL22 but did not express any of the other known Treg-attracting chemokines (CCL17, CCL20, or CXCL12). In addition, TNFα matured DC produced low levels of CCL5, but none of the CXCR3 ligands implicated in the attraction of Teff cells (Fig. 1A and B). Analogous to TNFα, exposure to PGE2 alone failed to enhance the spontaneous levels of CCL22 expression. Strikingly, however, the combination of PGE2 with TNFα resulted in dramatic elevation of CCL22 gene expression (Fig. 1A) and the secretion of CCL22 protein (Fig. 1B). In accordance with previous reports (26–28), IFNα strongly enhanced the production of CCL5 and all three CXCR3 ligands (CXCL9, CXCL10, and CXCL11; Fig. 1A).
Figure 1.
IFNα and PGE2 cross-regulate the production of Teff- and Treg-attracting chemokines by DC. Day 6 monocyte-derived immature DCs were exposed to PGE2 and/or IFNα either alone (□) or in the presence of TNFα (■) as a DC maturation-inducing agent. A and B, dominant effect of IFNα on the chemokine production profiles in maturing DC. A, expression of Treg-attracting (top) and Teff-attracting (bottom) chemokine genes in the differentially treated DC. *, the data are expressed as the ratios between the expression of the individual chemokine genes and HPRT1 (see Materials and Methods) and represent one of five experiments that all yielded similar results. B, secretion of CCL5, CXCL9, CXCL10, and CCL22 proteins by the differentially treated DC. PGE2-induced CCL22 production was significantly higher (P < 0.01) compared with the TNFα treatment alone.
In addition to the anticipated ability of IFNα to enhance the production of Teff-attracting chemokines, unexpectedly IFNα also proved to be highly effective in reducing the spontaneous DC production of CCL22 and counteracting the PGE2-induced enhancement of CCL22 production (Fig. 1A and B). PGE2 failed to significantly inhibit the early mRNA expression of IFNα-inducible chemokines (Fig. 1A), resulting in a dominant character of the IFNα-dependent regulation of the DC secretion of Treg- and Teff-attracting chemokines at 48 h (Fig. 1B). The expression of CXCL9, CXCL10, and CXCL11 genes was, however, partially inhibited by PGE2 at later time of activation (data not shown), suggesting that the PGE2 may limit the duration of the expression of the CXCR3 ligand genes.
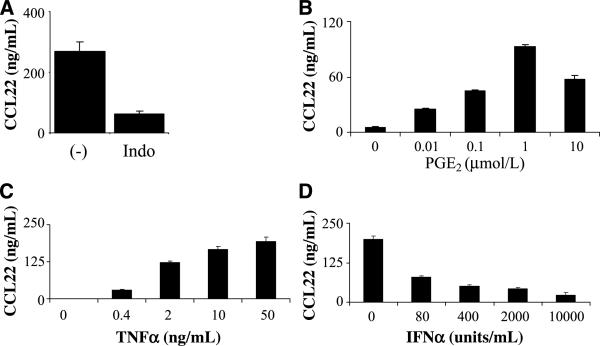
To determine if the low-level “spontaneous” CCL22 production by DC can be triggered by DC-produced endogenous PGE2, DCs were treated with TNFα in the presence or absence of indomethacin (an inhibitor of prostaglandin synthesis). In accordance with this possibility, indomethacin suppressed the spontaneous CCL22 production in TNFα-matured DC (Fig. 2A). The elimination of endogenous PGE2 revealed the wide range of dose-dependent CCL22-inducing effect of exogenously added PGE2 (Fig. 2B). The ability of PGE2 to promote the CCL22 production by DC critically depended on the presence of TNFα, as shown by the inability of PGE2 to promote the CCL22 production in the absence of TNFα and the dose dependence of the TNFα effect (Fig. 2C). A similar synergistic activity of PGE2 and TNFα in inducing CCL22 production was also observed in the case of macrophages generated from monocytes in the presence of GM-CSF alone (Supplementary Fig. S1). This CCL22-enhancing effect of PGE2 on the TNFα-maturing DC was counteracted by IFNα in a dose-dependent manner (Fig. 2D).
Figure 2.
Dose-dependent cross-regulation of CCL22 production by TNFα, PGE2, and IFNα. A, involvement of endogenous prostaglandins in the production of “baseline” levels of CCL22 by maturing DC. Please note the reduction (P < 0.05) of the baseline CCL22 production in the presence of prostaglandin synthesis inhibitor indomethacin (Indo; 50 μmol/L; added at the beginning of cultures). B, dose-dependent enhancement of CCL22 production in maturing DC by exogenous PGE2. Indomethacin (50 μmol/L) was present in all cultures to eliminate the endogenous PGE2 production. C, DCs were treated with 1 μmol/L PGE2 but with increasing doses of TNFα to reveal TNFα-dependent induction of CCL22 by PGE2. D, inhibition of CCL22 production by increasing doses of IFNα in the TNFα-treated (50 ng/mL) and PGE2-treated (1 μmol/L) DC. Data from one of two (C and D) or four (A and B) experiments that all yielded similar results.
In contrast to high levels of CCL22 production, in none of the conditions did DC produce significant levels of the additional chemokines similarly implicated in Treg attraction, CCL20 and CXCL12 (Fig. 1A; data not shown), suggesting that CCL22 is the major DC-associated chemokine responsible for their ability to attract and interact with Tregs.
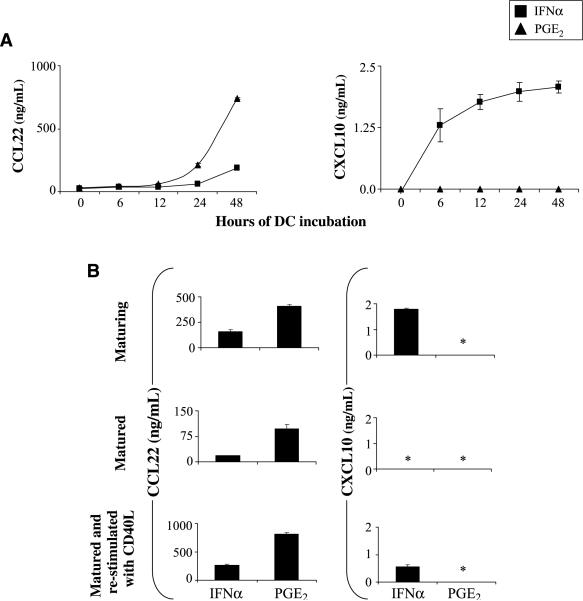
Stability of the elevated CCL22 production by PGE2-exposed DC
Consistent with the preferential activation of the Treg system at later stages of immune responses, the PGE2-induced CCL22 secretion was undetectable until 24h after DC stimulation but continued to accumulate throughout the following period (Fig. 3A). Moreover, PGE2-matured DC continued to produce high levels of CCL22 for 48 h after the removal of PGE2 from the cultures (Fig. 3B). In contrast, the IFNα-induced production of CXCL10 was evident within 6 h of DC activation (Fig. 3A), but its continued secondary secretion after the withdrawal of IFNα required restimulation with CD40L (Fig. 3B), the interaction with CD40L-expressing CD4+ Th cells (data not shown), or additional Toll-like receptor–mediated activation during maturation (see below).
Figure 3.
The ability of DC to produce Teff- and Treg-attracting chemokines is imprinted during DC maturation. A, kinetics of CXCL10 and CCL22 protein secretion by DC maturing in the presence of IFNα (■) or PGE2 (▲). TNFα was added to both types of cultures. Figure represents combined data from two experiments that yielded similar results. The difference in CCL22 production between IFNα-treated and PGE2-treated DC was significant at 24 h (P < 0.05) and 48 h (P < 0.01) of stimulation. B, stability of the maturation-induced chemokine profiles in DC matured with either IFNα or PGE2 in the presence of TNFα. Chemokine concentrations in the supernatants from the DC maturation cultures (top) and in the 48-h cultures of the DC harvested, washed, and replated in the absence (middle) or presence (bottom) of CD40L. In all three situations, the IFNα- versus PGE2-treated DC showed significant differences in CCL22 production (P < 0.01, 0.05, and 0.01, respectively). *, below 0.05 ng/mL.
Importantly, the differences in the abilities to produce these Teff-attracting versus Treg-attracting chemokines encoded during the DC maturation by IFNα and PGE2 were fully reproduced after subsequent CD40L stimulation in the absence of the original modulating factors. These data indicate that in analogy to the previously identified ability of maturing DC to “memorize” the conditions of their maturation and translate these signals into different levels of production of IL-12 and different ability to induce the functionally different T-cell responses (14), inflammatory factors may also prime DC for preferential interaction with functionally distinct T-cell subsets after leaving the peripheral tissues and migrating to the draining lymph nodes.
Preferential recruitment of FOXP3+ Treg cells by PGE2-exposed DC: key role of CCL22
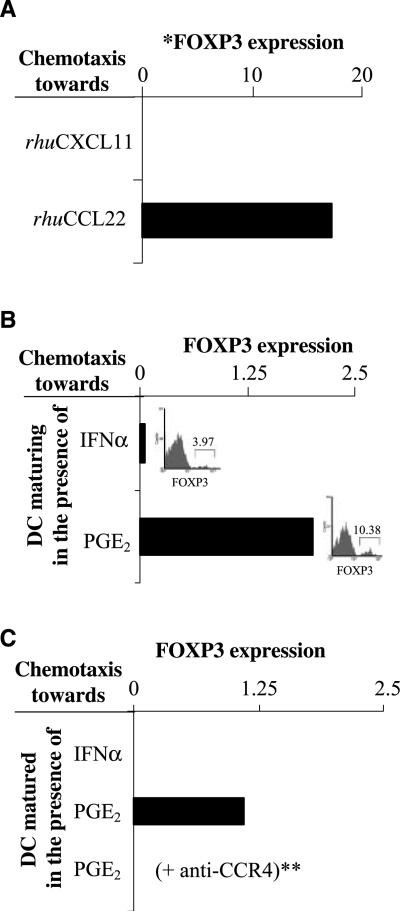
Because DC matured in the presence of PGE2 or IFNα had different abilities to produce the chemokines previously implicated in the preferential attraction of the regulatory versus Teff cells, we tested their ability to recruit FOXP3+ T cells, as opposed to other CD4+ T-cell subsets. Although the overall numbers of freshly isolated peripheral blood CD4+ T cells migrating in response to recombinant CCL22 were ~2-fold lower compared with the migration to CXCL10 (or to CXCL11, used as an alternative CXCR3 ligand), the CCL22-responsive population of CD4+ T cells was enriched in FOXP3+ cells (and CTLA4+ cells), as shown by Taqman analysis and intracellular staining (Fig. 4A; Supplementary Figs. S2–S3), consistent with previous reports showing high activity of CCL22 in the recruitment of Treg cells (3, 5).
Figure 4.
PGE2 imprints a stable Treg-attracting function in maturing DC: key role of CCR4 in the ability of DC to attract FOXP3+ T cells. Purified CD4+ T cells were loaded into the upper chamber of the Transwells and allowed to migrate toward recombinant CCR4 or CXCR3 ligands or the supernatants of IFNα- or PGE2-treated DC. The migrated CD4+ T cells from the bottom chambers were pooled (eight wells per group, with higher numbers needed for the intracellular staining experiments) and analyzed by Taqman for the presence of FOXP3+ cells. A, Taqman analysis of CD4+ T cells migrated in response to rhuCCL22 or to CXCL10 (see Supplementary Fig. S2 for the intracellular expression of FOXP3 protein in the individual CD4+ T cells). Similar data were obtained in two additional experiments. B, Taqman analysis of the CD4+ T cells migrated in response to the factors released during the TNFα-driven maturation of DC exposed to IFNα or PGE2. Insets, frequencies of FOXP3+ T cells in the CD4+ T-cell populations recruited by IFNα- or PGE2-treated DC supernatants. Similar data were obtained in three additional experiments. C, CD4+ T cells migrating in response to the supernatants from harvested and reseeded DC after maturation. *, all mRNA data are normalized for HPRT1. **, the CD4+ T cells were pretreated with CCR4 blocking antibody before addition to the upper chambers of the chemotaxis plate. Similar data were obtained using excess CCL22 to abrogate the CCL22 gradient.
In accordance with the differences in CCL22 production between the differentially matured DC (Figs. 1–3) and our observations that CCL22 represents the main DC-produced Treg-attracting chemokine (Fig. 1), the recruitment of FOXP3-expressing cells was a selective property of the PGE2-matured DC (Fig. 4B). Similar differences in Treg-attracting activity patterns were observed using not only the supernatants from the DCs undergoing maturation in the presence of PGE2 versus IFNα (Fig. 4B) but also the supernatants from the differentially matured DCs that were removed from these different environments and recultured for additional 24 h in the absence of any additional stimuli or modulators (Fig. 4C).
The recruitment of FOXP3+ cells was completely abrogated in the presence of CCR4 blocking antibody (Fig. 4C) or by the disruption of CCL22 gradient using excess CCL22 (data not shown). Because human DCs produce CCL22 but not an alternative CCR4 ligand, CCL17 (Fig. 1A), these data indicate that CCR4-CCL22 interaction, but not other chemokine systems similarly implicated in Treg attraction to different tissues (such as CXCL12-CXCR4 and CCL20-CCR6; refs. 3–8, 30), is responsible for the elevated ability of PGE2-matured DC to attract FOXP3+ T cells.
Elevated Treg-attracting activity of the clinical-grade DC preparations generated in the presence of PGE2
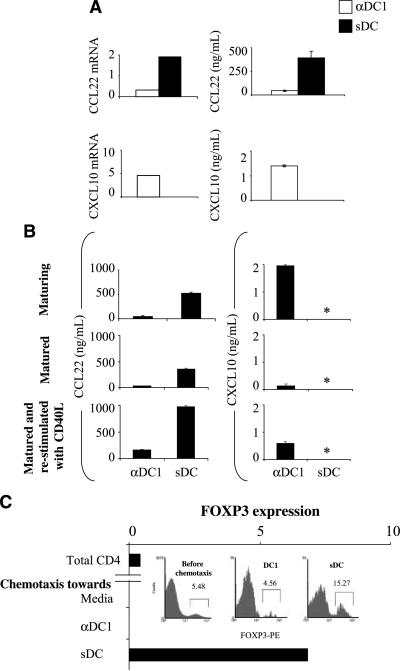
It has been recently shown that the vaccination of cancer patients with sDC matured in the presence of IL-1β, TNFα, IL-6, and PGE2 (35) can expand their FOXP3+ Treg cell population (20). In accordance with the possibility that PGE2 used in the process of their generation may preferentially promote the interaction of the vaccine-carrying sDC with Tregs, we observed that sDCs showed high production of Treg-attracting CCL22 (rather than Teff-attracting chemokines; Fig. 5A and B) and were highly effective in selectively attracting CD4+FOXP3+ T cells from freshly isolated bulk population of CD4+ T cells (Fig. 5C). In contrast, alternatively matured type 1–polarized DC (αDC1) obtained in the presence of IL-1β, TNFα, IFNγ, IFNα, and polyI:C (36) showed strongly reduced production of CCL22 (Fig. 5A and B). Accordingly, although αDC1s were more effective than sDCs in attracting high numbers of CD4+ T cells (Supplementary Fig. S2B), in contrast to sDCs, they did not preferentially recruit FOXP3+ T cells from the total population of CD4+ T cells (Fig. 5C, inset).
Figure 5.
Different clinically applied DC types display strong differences in the ability to attract FOXP3+ T cells. A, sDC (■) generated in the presence of IL-1β, TNFα, IL6, and PGE2 (35) and αDC1s (□) matured in the presence of IL-1β, TNFα, IFNα, IFNγ, and polyI:C (36) were tested for the expression of Teff- or Treg-attracting chemokine genes (left; also see Supplementary Fig. S5) and the secretion of the relevant chemokines (right; P < 0.05). Representative data from over 10 donors. B, stability of the chemokine production pattern in different types of clinically applied DC. Chemokine contents in the supernatants from DC maturation cultures (top) and in the 48-h cultures of the harvested, washed, and replated DC in the absence (middle) or presence (bottom) of CD40L. αDC1 and sDC showed significant differences in CCL22 production in all stages tested (P < 0.01). *, below 0.05 ng/mL. Similar data were obtained in two to four additional experiments. C, Taqman analysis of freshly isolated purified CD4+ T cells (before migration) and of the CD4+ T cells migrated in response to medium (negative control, spontaneous migration only), αDC1, or sDC supernatants. Data from one of five experiments that all yielded similar results. Inset, frequencies of FOXP3+ T cells in the CD4+ T-cell populations recruited by the supernatants from αDC1s or sDCs were determined by flow cytometry.
Interestingly, in contrast to the DC matured in the presence of TNFα plus IFNα alone that stopped the production of CXCL10 directly after removal of these factors (Fig. 3), αDC1 produced the Teff-attracting chemokines for a prolonged time period, with CXCL10 being detectable in the supernatants of the cells removed from the original maturation cocktail even in the absence of CD40L stimulation (Fig. 5B, middle).
Discussion
The current data indicate that the character of the inflammatory environment can affect the balance between Teff and Treg cell activation by instructing the maturing DC to adopt a stable propensity to interact with each of these T-cell types. Our results show that in analogy to the previously identified ability of maturing DC to memorize the conditions of their maturation and translate them into different ability to induce the functionally different T-cell responses (14, 36, 37), maturing DC can also be primed for preferential interaction with the functionally different T-cell subsets.
Although numerous chemokines, including CCL20, CCL22, and CXCL12, respectively, signaling via CCR20, CCR4, and CXCR4, are known to preferentially attract Treg to different tissues (refs. 3–8, 30 reviewed in ref. 38), our data show that the ability of human DC to attract FOXP3+ Treg cells is strictly CCR4dependent (Fig. 4C), implicating the key role of CCL22, the only DC-produced CCR4 ligand (see Fig. 1A).
Furthermore, our data indicate that PGE2, including the endogenously produced PGE2 (Fig. 2A) and the PGE2 produced by tumor tissues (Supplementary Fig. S4), is a potent inducer of the Treg-attracting propensity of human DCs. In contrast to this common mediator of chronic inflammation (21), IFNα, a factor produced at the early stages of intracellular infections and known to promote the production of Teff-attracting chemokines (26–28), proved to be a potent antagonist of Treg-attracting DC function. Although similar CCL22-antagonistic function has been reported in the case of IFNγ (22), the factor produced by NK and T cells, the current data indicate that the suppression of Treg recruitment can be also driven by the factors released from directly infected stromal cells at the earliest stages of acute infection, facilitating the early induction of the pathogen-specific immunity.
Interestingly, the IFNα-induced production of CXCL10 (and other CXCR3 ligands; data not shown) was evident within 6 h of DC activation and was terminated following the elimination of the IFNα signal. In contrast, CCL22 induction occurred with substantial delay and was produced at high concentrations over prolonged period of time. This particular kinetics of the PGE2-induced production of CCL22 and the resulting Treg attraction is consistent with the role of PGE2 in preserving tissue homeostasis and limiting the duration of the inflammatory type responses.
The ability of PGE2 to prime DC for elevated attraction of Treg cells adds to the previously postulated ability of this agent to promote the de novo induction of Treg cells (9–11), as well as to its previously defined ability to suppress the functions of Th1-type cells (39–42), and to promote the recruitment of Th2 cells (22–24). Although the ability of PGE2 to promote the attraction of Tregs is likely to be advantageous in limiting the duration of physiologic immune responses and the associated tissue damage, it is also likely to contribute to the limited effectiveness of responses against PGE2-overproducing cancers (43–46), able to imprint high CCL22-producing function in DC (Supplementary Fig. S4).
Importantly, the PGE2-induced Treg-attracting phenotype persisted in DC even after the removal of PGE2, with the second wave of the CCL22 production being further elevated after the secondary stimulation of the PGE2-exposed DC with CD40L. Similarly, the elevated production of CXCL10 by IFNα-matured DC could also be observed following CD40L-mediated restimulation of DCs in the absence of IFNα. This ability of DC to memorize the conditions of their maturation, reflecting them in their subsequent chemokine production, is likely to have implications for the therapeutic use of DC to preferentially activate different T-cell subsets. Although PGE2-matured DCs have been shown to expand the numbers of FOXP3+ Treg cells in cancer patients (20), it remains to be seen whether such undesirable effects can be reduced in the clinical trials involving αDC1s or other DC types obtained in the absence of PGE2.
In summary, the current data show that the inflammatory factors present during DC maturation imprint the differential ability of mature DC to interact with distinct T-cell subsets. Our data help to explain the hyperactivation of the Treg system in the setting of chronic infections and cancer and facilitate the design of the immunotherapies aimed at the selective activation of the inflammatory versus regulatory type of immune cells.
Supplementary Material
Acknowledgments
Grant support: NIH grants CA095128, CA114931, CA101944, and EA055944 (P. Kalinski) and the institutional funds in support of CA137214.
We thank Dr. Eva Wieckowski, Mary Jo Buffo, and Seth Shaltes for their assistance with intracellular staining and flow cytometry; Erik Berk for providing monocytes; and Robbie Mailliard for technical advice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 2.Taams LS, Palmer DB, Akbar AN, Robinson DS, Brown Z, Hawrylowicz CM. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology. 2006;118:1–9. doi: 10.1111/j.1365-2567.2006.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–94. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 5.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–51. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q, Bromley SK, Means TK, et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204:1327–34. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 10.Liu VC, Wong LY, Jang T, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar MV, Steinman RM, Sapp M, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–80. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–7. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J Exp Med. 2001;194:1767–75. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 1999;189:803–10. doi: 10.1084/jem.189.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–70. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–52. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda E, Sugiura T, Okada K, Zeki K, Yamashita U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J Immunol. 2001;166:1650–8. doi: 10.4049/jimmunol.166.3.1650. [DOI] [PubMed] [Google Scholar]
- 23.Lebre MC, Burwell T, Vieira PL, et al. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83:525–35. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 24.McIlroy A, Caron G, Blanchard S, et al. Histamine and prostaglandin E up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-γ-induced CXCL10 production by immature human dendritic cells. Immunology. 2006;117:507–16. doi: 10.1111/j.1365-2567.2006.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang KS, Georgiev P, Recher M, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006;116:2456–63. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahalingam S, Chaudhri G, Tan CL, John A, Foster PS, Karupiah G. Transcription of the interferon γ (IFN-γ)-inducible chemokine Mig in IFN-γ-deficient mice. J Biol Chem. 2001;276:7568–74. doi: 10.1074/jbc.M005773200. [DOI] [PubMed] [Google Scholar]
- 28.Carr DJ, Campbell IL. Herpes simplex virus type 1 induction of chemokine production is unrelated to viral load in the cornea but not in the nervous system. Viral Immunol. 2006;19:741–6. doi: 10.1089/vim.2006.19.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–86. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 31.Mahalingam S, Foster PS, Lobigs M, Farber JM, Karupiah G. Interferon-inducible chemokines and immunity to poxvirus infections. Immunol Rev. 2000;177:127–33. doi: 10.1034/j.1600-065x.2000.17720.x. [DOI] [PubMed] [Google Scholar]
- 32.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 33.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 34.Okada N, Gao JQ, Sasaki A, et al. Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host's immune system: implications for chemokine-based cancer immunotherapy. Biochem Biophys Res Commun. 2004;317:68–76. doi: 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 36.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. α-Type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 37.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez-Ramos JC, Lloyd C, Kapsenberg ML, Gonzalo JA, Coyle AJ. Non-redundant functional groups of chemokines operate in a coordinate manner during the inflammatory response in the lung. Immunol Rev. 2000;177:31–42. doi: 10.1034/j.1600-065x.2000.17713.x. [DOI] [PubMed] [Google Scholar]
- 39.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 40.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 41.Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 42.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–9. [PubMed] [Google Scholar]
- 43.Inaba T, Sano H, Kawahito Y, et al. Induction of cyclooxygenase-2 in monocyte/macrophage by mucins secreted from colon cancer cells. Proc Natl Acad Sci U S A. 2003;100:2736–41. doi: 10.1073/pnas.0435410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 45.Uchida K, Schneider S, Yochim JM, et al. Intratumoral COX-2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidine-based chemotherapy. Clin Cancer Res. 2005;11:3363–8. doi: 10.1158/1078-0432.CCR-04-1650. [DOI] [PubMed] [Google Scholar]
- 46.Williams C, Shattuck-Brandt RL, DuBois RN. The role of COX-2 in intestinal cancer. Ann N Y Acad Sci. 1999;889:72–83. doi: 10.1111/j.1749-6632.1999.tb08725.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.