Abstract
The ability of the ocular surface to mount an immune response is in part attributed to a family of proteins called toll-like receptors (TLRs). The latter are evolutionary conserved receptors that recognize and respond to various microbes and endogenous ligands. In addition to their recognition function, TLR activation triggers a complex signal transduction cascade that induces the production of inflammatory cytokines and co-stimulatory molecules, thus initiating innate and adaptive immunity. Toll-like receptor expression at the ocular surface is modulated during infection (e.g. Herpes simplex, bacterial keratitis and fungal keratitis) as well as during various inflammatory conditions (allergic conjunctivitis and dry eye syndrome). Here recent findings regarding TLR expression and their involvement in various ocular surface diseases are discussed.
Keywords: Toll-like receptors, cornea, conjunctiva, inflammation, infection
1. Introduction
Toll-like receptors (TLRs) are a family of highly conserved glycoprotein pattern recognition receptors that recognize conserved motifs on pathogen associated molecular patterns (PAMPs) on bacteria, viruses, fungi and protozoa. TLRs are expressed on a wide variety of cell types including epithelia, endothelia, antigen presenting cells and lymphocytes. They are type I transmembrane glycoproteins which have an extracellular leucine-rich domain and a cytoplasmic domain that is homologous to the signaling domain of the interleukin (IL)-1 receptor hence is referred to as the Toll/IL-1 receptor (TIR) domain. The latter mediates activation of intracellular signaling pathways, leading to functional changes including cytokine, chemokine and adhesion molecule expression.
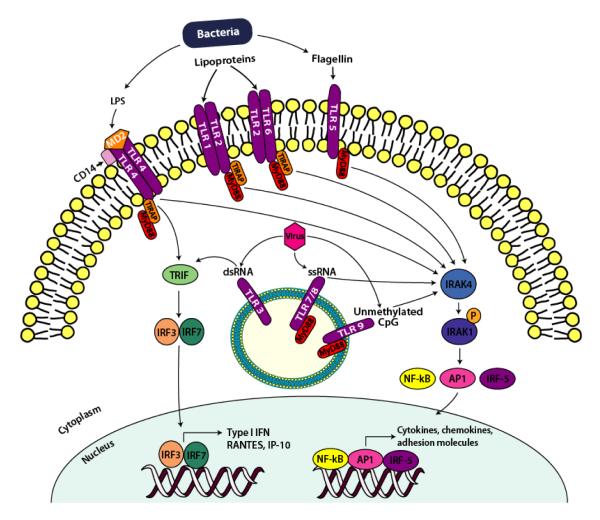
To date, 10 functional human TLRs have been identified; their microbial ligands and signaling pathways are depicted in figure 1. TLR1, 2, 4, 5, 6, and 10 are typically located at the cell surface.TLR2 forms heterodimers with TLR1 and with TLR6 and recognizes a variety of microbial lipoproteins. TLR2/6 and TLR2/1 heterodimers recognize bacterial diacyl and triacyl lipopeptides respectively (von Aulock et al., 2003; Takeda et al., 2002). TLR4 forms a complex with MD-2 and CD14 and recognizes lipopolysaccharide (LPS) from Gram-negative bacteria (Beutler 2000), and TLR5 recognizes flagellin, a component of bacterial flagella (Hayashi et al., 2001). TLR10 is able to dimerize with TLR1 and TLR2, but the microbial ligand for this receptor has yet to be identified (Hasan et al., 2005). TLR 3, 7, 8, and 9 are typically located intracellularly, on endosomal membranes and recognize nucleic acids. TLR3 recognizes double stranded RNA, a by-product of viral replication (Alexopoulou et al., 2001) whereas TLR7 and 8 recognize viral single stranded RNA (Diebold et al., 2004; Heil et al., 2004). TLR9 responds to unmethylated cytosine-phosphate-guanosine dinucleotide (CpG) motifs found in both bacterial and viral DNA (Hemmi et al., 2000; Tabeta et al., 2004).
Figure 1.
Simplified Overview of TLR Signaling. Cell surface TLR2, 4 and 5 recognize bacterial PAMPs lipoproteins, LPS and flagellin respectively, whereas intracellular TLR3, 7/8, and 9 recognize microbial dsRNA, ssRNA and unmethlylated CpG motifs respectively from either replicating or infecting viruses or bacteria in the endosome of the cell. The activation of TLRs initiates a MyD88-dependent (all TLRs except TLR3) or TRIF-dependent (TLR3 and TLR4) pathway. The MyD88-dependent pathway utilizes adapter molecule TIRAP (except TLR7, 8 and 9) leading to IRAK-4 and IRAK-1 recruitment, activated IRAK-4 phosphorylates IRAK-1 which ultimately leads to the activation of transcription factors AP-1, NFκB and IRF-5. TLR3 and TLR4 signal via a MyD88-independent pathway that is mediated via the adaptor protein, TRIF, which leads to the activation of transcription factors IRF-3 and IRF-7 that induce the expression of type I IFN genes.
Although TLRs were first recognized for their capacity to bind PAMPs recently a number of endogenous ligands have come to light. Many of these are molecules indicative of tissue trauma, such as intracellular components of ruptured cells, nucleic acids, heat shock proteins and extracellular matrix breakdown products such as hyaluronan fragments, fibrinogen and high-mobility group box 1 proteins (Kluwe et al., 2009). Thus, TLRs may be part of a surveillance system to monitor tissue injury and progress of re-modeling as well as infection. On the downside, TLR activation by endogenous ligands is also associated with disease; activation of TLR9 by endogenous DNA is implicated in the development of autoimmune disorders such as systemic lupus erythematosus in both humans and murine models of the disease (Lamphier et al., 2006).
With the exception of aforementioned self-nucleic acid signaling via TLR9, endogenous TLR ligands trigger TLR2 or TLR4. Owing to similarities among the cytokine effects of these endogenous ligands and TLR2/4 microbial agonists it has been suggested that contamination with bacterial LPS or lipoprotein is actually responsible for at least some of the effects attributed to endogenous ligands (Tsan and Gao 2007). Thus, studies claiming identification of an endogenous TLR ligand need to be scrutinized to ensure adequate controls were in place to account for possible bacterial product contamination.
All TLRs, except TLR3, signal via the adaptor molecule myeloid differentiation protein 88 (MyD88) which associates with the TLR cytoplasmic domain via a homophilic interaction between the TIR domains (fig. 1). IL-1R-associated kinase (IRAK)-4 and IRAK-1 are recruited, activated IRAK-4 phosphorylates IRAK-1 which ultimately leads to the activation of transcription factors activating protein (AP)-1, nuclear factor κB (NFκB) and interferon regulatory factor (IRF)-5. This stimulates the expression of multiple genes such as cytokines, chemokines and adhesion molecules. TLR3 signals via a MyD88 independent pathway that is mediated via the adaptor protein TIR domain-containing adaptor protein-inducing interferon (IFN)-β (TRIF), thus leading to the activation of transcription factors IRF-3 and IRF-7 that induce expression of IFN-α/ β and IFN inducible genes such as RANTES and interferon-inducible protein (IP)-10. Figure 1 shows a general overview of TLR signaling, for comprehensive details of the pathways the reader is referred to a review article by Albiger et al. (2007).
2. Expression of TLRs at the Ocular Surface
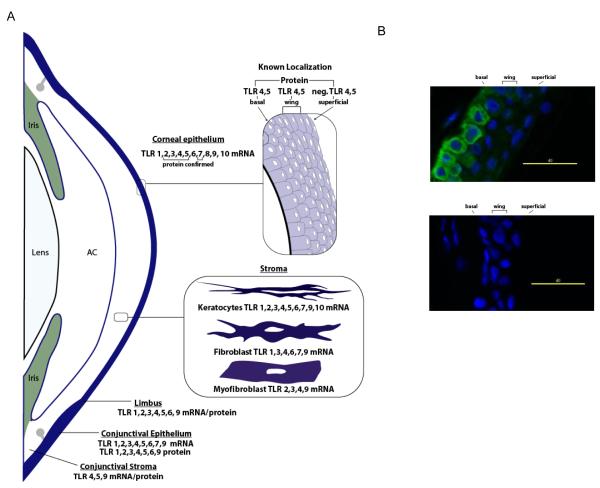
A summary of findings regarding cornea and conjunctival TLR expression from several different published sources cited below is presented in figure 2. The first report of the localization of TLRs to the ocular surface came in 2001 when Song et al. showed that freshly isolated and telomerase immortalized human corneal epithelial cells (HCEC) express TLR4. Subsequently the expression of mRNA for TLRs 1-10 has been detected in the corneal epithelium from subjects undergoing various ocular surgeries and from cadaver corneas, although not all subjects expressed all TLRs and the relative expression between subjects was variable, with TLR7 and 8 tending to be lower (Jin et al., 2007; Redfern RL et al., IOVS 2006; 47: ARVO E-Abstract 4372; Ueta et al., 2005; Wu et al., 2007). Similar results have been observed with primary cultured HCEC and cell lines (Kumar et al., 2004; Redfern RL et al., IOVS 2006; 47: ARVO E-Abstract 4372; Wu et al., 2007). Expression at the protein level has been confirmed for TLR2, 3, 4, 5 and 7 (Hozono et al., 2006; Kumar et al., 2006a; Li et al., 2005; Song et al., 2001; Ueta et al., 2004, 2005; Wu et al., 2007; Zhang et al., 2003).
Figure 2.
Expression of TLRs at the Ocular Surface. (A) This summary is based on published data from multiple sources discussed in the text for human tissue sections and primary cultured cells. AC: anterior chamber, neg: negligible expression. (B) TLR 5 expression. TLR5 (green) is localized to basal and wing epithelial cells in normal human corneas (top image).The isotype control antibody revealed no background staining (bottom image). DAPI was used to stain nuclei (blue).
Studies of the distribution and functionality of some TLRs at the ocular surface has produced contrasting results in a number of instances. Ueta et al. (2004, 2005) observed intracellular TLR2 expression in HCEC that was unresponsive to peptidoglycan. However, Kumar et al. (2004, 2006b) found cell surface TLR2 expression, stimulation of which activated NFκB and upregulated cytokine and antimicrobial peptide (human β-defensin-2, hBD-2) expression. Similarly, Kumar et al. (2006a) observed functional (as determined by NFκB activation and IL-6 and IL-8 secretion) intracellular expression of TLR3 by HCEC. However Ueta et al. (2005) reported that TLR3, while functional, was expressed at the cell surface. TLR3 is commonly found intracellularly on endosomal membranes, although surface expression has been documented for other cell types including fibroblasts (Matsumoto et al., 2003) and cytokine exposed keratinocytes (Begon et al., 2007). Several studies support expression of functional TLR4 by HCEC (McNamara et al., 1999; Song et al., 2001; Wu et al., 2007). However, others report intracellular expression of TLR4 that was unresponsive to LPS, leading to the suggestion that this would contribute to an “immunosilent environment” to prevent unnecessary responses to commensal flora (Kumar et al., 2006b; Ueta et al., 2004).
As these aforementioned studies have been carried out primarily with cultured cells, the variability in the resulting data may relate to donor variation (for primary cultures), culture conditions and differences in how cell lines were derived. Notably Blais et al. (2005) observed that while LPS alone had little effect on IL-6 and IL-8 secretion, addition of CD14 or LPS binding protein increased their secretion, suggesting that culture conditions can have a significant influence on the responsiveness of cells to LPS. Furthermore, lack of MD-2 is responsible for the inability of some HCEC to respond to LPS (Zhang et al., 2009).
Blais et al. (2005) found TLR4 and MD-2 expression in the basal and wing but not the superficial epithelial cells of human corneal tissue sections. Zhang et al. (2003) reported a similar distribution of TLR5, a finding confirmed by Hozono et al. (2006) and by our lab (fig. 2). This suggests that TLR4 and 5 will only be activated when there is a breach in the epithelium thus preventing inappropriate inflammatory responses when the epithelium is intact (Zhang et al., 2003). Interestingly, Hozono et al. (2006) showed that flagellin from ocular pathogenic bacteria, but not that from ocular non-pathogens or intestinal pathogens, activated gene transcription and cytokine production in HCEC; however the mechanism underlying this is not understood.
Few studies have specifically addressed TLR expression in corneal layers other than the epithelium. Ebihara et al. (2007) detected TLR2 and 4, but not 3 or 9 in keratocytes from human cadaver corneas whereas we observed expression of all TLR mRNAs except 8 (Redfern RL et al., IOVS 2006;47: ARVO E-Abstract 4372) in stromal cells, but we did not differentiate between keratocytes and resident immune cells (Hamrah & Dana, 2007). We also found that cultured corneal fibroblasts express TLR1, 3, 4, 6, 7, and 9 and functional studies have shown that TLR3 (Liu et al., 2008) and TLR4 (Kumagai et al., 2005) activation in corneal fibroblast results in cytokine secretion. Such stromal TLR expression is expected to provide immediate surveillance against microbial infection following a breach in the ocular surface that penetrates the entire epithelial barrier.
In regards to the conjunctiva, immunohistochemistry revealed the expression of TLR2, 4, and 9 in both the epithelium and stroma, with staining being more intense in the stroma (Bonini et al., 2005). These findings were confirmed by Li et al. (2007) who additionally observed staining for TLR1, 3 and 5. Studies with samples collected by impression cytology and in cultured cells show that conjunctival epithelial cells typically express TLR1-6 and 9, have variable expression of TLR7 and do not express TLR 8 or 10 (Cook et al., 2005; Redfern RL et al., IOVS 2006;47: ARVO E-Abstract 4372). Similar results were also observed for limbal epithelial cells (Li et al., 2007). Activation of TLR1/2, 3, 4 and 5 has been shown to trigger primary conjunctival epithelial cell cytokine secretion (Li et al., 2007; Chung et al., 2009). However, Talreja et al. (2005) found that in a conjunctival epithelial cell line TLR4 agonists, LPS was unable to stimulate cytokine secretion due to lack of expression of MD-2.
In summary, despite contrasting data from some laboratories, the current literature indicates the expression of multiple TLRs by corneal and conjunctival cells. They are capable of responding to invading pathogens providing a valuable defense mechanism to reduce microbial infection, however, TLR activation has the potential to do more harm than good, as it can lead to a robust inflammatory response which may contribute to disease processes as discussed below and summarized in table 1.
Table 1.
Association of TLRs with Ocular Surface Diseases
| Disease | TLR |
|---|---|
| Herpes Simplex keratitis | TLR2,3,4,7,9 |
| Pseudomonas keratitis | TLR4,5,9 |
| Fungal keratitis | TLR2,4 |
| Vernal keratoconjunctivitis | TLR4,9 |
| Atopic Keratoconjunctivitis |
TLR2 |
| Sjögren’s syndrome | TLR1,2,3,4 |
| Non- Sjögren’s syndrome | TLR2,4,5,9 |
4. TLRs in Ocular Surface Disease
4.1 Infection
Given that the major function of TLRs is pathogen recognition, it follows that these receptors play an important role in the ocular surface immune response to infectious agents. TLR activation by pathogens on the ocular surface would be expected to result in production of cytokines and chemokines important for stimulating immune and inflammatory cell infiltration into the area to alleviate the microbial load and resolve the infection. Whereas TLR activation may facilitate activation of the acquired immune response by enhancing MHCII and co-stimulatory molecule expression on antigen presenting cells resident in the cornea and conjunctiva (Hamrah & Dana 2007; Yamagami et al., 2007) which may lead to activation of the acquired immune response. These two arms of the immune response may protect the ocular surface from microbial infection however the sequelae from the inflammatory response may result in damage to the ocular surface over and above that from the initial infection. Involvement of TLRs in various ocular infections is discussed here.
4.1.1 Herpes Simplex Keratitis
Herpes simplex virus (HSV)-1 is a frequent cause of corneal blindness that commonly necessitates a corneal transplant and is one of the leading cause of unilateral infectious corneal blindness worldwide (Liesegang, 2001). In the US and France, the incidence of HSV-1 new cases of ocular infection is estimated to be 8.4–13.2 per 100,000 person annually with an overall incidence, including recurrences, of 20.7–31.5 episodes per 100,000 persons (Labetoulle et al., 2005; Liesegang et al.,1989). Infection of the corneal epithelium and stroma leads to a robust inflammatory response which may produce sight-threatening keratitis. Although this response helps reduce viral load it also contributes to ocular surface damage, resulting in corneal scarring and vision loss. Notably, the absence of TLR2 or TLR4 and to a lesser extent TLR9 resulted in significantly diminished vision impairing HSV corneal lesions compared to wild-type (WT) mice (Sarangi et al., 2007). Furthermore, mice lacking the adapter molecule MyD88, which is required for signaling of all TLRs (except TLR3) were resistant to lesion development, but they were unable to control the HSV infection and most succumbed to lethal encephalitis (Sarangi et al., 2007). These findings suggest that TLR participation in particular, contributes to reducing the viral load but also promotes sight-threatening inflammation in HSV infection.
Jin et al. (2007) showed that almost all TLRs were expressed in human corneas with active HSV infection, but in particular TLR4, 7, 8 and 9 mRNA expression and TLR2 and TLR9 protein were upregulated relative to healthy corneas. Also, corneas with prior HSV infection showed an upregulation of TLR7 and downregulation of TLRs 2-10. Unfortunately as the authors did not correlate TLR expression with particular cell types it is difficult to fully interpret these observations.
Infection of corneal epithelial cells with HSV-1 (KOS strain) caused two peaks of activation of NFκB and MAPK (Li DQ et al., 2006). The first (1-4hrs post-infection) was associated with increased expression of IL-6, IL-8, TNFα and IFNβ. While the second phase (8hrs post-infection), was associated with enhanced expression of TLR7 but downregulation of TLR3, results in keeping with Jin et al. (2007). These observations suggest that TLRs may function sequentially; with TLR3 being activated first and TLR7 then being upregulated as a consequence of the infection (Li et al., 2006). Cook et al. (2004) have suggested that persistent TLR activation by HSV antigens and DNA may lead to prolonged expression of cytokines/chemokines and contribute to pathology after the active infection has subsided.
Together these data suggest that TLRs 3, 4, 7 and 9 participate in the epithelial response to HSV infection and activation of specific TLRs leads to the production of antiviral molecules that can directly participate in protecting the ocular surface. That TLR activation is important for resolution of viral infection is demonstrated by the success of Imiquimod, a TLR7 agonist, FDA approved for treating human papilloma viral infections which notably also has had some success in treating HSV infections in clinical studies (Miller et al., 2008). However, while activation of some TLRs is clearly beneficial, excessive inflammation at the ocular surface can also occur which may result in sight-threatening inflammation in attempts to control the viral infection.
4.1.2 Bacterial Keratitis
Pseudomonas aeruginosa (PA) and Staphylococcus aureus (SA) are two of the most common isolates from patients with microbial keratitis (Pachigolla et al., 2007), with PA being the most common cause of bacterial keratitis in extended-wear contact lens users (Green et al., 2007). Several studies have found that contact lens wear is the highest risk factor for developing serious bacterial keratitis (Kerautret et al., 2006). In the US, the overall annual rate of microbial keratitis with visual acuity loss in silicone hydrogel contact lens wearers is 3.6 per 10,000 (Schein et al., 2005). Notably, the number of ocular isolates of methicillin-resistant Staphylococcus aureus (MRSA) has increased from 4.1% in 1998 to 1999 to 16.7% in 2005 to 2006 (Freidlin et al., 2007). If not treated promptly microbial keratitis can lead to epithelial defects, stromal ulceration, scarring and vision impairment. Cultured corneal and conjunctival epithelial cells respond to ocular surface pathogens, their extracts and known PAMPs by the production of cytokines and chemokines characteristic of TLR activation which in vivo, is expected to recruit immune and inflammatory cells to destroy the invading pathogen. Furthermore the activation of ocular surface epithelial cells by pathogens and TLR agonists also leads to the production of antimicrobial peptides such as hBD-2 and the cathelicidin LL-37 (Kumar et al., 2006b, 2007; McNamara et al., 1999; Redfern RL et al., IOVS 2006; 47: ARVO E-Abstract 4372). These peptides can kill pathogens by causing membrane disruption through an electrostatic interaction of the positively charged peptide and the negatively charged microbial membrane (Radek & Gallo 2007). Thus, in addition to facilitating effector cell recruitment, TLR activation at the ocular surface is expected to lead to local production of antimicrobial peptides that can assist in eliminating pathogens.
Several studies have found that SA can cause severe keratitis in infected individuals and in animal models as characterized by bacterial invasion of the underlying stroma and intense neutrophil infiltration which results in corneal opacification and potentially loss of vision (Hume et al., 2001; Girgis et al., 2003; Hume et al., 2005; Bourcier et al. 2003, Sloop et al., 1999). In an experimental mouse model of SA keratitis, exposure of corneal epithelium to SA increased neutrophil recruitment to the corneal stroma, corneal thickness and corneal haze in normal C57Bl/6 mice, mice deficient TLR4 or TLR9, but not in mice deficient in TLR2 or MyD88, suggesting that S. aureus-induced corneal inflammation is mediated by TLR2 and MyD88 (Sun et al., 2006). The same group also reported that UV killed SA and Pam3Cys (TLR2 synthetic ligand) stimulated the phosphorylation of MAP kinases, JNK, p38 MAPK and ERK, and the blockade of JNK, but not that of p38 or ERK phosphorylation, had an inhibitory effect on IkB alpha degradation and CXC chemokine production (Adhikary et al., 2008). Furthermore they also found that corneal inflammation was significantly impaired in mice deficient in JNK1 mice compared with control mice, suggesting that JNK has an essential role in TLR2-induced corneal inflammation.
Extensive study of the underlying mechanism of the pathogenesis of PA keratitis in experimental models has revealed that mice can be divided in two groups based upon their immune response to the pathogen (Hazlett, 2004). BALB/c mice are resistant to PA infection as they mount a Th2 based response that facilitates recovery and corneal healing. While C57BL/6 mice are susceptible to PA infection as they mount a Th1 based immune response leading to corneal perforation. Comparison among these mouse strains provides a unique opportunity to understand the immune response to PA and involvement of TLRs. In 2005 Huang et al., reported that silencing TLR9 by siRNA in C57BL/6 mice resulted in less severe inflammation, reduced PMN infiltration but consequently increased bacterial load. These data suggested that TLR9 activation is required to adequately eliminate bacteria but that it also contributes to corneal destruction. Subsequently, the same group also reported that corneal TLR4 expression is increased in PA infection and deficiency of this receptor in BALB/c mice resulted in a susceptible rather than resistant phenotype (Huang et al., 2006a). These observations suggest that TLR4 is critical for resistance to PA keratitis.
Additional animal studies have shown that single Ig IL-1R-related molecule (SIGIRR) and ST2 are also required for resistance to PA infection (Huang et al., 2006b, 2007). SIGIRR and ST2 are negative regulators of TLR signaling which act by sequestering MyD88 and IRAK. Blocking SIGIRR or ST2 activity was associated with more serious clinical disease, indicating that while TLR signaling is required for resistance to PA keratitis, if its activity cannot be adequately regulated by SIGIRR/ST2 then ocular surface damage ensues. Thus, TLR participation in PA keratitis is essential for eliminating the organism, but is problematic as it contributes to corneal destruction. Such duality makes it difficult to envision modulating TLR activity as a means to control corneal damage.
In an interesting in vitro study, Maltseva et al., (2007) reported that a MyD88 dependent increase in corneal epithelial hBD-2 expression caused by exposure to PA supernatant was abrogated by the presence of a contact lens, thus giving new insight into the mechanism by which contact lens wear predisposes to PA keratitis. Additional in vivo studies have shown that defensins and LL-37 play an important role in protecting the ocular surface from PA infections. In particular, mice deficient in cathelicidin-related antimicrobial peptide (CRAMP), the murine homologue of LL-37are more susceptible to PA keratitis, had significantly delayed bacterial clearance and an increased number of infiltrating neutrophils in the cornea (Huang LC et al., 2007). A similar finding was reported in BALB/c mice following knock down of mBD-2 or mBD-3, but not of mBD-1 or mBD-4, by siRNA (Wu et al., 2009a, 2009b). Furthermore Wu et al. also found that silencing mBD2, mBD3 or both defensins resulted in a significant upregulation of TLR2, TLR4 and MyD88 but not TLR5 or TLR9 (Wu et al., 2009b). A recent study by Kumar et al. (2008) also has revealed an interesting therapeutic possibility. They observed that pre-treatment with the TLR5 agonist flagellin markedly reduced the severity of subsequent PA infection in C57BL/6 mice. This was in part due to induction of corneal expression of the antimicrobial molecules, nitric oxide and cathelin-related antimicrobial peptide (the murine homologue of LL-37). They also observed similar results in vitro, as flagellin pre-treatment enhanced PA induced expression of hBD-2 and LL-37 in HCEC (Kumar et al., 2007). These observations raise the possibility of utilizing TLR activation as a prophylactic means of preventing an overwhelming inflammatory response and corneal destruction in PA keratitis.
4.1.3 Fungal Keratitis
Fungal keratitis is characterized by a severe inflammatory response initiated by virulence factors which is then exacerbated by the host response potentially leading to destruction of the cornea and poor visual outcome. Fungal keratitis typically occurs in tropical or subtropical climates such as in southern Florida, and Fusarium is the most common isolate (41%) followed by Candida (14%) and Apergillus (12%) (Iyer et al., 2006). In fact, fungal keratitis can account for up to 52% of microbial keratitis cases in CL wearers (Tuli et al., 2007) in Florida. A study from the Massachusettes Eye and Ear Infirmary in the Boston area, found that the number cases of fungal infections has doubled from 1999-2002 to 2004-2007 with approximately 1.0 case per month (Jurkunas et al., 2009). In early 2006, multiple reports of Fusarium keratitis among CL wearers were submitted to the Centers for Disease Control and Prevention which were later found to be associated with Bausch & Lomb ReNu (Rochester, NY) brand contact lens solution (Chang et al., 2006). Since the recall of this solution, the number of cases has dropped (Chang et. al., 2006) but in some cities fungal keratitis is the leading cause of microbial infections. In a three year retrospective study of patients with microbial keratitis in Brazil, Fusarium was the most common isolate from patients with microbial keratitis but ocular trauma was found to be the major risk factor (Furlantto et al., 2010) in these cases.
Little is known of the precise role of TLRs in fungal keratitis but they have been implicated in fungal recognition and subsequent cytokine production. Known fungal TLR ligands include zymosan which activates TLR2/TLR6 heterodimers, whereas mannan activates TLR4 (Roeder et al., 2004). Interestingly while TLR4 activation induces chemokine release and leukocyte recruitment, TLR2 activation results in the production of anti-inflammatory IL-10 and T-regulatory cell proliferation which may represent an attempt to circumvent host defense mechanisms (Netea et al., 2006).
As noted above, members of the genus Fusarium are the most frequently isolated organisms in patients with fungal keratitis (Iyer et al., 2006). Inactivated hyphae of Fusarium solani upregulate the expression of TLR2, 3, 4, and 6 mRNA, TLR2 and 4 protein and increase secretion of IL-6 and IL-8 in HCEC (Jin et al., 2008). Further, a recent in vivo study of contact lens associated Fusarium keratitis has shown that mice deficient in TLR4 but not TLR2 have impaired responses to Fusarium indicating that TLR4 plays a role in controlling growth and replication of the pathogen (Sun et al., 2009).
Aspergillus fumigatus is another fairly common culprit in fungal keratitis. Guo and Wu (2009) found that exposure of HCEC to Aspergillus fumigatus antigens upregulated TLR2 and TLR4 and stimulated the release of IL-1β and IL-6. Furthermore, they recently reported that exposure of HCEC to Aspergillus fumigatus antigens resulted in the release of IL-10 which was inhibited by treatment with TLR2, and TLR4 antibodies (Zhao et al., 2009). Together, these results suggest that TLR2 and TLR4 are involved in the ocular surface response to fungal pathogens but much remains to be understood of their specific roles.
In summary, TLRs play a critical role in recognizing and responding to various microbes on the ocular surface. The absence of specific TLRs can result in uncontrolled microbial infection which can be fatal in some animal models whereas excessive TLR activation can stimulate sight-threatening inflammation. Further understanding of the involvement of specific TLRs would shed light on how a balance between microbial clearance and an appropriate inflammatory response can be achieved, allowing for the development of potential therapeutic paradigms to optimize anti-microbial effects while minimizing damaging inflammatory responses.
4.2 Ocular Surface Inflammation
As activation of TLRs leads to the production of inflammatory cytokines it is reasonable to hypothesize that these receptors may play a role in mediating some of the events in inflammatory ocular surface disorders. In such a scenario it is envisaged that TLR activation would, most likely, be via various endogenous ligands and/or normal flora bacteria rather than pathogens. For example, a sudden excess of endogenous ligand may lead to TLR over-activation, or a breach of the superficial epithelial layers may provide access to TLRs normally hidden from ocular surface commensals. The involvement of TLRs in two ocular surface disorders characterized by inflammation, allergic conjunctivitis and dry eye, are discussed here.
4.2.1 Allergic Conjunctivitis
Allergic conjunctivitis (AC) refers to hypersensitivity disease affecting the eye lids, conjunctiva and sometimes the cornea. The term covers various clinical forms including seasonal allergic conjunctivitis, vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC). A number of studies have linked TLRs with systemic allergic disease (Bauer et al., 2007) thus it is reasonable to suggest that these versatile receptors may have a role in ocular allergy too. Bonini et al. (2005) evaluated TLR2, 4, 9 expression in conjunctival biopsy specimens from 10 patients with a normal ocular surface undergoing cataract surgery and 9 patients with VKC. All three TLRs were detected by immunostaining in the conjunctival epithelium and stroma of both normal and VKC patients, with staining being more intense in the stroma. Comparing the two groups, TLR4 expression was increased, TLR9 was decreased and there was no significant change in TLR2 expression in VKC versus normal conjunctiva. In VKC stroma some TLR4 staining was localized to CD4+ve T-cells, eosinophils and mast cells; also some TLR9 staining co-localized to CD4+ve T-cells and eosinophils but not mast cells. Positive staining was also found on cells with fibroblast-like morphology. It remains to be determined if these changes in TLR expression are the “cause or effect”. Signaling through TLR4 has been shown to induce a Th2 mediated allergic response in a mouse model (Eisenbarth et al., 2002) while increased expression of TLR4 may be accounted for by cells infiltrating the conjunctiva in response to VKC and it is possible that enhanced TLR4 expression and activation may lead to overproduction of pro-inflammatory cytokines and chemokines which exacerbate the ongoing inflammatory response.
Cook et al. (2005) noted increased immunostaining for TLR2 in conjunctival impression cytology samples from patients with AKC. Their accompanying in vitro investigation revealed that SA and IFNγ upregulated TLR2 expression in cultured conjunctival epithelial cells and that SA and TLR2 agonists stimulated IL-8 and TNFα production. As SA colonization is common in AKC, they suggested that SA maybe responsible for TLR2 upregulation and activation, and that TLR2 mediated cytokine production may contribute to the ongoing inflammatory response. The upregulation of TLR2 in patients with AKC raises an interesting possibility that TLR2 can also be activated by endogenous ligands. For example, HSP70, an endogenous ligand for TLR2 is expressed in the conjunctival epithelium in patients with AKC (Berra et al., 1994) and conceivably could contribute to inflammation by activating over-expressed TLR2.
In seeking to understand the mechanisms underlying ocular allergy Fukushima et al. (2006) treated mice with TLR2 agonist, Pam3CSK4 to determine if it could affect the development of experimental immune-mediated blepharoconjunctivitis induced by short ragweed pollen. Interestingly, treatment during the efferent phase significantly suppressed eosinophil infiltration and this was attributed to apoptosis of CD4+ T-cells. Previously, in a similar mouse model, it had been observed that oligonucleotides with unmethylated CpG motifs (TLR9 agonist) administered after ragweed sensitization inhibited the immediate hypersensitivity response and later infiltration of inflammatory cells and also induced a ragweed specific Th1 response (Magone et al., 2000; Miyazaki et al., 2000). These observations suggest that the development of AC may be modulated by TLR agonists. Indeed a large body of evidence has accumulated indicating that TLR9 ligands are effective in the prevention and treatment of animal models of a variety of allergic disorders and a number of TLR9 agonists are in clinical trials for allergic rhinitis and asthma (Hayashi & Raz, 2006; Kanzler et al., 2007).
4.2.2 Dry eye
Dry eye is a common multi-factorial disorder in which inflammation plays a major role. The core mechanisms are driven by tear hyperosmolarity (Gilbard et al., 1978; Farris 1994; Bron et al., 2002) and tear film instability which stimulates an increase in proinflammatory cytokines (Afonso et al., 1999; Pflugfelder et al., 1999; Solomon et al., 2001) at the ocular surface. The resulting inflammation exacerbates tear film instability and hyperosmolarity creating a vicious cycle (2007 Report of the Dry Eye Workshop). At its most severe (dry eye associated with the autoimmune disorder Sjögren’s syndrome (SS)) dry eye carries an increased risk of corneal ulceration (Vivino et al., 2001) and infection (Derk and Vivino 2004) which may result in vision loss. In SS, the activity of self-reactive lymphocytes leads to damage and destruction of the salivary and lacrimal glands leading to severe dry mouth and dry eye. Kawakami et al. (2007) studied TLR expression by immunohistochemistry in labial salivary glands and observed increased expression of TLR2, 3 and 4 and also the adaptor molecule MyD88 in samples from patients with SS. Staining was localized to acinar cells, ductal epithelial cells and infiltrating mononuclear cells. They also observed that TLR agonists stimulated production of IL-6 and expression of CD54 (ICAM-1) and thus concluded that TLR activation may contribute to the inflammatory microenvironment in SS. Spachidou et al., (2007) also reported TLR1-4 expression and upregulation of CD54, CD40 and MHC I in response to TLR activation in epithelial cells from labial salivary gland biopsies. Furthermore, expression of TLR1, 2 and 4 was significantly greater in cells from SS patients, suggesting the active participation of TLRs in the pathophysiology of SS. A gene array study also revealed upregulation of TLR8 and 9 in labial salivary glands from SS patients (Gottenberg et al., 2006). In a mouse model of SS, Killedar et al., (2006) observed that TLR3 and 7 and several of their downstream effectors were upregulated in the submandibular glands early in the disease course. Together these observations point to a role for TLRs in salivary gland inflammation in SS and considering similarities between the two secretory systems similar findings are expected in the lacrimal glands. In keeping with this, TLR4 mRNA expression was increased in the lacrimal glands and cornea in the MRL/lpr mouse model of (Christopherson PL, et al. IOVS 2005; 46: ARVO E-Abstract 4462)
Although SS causes severe dry eye, non-SS dry eye is more prevalent. In a pilot study, (Barabino S, et al.IOVS 2006; 47: ARVO E- Abstract 5594) TLR2 but not TLR4 mRNA was upregulated in conjunctival cells in patients with dry eye. However they did not observe a concomitant increase in TLR2 protein expression. In our own preliminary studies we have observed that while dry-eye related cytokines (IL-1α and β, TNFα and TGFβ) had no effect on ocular surface epithelial cell TLR expression, hyperosmotic culture media increased the expression of TLR4, decreased that of TLR9 but had no effect on TLR5 whereas a desiccating environment upregulated HCEC expression of TLR4 and 5 but downregulated TLR9 (Redfern R.L., Optom. Vis. Sci. 2007: 83: E-Abstract 065127). These preliminary data indicate that dry eye conditions differentially modulate TLR expression and are suggestive of TLR participation in the pathophysiology of non-SS as well as SS dry eye.
As noted earlier, it is unknown if altered ocular surface TLR expression in inflammatory conditions is cause or effect. However, regardless of the etiology a change in expression pattern may have both beneficial and detrimental effects. Upregulated expression may confer an enhanced ability for pathogen recognition, whereas reduced expression may lead to an inadequate response and therefore increased risk of infection. However, the latter may be compensated for by the fact that many pathogens are recognized in more than one way, by interactions with multiple TLRs and interaction with other PRRs. For example, mice deficient in TLR4 still respond to PA (Huang et al., 2006a), presumably in part through the activation of TLR5 by PA flagellin. Enhanced TLR expression may lead to inappropriate and exacerbated inflammatory responses thus contributing to disease processes such as allergy and dry eye, whereas reduced expression would be expected to be anti-inflammatory. In general, activation of the various TLRs leads to a similar response by ocular surface epithelial cells i.e. cytokine/chemokine and antimicrobial peptide production. Therefore it is possible that downregulation of the expression of some TLRs is a compensatory response for the upregulation of other TLRs, to try to minimize hyper-responsiveness. Much is yet to be learned about the contribution of TLRs to ocular allergy and dry eye. In particular, studies investigating endogenous TLR ligands (e.g. heat and stress shock proteins, high mobility group box 1 protein etc) are currently lacking but will be very important in furthering our understanding of the role of TLRs in the pathophysiology of ocular surface inflammation.
Summary.
As discussed here current evidence indicates that TLRs are important molecules expressed at the ocular surface. They have a primary role in detecting the presence of various pathogens leading to activation of innate immune responses such as production of antimicrobial peptides and recruitment of immune and inflammatory cells by chemokine and cytokine production. Unfortunately, TLR action may be a double edge-sword as the consequence of their activation may contribute to ocular surface destruction during infection and the exacerbation of various ocular surface inflammatory conditions such as allergy and dry eye.
Acknowledgements
The authors thank Kim Thompson of the University of Houston College of Optometry (UHCO) audio-visual department for drawing figures 1 and 2. The authors’ own studies reported herein were supported by NIH grants EY13175 (AMM), EY07024 and EY18113 (RLR), EY07551 (UHCO CORE grant). RLR is also a recipient of the William C. Ezell Fellowship.
Abbreviations
- TLR
Toll-like receptor
- IL
interleukin
- PA
Pseudomonas aeruginosa
- SA
Staphylococcus aureus
- LPS
lipopolysaccharide
- NFκB
nuclear factor κB
- MyD88
myeloid differentiation protein 88
- IFN
interferon
- HCEC
human corneal epithelial cells
- HSV
herpes simplex virus
- VKC
vernal keratoconjuctivitis
- SS
Sjögren’s syndrome
Footnotes
Commercial interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–7. doi: 10.1189/jlb.1107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso AA, Obrin L, Monroy DC, et al. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Henriques-Normark B, et al. Role of the innate immune system in host defense against bacterial infections: Focus on Toll-like receptors. J Int Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Bauer S, Hangel D, Yu P. Immunobiology of toll-like receptors in allergic disease. Immunobiol. 2007;212:521–533. doi: 10.1016/j.imbio.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Begon E, Michel L, Flageul B, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17:497–506. doi: 10.1684/ejd.2007.0264. [DOI] [PubMed] [Google Scholar]
- Berra A, Dutt JE, Nouri M, Foster CS. Heat shock protein expression in human conjunctiva. Invest Ophthalmol Vis Sci. 1994;35:352–7. [PubMed] [Google Scholar]
- Beutler B. TLR4: Central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- Blais DR, Vascotto SG, Griffith M, et al. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–4244. doi: 10.1167/iovs.05-0543. [DOI] [PubMed] [Google Scholar]
- Bonini S, Micera A, Iovieno A, et al. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmol. 2005;112:1528–1534. doi: 10.1016/j.ophtha.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Yokoi N, et al. Using osmolarity to diagnose dry eye: a compartmental hypothesis and review of our assumptions. Adv Exp Med Biol. 2002;506:1087–1095. doi: 10.1007/978-1-4615-0717-8_153. [DOI] [PubMed] [Google Scholar]
- Chang DC, Grant GB, O’Donnell K, et al. Fusarium Keratitis Investigation Team. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;23:953–63. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–5. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Chung SH, Kweon MN, Lee HK, Choi SI, Yang JY, Kim EK. Toll-like receptor 4 initiates an innate immune response to lipopolysaccharide in human conjunctival epithelial cells. Exp Eye Res. 2009;88:49–56. doi: 10.1016/j.exer.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Cook EB, Stahl JL, Esnault S, et al. Toll-like receptor 2 expression on human conjunctival epithelial cells: a pathway for Staphylococcus aureus involvement in chronic ocular proinflammatory responses. Ann Allergy Asthma Immunol. 2005;94:486–497. doi: 10.1016/S1081-1206(10)61120-9. [DOI] [PubMed] [Google Scholar]
- Derk CT, Vivino FB. A primary care approach to Sjögren’s syndrome. Helping patients cope with sicca symptoms. Postgrad Med. 2004;116:49–54. doi: 10.3810/pgm.2004.09.1587. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Ebihara N, Yamagami S, Chen L, et al. Expression and function of Toll-like receptor-3 and -9 in human corneal myofibroblasts. Invest Ophthalmol Vis Sci. 2007;48:3069–3076. doi: 10.1167/iovs.06-0968. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, et al. Lipopolysaccharide enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris RL. Tear osmolarity – a new gold standard? Adv Exp Med Biol. 1994;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]
- Freidlin J, Acharya N, Lietman TM, Cevallos V, Whitcher JP, Margolis TP. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthalmol. 2007;144:313–5. doi: 10.1016/j.ajo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Yamaguchi T, Ishida W, et al. TLR2 agonist ameliorates murine experimental allergic conjunctivitis by inducing CD4 positive T-cell apoptosis rather than by affecting the Th1/Th2 balance. Biochem Biophys Res Communs. 2006;339:2048–1055. doi: 10.1016/j.bbrc.2005.11.114. [DOI] [PubMed] [Google Scholar]
- Furlanetto R, Andreo EG, Finotti IG, Arcieri ES, Ferreira MA, Rocha FJ. Epidemiology and etiologic diagnosis of infectious keratitis in Uberlandia, Brazil. Eur J Ophthalmol. 2010 Feb 20; doi: 10.1177/112067211002000312. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Farris RL, Santamaria J., III Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- Girgis DO, Sloop GD, Reed JM, O’Callaghan RJ. A new topical model of Staphylococcus corneal infection in the mouse Invest. Ophthalmol. Vis. Sci. 2003;44:1591–1597. doi: 10.1167/iovs.02-0656. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc Natl Acad Sci USA. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–7. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- Guo H, Wu X. Innate responses of corneal epithelial cells against Aspergillus fumigatus challenge. FEMS Immunol Med Microbiol. 2009;56:88–93. doi: 10.1111/j.1574-695X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Dana MR. Corneal-antigen presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- Hasan U, Chaffols C, Gaillard C, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Raz E. TLR9-based immunotherapy for allergic disease. Am J Med. 2006;119:897.e1–897.e6. doi: 10.1016/j.amjmed.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hozono Y, Ueta M, Hamuro J, et al. Human corneal epithelial cells responds to ocular-pathogenic, but not to nonpathogenic-flagellin. Biochem Biophys Res Communs. 2006;347:238–247. doi: 10.1016/j.bbrc.2006.06.088. [DOI] [PubMed] [Google Scholar]
- Huang X, Barrett RP, McClellan SA, et al. Silencing Toll-like receptor-9 in pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- Huang X, Du W, McClellan SA, et al. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006a;47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- Huang X, Hazlett LD, Du W, et al. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R and TLR4 signaling. J Immunol. 2006b;177:548–556. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- Huang LC, Reins RY, Gallo RL, McDermott AM. Cathelicidin-deficient (Cnlp −/− ) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4498–508. doi: 10.1167/iovs.07-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Du W, Barrett RP, et al. ST2 is essential for TH2 responsiveness and resistance to pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4626–4633. doi: 10.1167/iovs.07-0316. [DOI] [PubMed] [Google Scholar]
- Hume EB, Cole N, Khan S, Garthwaite LL, Aliwarga Y, Schubert TL, Willcox MD. A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunol. Cell Biol. 2005;83:294–300. doi: 10.1111/j.1440-1711.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- Hume EB, Dajcs JJ, Moreau JM, Sloop GD, Willcox MD, O’Callaghan RJ. Staphylococcus corneal virulence in a new topical model of infection. Invest. Ophthalmol. Vis. Sci. 2001;42:2904–2908. [PubMed] [Google Scholar]
- Iyer SA, Tuli SS, Wagoner RC. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006;32:267–71. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- Jin X, Qin Q, Chen W, et al. Expression of Toll-like receptors in healthy and herpes simplex virus-infected cornea. Cornea. 2007;26:847–852. doi: 10.1097/ICO.0b013e318093de1f. [DOI] [PubMed] [Google Scholar]
- Jin X, Qin Q, Lin Z, Chen W, Qu J. Expression of toll-like receptors in the Fusarium solani infected cornea. Curr Eye Res. 2008;33:319–24. doi: 10.1080/02713680802008238. [DOI] [PubMed] [Google Scholar]
- Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea. 2009;28:638–43. doi: 10.1097/ICO.0b013e318191695b. [DOI] [PubMed] [Google Scholar]
- Kanzler H, Barrat FJ, Hessel EM, et al. Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- Kerautret J, Raobela L, Colin J. Serious bacterial keratitis: a retrospective clinical and microbiological study. J Fr Ophtalmol. 2006 Oct;29(8):883–8. doi: 10.1016/s0181-5512(06)70108-5. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nakashima K, Tamai M, et al. Toll-like receptor in salivary glands from patients with Sjögren’s syndrome: functional analysis by human salivary gland cell line. J Rheumatol. 2007;34:1019–1026. [PubMed] [Google Scholar]
- Killedar SY, Eckenrode SE, McIndoe RA, et al. Early pathogenesis events associated with Sjögren’s syndrome (SjS)-like disease of the nod mouse using microarray analysis. Lab Invest. 2006;86:1243–1260. doi: 10.1038/labinvest.3700487. [DOI] [PubMed] [Google Scholar]
- Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med. 2009;87:125–38. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N, Fukuda K, Fujitsu Y, et al. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest Opthalmol Vis Sci. 2005;46:114–120. doi: 10.1167/iovs.04-0922. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu F-SX. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: Role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest Ophthalmol Vis Sci. 2004;45:3513–3522. doi: 10.1167/iovs.04-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu F-SX. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006a;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu F-SX. Toll-like receptor 2-mediated expression of β-defensin-2 in human corneal epithelial cells. Microb Infect. 2006b;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yin J, Zhange J, et al. Modulation of corneal epithelial innate immune response to Pseudomonas infection by flagellin pretreatment. Invest Opthalmol Vis Sci. 2007;48:4664–4670. doi: 10.1167/iovs.07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Hazlett LD, Yu FS. Flagellin suppresses inflammatory response and enhances bacterial clearance in murine model of pseudomonas keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–895. doi: 10.1016/j.ophtha.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Lamphier MS, Sirois CM, Verma A, et al. TLR9 and the recognition of self and non-self nucleic acids. Ann NY Acad Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shen J, Beuerman RW. Expression of toll-like receptors in human limbal and conjunctival epithelial cells. Mol Vis. 2007;8:813–22. [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang J, Kumar A, et al. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 2006;117:167–176. doi: 10.1111/j.1365-2567.2005.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang J. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kimura K, Yanai R, Chikama T, Nishida T. Cytokine, chemokine, and adhesion molecule expression mediated by MAPKs in human corneal fibroblasts exposed to poly(I:C) Invest Ophthalmol Vis Sci. 2008;49:3336–44. doi: 10.1167/iovs.07-0972. [DOI] [PubMed] [Google Scholar]
- Magone MT, Chan C-C, Beck L, et al. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur J Immunol. 2000;30:1841–1850. doi: 10.1002/1521-4141(200007)30:7<1841::AID-IMMU1841>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Maltseva IA, Fleiszig SMJ, Evans DJ, et al. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human β-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res. 2007;85:142–153. doi: 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Tanabe M, et al. Subcellular localization of toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21:69–87. doi: 10.1358/dnp.2008.21.2.1188193. Review. [DOI] [PubMed] [Google Scholar]
- Miyazaki D, Liu G, Clark L, et al. Prevention of acute allergic conjunctivitis and late-phase inflammation with immunostimulatory DNA sequences. Invest Ophthalmol Vis Sci. 2000;41:3850–3855. [PubMed] [Google Scholar]
- Netea MG, Ferwerda G, van der Graaf CA, Van der Meer JW, Kullberg BJ. Recognition of fungal pathogens by toll-like receptors. Curr Pharm Des. 2006;12:4195–201. doi: 10.2174/138161206778743538. Review. [DOI] [PubMed] [Google Scholar]
- Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens. 2007;33:45–9. doi: 10.1097/01.icl.0000234002.88643.d0. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. Toll-like receptors as key mediators in innate antifungal immunity. Med Mycol. 2004;42:485–98. doi: 10.1080/13693780400011112. [DOI] [PubMed] [Google Scholar]
- Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–38. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein OD, McNally JJ, Katz J, Chalmers RL, Tielsch JM, Alfonso E, Bullimore M, O’Day D, Shovlin J. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005 Dec;112(12):2172–9. doi: 10.1016/j.ophtha.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Sloop GD, Moreau JM, Conerly LL, Dajcs JJ, O’Callaghan RJ. Acute inflammation of the eyelid and cornea in Staphylococcus keratitis in the rabbit. Invest. Ophthalmol. Vis. Sci. 1999;40:385–391. [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, et al. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2282–2292. [PubMed] [Google Scholar]
- Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and Toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- Spachidou MP, Bourazopoulou E, Maratheftis CI, et al. Expression of functional toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2007;147:497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–32. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chandra J, Mukherjee PK, Szczotka-Flynn L, Ghannoum M, Pearlman E. A murine model of contact lens associated Fusarium keratitis. Invest. Ophthalmol. Vis. Sci. 2009 doi: 10.1167/iovs.09-4237. First published as doi:10.1167/iovs.09-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Aci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by toll-like receptors. J Endotoxin Res. 2002;8:459–463. doi: 10.1179/096805102125001073. [DOI] [PubMed] [Google Scholar]
- Talreja J, Dileepan K, Puri S, et al. Hman conjunctival epithelial cells lack lipopolysaccharide responsiveness due to deficient expression of MD2 but respond after interferon-γ priming or soluble MD2 supplementation. Inflammation. 2005;29:170–181. doi: 10.1007/s10753-006-9014-y. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Baochong Gao. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. Review. [DOI] [PubMed] [Google Scholar]
- Tuli SS, Iyer SA, Driebe WT. Jr. Fungal keratitis and contact lenses: an old enemy unrecognized or a new nemesis on the block? Eye Contact Lens. 2007;33:415–7. doi: 10.1097/ICL.0b013e318157e999. [DOI] [PubMed] [Google Scholar]
- Ueta M, Nochi T, Jang MH, Park EJ, Igarashi O, Hino A, Kawasaki S, Shikina T, Hiroi T, Kinoshita S, Kiyono H. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–47. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- Ueta M, Hamuro J, Kiyono H, et al. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–294. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- von Aulock S, Morath S, Hareng L, et al. Lipoteichoic acid from Staphylococcus aureus is a potent stimulus for neutrophil recruitment. Immunobiol. 2003;208:413–422. doi: 10.1078/0171-2985-00285. [DOI] [PubMed] [Google Scholar]
- Vivino FB, Minerva P, Huang CH, et al. Corneal melt as the initial presentation of primary Sjögren’s syndrome. J Rheumatol. 2001;28:379. [PubMed] [Google Scholar]
- Wu X, Gao J, Ren M. Expression profiles and function of Toll-like receptors in human corneal epithelia. Chin Med J. 2007;120:893–897. [PubMed] [Google Scholar]
- Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol. 2009a;182:1609–16. doi: 10.4049/jimmunol.182.3.1609. [DOI] [PubMed] [Google Scholar]
- Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J Immunol. 2009b;183:8054–60. doi: 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Yokoo S, Amano S, et al. Characterisation of bone marrow derived cells in the substantia propria of the human conjunctiva. Invest Ophthalmol Vis Sci. 2007;48:4476–4481. doi: 10.1167/iovs.06-1543. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu K, Ambati B, et al. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kumar A, Wheater M, Yu FS. Lack of MD-2 expression in human corneal epithelial cells is an underlying mechanism of lipopolysaccharide (LPS) unresponsiveness. Immunol Cell Biol. 2009;87:141–8. doi: 10.1038/icb.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15:155–68. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]