Abstract
Purpose
To review the cases of viral retinitis following intravitreal steroid administration at a single center, to estimate the incidence, and to propose risk factors for its occurrence.
Design
Retrospective, observational case series
Methods
736 intravitreal triamcinolone (IVTA) injections were given in the clinic and operating room by three retina specialists at a single academic medical center, between September 2002 to November 2008. Inclusion criteria were simply a history of one or more IVTA injections during the period. The overall incidence of viral retinitis following IVTA was calculated. Subsequently, a chart audit was performed to estimate the number of patients with immune-altering conditions who had received IVTA during the time period, and the incidence within this subgroup was calculated.
Results
Viral retinitis developed following IVTA injection in three patients, yielding an overall incidence of 3/736 or 0.41%. An estimated 334 injections were given to patients with an immune-altering condition, including diabetes. All three of the patients who developed viral retinitis following IVTA possessed abnormal immune systems, yielding an incidence rate of 3/334 or 0.90% within this subgroup.
Conclusions
Our high reported incidence for this potentially devastating complication can be attributed to multiple factors, including coexisting medical immunocompromising comorbidities, a higher dose with a longer duration of local immunosuppression in the vitreous, multiple injections, as well as previous viral retinitis. Caution with a high index of clinical suspicion and frequent follow-up is advised in patients receiving IVTA with potentially immune-altering conditions, even after apparent immune recovery.
Introduction
Viral retinitis is a devastating condition with disastrous consequences. Depending on the etiologic agent and immune status of the victim, the infection can often rapidly progress to involve the entire retina, including the macula, ultimately leading to severe loss of vision. Cytomegalovirus (CMV) was the first viral retinitis to be described and remains the most common etiologic agent. It generally affects severely immunocompromised patients, such as human immunodeficiency virus (HIV) positive individuals with CD4 T-lymphocyte counts below 50 cells/mm3 or rarely, those with iatrogenic immunosuppression following chemotherapy, organ transplantation, or systemic corticosteroids for other indications1-5. Acute retinal necrosis (ARN) was initially reported by Urayama et al in 1971 and is most common in young immunocompetent adults6. Freeman et al demonstrated herpes group virus in endoretinal biopsy specimens in the acute phase of the disease, with varicella zoster virus (VZV) or herpes simplex virus (HSV) being the usual culprits7. Patients left untreated can suffer contralateral eye involvement within one month in up to a third of cases. More recently, a highly destructive, rapidly progressive variant of ARN that occurs solely in severely immunocompromised individuals has been described. Progressive outer retinal necrosis (PORN) syndrome is caused by the same viruses as ARN, and leads to rapid involvement of the central and peripheral retina by full thickness necrosis, ultimately leading to severe vision loss from retinal detachment or optic atrophy6. Although the virulence and rate of destruction of viral retinitis can vary, prompt appropriate treatment is the key to a favorable visual outcome. However, the therapeutic agents employed are often associated with frequent and severe systemic side effects, namely bone marrow suppression and nephrotoxicity. Patients, especially those that are immunocompromised, often require lifelong prophylaxis with these toxic agents to avoid recurrent infections in the same and contralateral eye.
In recent years, case reports have surfaced identifying immunocompetent patients who developed viral retinitis following the administration of intravitreal steroids8-14. As the popularity of intravitreal triamcinolone acetonide injection (IVTA) and the indications for its use have grown over the years, deleterious side effects such as elevated intraocular pressure, posterior subcapsular cataracts, vitreous hemorrhage, and endophthalmitis have become well known to ophthalmologists, and intravitreal steroids are often avoided in patients deemed to be at higher risk of these complications15-19. Viral retinitis however, has received far less attention as a potential consequence of the medication. In 2005, Saidel et al first described CMV retinitis (diagnosed by vitreous fluid polymerase chain reaction (PCR)) four months following IVTA in a patient treated for clinically significant macular edema (CSME) from diabetes 8. Delyfer et al reported two immunocompetent patients who developed CMV retinitis following IVTA use, for exudative age-related macular degeneration (ARMD) in one case and macular edema resulting from a central retinal vein occlusion (CRVO) in the second9. The cases were diagnosed via anterior chamber paracentesis and PCR. A recent paper by Ufret-Vincenty et al presented a patient diagnosed with CMV retinitis (by clinical appearance) following placement of a Fluocinolone Acetonide (Retisert) implant for vitritis and macular edema secondary to Behcet's disease10. The patient had not received systemic immunosuppressive medication for five months prior to the development of retinitis. Other papers have described ARN due to presumed HSV (on the basis of positive serum antibodies) following IVTA in previously immunocompetent patients, although PCR was not performed on aqueous or vitreous fluid11,12.
Although reports of viral retinitis following IVTA have begun to surface in the literature, no information on the incidence of this potentially severe complication exists. Furthermore, minimal discussion of risk factors predisposing individuals to viral retinitis following IVTA has been presented. Our purpose is to review the cases of viral retinitis following intravitreal steroid administration at a single center, to estimate the incidence, and to propose risk factors for its occurrence. Following are three cases of primary or reactivated viral retinitis that developed following IVTA from our institution in patients with immune-altering medical comorbidities.
Methods
We retrospectively reviewed the charts of patients from our institution who had received IVTA from three retina specialists during the period September 2002 to November 2008. No patients received IVTA at our institution prior to 2002. All cases were included, regardless of diagnosis code or indication for the treatment. The most common diagnoses were cystoid macular edema, macular edema, and diabetic macular edema. Settings for the injections included the clinic and operating room. The overall incidence of this complication was calculated as the number of viral retinitis cases divided by the total number of IVTA injections administered. Subsequently, a chart audit was performed to estimate the number of patients with altered immunity who had received IVTA during the time period. Responsible conditions included diabetes mellitus (DM), HIV/AIDS (Acquired Immunodeficiency Syndrome), or use of long-term oral steroids or steroid-sparing immunomodulatory or chemotherapeutic agents.
Results
A total of 736 IVTA injections were given over this period. Viral retinitis subsequently developed in three patients. The overall incidence of viral retinitis following IVTA at our institution was 3/736 or 0.41%. Of the 736 total injections, an estimated 334 were given to patients with an immune-altering condition, including diabetes. All three of the patients at our center who developed viral retinitis following IVTA possessed abnormal immune systems. The calculated incidence of viral retinitis following IVTA among this subgroup at our institution was 3/334 or 0.90%. Table 1 describes the clinical data for the three patients. The mean age of the patients who developed viral retinitis was 59.3 years (range 43-73). Two were male and one was female. Indications for IVTA were macular edema from various causes. The patients received either one or two injections, and the dose of IVTA administered was 20 mg/0.1 cc in all three cases. Two patients had been previously vitrectomized. The mean interval to the development of retinitis following the final IVTA was sixteen weeks (range 10-26).
Table 1.
Characteristics of Patients who Developed Viral Retinitis Following Intravitreal Triamcinolone Injection
| Age | Medical Comorbidities | Indication for IVTA | Number of Injections | Dose (mg/0.1cc) | Previous Vitrectomy | Interval to Retinitis (weeks) | Etiology | Diagnosis Confirmation | Prior Retinitis | Pre-IVTA VA | Post-retinitis VA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 62 | NIDDM | CME from BRVO | 2 | 20 | yes | 26 | CMV | Vitreous PCR | none | 20/200 | 20/400 |
| 43 | HIV | CME from IRU | 1 | 20 | no | 13 | CMV | Prior CMV retinitis, clinical appearance | CMV | 20/40 | 20/40 |
| 73 | Metastatic ovarian cancer on chemotherapy | IRF from ERM | 1 | 20 | yes | 10 | VZV | Response to treatment | none | 20/200 | 20/400 |
NIDDM – non-insulin dependent diabetes mellitus, HIV – human immunodeficiency virus, CME – cystoid macular edema, BRVO – branch retinal vein occlusion, IRU – immune recovery uveitis, IRF – intra-retinal fluid, ERM – epiretinal membrane, CMV – cytomegalovirus, VZV – varicella zoster virus, PCR – polymerase chain reaction, IVTA – intravitreal triamcinolone, VA – visual acuity
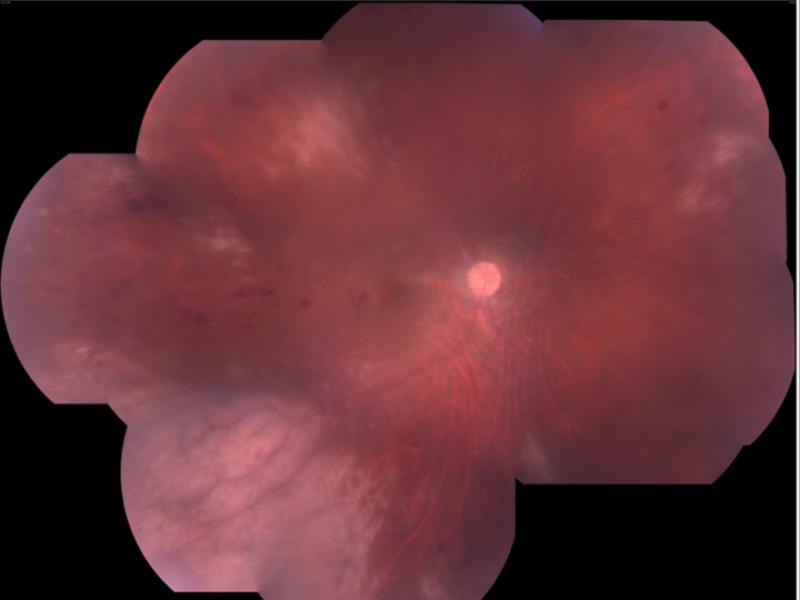
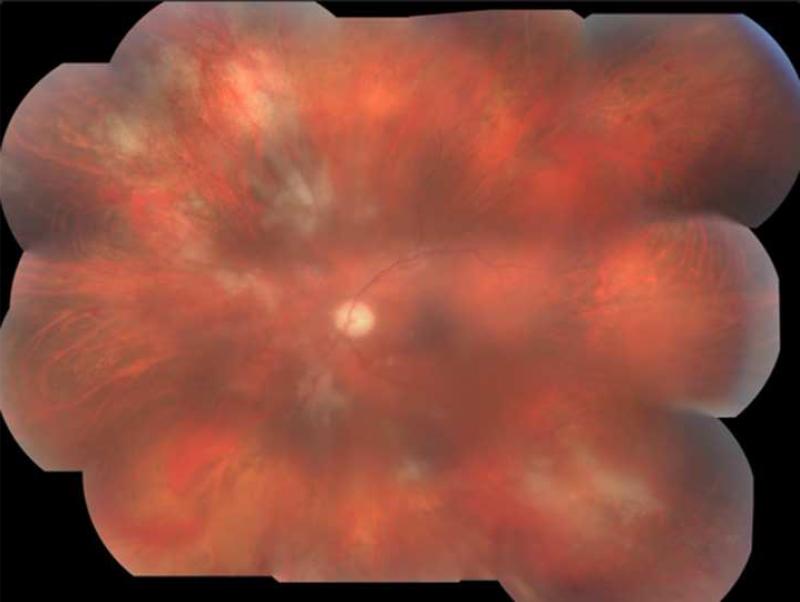
Patient one was a diabetic male who received IVTA for recurrent macular edema secondary to a branch retinal vein occlusion (BRVO) that had been refractory to grid laser and anti-VEGF therapy. Six months following a second IVTA during a vitrectomy with epiretinal membrane peel, the patient presented with 3+ anterior chamber cell, keratic precipitates, vitritis, and foci of retinal whitening and hemorrhage in the superotemporal and inferotemporal periphery (Fig. 1). Although initial vitreous biopsy yielded negative cultures and PCR for CMV, HSV, and VZV, a clinical diagnosis of ARN was made and Valacyclovir 1g three times daily was initiated. However, progressive worsening of the retinitis led to retinal detachment requiring vitrectomy with endolaser and silicone oil. Repeat PCR analysis of vitreous fluid at this time was positive for CMV, and therapy was switched to Valgancyclovir 900mg twice daily. CD4 T-lymphocyte count was 307 cells/uL with no history of immunodeficiency other than diabetes. Valgancyclovir was continued for six more weeks, at which time all retinitis lesions were healed.
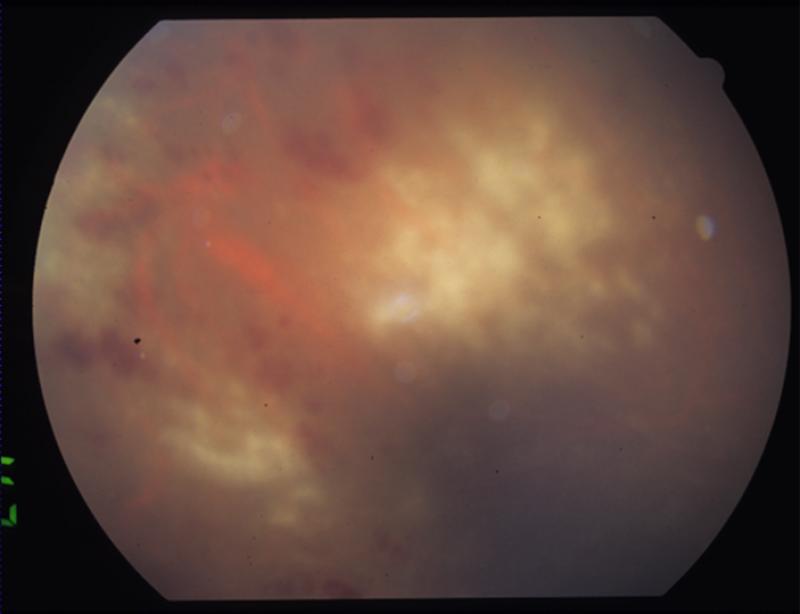
FIGURE 1.
Patient 1 with active CMV retinitis. (Top left) The patient's fundus is shown, with active CMV retinitis and hemorrhage in the superotemporal periphery twenty-six weeks after a second intravitreal triamcinolone (IVTA) injection. (Top right) The view of the macula is obstructed due to vitritis. Active CMV retinitis is visible in the superotemporal and inferotemporal periphery. (Bottom left) A wide angle view of the fundus shows a large area of inferotemporal CMV retinitis and a smaller focus superotemporally.
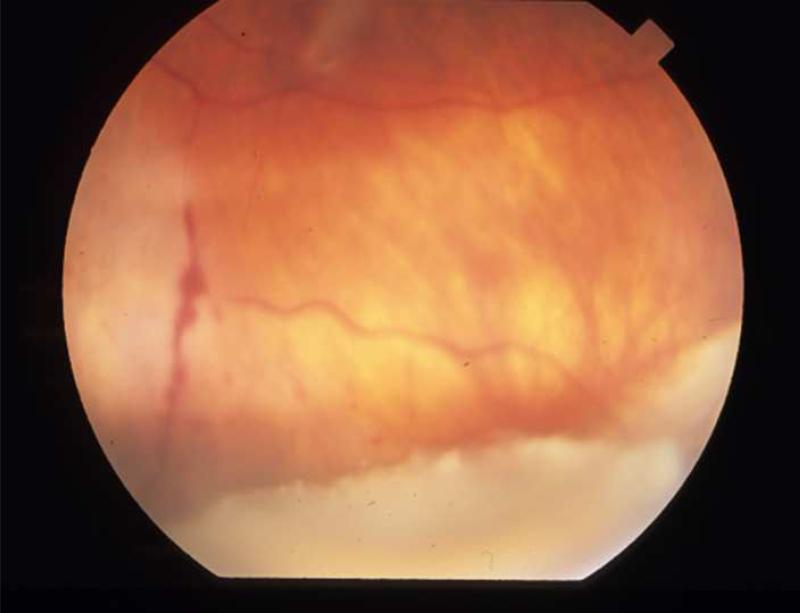
Patient two was an HIV positive male with a history of CD4 count below 50 cells/uL and CMV retinitis, after which highly active antiretroviral therapy (HAART) was instituted. Within months, his CD4 count had risen to 325 and macular edema and vitritis had developed, leading to a diagnosis of immune recovery uveitis (IRU). As no CMV lesions were present, the maintenance dose of Valgancyclovir 450mg twice daily was discontinued and IVTA was given for the macular edema. The patient returned three months later with 3+ vitreous cell and apparent reactivation of CMV retinitis despite CD4 count of 417 cells/uL (Fig. 2). Valgancyclovir was begun at treatment dose of 900mg twice daily, and therapy is currently ongoing.
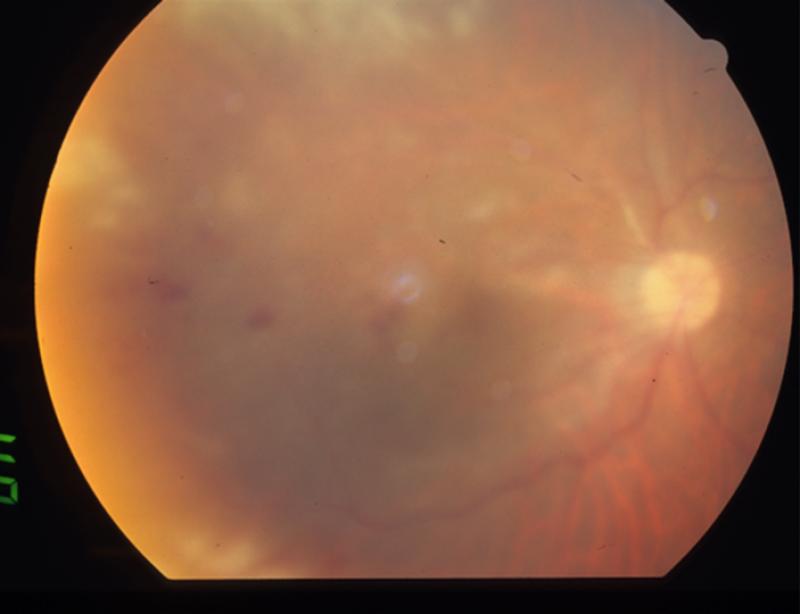
FIGURE 2.
Patient 2 with reactivated CMV retinitis. (Top left) The patient's fundus shows an area of healed CMV retinitis inferior to the optic nerve prior to IVTA and subsequent reactivation. (Top right) Thirteen weeks following IVTA, the fundus shows multiple areas of retinal whitening involving the posterior pole and periphery. The original healed CMV scar is visible inferior to the optic nerve.
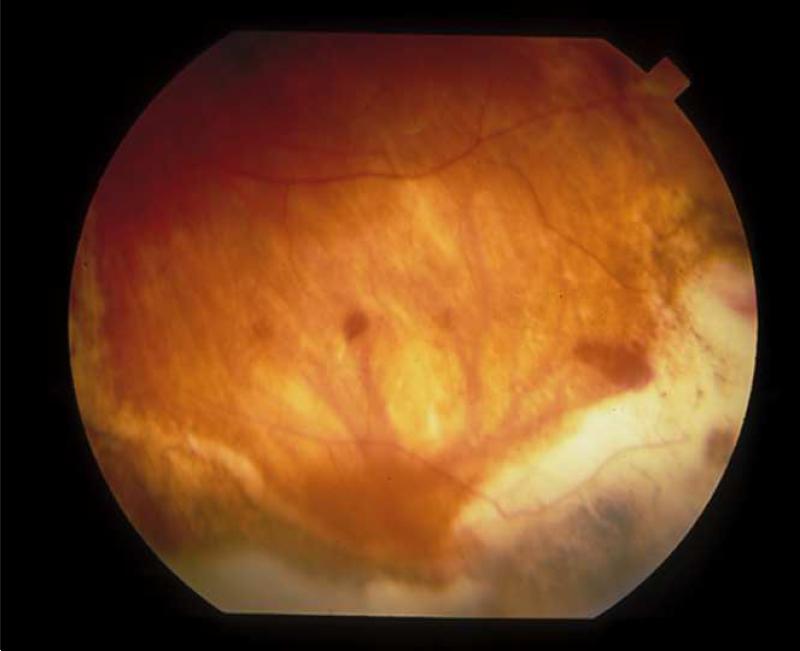
Patient three had a vision of 20/200 and an epiretinal membrane (ERM) and had received IVTA intraoperatively at the end of a vitrectomy and ERM removal. Ten weeks following the IVTA, the patient presented with 2+ cell in the anterior chamber and new retinitis suspicious for acute retinal necrosis (ARN) (Fig. 3). Medical comorbidities included metastatic ovarian cancer status post resection and radiation with active chemotherapy. Also of note was a history of VZV keratouveitis in June 2005 with recurrent anterior uveitis in October 2006. Foscarnet 2.4mg/0.1cc was given intravitreally and Valacyclovir 1g three times daily was initiated. Within two months, the anterior chamber was quiet, retinitis had resolved, and Valacyclovir was decreased to a maintenance dose to be continued indefinitely (Fig. 3).
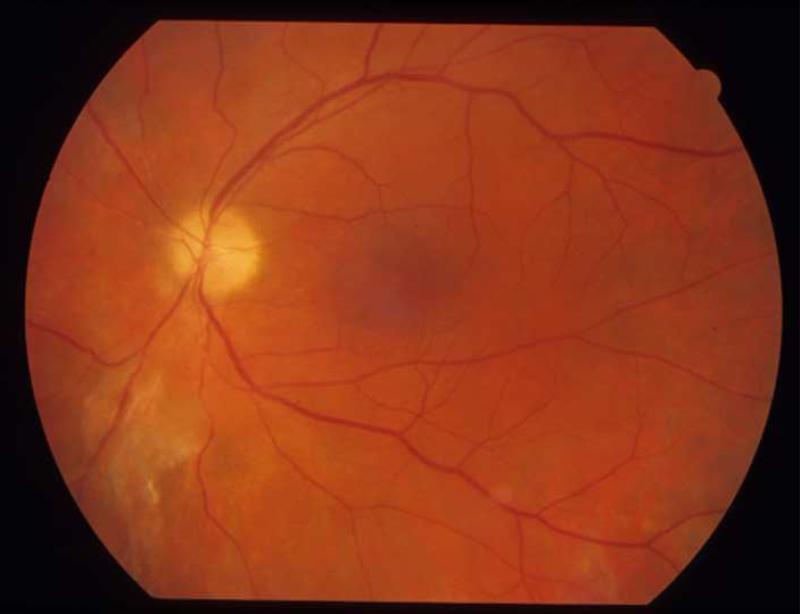
FIGURE 3.
Patient 3 with triamcinolone and ARN from VZV. (Top left) Residual triamcinolone is visible ten weeks following IVTA injection, along with inferior active retinitis due to ARN. (Top right) Healed ARN with chorioretinal scarring remains ten weeks later, following treatment.
Discussion
We report a single center series of cases of viral retinitis following IVTA and an estimate of the incidence of this complication. The incidence of 0.90% in the immune-altered subset is more than double our overall incidence of 0.41%. These incidences may seem high given the small number of case reports published thus far, and are likely higher than the experience of most retina specialists who employ IVTA in their clinical practices, many of whom may not be familiar with this complication. The Diabetic Retinopathy Clinical Research Network (DRCRnet) evaluated the safety and efficacy of two doses of preservative-free intravitreal triamcinolone versus focal/grid photocoagulation for the treatment of diabetic macular edema20. Five hundred and ten eyes received a total of 1649 intravitreal triamcinolone injections in 1mg and 4mg doses. No cases of viral retinitis were reported. The higher incidence at our institution likely represents an increased susceptibility to this complication in our patient population. As previously described, all three patients had coexisting systemic immune-altering conditions. One was HIV positive, one was receiving systemic chemotherapy for metastatic ovarian cancer, and another was diabetic. Furthermore, the HIV positive individual had previous viral retinitis during time periods when his CD4 T-lymphocyte count was below 50 cells/uL and experienced reactivation following IVTA despite improved CD4 counts and concomitant antiviral prophylaxis.
The HIV positive individual developed reactivation of CMV retinitis following IVTA for immune recovery uveitis macular edema. Immune recovery uveitis is relatively common in patients with CMV retinitis, with Jabs et al reporting a prevalence of 15.5%21. Our group has previously described the effective use of IVTA to treat immune recovery uveitis macular edema in seven patients, none of which experienced CMV reactivation over a minimum follow-up period of nine months22. However, viral retinitides are known to remain latent following infection, and subsequent local immunosuppression with IVTA may allow these dormant organisms to proliferate and cause reinfection despite presumed immune recovery. Delessandro and Bottaro reported a case of CMV reactivation following posterior subtenon's triamcinolone for immune recovery uveitis23. Our group previously reported a patient with AIDS who developed newly active CMV retinitis nineteen months after the initiation of HAART therapy, despite a documented median CD4 count of 204/uL (range 125-284/uL) during the treatment period24. In this particular patient, lymphoproliferative assays revealed that the CMV-specific T-cell response was found to be negligible. Furthermore, Jacobson et al described active CMV retinitis in patients with CD4 cell counts of greater than 195/uL during the early stages of response to antiretroviral therapy25. In addition, there have been previous reports of reactivation of CMV retinitis in patients despite high CD4 cell counts26-28. On the other hand, our group also observed four AIDS patients who were nonresponders to HAART, yet remained free of CMV retinitis recurrence despite CD4 cell count less than 50/uL and lack of anti-CMV therapy24. These patients illustrate that an arbitrary cutoff value cannot be established to predict protection from CMV retinitis.
The propensity for intraocular steroids to cause infection secondary to local immunosuppression is supported by data from a retrospective, multicenter case series of 922 IVTA injections that reported an endophthalmitis incidence rate of 0.87%29. In contrast, a retrospective, multicenter, consecutive case series of 12,585 intravitreal bevacizumab and 14,320 ranibizumab injections from four clinical centers found an endophthalmitis rate of only 0.02%30. A high index of suspicion may be warranted in patients with histories of viral retinitis prior to IVTA, regardless of apparent immune recovery or appropriate antiviral prophylaxis.
The role of diabetes mellitus as an immune-altering condition may often be overlooked in clinical practice. Diabetics are frequent victims of macular edema due to their damaged vascular endothelium and increased capillary permeability. The use of IVTA to treat diabetic macular edema has grown considerably in popularity in recent years, especially in patients with diffuse macular edema for which grid or focal laser therapy is impractical or ineffective. Similar to one of our diabetic patients who developed viral retinitis following IVTA with no other immunocompromising conditions, many of the case reports of viral retinitis following IVTA in supposedly immunocompetent patients occurred in diabetics8,9,14. Patient one in our study had been diagnosed with non-insulin-dependent diabetes mellitus only months prior to his initial presentation with a BRVO. Due to the very recent diagnosis, no hemoglobin A1c values were available, but the patient's morning fasting glucose levels varied between the mid 100s and low 200s during subsequent follow-up visits in the retina clinic. These values suggested not only poorly-controlled diabetes, but also raised the possibility that the patient maintained high blood glucose levels for an extended period of time prior to an official diagnosis of diabetes. This may have contributed to previously undiagnosed diabetic microangiopathy that not only predisposed the patient to a vascular occlusive event, but subsequently to persistent macular edema and vulnerability to viral retinitis following IVTA.
Another possible risk factor for viral retinitis following IVTA could be the dose given. Whereas most case reports of this complication, as well as the DRCRnet study previously referenced, employed a dose of 1mg/0.1cc or 4mg/0.1cc, the physicians at our institution most frequently administer 20mg decanted triamcinolone because of its presumed longer and stronger effect, potentially leading to fewer cumulative reinjections over time. Simple decantation allows the injection of a higher dose in a small volume after the removal of a significant portion of the benzyl alcohol preservative as verified by high-performance liquid chromatography31. Our group has directly compared the effect on intraocular pressure (IOP) of the standard 4mg versus the decanted 20mg IVTA and did not find a higher risk of IOP elevation with the higher dose32. Jonas et al similarly used a high dose of 20 to 25mg and have not reported a higher incidence of ocular adverse effects following their injections15. Furthermore, they have reported no cases of viral retinitis following high dose IVTA. His group has described measurable concentrations of triamcinolone following 25mg IVTA up to 1.5 years after the injection in nonvitrectomized eyes, despite the disappearance of clinically visible crystals after nine months33. Jonas’ group has also reported measurable concentrations of triamcinolone in silicone oil up to eight months after vitrectomy with silicone oil instillation and simultaneous 25mg IVTA34. Our group reported that the median time to disappearance of triamcinolone following 20mg injection in the vitrectomized eye was 113 days35. On the other hand, Beer and associates examined the pharmacokinetics of IVTA of the standard 4mg dose and found a mean elimination half-life of 18.6 days in nonvitrectomized patients, suggesting persistent measurable concentrations for approximately three months36. The half-life in vitrectomized patients was much shorter, only 3.2 days. It is apparent that a higher dose of IVTA remains in the vitreous for a much longer period of time, regardless of the vitrectomy status of the patient. However, the benefit of a longer duration of action of the higher dose of IVTA may come with the risk of prolonged local immunosuppression and susceptibility to primary infection or reactivation of endogenous latent viral retinitis. Patient one in our series developed viral retinitis sometime between two and six months following 20mg IVTA despite a previous vitrectomy. Although the viral retinitis was not detected until an exam six months following the second IVTA, we suspect that the retinitis developed shortly after a visit two months following the second injection. The combination of retinitis involving the superotemporal and inferotemporal periphery, along with already decreased visual acuity from macular edema, may have allowed the patient to remain asymptomatic, even at the time that the retinitis was finally detected. The other two patients both developed viral retinitis within three months of IVTA. Multiple IVTA and the resulting prolonged immunosuppression may also have increased patient one's vulnerability to the development of viral retinitis.
In conclusion, we have presented a single center series of cases of viral retinitis following IVTA. Our high reported incidence for this potentially devastating complication can be attributed to multiple factors. Coexisting medical immune-altering comorbidities, a higher dose with a longer duration of local immunosuppression in the vitreous, multiple injections, as well as previous viral retinitis, alone or in combination could account for the relatively high incidence obtained in our series. Caution with a high index of clinical suspicion and frequent follow-up is advised in patients receiving IVTA with potentially immunocompromising conditions, even after apparent immune recovery. Furthermore, ongoing antiviral prophylaxis both before and after IVTA injection may be prudent in patients with a history of viral retinitis.
Table 2.
Published Cases of Viral Retinitis Following Intravitreal Triamcinolone
| Author | Dose (mg/0.1cc) | Indication for IVTA | Etiology | Diagnosis Confirmation | Interval to Retinitis | Medical Comorbidities |
|---|---|---|---|---|---|---|
| MA Saidel et.al. | 4 | CSME | CMV | Vitreous PCR | 4 months | NIDDM |
| TY Toh et.al. | 4 | Exudative AMD (w/PDT) | HSV | Serum IgG and IgM | 5 months | None |
| T. Aggermann et.al. | 4 | CME due to CRVO | HSV | Serum IgG | 3 weeks | None |
| MN Delyfer et.al. | 20 | Exudative AMD (w/PDT) | CMV | AC PCR | 3 months | NIDDM |
| MN Delyfer et.al. | 8 × 3 | CME due to CRVO | CMV | AC PCR | 3 months | NIDDM |
| RL Ufret-Vincenty et.al. | Retisert × 2 | CME/vitritis due to Behcet's | CMV | clinical | 5 months after Retisert #2 | Behcet's Disease |
| T. Sekiryu et.al. | 4 | CME due to BRVO | CMV | AC PCR | 7 months | NIDDM |
| YS Park et.al. | 4 | CME due to CRVO | CMV | AC PCR | 4 months | None |
CSME – clinically significant macular edema, AMD – age-related macular degeneration, PDT – photodynamic therapy, CME – cystoid macular edema, CRVO – central retinal vein occlusion, BRVO – branch retinal vein occlusion, CMV – cytomegalovirus, HSV – herpes simplex virus, PCR – polymerase chain reaction, AC – anterior chamber, NIDDM – non insulin-dependent diabetes mellitus
Acknowledgements/Disclosure
A. Funding/Support: Support provided by NIH grant EY07366 (WRF), an unrestricted grant from Research to Prevent Blindness (UCSD), and a Heed Ophthalmic Fellowship (SFO).
B. Financial Disclosures: The authors indicate no financial conflict of interest in this study. Unrelated financial disclosures include the following: consultant to Opko Miami, FL (WRF), consultant to OD OS (WRF), grant support from Genentech (WRF), grant support from Geneneron (WRF), grant support from Sirion (WRF).
C. Author Contributions: design of study (AMS, SFO, WRF), conduct of study (AMS, SFO, WRF), data collection (AMS, SFO, WRF), data analysis (AMS, SFO, WRF), data interpretation (AMS, SFO, WRF), preparation and review of the manuscript (AMS, SFO, WRF).
D. IRB approval was obtained from the University of California, San Diego (UCSD) prior to the study.
Biography
 Ankur M. Shah, MD is a third year ophthalmology resident at the University of California, San Diego, where he serves as chief resident. He received a Bachelor of Science degree from the University of Michigan and his medical degree from Northwestern University. His research interests include medical retina and uveitis.
Ankur M. Shah, MD is a third year ophthalmology resident at the University of California, San Diego, where he serves as chief resident. He received a Bachelor of Science degree from the University of Michigan and his medical degree from Northwestern University. His research interests include medical retina and uveitis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiegand TW, Young LH. Cytomegalovirus retinitis. Int Ophthalmol Clin. 2006;46:91–110. doi: 10.1097/00004397-200604620-00010. [DOI] [PubMed] [Google Scholar]
- 2.Kuo IC, Kempen JH, Dunn JP, et al. Clinical characteristics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. Am J Ophthalmol. 2004;138:338–46. doi: 10.1016/j.ajo.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Crippa F, Corey L, Chuang EL, et al. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:214–9. doi: 10.1086/318447. [DOI] [PubMed] [Google Scholar]
- 4.Benz MS, Glaser JS, Davis JL. Progressive outer retinal necrosis in immunocompetent patients treated initially for optic neuropathy with systemic corticosteroids. Am J Ophthalmol. 2003;135:551–3. doi: 10.1016/s0002-9394(02)01978-5. [DOI] [PubMed] [Google Scholar]
- 5.Browning DJ. Acute retinal necrosis following epidural steroid injections. Am J Ophthalmol. 2003;136:192–4. doi: 10.1016/s0002-9394(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DA, Pyatetsky D. Necrotizing herpetic retinopathies. Focal Points: Clinical Modules for Ophthalmologists. 2008;26(10) [Google Scholar]
- 7.Freeman WR, Thomas EL, Rao NA, et al. Demonstration of herpes group virus in acute retinal necrosis syndrome. Am J Ophthalmol. 1986;102(6):701–9. doi: 10.1016/0002-9394(86)90396-x. [DOI] [PubMed] [Google Scholar]
- 8.Saidel MA, Berreen J, Margolis TP. Cytomegalovirus retinitis after intravitreal triamcinolone in an immunocompetent patient. Am J Ophthalmol. 2005;140:1141–3. doi: 10.1016/j.ajo.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 9.Delyfer MN, Rougier MB, Hubschman JP, et al. Cytomegalovirus retinitis following intravitreal injection of triamcinolone: report of two cases. Acta Ophthalmol Scand. 2007;85:681–3. doi: 10.1111/j.1600-0420.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 10.Ufret-Vincenty RL, Singh RP, Lowder CY, Kaiser PK. Cytomegalovirus retinitis after fluocinolone acetonide (Retisert™) implant. Am J Ophthalmol. 2007;143:334–5. doi: 10.1016/j.ajo.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Aggermann T, Stolba U, Brunner S, Binder S. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica. 2006;220:131–3. doi: 10.1159/000090579. [DOI] [PubMed] [Google Scholar]
- 12.Toh TY, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Royal Australian and New Zealand College of Ophthalmologists. 2006:380–2. doi: 10.1111/j.1442-9071.2006.01229.x. [DOI] [PubMed] [Google Scholar]
- 13.Park YS, Byeon SH. Cytomegalovirus retinitis after intravitreal triamcinolone injection in a patient with central retinal vein occlusion. Korean J Ophthalmol. 2008;22(2):143–4. doi: 10.3341/kjo.2008.22.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekiryu T, Tomohiro I, Kaneko H, Saito M. Cytomegalovirus retinitis after intravitreal triamcinolone acetonide in an immunocompetent patient. Jpn J Ophthalmol. 2008;52:414–6. doi: 10.1007/s10384-008-0576-0. [DOI] [PubMed] [Google Scholar]
- 15.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for the treatment of diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 16.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003;136:384–6. doi: 10.1016/s0002-9394(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 17.Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitis cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001;108:765–72. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 18.Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136:419–25. doi: 10.1016/s0002-9394(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 19.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–3. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–59. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabs DA, Van Natta ML, Kempen JH. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 22.Morrison VL, Kozak I, LaBree LD, et al. Intravitreal triamcinolone acetonide for the treatment of immune recovery uveitis macular edema. Ophthalmology. 2007;114:334–9. doi: 10.1016/j.ophtha.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Dalessandro L, Bottaro E. Reactivation of CMV retinitis after treatment with subtenon's corticosteroids for immune recovery uveitis in a patient with AIDS. Scand J Infect Dis. 2002;34:780–2. doi: 10.1080/00365540260348644. [DOI] [PubMed] [Google Scholar]
- 24.Song MK, Schrier RD, Smith IL, Plummer DJ, Freeman WR. Paradoxical activity of CMV retinitis in patients receiving highly active antiretroviral therapy. Retina. 2002;22:262–7. doi: 10.1097/00006982-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson MA, Zegans M, Pavan PR, et al. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–5. doi: 10.1016/S0140-6736(96)11431-8. [DOI] [PubMed] [Google Scholar]
- 26.Petit N, Zandotti C, Riss JM, et al. Relapse of CMV retinitis in an AIDS patient with high CD4 counts. J Antimicrob Chemother. 1998;41:666–7. doi: 10.1093/jac/41.6.666. [DOI] [PubMed] [Google Scholar]
- 27.Angus BJ, et al. HIV-associated CMV retinitis occurring in the setting of a high CD4 count: what about AIDS definitions? Int J STD AIDS. 1994;5:71–2. doi: 10.1177/095646249400500119. [DOI] [PubMed] [Google Scholar]
- 28.Frame RD, Hutchins RK, Skahan KJ, et al. Progression of cytomegaloviral retinitis in a patient responding to highly active antiretroviral therapy. Invest Ophthalmol Vis Sci. 1999;40(4):S873. [Google Scholar]
- 29.Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–6. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 30.Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28:1395–9. doi: 10.1097/IAE.0b013e3181884fd2. [DOI] [PubMed] [Google Scholar]
- 31.Morrison VL, Koh HJ, Cheng L, et al. Intravitreal toxicity of the Kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006;26:339–44. doi: 10.1097/00006982-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Tammewar AM, Cheng L, Kayikcioglu OR, et al. Comparison of 4 mg versus 20 mg intravitreal triamcinolone acetonide injections. Br J Ophthalmol. 2008;92:810–3. doi: 10.1136/bjo.2007.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:560–2. doi: 10.1016/j.ajo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Jonas JB. Concentration of intravitreally injected triamcinolone acetonide in intraocular silicone oil. Br J Ophthalmol. 2002;86:1450–1. doi: 10.1136/bjo.86.12.1450-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosobucki BR, Freeman WR, Cheng L. Photographic estimation of the duration of high dose intravitreal triamcinolone in the vitrectomised eye. Br J Ophthalmol. 2006;90:705–8. doi: 10.1136/bjo.2005.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]