Abstract
Background
Identifying persons at risk for sudden cardiac death (SCD) is challenging. A comprehensive evaluation may reveal clues about the clinical, anatomic, genetic and metabolic risk factors for SCD.
Methods
Seventy-one SCD victims (25–60 years-old) without an initially apparent cause of death were evaluated at the Hennepin County Medical Examiner’s office from August, 2001 to July, 2004. We reviewed their clinic records conducted next-of-kin interviews and performed autopsy, laboratory testing and genetic analysis for mutations in genes associated with the long-QT syndrome.
Results
Mean age was 49.5±7 years, 86% were male and 2 subjects had history of coronary heart disease (CHD). Coronary risk factors were highly prevalent in comparison to individuals of the same age group in this community (e.g. smoking 61%; hypertension 27%; hyperlipidemia 25%) but inadequately treated. On autopsy, 80% of the subjects had high-grade coronary stenoses. Acute coronary lesions and previous silent myocardial infarction (MI) were found in 27% and 34%, respectively. Further, 32% of the subjects had recently smoked cigarettes and 50% had ingested analgesics. Possible deleterious mutations of the ion channel genes were detected in 5 (7%) subjects. Of these, 4 were in the sodium channel gene SCN5A.
Conclusions
Overwhelming majority of the SCD victims in the community had severe subclinical CHD, including undetected previous MI. Traditional coronary risk factors were prevalent and under-treated. Mutations in the long-QT syndrome genes were detected in a few subjects. These findings imply that improvements in the detection and treatment of subclinical CHD in the community are needed to prevent SCD.
Keywords: death, sudden, epidemiology, genetics, pathology, coronary disease
Sudden cardiac death (SCD) is responsible for over 50% of all deaths due to cardiovascular disease, amounting to more than 300,000 deaths each year in the United States (1–3). Although advances have been made in preventing SCD in certain subsets of patients, identifying the high-risk individual in the general population is still challenging (4). Coronary heart disease (CHD) underlies the majority of SCDs in adults (5,6). Recently, genetic predisposition to SCD has also been suggested (7–10). However, predicting arrhythmic events on the basis of CHD alone is not feasible in individuals and specific mutations increasing the SCD risk in the community setting have not yet been identified. Furthermore, transient external factors (e.g. toxins) that help trigger SCD in individuals with abnormal anatomic or genetic substrate have often been elusive.
Many prior evaluations of SCD have either concentrated on high-risk populations (e.g. known CHD or heart failure) or have pursued a single-modality investigation strategy (e.g. autopsy alone). However, the interaction of an abnormal substrate and transient factors in SCD may require a multi-faceted approach, which includes clinical, anatomic, genetic and metabolic evaluations. The objective of the present study is to fill this gap in our knowledge by conducting a comprehensive evaluation of victims (age 25–60 years) of out-of-hospital SCD, in whom the etiology of the fatal event was not initially readily apparent. We reviewed the subjects’ clinic records, conducted next-of-kin interviews and performed autopsy, laboratory testing and genetic analysis for mutations in genes associated with the long-QT syndrome.
METHODS
Setting
Hennepin is the largest county (population > 1.2 million) in the seven-county metropolitan area of Minneapolis-St. Paul, Minnesota and includes the city of Minneapolis, as well as suburban and rural areas. By statute, all sudden or unexpected deaths in Hennepin County must be reported to the office of the county Medical Examiner (ME). Deaths due to other causes (e.g. violence, burns, foul-play etc.) and deceased persons with special circumstances (e.g. inmates, organ donors etc.) also need to be reported (complete list at www.hennepin.us) to the ME office. Thus, the ME receives reports from the police, ambulance services, funeral homes, emergency departments, physician practices and victims’ families. In general, autopsy is considered for all sudden death victims < 60 years-old with no obvious cause of death on presentation. Barring some special or legal circumstances, older individuals and those with a clinically-apparent cause of death (e.g. cancer, known CHD, chronic alcoholism etc.) are not routinely autopsied. It is also the office’s policy to include the family of the victim in making the decision for autopsy.
Study patients
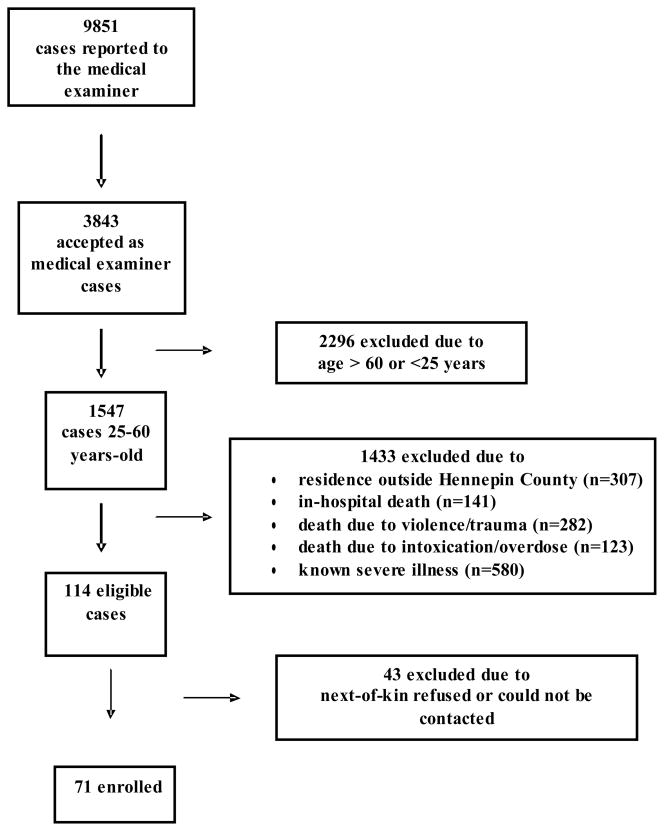
This study was approved by the University of Minnesota Institutional Review Board. The protocol was part of the Minnesota Heart Survey, which is a population-based, longitudinal study, designed to monitor and explain cardiovascular mortality, morbidity and risk factors in the Minneapolis-St. Paul metropolitan area (11). From August, 2001 to July, 2004, individuals between the ages 25–60 years, who died suddenly and unexpectedly within 24 hours of symptom onset (or being last seen alive), outside of a hospital (or pronounced dead in the emergency department) and evaluated by the Hennepin County ME were eligible to be included in the present study (Figure 1). Those who died due to an apparent external cause (e.g. trauma, violence, suicide), drug or substance overdose, or other known severe illness (e.g. cancer, cardiomyopathy, CHD, alcoholic cirrhosis, end-stage kidney disease, seizure-related deaths, pulmonary embolus, obstructive pulmonary disease etc.) were excluded. Thus, 114 cases without an initially apparent cause of death met the study inclusion criteria (Figure 1). The families of the victims were invited to participate in the study by a trained staff member at the ME’s office. In 43 cases, the next-of-kin declined the invitation and 71 cases were enrolled. Victims, whose next-of-kin declined study participation, were similar to those who were enrolled in age, gender, race and final autopsy diagnosis.
Figure 1.
Selection of 71 sudden death victims without an initially apparent cause of death
Data collection
The circumstances surrounding the index event, including potential triggers, prior illnesses, cardiovascular risk factors, co-morbid conditions and medications were obtained from structured interviews with the family of the deceased and the witnesses of the event. The structured interviews were conducted by a trained staff member at the ME’s office 2–3 months after the event to avoid the acute period of grief, but before the memories of the event were blurred. Additional clinical information was obtained from medical records. Duplicate interviews and duplicate chart abstractions were performed in 5% of the cases to assure reproducibility of the interviews and the reliability of the chart data.
Autopsy Evaluation of the Hearts and Definitions
Hearts were weighed and the size and shape of cardiac chambers were ascertained. The coronary arteries were cut in 4–5 mm cross sections for the assessment of the lumen size. Significant CHD was defined as ≥75% reduction of luminal cross-sectional area in at least 1 major epicardial artery (12). An acute plaque rupture consisted of a luminal platelet-fibrin thrombus continuous with an underlying lipid-rich core. The connection between the thrombus and the lipid core was through a disrupted thin fibrous cap infiltrated by macrophages (13). Both ventricles were sliced from apex to base at intervals to yield 4 cross-sectional specimens with the last being near the tips of the papillary muscles. Sampling for histologic studies was done with full-thickness tissue blocks from the posterobasal, mid anterior, mid lateral and mid septal regions of both ventricles. Portions of related valves were included in these blocks. From each block, hematoxylin-eosin, trichrome, and elastic-van Gieson stained slides were prepared and examined. In addition to the ME, all hearts were also examined at the Jesse Edwards Cardiovascular Pathology Unit (St. Paul, Minnesota) a specialized cardiac pathology center.
Acute myocardial infarction (MI) was diagnosed by the presence of coagulation necrosis of myocytes with or without an associated inflammatory infiltrate (14). Healed MI was identified by focal macroscopic replacement of the myocardium by scarring, with histological confirmation. Myocardial hypertrophy was defined as increased heart weight for body surface area and/or > 16 mm thickness of compact myocardium of the left ventricle.
Blood and tissue analysis
Routine toxicology qualitative screening of urine, blood, liver tissue and heart tissue for drugs was performed by both selective immunoassays (Microgenics Corporation, Pleasanton, CA) and by high-pressure liquid chromatography (Remedi HS Drug Profiling System, Bio-Rad Diagnostics Group) for a wider range (library of over 900 drugs and metabolites) of prescription and non-prescription drugs and their metabolites. Any drugs identified by screening were then quantified/confirmed utilizing either high-pressure liquid chromatography or by gas chromatography mass spectrometry on a Hewlett-Packard 5972 mass selective detector and a 5890 gas chromatograph equipped with a 30-m DB-5 capillary column (Agilent Technologies, Palo Alto, CA). A Unix-based Target Thru-Put operating software computer system was used for data compilation. Standards and deuterated internal standards were obtained from Radian Corp (Austin, TX), and appropriate quality control procedures were followed.
Genetic analysis
An approximate 1 cm3 piece of liver was obtained at the time of autopsy and stored at −20ºC by the ME. After written consent of the next-of-kin, the frozen tissue was transferred to the University of Minnesota genetics laboratory for gene analysis. Deoxyribonucleic acid was extracted using standard protocol, and stored at 4ºC.
Five genes associated with long QT syndrome were analyzed: KCNQ1, KCNH2, KCNE1, KCNE2, and SCN5A. Polymerase chain reaction sequencing primers were designed for KCNQ1, KCNH2, KCNE1 and KCNE2 to analyze all exon and adjacent intron sequences. Polymerase chain reaction sequencing primers for SCN5A were from published data (15,16). Gene sequence analysis with DNA extracted from the liver samples was performed in the Advanced Genomic Analysis Center at the University of Minnesota, using standard methods (www.agac.umn.edu). A sequence change was classified as a possible deleterious mutation using the following criteria: 1- found in a single sample; 2- resulted in an amino acid change in the protein; and 3- previously unreported as a polymorphic variant (see databases for individual genes). Functional studies of individual mutations were not performed.
RESULTS
Clinical characteristics and circumstances of SCD
The study participants were 49.5±7 years-old (range 27–60 years) and 86% were male (Table 1). History of hypertension, diabetes mellitus and/or hyperlipidemia were common. Smoking and obesity were highly prevalent. Overall, 61% of the participants were current (48%) or past (13%) smokers and 80% had a body mass index in the overweight or obese range (Table 1). Only 2 participants had a history of CHD; 1 with a documented previous MI. Family history of MI and sudden death were present in 16 and 4 participants, respectively. Of the 71 SCDs, 46 (65%) occurred at home and 20 (28%) were witnessed. Resuscitation was attempted in 11 (15%) victims.
Table 1.
Baseline demographic and clinical characteristics of the sudden death victims and of the reference population *†
| Demographic variables | Study subjects n=71 | Reference population n=2380 |
|---|---|---|
| Age (years) | 49.5 ± 7 | 44±9 |
| 25–29, n (%) | 2 (3%) | |
| 30–39, n (%) | 4 (6%) | |
| 40–49, n (%) | 25 (35%) | |
| 50–59, n (%) | 40 (56%) | |
| Male, n (%) | 61(86%) | (46%) |
| Ethnicity, n (%) | ||
| White | 68 (96%) | (89%) |
| Black | 1(1%) | (4%) |
| Hispanic | 1(1%) | (3%) |
| Asian | 1(1%) | (4%) |
| Marital Status, n (%) | ||
| Single | 17(24%) | |
| Married | 36 (51%) | |
| Divorced | 16(23%) | |
| Widowed | 2(3%) | |
| Employed, n (%) | 64 (90%) | |
| Education, n (%) | ||
| ≤ 12 Years | 22 (31%) | (17%) |
| 12–15 Years | 23 (32%) | (29%) |
| ≥16 Years | 26 (37%) | (54%) |
| Clinical variables | ||
| Hypertension, n (%) | 19 (27%) | (16%) |
| Diabetes mellitus, n (%) | 5 (7%) | (6%) |
| Hyperlipidemia, n (%) | 18 (25%) | (18%) |
| Smoking Status, n (%) | ||
| Current | 34 (48%) | (12%) |
| Past | 9 (13%) | |
| Never | 13 (18%) | |
| Unknown | 15 (21%) | |
| Body mass index (kg/m2) | 30.8±8 | 28±6 |
| Obese (BMI ≥30), n (%) | 31 (44%) | (32%) |
| Overweight (BMI 25–30), n (%) | 26 (37%) | |
| History of CHD, n (%) | 2 (3%) | (1%) |
| History of stroke, n (%) | 1 (1%) | (1%) |
| History of mental health disorders, n (%) | 11(15%) | |
continuous variables are displayed as mean ± SD
reference population= 25–60 year-old individuals living in Hennepin County, Minnesota
Abbreviations: BMI= body mass index; CHD= coronary heart disease
Clinic records and electrocardiogram results
Fifty-one (72%) subjects had previous clinic records available. Of these, 14 had a clinic visit within 1 month of the SCD. Of the 14 patients, 12 reported not feeling well. Blood pressure and lipid measurements were found in 47 and 31 subjects, respectively. Hypertension and dyslipidemia were diagnosed in 19 and 18 subjects, but treatment with a medication was noted in 15 and 9, respectively.
An electrocardiogram (ECG) had been performed in 24 subjects, within 3.8±3.9 years of the SCD. Of these, 14 were completely normal. The remaining 10 ECGs showed prior inferior MI (n=1), left bundle branch block (n=1), atrial fibrillation (n=1), left ventricular hypertrophy (n=2) and non-specific ST segment changes (n=3). Prolonged QT interval was found in 2 ECGs, in 54 and 58 year-old men (QTc 0.49 ms and 0.52 ms) who were not taking any QT-prolonging medications.
Autopsy Results
Of the 71 subjects, 58 (82%) had high-grade coronary stenosis (defined as ≥1 coronary artery with ≥75% obstruction) on autopsy (Table 2). Additional 8 subjects (11%) had moderate coronary disease (i.e. 50%–74% obstruction). Of the 58 subjects with significant CHD, 18, 18 and 22 had high-grade stenoses in 1,2 and 3 coronary arteries, respectively. Plaque rupture and/or thrombus formation was found in 19 subjects (27%) whereas pathological changes consistent with acute MI were present in 4 (6%). Previous (recent or old) MI was detected in 24 subjects (34%). All but 1 of these events were previously undiagnosed.
Table 2.
Abnormalities detected at autopsy in the 71 individuals who died suddenly
| Coronary heart disease | |
| Coronary heart disease (≥ 75% obstruction), n (%) | 58 (82%) |
| No. severely obstructed coronary arteries, n (%) | |
| 1 vessel | 18 (25%) |
| 2 vessel | 18 (25%) |
| 3 vessel | 22 (31%) |
| Left main coronary disease, n (%) | 7 (10%) |
| Severity of the most significant lesion, n (%) | |
| <50% | 5 (7%) |
| 50%–74% | 8 (11%) |
| 75%–89% | 12 (17%) |
| ≥90% | 46 (65%) |
| Acute thrombus or ruptured plaque, n (%) | 19 (27%) |
| Acute myocardial infarction, n (%) | 4 (6%) |
| Recent myocardial infarction (≤ 4 weeks), n (%) | 8 (11%) |
| Old myocardial infarction, n (%) | 18 (25%) |
| Cardiac anatomic findings | |
| Left ventricular hypertrophy, n (%) | 41 (58%) |
| Interstitial fibrosis, n (%) | 39 (55%) |
| Cardiomegaly, n (%) | 42 (59%) |
| Heart weight* (grams) | 497±131 |
| range | 255–960 |
| LV thickness* (cm) | 1.47±0.2 |
| range | 0.9–2.0 |
| Other autopsy diagnoses | |
| Active lymphocytic myocarditis, n (%) | 2 (3%) |
| RV cardiomyopathy, n (%) | 2 (3%) |
| Anomalous coronary artery, n (%) | 1 (1%) |
| Hypertrophic cardiomyopathy, n (%) | 1 (1%) |
| Severe aortic valve stenosis, n (%) | 1 (1%) |
| Acute aortic dissection, n (%) | 1 (1%) |
| Coronary artery dissection, n (%) | 1 (1%) |
| Pericardial tamponade, n (%) | 1 (1%) |
mean±D
Left ventricular (LV) hypertrophy and cardiomegaly were present in the majority of subjects with maximal LV thickness ranging up to 20 mm and cardiac weight up to 960 grams (normal < 450 grams) (Table 2). Among other significant pathologic abnormalities that may be associated with SCD were active focal myocarditis (n=2), aortic dissection (n=1), coronary artery dissection (n=1), severe aortic valve stenosis (n=1), hypertrophic cardiomyopathy (n=1) and right ventricular cardiomyopathy (n=2).
Toxicology Results
Toxicology results are summarized in Table 3. At the time of SCD, many of the subjects had recently ingested habitual substances or over-the-counter medications. Almost 1/3rd of the subjects had recently smoked cigarettes (or exposed to second hand smoke) and 50% had ingested aspirin, acetaminophen, ibuprofen or opiate analgesics within 6–12 hours before the fatal event (Table 3). One subject had cocaine metabolites in his urine and another had tetrahydrocannabinol. However, the blood and tissue samples in these individuals were negative suggesting that these substances were used hours before the SCD.
Table 3.
Results of toxicology in the 71 sudden death victims
| Habitual Substances | |
| Caffeine | 16 (23%) |
| Ethanol | 10 (14%) |
| Nicotine | 23 (32%) |
| Over the Counter Medications | |
| Acetaminophen | 24 (34%) |
| Salicylates | 12 (17%) |
| Fexofenadine | 5 (7%) |
| Ibuprophen | 4 (6%) |
| Ranitidine | 4 (6%) |
| Diphenhydramine | 3 (4%) |
| Doxylamine | 2 (3%) |
| Phenylpropanolamine | 2 (3%) |
| Ephedrine/pseudoephedrine | 1 (1%) |
| Prescription Medications | |
| Opiates | 8 (11%) |
| Beta adrenergic blockers | 4 (6%) |
| Selective Serotonin Uptake Inhibitors | 3 (4%) |
| Clozapine | 2 (3%) |
| Lithium | 1(1%) |
| Flecainide | 1 (1%) |
| Verapamil | 1 (1%) |
| Sildenafil | 1 (1%) |
Cardiac biomarkers creatine kinase MB mass and cardiac troponin I were measured in 30 subjects. Creatine kinase MB (mean±SD= 63±82 ng/ml; range 0.5–274.1) was abnormal in 21 (normal ≤5 ng/ml) subjects, whereas cardiac troponin I (mean±SD= 3.2±4.9 ng/ml; range 0–20.4) was abnormal in 23 (normal <0.1 ng/ml).
Genetic analysis
Sequence changes that were characterized as possible deleterious missense mutations were identified in 4 cases (6%) and are listed in Table 4. One other case had a sequence change resulting in an amino acid substitution, but this mutation has been reported as a rare variant in healthy controls (case 5, mutation P2006A) (17,18). Mean age of the 4 cases with a possible deleterious missense mutation was 43±12. Three of the 4 were male and all were Caucasian. All 4 cases had other heart disease, including significant CHD (Table 4). Of the 2 cases with prolonged QT interval on ECG, one had a missense mutation in SCN5A gene.
Table 4.
Clinical and genetic data on the 5 sudden death victims with mutations in the genes encoding ion channels
| Case | Age | Sex | Race | Autopsy Findings | Gene | Nucleotide Change | Amino Acid Change | Other Factors | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | M | Caucasian | Lymphocytic myocarditis | KCNH2 | 877g→t | A293S | Family history of SCD; Doxylamine; THC | Unpublished |
| 2 | 38 | F | Caucasian | Coronary artery dissection; CHD | SCN5A | 2162t→c | L721P | Hypertension | Unpublished |
| 4501c→g | L1501V | Hyperlipidemia | Splawski et al.(41) | ||||||
| 3 | 49 | M | Caucasian | Severe CHD | SCN5A | 2944t→c | C982R | Diabetes | Unpublished |
| 4 | 56 | M | Caucasian | Severe CHD | SCN5A | 95a→g | Q32R | Hypertension; Opiates | Unpublished |
| 5 | 59 | M | Caucasian | Severe CHD | SCN5A | 6016c→g | P2006A | Hypertension; Hyperlipidemia; Opiates; Burpropion | Wang et al. (44) |
Abbreviations: CHD= coronary heart disease; MI= myocardial infarction; THC= tetrahydrocannabinol Change the references to numbers
In 4 of the 5 cases the mutation was in the SCN5A gene. The remaining mutation was in the KCNH2 gene. No deleterious mutations were identified in the KCNQ1, KCNE1, or KCNE2 genes. Four of the 5 cases were heterozygous for a single mutation, and 1 individual had two mutations in the same (SCN5A) gene. The L1501V mutation in case 2 has been reported in previous studies but the L721P mutation in the same individual is novel; chromosome phase of these two mutations was not determined.
DISCUSSION
The objective of the present investigation was to perform a detailed examination of adult SCD victims without an initially apparent cause of death, to uncover potential clinical, anatomic, metabolic and genetic risk factors for SCD. We found that more than 80% of the subjects had previously-undiagnosed but anatomically-severe CHD on autopsy, including 27% with acute ischemic lesions and 34% with previous silent MI. Many also had signs of chronic cardiac disease characterized by left ventricular hypertrophy (58%), interstitial fibrosis (55%) and cardiomegaly (59%). Notably, ~6% of the subjects had mutations in sodium channel gene SCN5A. We also found that, many of the subjects had ingested acetaminophen, aspirin, ibuprofen or prescription analgesics or had smoked cigarettes shortly before the fatal event.
The incidence and extent of CHD at autopsy in our cohort was similar to those reported in previous series (1–3, 19,20). Although, the majority of the SCD subjects do not have a clinical history of CHD at the time of death (21), ~80% (range 60%–100%) have high-grade coronary stenoses at autopsy (12,19,22–27). A substantial proportion (range 10%–80%) of these individuals also have acute coronary lesions (12,13,14,27), which are more common among younger victims with cardiac risk factors and in those who reported chest discomfort prior to death (28). Previously, Spaulding et al. found severe CHD in ~70% of cardiac arrest survivors, including total occlusion of a coronary artery in 40% (29). Our results demonstrate that the high prevalence of CHD among SCD victims has not changed since these previous studies.
More than 30% of our cohort had had previous, clinically-silent MI. Myocardial scar after a MI creates a substrate for reentrant arrhythmias such as ventricular tachycardia, increasing the risk of SCD. Other myocardial structural abnormalities such as left ventricular hypertrophy and interstitial fibrosis, found in the majority of our cohort, have also been associated with ventricular tachycardia in ischemic and non-ischemic heart disease (30,31).
Possible deleterious missense mutations in the genes encoding ion channel proteins were found in ~6% of our study cohort. The majority of these were in the sodium channel gene SCN5A, implicated in the pathophysiology of long-QT syndrome 3 and Brugada syndrome. Previously, Chugh et al. identified a mutation in KCNH2 in 2 of 12 adult victims of SCD (32) and Tester et al. showed that 30% of 49 victims of sudden unexplained death harbored a mutation in 1 of the genes implicated in long-QT syndrome (33). Futher, Ackerman et al. reported that approximately 5% of the general population harbor a rare missense variant of the cardiac sodium channel (34). Splawski et al. found that a single nucleotide sequence variant in the SCN5A gene was associated with a small increase in arrhythmia risk in African Americans, in the context of other potentially proarrhythmic influences (35). Most notably, Albert et al. recently found mutations of SCN5A gene in 10% of the women who died suddenly vs. 1.6% of the controls in the Nurses’ Health Study (36). Cumulatively, our results and previous studies and suggest that mutations in genes causing long-QT syndrome take part in a minority of SCD cases in the community. Indeed, these variants may not produce sudden death on their own but may predispose individuals to SCD in conjunction with a second offender such as drug effects or electrolyte imbalance (37). The higher prevalence of mutations observed in the study by Tester et al. may be because of a younger study cohort (33), as long-QT syndrome tends to be more expressive during adolescence vs. adulthood. Thus, long-QT syndrome mutations may be more prevalent in a younger SCD cohort vs. an older one.
It is not often possible to evaluate the symptoms of the SCD victim prior to death since the majority of deaths are unwitnessed and the history obtained from the relatives has limitations. In these cases, post-mortem toxicology screen may provide some important clues. In the present study 50% of subjects took analgesic and anti-inflammatory medications shortly before death, suggesting that they were not feeling well. Further, more than 30% of the subjects had smoked within hours before death, which may also represent feeling distressed. It is also possible that smoking may have been a trigger of SCD in some cases. Indeed, cigarette smoking promotes platelet aggregation and elevates plasma epinephrine concentrations increasing the likelihood of coronary vasospasm and thrombus formation (38). Also, previously, Burke et al. (13) showed that cigarette smoking was more common among SCD victims with acute coronary lesions, suggesting that smoking could trigger plaque rupture and coronary thrombosis.
Traditional coronary risk factors have previously been associated with SCD, particularly when a clinical history of CHD is absent (5). In our study cohort, smoking and obesity were very common and the prevalence of hypertension, diabetes mellitus and hyperlipidemia was higher than expected for age in the community (11,39). However, almost 30% of our cohort did not have any clinic records and almost half of those who were seen in clinic did not have serum lipid measurements. Further, medicine therapy for hypertension and hyperlipidemia was not initiated in many patients with these conditions. Whether SCD could have been averted with treatment in the participants of this study is unknown. However, there is evidence that beta blockers reduce SCD in patients with previous MI (> 30% of our cohort) and statins are associated with reduction of ventricular tachycardia and SCD (40–42). These data underscore the need for improvement in the detection of old silent MIs and administering appropriate post-MI therapy (43).
The strengths of our study include the meticulous multi-modality evaluation of SCD victims. Indeed, few studies have reported simultaneous evaluation of victims with next-of-kin interviews, revision of clinic records, autopsy, toxicology and genetic analysis. The community origin of our study population is also considered a strength. Our cohort was constituted from all victims of SCD who met the inclusion criteria and came from a defined geographic area. Our study also has some limitations. First, there was no control group to compare the incidence of the abnormalities detected in SCD victims and the sample size was relatively small. However, our results were similar to previous SCD series. Also, we could not assess the functional significance of the genetic variants detected among SCD victims in this study. Family screening to check co-segregation and potential effects on surface ECG could not be performed. However, the detected mutations are likely deleterious because they were present in 1 case only, had not been reported in large surveys of long-QT gene variants or had been reported as pathologic mutations in previous analyses (Table 4). Furthermore, the C982R missense mutation is similar to other mutations that are considered pathologic in this gene.
In conclusion, great majority of younger SCD victims without an initially apparent cause of death, have significant CHD on autopsy, including old undetected MI. Traditional coronary risk factors were prevalent and under-treated. Many of the subjects had ingested analgesics or had smoked cigarettes shortly before the fatal event, suggesting that they were not feeling well. Also, ~6% of the subjects had mutations in sodium channel gene SCN5A. These findings imply that significant improvements in the detection and treatment of subclinical CHD in the community are needed to prevent SCD.
Acknowledgments
This work was funded by National Heart Lung and Blood Institute (R01-HL-65755, Community Surveillance of Coronary Heart Disease and Sudden Death; Minnesota Heart Study). Dr. Adabag is supported, in part, by VA Clinical Science R&D Service (Grant no. 04S-CRCOE 001), Washington, DC. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Abbreviation List
- SCD
Sudden cardiac death
- CHD
Coronary heart disease
- ME
Medical examiner
- MI
Myocardial infarction
- ECG
Electrocardiogram
- LV
Left ventricular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng Z, Croft JB, Giles WH, Mensah GA. Sudden Cardiac Death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Zipes DP, Wellens HJJ. Sudden Cardiac Death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 4.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: structure, function and time-dependence of risk. Circulation. 1992;85:I2–I10. [PubMed] [Google Scholar]
- 5.Kannel WB, Gagnon D, Cupples LA. Epidemiology of sudden coronary death: population at risk. Can J Cardiol. 1990;6:439–444. [PubMed] [Google Scholar]
- 6.Kuller L, Lilienfeld A, Fisher R. Sudden and unexpected deaths in young adults. An epidemiological study. JAMA. 1966;198:248–252. [PubMed] [Google Scholar]
- 7.Friedlander Y, Siscovick DS, Weinmann S, et al. Family History as a Risk Factor for Primary Cardiac Arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 8.Jouven X, Desnos M, Guerot M, Ducimetière P. Predicting Sudden Death in the Population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 9.Dekker LR, Bezzina CR, Henriques JP, et al. Familial Sudden Death Is an Important Risk Factor for Primary Ventricular Fibrillation: a case-control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 10.Arking DE, Chugh SS, Chakravarti A, Spooner PM. Genomics in sudden cardiac death. Circ Res. 2004;94:712–723. doi: 10.1161/01.RES.0000123861.16082.95. [DOI] [PubMed] [Google Scholar]
- 11.McGovern PG, Jacobs DR, Jr, Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997:the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Davies MJ. Anatomic features in victims of sudden coronary death: coronary artery pathology. Circulation. 1992;85:I19–I24. [PubMed] [Google Scholar]
- 13.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 14.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions and myocardial infarction. Circulation. 1995;92:1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Li Z, Shen J, Keating MT. Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics. 1996;34:9–16. doi: 10.1006/geno.1996.0236. [DOI] [PubMed] [Google Scholar]
- 16.Syrris P, Murray A, Carter ND, McKenna WM, Jeffery S. Mutation detection in long QT syndrome: a comprehensive set of primers and PCR conditions. J Med Genet. 2001;38:705–71. doi: 10.1136/jmg.38.10.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman MJ, Splawski I, Makielski JC, et al. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;1:600–607. doi: 10.1016/j.hrthm.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Arnestad M, Crotti L, Rognum TO, et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 19.Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In: Braunwald E, Zipes DP, Libby P, editors. Heart Disease: A Textbook of Cardiovascular Medicine. W.B Saunders Company; 2001. pp. 890–931. [Google Scholar]
- 20.Myerburg RJ, Kessler KM, Castellanos A. Sudden Cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 21.De Vreede-Swaggemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. NEJM. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 23.Schmermund A, Schwartz RS, Adamzik M, et al. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with ‘healthy’ subjects dying out of hospital. Atherosclerosis. 2001;155:499–508. doi: 10.1016/s0021-9150(00)00598-0. [DOI] [PubMed] [Google Scholar]
- 24.Reichenbach DD, Moss NS, Meyer E. Pathology of the heart in sudden cardiac death. Am J Cardiol. 1977;39:865–872. doi: 10.1016/s0002-9149(77)80041-6. [DOI] [PubMed] [Google Scholar]
- 25.Warnes CA, Roberts WC. Sudden coronary death: relation of amount and distribution of coronary narrowing at necropsy to previous symptoms of myocardial ischemia, left ventricular scarring and heart weight. Am J Cardiol. 1984;54:65–73. doi: 10.1016/0002-9149(84)90305-9. [DOI] [PubMed] [Google Scholar]
- 26.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Michalodimitrakis M, Mavroforou A, Giannoukas AD. Lessons learnt from the autopsies of 445 cases of sudden cardiac death in adults. Coron Artery Dis. 2005;16:385–389. doi: 10.1097/00019501-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Leach IH, Blundell JW, Rowley JM, Turner DR. Acute ischemic lesions in death due to ischemic heart disease. An autopsy study of 333 cases of out-of-hospital death. Eur Heart Journ. 1995;16:1181–1185. doi: 10.1093/oxfordjournals.eurheartj.a061073. [DOI] [PubMed] [Google Scholar]
- 29.Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–33. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 30.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 31.Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and Frequency of Arrhythmias in Hypertrophic Cardiomyopathy in Relation to Delayed Enhancement on Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 32.Chugh SS, Senashova O, Watts A, et al. Postmortem molecular screening in unexplained sudden death. J Am Coll Cardiol. 2004;43:1625–1629. doi: 10.1016/j.jacc.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 33.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J Am Coll Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Ackerman MJ, Splawski I, Makielski JC, et al. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;1:600–7. doi: 10.1016/j.hrthm.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Splawski I, Timothy KW, Tateyama M, et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 36.Albert CM, Nam EG, Rimm EB, et al. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. doi: 10.1161/CIRCULATIONAHA.107.736330. [DOI] [PubMed] [Google Scholar]
- 37.Noseworthy P, Newton-Cheh C. Genetic Determinants of Sudden Cardiac Death. Circulation. 2008;118:1854–1863. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- 38.Hung J, Lam JY, Lacoste L, Letchacovski G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–2436. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- 39.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics 2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP AVID Investigators. Are lipid-lowering drugs also antiarrhythmic drugs? An analysis of the Antiarrhythmic versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol. 2003;42:81–87. doi: 10.1016/s0735-1097(03)00498-4. [DOI] [PubMed] [Google Scholar]
- 42.Levantesi G, Scarano M, Marfisi R, et al. Meta-analysis of effect effect of statin treatment on risk of sudden death. Am J Cardiol. 2007;100:1644–1650. doi: 10.1016/j.amjcard.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Chase D, Roderick PJ, Burnley H, Gallagher PJ, Roberts PR, Morgan JM. Is there unmet need for implantable cardioverter defibrillators? Findings from a post-mortem series of sudden cardiac death. Europace. 2008;10:741–746. doi: 10.1093/europace/eun114. [DOI] [PubMed] [Google Scholar]
- 44.Wang DW, Desai RR, Crotti L, et al. Cardiac sodium channel dysfunction in sudden infant death syndrome. Circulation. 2007;115:368–76. doi: 10.1161/CIRCULATIONAHA.106.646513. [DOI] [PubMed] [Google Scholar]