Abstract
Wingless (Wg)/Wnt has been proposed to exert various functions as a morphogen depending on the levels of its signalling. Therefore, not just the concentration of Wg/Wnt, but also the responsiveness of Wg/Wnt-target cells to the ligand, must have a crucial function in controlling cellular outputs. Here, we show that a balance of ubiquitylation and deubiquitylation of the Wg/Wnt receptor Frizzled determines the cellular responsiveness to Wg/Wnt both in mammalian cells and in Drosophila, and that the cell surface level of Frizzled is regulated by deubiquitylating enzyme UBPY/ubiquitin-specific protease 8 (USP8). Although ubiquitylated Frizzled underwent lysosomal trafficking and degradation, UBPY/USP8-dependent deubiquitylation led to recycling of Frizzled to the plasma membrane, thereby elevating its surface level. Importantly, a gain and loss of UBPY/USP8 function led to up- and down-regulation, respectively, of canonical Wg/Wnt signalling. These results unveil a novel mechanism that regulates the cellular responsiveness to Wg/Wnt by controlling the cell surface level of Frizzled.
Keywords: deubiquitylating enzyme, Frizzled, ubiquitylation, Wingless, Wnt
Introduction
Wingless (Wg)/Wnt proteins are secreted lipoglycoprotein ligands that control various aspects of development, including cell proliferation, migration, fate specification, and polarity formation (Veeman et al, 2003; Logan and Nusse, 2004; Klein and Mlodzik, 2005; Clevers, 2006). Wg/Wnt signals are transmitted through three major pathways in target cells: the canonical, planar cell polarity, and Ca2+ pathways (Schulte and Bryja, 2007). As a morphogen, Wg/Wnt regulates tissue patterning depending on the strength of the canonical pathway signalling generated in target cells. Morphogens are secreted by restricted groups of cells and transported through tissues to form a graded distribution (Kornberg and Guha, 2007). In the Drosophila wing disc, Wg is expressed in the dorso-ventral border and diffuses gradually in the wing pouch. In regions proximal to the border, sensory organ precursor (SOP) cells are induced by strong Wg signals to differentiate into mechanosensory and chemosensory bristles at the adult wing margin (Couso et al, 1994). On the other hand, the threshold of Wg signals required for the expression of Distal-less (Dll) and vestigial (vg) [QE] is lower. Thus, their expression expands to regions more distal to the border, and induces proliferation of the distal cells (Zecca et al, 1996; Neumann and Cohen, 1997).
Forced expression of DFrizzled-2 (DFz2), the high-affinity Wg receptor in Drosophila, in the wing disc results in excess Wg signalling and misregulated extracellular Wg distribution, and induces ectopic sensory bristles in regions distal to the border (Cadigan et al, 1998), suggesting that Wg signalling is controlled by the cell surface Fz level of target cells. Therefore, regulation of the Fz level is crucial for proper response to Wg. Endocytosis is one of the central mechanisms that control the amount of plasma membrane proteins. Fz endocytosis and subsequent lysosomal trafficking have been reported in Drosophila and mammalian cells (Chen et al, 2003; Piddini et al, 2005; Blitzer and Nusse, 2006; Rives et al, 2006; Seto and Bellen, 2006; Yamamoto et al, 2006). However, it is unclear whether Fz endocytosis affects Wg signalling negatively or positively (Kikuchi and Yamamoto, 2007; Gagliardi et al, 2008). In addition, although the involvement of various proteins including β-arrestin 2, Dishevelled-2 (Dvl-2), protein kinase C, Arrow (Arr)/LRP 5 and 6, and AP2 in clathrin- and caveolin-mediated Fz endocytosis has been shown (Chen et al, 2003; Yamamoto et al, 2006; Yu et al, 2007), regulatory mechanisms underlying lysosomal trafficking of endocytosed Fz are not clear. Accordingly, regulation of the cell surface Fz level by endocytosis and lysosomal degradation is largely unknown.
Mechanisms of lysosomal trafficking of receptor tyrosine kinases (RTKs) have been extensively studied (Gruenberg and Stenmark, 2004; Saksena et al, 2007). On ligand binding, activated RTKs undergo rapid endocytosis and are transported to the early endosome. RTKs are subsequently incorporated into lumenal vesicles that invaginate from the endosomal limiting membrane. Finally, fusion of such late endosomes, referred to as multivesicular bodies (MVBs), with the lysosome delivers RTKs into the lysosomal lumen. In this trafficking process, ubiquitylation of RTKs serves as a sorting signal that targets the receptors from the early endosome to the lysosome through the MVB. On the endosomal membrane, ubiquitylated RTKs are sorted by four protein complexes termed as endosomal sorting complex required for transport (ESCRT)-0, I, II, and III, which recognize the ubiquitin (Ub) moieties of RTKs and direct the cargo proteins into the invaginating MVB vesicle (Gruenberg and Stenmark, 2004; Saksena et al, 2007).
UBPY/Ub-specific protease 8 (USP8), a deubiquitylating enzyme of the USP family, participates in the endosomal sorting of ubiquitylated RTKs through interaction with ESCRT-0 and ESCRT-III (Mizuno et al, 2005; Row et al, 2007). UBPY deubiquitylates ligand-activated epidermal growth factor receptor (EGFR) on the endosome and regulates its lysosomal traffic, although it is under debate whether deubiquitylation promotes or suppresses EGFR degradation (Mizuno et al, 2005; Row et al, 2006; Niendorf et al, 2007; Komada, 2008). In this study, we show that Fz undergoes ubiquitylation-dependent trafficking to the lysosome and that UBPY facilitates Wg/Wnt signalling by suppressing the trafficking/degradation of Fz and increasing its cell surface level through its recycling, providing a novel mechanism that regulates the cellular responsiveness to Wg/Wnt.
Results
Drosophila UBPY is required for sensory bristle formation in the Drosophila wing
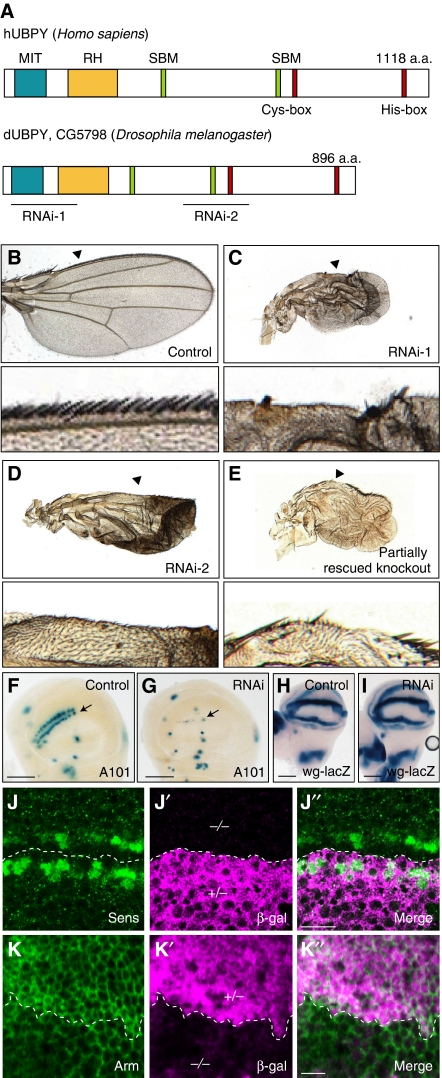
To find a new regulator of Wg signalling at the level of protein localization, transport, or degradation, we screened ∼400 genes implicated in membrane trafficking in the genome-wide Drosophila RNA interference (RNAi) library (http://www.shigen.nig.ac.jp/fly/nigfly/about/aboutRnai.jsp). We induced double-strand RNAs (dsRNAs) corresponding to 500 nucleotides in the open reading frames of such genes in the wing pouch by crossing with the scalloped (sd)-Gal4 driver, and examined the formation of sensory bristles at the wing margin. This screen led to the identification of nine genes, knockdown of which resulted in various defects in bristle formation (data not shown). Among them, depletion of an orthologue of the mammalian deubiquitylating enzyme UBPY/USP8 (Figure 1A) (Naviglio et al, 1998; Kato et al, 2000) caused loss of sensory bristles (Figure 1B and C). Drosophila UBPY (dUBPY, CG5798) exhibits an overall amino-acid sequence identity of 28% to human UBPY, and harbours the Cys- and His-box motifs that form the catalytic core, as well as most of the hallmark features of mammalian UBPY (Figure 1A) (Komada, 2008). Real-time PCR analysis showed that the dsRNA for dUBPY, when induced in whole fly bodies by actin-Gal4, suppresses the expression of dUBPY mRNA to ∼30% of that in controls, verifying the effectiveness of this in vivo RNAi system (data not shown).
Figure 1.
dUBPY regulates Wg signalling in the wing disc. (A) Domain structures of human hUBPY and Drosophila dUBPY. Positions of the Cys- and His-boxes, MIT domain, rhodanese homology (RH) domain, and SH3-binding motifs (SBMs), as well as the dsRNA-target sites used in this study, are indicated. (B–E) Top panels show wings from (B) control (sd-Gal; UAS-GFP RNAi), (C) RNAi-1 (sd-Gal; UAS-lacZ/UAS-dUBPY RNAi-1), (D) RNAi-2 (sd-Gal4; UAS-lacZ/UAS-dUBPY RNAi-2), and (E) partially rescued knockout (UAS-dUBPY; UBPY K.O.) flies. Bottom panels show high-magnification images of the wing margins indicated by arrowheads in top panels. (F–I) Activity staining of β-gal derived from neuralized-lacZ (A101) (F, G) and wg-lacZ (H, I) in control (F, H) and dUBPY RNAi (G, I) wing discs. Arrows indicate the positions of the dorso-ventral border (F, G). (J–K″) Anti-Sens (J) and anti-Arm (K) staining of dUBPY knockout clones, which are negative for β-gal (J′, K′, −/−), in the wing disc. J″ and K″ are merged images. Bars, 100 μm (F–I); 10 μm (J″, K″).
The possibility that the observed wing defect was due to an off-target effect of the dsRNA was excluded by the following experiments. First, knockdown by a second set of dsRNA (Figure 1A) also caused the loss of sensory bristles (Figure 1D). Second, we generated dUBPY knockout mutant flies (Supplementary Figure S1). As the knockout caused embryonic lethality, partially rescued knockout strains were developed. These adults exhibited the same loss of sensory bristles as seen in the knockdown flies (Figure 1E). Accordingly, we concluded that this phenotype results from the dysfunction of dUBPY.
dUBPY facilitates canonical Wg signalling in Drosophila
To elucidate the cause of the loss of sensory bristles on dUBPY knockdown, we examined the specification of SOP cells, which can be marked by neuralized-lacZ (A101) (Campuzano and Modolell, 1992), along the dorso-ventral border in the wing disc (Figure 1F, arrow). In the dUBPY knockdown wing disc, expression of neuralized-lacZ was barely detectable in this region (Figure 1G, arrow). As SOP cells are specified by sequential activation of the Notch and Wg signalling pathways (Couso et al, 1994; de Celis and Bray, 1997), expression of the Notch-target genes Cut, vgBE-lacZ, and wg-lacZ (Kim et al, 1996) was examined. These genes were normally expressed in the knockdown disc (Figure 1H and I; Supplementary Figure S2A–D), suggesting that Notch signalling is not compromised by knockdown of dUBPY. In contrast, the number of cells expressing the Wg-target protein Senseless (Sens; Nolo et al, 2000) and the amount of Armadillo (Arm)/β-catenin protein, which accumulates intracellularly in response to Wg signal, were obviously reduced in the dUBPY knockout clones in the wing disc (Figure 1J–K″). These results suggested that canonical Wg signalling is impaired in the absence of dUBPY and thus that dUBPY is a positive regulator of Wg signalling in Drosophila. As the level of wg expression was not affected in the dUBPY knockdown wing disc (Figure 1H and I), and as the amount and distribution of extracellularly secreted Wg were indistinguishable between the control and dUBPY knockdown discs (Supplementary Figure S2E and F), dUBPY was suggested to participate in Wg signalling in Wg-target cells.
UBPY facilitates canonical Wnt signalling in mammalian cells
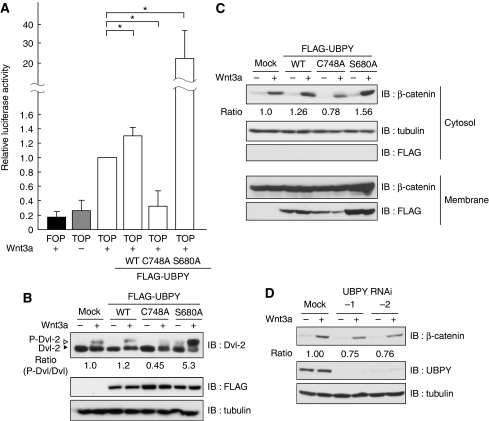
To biochemically address how UBPY regulates Wg/Wnt signalling, we used mammalian cells in culture. First, we examined whether UBPY is required for the Wnt canonical pathway in mammals. The activity of the TCF/LEF transcription factors that induces Wnt-target gene expression (MacDonald et al, 2009) was tested using the TOP-FLASH luciferase reporter gene assay in HEK293T cells. The level of Wnt-target expression induced by Wnt3a was slightly up-regulated by overexpression of wild-type UBPY (UBPYWT) (Figure 2A). However, UBPYC748A,a catalytically inactive mutant that acts in a dominant-negative manner (Mizuno et al, 2005), suppressed expression to the same level as seen in unstimulated cells (Figure 2A). In contrast, overexpression of UBPYS680A, a constitutively active mutant that does not undergo 14-3-3-mediated catalytic inhibition (Mizuno et al, 2007), caused a significant increase in Wnt3a-induced target expression (Figure 2A).
Figure 2.
UBPY facilitates canonical Wnt signalling in mammalian cells. (A) HEK293T cells were transfected with control FOP-FLASH or TOP-FLASH luciferase together with the indicated FLAG-UBPY constructs, and treated with Wnt3a overnight. Relative luciferase activity in the cell lysates is shown as mean±s.d. (n=4, *P<0.02, t-test). (B) NIH3T3 cells were transfected with the indicated FLAG-UBPY constructs, treated with Wnt3a for 1.5 h, and the lysates were immunoblotted with indicated antibodies. Positions of phosphorylated (P-Dvl-2) and non-phosphorylated Dvl-2 are indicated (top). The ratios of P-Dvl-2 to Dvl-2, which are normalized to that in mock-transfected cells stimulated with Wnt3a, are indicated below the top panel. (C) HEK293T cells were transfected with the indicated FLAG-UBPY constructs, treated with Wnt3a overnight, and their lysates were separated into cytoplasmic and membrane fractions. Each fraction was immunoblotted with indicated antibodies. FLAG-UBPY was solely recovered in the membrane fraction as described earlier (Mizuno et al, 2005). (D) HeLa cells were transfected with or without UBPY siRNAs and treated with Wnt3a for 5 h in the presence of cycloheximide. Cell lysates were immunoblotted with indicated antibodies. Relative intensity of the β-catenin bands is indicated (C, D).
To examine whether UBPY regulates the Wnt canonical pathway at the level of Fz receptors, phosphorylation of Dvl-2, which is induced by its direct interaction with activated Fz (MacDonald et al, 2009), was examined. Although the level of Wnt3a-induced Dvl-2 phosphorylation was similar between mock- and UBPYWT-transfected NIH3T3 cells, it was lower in UBPYC748A-expressing cells (Figure 2B). Conversely, when UBPYS680A was expressed, Dvl-2 phosphorylation was even detected in unstimulated cells, and its level after Wnt3a stimulation was much higher than in control cells (Figure 2B). As the canonical pathway signalling relies on stabilization of β-catenin acting downstream of Dvl (MacDonald et al, 2009), regulation of the cytoplasmic β-catenin level by UBPY was also examined. HEK293T cells transfected with UBPYWT, UBPYC748A, or UBPYS680A were stimulated with Wnt3a, and their cytoplasmic fractions were immunoblotted with anti-β-catenin antibody. Compared with cells expressing UBPYWT, the level of cytoplasmic β-catenin was decreased in UBPYC748A-expressing cells and increased in UBPYS680A-expressing cells (Figure 2C). Similarly, RNAi-mediated knockdown of endogenous UBPY in HeLa cells also resulted in a reduced level of β-catenin accumulation on Wnt3a stimulation (Figure 2D). Collectively, these results suggested that the UBPY-mediated regulation of canonical Wg/Wnt signalling is conserved in mammalian cells, and that Fz is a target of UBPY.
Fz undergoes ubiquitylation and deubiquitylation in mammalian cells
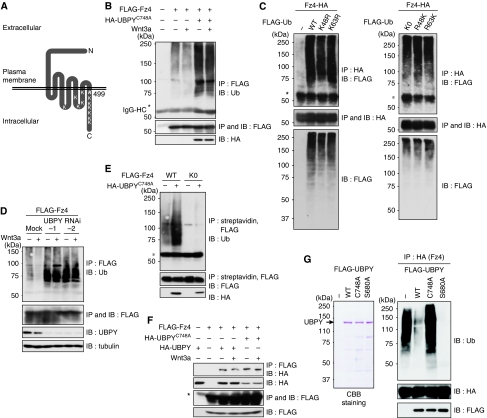
Mammalian UBPY deubiquitylates ligand-activated RTKs on the endosome (Mizuno et al, 2005; Row et al, 2006). However, it remains unknown whether Fz undergoes ubiquitylation. To examine this question, Frizzled-4 (Fz4; Figure 3A), a widely expressed member of the mammalian Fz family, was tagged with the FLAG epitope and expressed in HeLa cells. The cells were treated with or without Wnt3a for 15 min and lysed using a hot lysis method to disrupt Fz4 interaction with other ubiquitylated proteins. FLAG-Fz4 was immunoprecipitated from the lysates with anti-FLAG antibody and immunoblotted with anti-Ub antibody. Fz4 was detected as high-molecular-weight band shifts, suggesting that Fz4 undergoes ubiquitylation (Figure 3B). When FLAG-tagged Ub was co-transfected with HA-tagged Fz4, ubiquitylated Fz4 was detected by anti-FLAG immunoblotting of anti-HA immunoprecipitate (Figure 3C, left, top). To elucidate whether Fz is conjugated with poly-Ub or mono-Ub, we used several Ub mutants. As Lys48 and Lys63 are the major sites used for poly-Ub chain formation (Haglund and Dikic, 2005), we co-expressed UbK48R and UbK63R, in which Lys48 and Lys63, respectively, are replaced to Arg, with HA-Fz4. Fz4 was similarly ubiquitylated in cells expressing these mutants (Figure 3C, left, top). We further examined additional Ub mutants: UbK0, UbR48K, and UbR63K. UbK0 harbours Lys-to-Arg replacement at all seven Lys residues. UbR48K and UbR63K are mutants in which all Lys residues except for Lys48 and Lys63, respectively, are replaced with Arg. The pattern of Fz4 ubiquitylation was similar among cells expressing these Ub mutants (Figure 3C, right) and wild-type Ub (Figure 3C, left). These results suggested that none of the Lys residues in Ub (including Lys48 and Lys63) are required for Fz4 ubiquitylation. We thus suggest that Fz4 is monoubiquitylated at multiple sites.
Figure 3.
Fz4 undergoes ubiquitylation and deubiquitylation in mammalian cells. (A) Schematic structure of mouse Fz4. Positions of the 12 Lys residues facing the cytoplasm are indicated. (B) HeLa cells were transfected with FLAG-Fz4 with or without HA-UBPYC748A, and treated with Wnt3a for 15 min. FLAG-Fz4 was immunoprecipitated and immunoblotted with indicated antibodies. (C) HeLa cells were transfected with HA-Fz4 with the indicated FLAG-Ub constructs. HA-Fz4 was immunoprecipitated and immunoblotted with indicated antibodies. (D) HeLa cells were transfected with FLAG-Fz4 with or without UBPY siRNAs, and treated with Wnt3a for 1 h. FLAG-Fz4 was immunoprecipitated and immunoblotted with indicated antibodies. (E) HeLa cells were transfected with FLAG-tagged Fz4 or Fz4K0 with or without HA-UBPYC748A, and labelled with biotin on the cell surface. Biotinylated FLAG-Fz4 proteins were immunoprecipitated and immunoblotted with indicated antibodies. (F) HeLa cells were transfected with FLAG-Fz4 with HA-tagged UBPYWT or UBPYC748A, and treated with Wnt3a for 1 h. FLAG-Fz4 was immunoprecipitated and immunoblotted with indicated antibodies. Total lysates were also immunoblotted with indicated antibodies (B–F, lower panels). Asterisks indicate the IgG heavy chain used for immunoprecipitation (B, C, E, F). (G) FLAG-UBPY proteins, expressed in COS-7 cells and immunopurified with anti-FLAG beads, were stained with Coomassie Brilliant Blue (CBB) after electrophoresis (left). HA-Fz4 was expressed in HeLa cells, immunoprecipitated with anti-HA antibody, and incubated with the purified UBPY proteins. The reaction mixtures were immunoblotted with indicated antibodies (right).
The level of Fz4 ubiquitylation was drastically elevated when dominant-negative UBPYC748A was co-expressed (Figure 3B) or UBPY was depleted using RNAi (Figure 3D). We also constructed a Fz4K0 mutant in which all 12 of the Lys residues facing the cytoplasm (Figure 3A) were replaced by Arg, and expressed it in HeLa cells. Cell surface FLAG-Fz4K0 was labelled with biotin and sequentially precipitated with streptavidin and anti-FLAG antibody. Immunoblotting of precipitated FLAG-Fz4K0 with anti-Ub antibody showed that this mutant is no longer ubiquitylated on the cell surface even when UBPYC748A is expressed (Figure 3E). In addition, interaction between Fz4 and UBPY was observed by co-immunoprecipitation of HA-tagged UBPY proteins with FLAG-Fz4 from cell lysates (Figure 3F). Finally, we examined whether UBPY deubiquitylates Fz4 in vitro. FLAG-tagged UBPY proteins were immunopurified from transfected cells using anti-FLAG antibody (Figure 3G, left) and incubated with ubiquitylated HA-Fz4, which was also immunoprecipitated from transfected cells. As expected, wild-type UBPY, but not catalytically inactive UBPYC748A, deubiquitylated Fz4 (Figure 3G, right). In addition, constitutively active UBPYS680A exhibited higher activity than wild-type UBPY (Figure 3G, right). Collectively, these data showed that Fz4 is a substrate for UBPY. However, ubiquitylation (Figure 3B and D) and UBPY binding (Figure 3F) of Fz4 were not enhanced by Wnt3a, suggesting that unlike RTKs, the level of Fz ubiquitylation is not regulated by ligand stimulation.
UBPY regulates degradation and cell surface level of Fz in mammalian cells
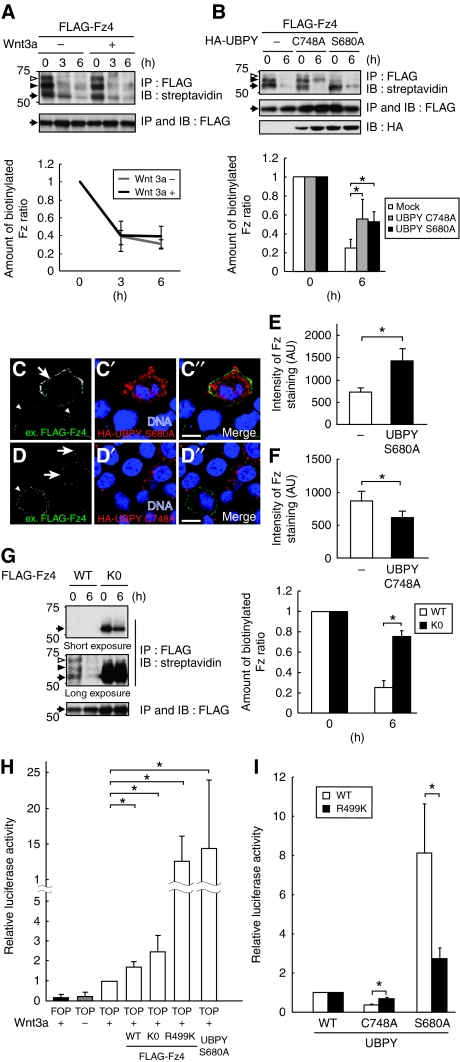
On the basis of the finding that Fz4 undergoes ubiquitylation, we next investigated the degradation of Fz4. We first compared the rate of Fz4 degradation in unstimulated and Wnt-stimulated cells. HeLa cells were transfected with FLAG-Fz4, and cell surface proteins were pulse labelled with biotin and chased in the presence or absence of Wnt3a. Immunoprecipitation with anti-FLAG antibody followed by blotting with peroxidase-conjugated streptavidin detected cell surface FLAG-Fz4 mainly as a ladder of ∼55-, 63-, and 70-kDa bands (Figure 4A, top, arrow, closed arrowhead, and open arrowhead). These bands probably represent Fz4 proteins conjugated with zero, one, and two Ub molecules, respectively, because the 63- and 70-kDa forms were not detected when non-ubiquitylatable Fz4K0 was expressed (Figure 4G, top). The 63-kDa band was faint in Figure 3 because of a lower amount of anti-Ub antibody bound to mono- than to multiply ubiquitylated proteins. The intensities of these bands were summed, and the ratio of the intensity at 3 and 6 h of chase to that at 0 h was determined (Figure 4A, bottom). This comparison showed that the degradation rate of biotinylated FLAG-Fz4 is similar in unstimulated and Wnt-stimulated cells, suggesting that ligand stimulation does not accelerate Fz degradation. This was consistent with the results showing that Wnt did not enhance Fz ubiquitylation (Figure 3B and D). To determine how Fz is degraded, we examined the effects of a lysosome inhibitor, bafilomycin A1, and a proteasome inhibitor, MG132. Degradation of biotinylated cell surface FLAG-Fz4 was delayed by treating cells with bafilomycin A1, but not with MG132, suggesting that Fz4 is degraded in the lysosome (Supplementary Figure S3).
Figure 4.
UBPY regulates degradation and cell surface level of Fz4 in mammalian cells. (A) HeLa cells were transfected with FLAG-Fz4, labelled with biotin on the cell surface, and treated with or without Wnt3a for 3 or 6 h. FLAG-Fz4 was immunoprecipitated and blotted with streptavidin and anti-FLAG antibody. Arrows, closed arrowheads, and open arrowheads indicate Fz proteins conjugated with zero, one, and two Ub molecules, respectively. (B) HeLa cells were transfected with FLAG-Fz4 with HA-tagged UBPYC748A or UBPYS680A, labelled with biotin, and chased for 6 h. FLAG-Fz4 was immunoprecipitated and blotted with streptavidin and anti-FLAG antibody. Total lysates were immunoblotted with anti-HA antibody. (C–D″) HEK293T cells were transfected with Fz4, which was FLAG-tagged extracellularly, together with HA-tagged UBPYS680A (C–C″) or UBPYC748A (D–D″). Living cells were stained with anti-FLAG antibody (C, D). After fixation, cells were further stained with anti-HA antibody and TO-PRO-3 (C′, D′). Arrows indicate cells expressing UBPYS680A or UBPYC748A. Arrowheads indicate cells expressing no ectopic UBPY. C″ and D″ are merged images. Bars, 10 μm. (E, F) Anti-FLAG fluorescence intensity of FLAG-Fz4-expressing cells in the experiments in C–C″ and D–D″ was quantified and shown as mean±s.d. (n [field of view]=10–25, *P<0.01, t-test). (G) HeLa cells were transfected with FLAG-tagged Fz4 or Fz4K0, labelled with biotin, and chased for 6 h. FLAG-Fz4 proteins were immunoprecipitated and blotted with streptavidin and anti-FLAG antibody. The intensity of the biotinylated Fz4 bands was quantified, and the ratio of the intensity after 3 or 6 h of chase to that at 0 h is shown as mean±s.d. (n=5, *P<0.01, t-test) (A, B, bottom; G, right). (H) HEK293T cells were transfected with FOP-FLASH or TOP-FLASH luciferase together with the indicated Fz4 or UBPY constructs, and treated with Wnt3a overnight. Relative luciferase activity in the cell lysates is shown as mean±s.d. (n=3, *P<0.02, t-test). (I) HEK293T cells were transfected with TOP-FLASH luciferase together with the indicated Fz4 and UBPY constructs and treated with Wnt3a overnight. Relative luciferase activity in UBPYC748A- and UBPYS680A-expressing cells to that in UBPYWT-expressing cells is shown (mean±s.d.; n=3, *P<0.02, t-test).
When dominant-negative UBPYC748A was overexpressed, non-ubiquitylated Fz4 was lost (Figure 4B, top, arrow), but degradation of ubiquitylated Fz4 was delayed (Figure 4B, top, closed, and open arrowheads) during the chase period. This delayed degradation of ubiquitylated Fz will be discussed below (see Discussion). More importantly, Fz4 degradation was also inhibited when constitutively active UBPYS680A was expressed (Figure 4B). In this case, however, non-ubiquitylated Fz4 accumulated (Figure 4B, top, arrow), suggesting that deubiquitylated Fz escapes from degradation. After endocytosis, non-ubiquitylated plasma membrane proteins are recycled back to the cell surface (Gruenberg and Stenmark, 2004). To test whether deubiquitylated Fz4 is returned to the cell surface in UBPYS680A-expressing cells, we examined the cell surface level of Fz4 in two experiments. First, we performed surface immunostaining of living HEK293T cells transfected with N-terminally (extracellularly) FLAG-tagged Fz4 together with HA-tagged UBPY proteins. Cells were incubated with anti-FLAG antibody at 4°C to block endocytosis, and cell surface Fz4 levels were quantified by measuring the anti-FLAG fluorescence intensity of UBPY-transfected and -untransfected cells in the same microscopic fields, typical examples of which are shown in Figure 4C–C″ and D–D″. In UBPYS680A-expressing cells, the level of surface anti-FLAG staining was increased to ∼190% of that in untransfected cells, suggesting that Fz4 accumulates on the cell surface when deubiquitylated by UBPY (Figure 4C–C″ and E). In UBPYC748A-expressing cells, in contrast, the surface Fz4 level was decreased to ∼70% of that in the control (Figure 4D–D″ and F), suggesting that the ubiquitylated Fz4 in these cells (Figure 4B, closed and open arrowheads) accumulated intracellularly. Second, we transfected HEK293T cells with FLAG-Fz4 together with UBPYC748A or UBPYS680A, and labelled their surface with biotin. Cell surface FLAG-Fz4 was immunoprecipitated with anti-FLAG antibody and detected with streptavidin after electrophoresis. Consistent with the results of surface staining (Figure 4C–F), quantification of the bands corresponding to Fz4 showed that its surface level was increased to ∼160% and decreased to ∼70% of that in the control by overexpressing UBPYS680A and UBPYC748A, respectively (Supplementary Figure S4). As these experiments were performed in the absence of Wnt stimulation, they suggested that the steady-state level of Fz is regulated by UBPY.
Consistent with the notion that ubiquitylation drives Fz degradation, the steady-state surface level of non-ubiquitylatable FLAG-Fz4K0 was markedly higher than that of wild-type Fz4 (Figure 4G, 0 h), and its degradation was strongly suppressed (Figure 4G, 6 h). However, Wnt3a-dependent TOP-FLASH activity was not significantly elevated in FLAG-Fz4K0-transfected HEK293T cells compared with cells expressing wild-type Fz4 (Figure 4H), which, we reasoned, may be due to the absence of cytoplasmic Lys499 (Figure 3A), a critical residue for Wnt signalling (Umbhauer et al, 2000), in Fz4K0. We, therefore, constructed a Fz4R499K mutant in which all cytoplasmic Lys residues except for Lys499 are replaced to Arg, and confirmed that Fz4R499K still does not undergo ubiquitylation (data not shown). As expected, Fz4R499K markedly enhanced Wnt signalling: its overexpression increased TOP-FLASH ∼eight-fold compared with wild-type Fz4, comparable with the level observed when constitutively active UBPYS680A was expressed (Figure 4H). By using Fz4R499K, we further examined the significance of UBPY-mediated deubiquitylation of Fz in Wnt signalling. If UBPY regulates Fz by changing the ubiquitylation level, Wnt signalling through non-ubiquitylatable Fz4R499K should no longer be affected by altered UBPY activity. To test this hypothesis, we co-expressed Fz4R499K with UBPYC748A or UBPYS680A and measured the TOP-FLASH activity. The results clearly showed that the effects of UBPYC748A and UBPYS680A on Wnt signalling are much weaker when Fz4R499K was expressed than when wild-type Fz was expressed (Figure 4I). Collectively, these results suggested that Fz undergoes ubiquitylation-dependent degradation, which is negatively regulated by UBPY to increase its cell surface level and cellular responsiveness to Wnt.
dUBPY regulates cell surface level of Fz in Drosophila
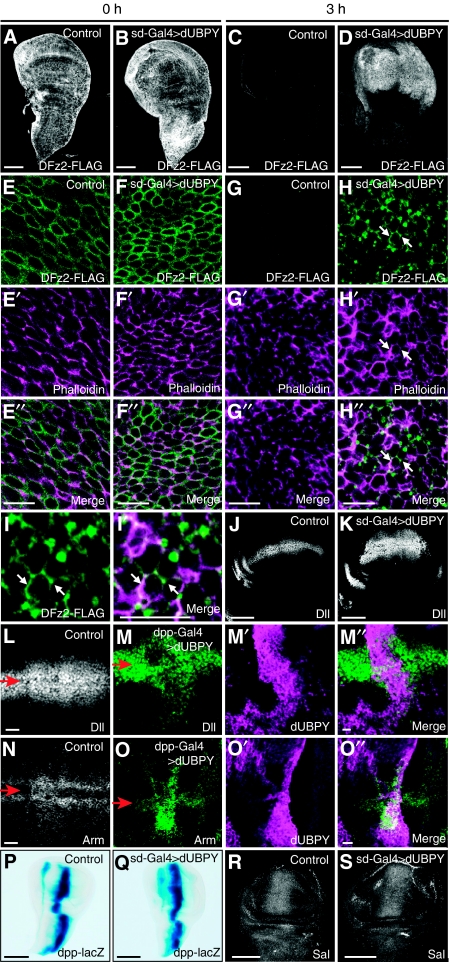
To investigate whether UBPY regulates the cell surface level of Fz in vivo, we examined the effect of dUBPY overexpression or knockout in the Drosophila wing disc. As there is no immunohistology-grade antibody available for Fz proteins, a transgenic Drosophila strain bearing FLAG-tagged DFz2 (Piddini et al, 2005) was used. When induced by heat shock, DFz2-FLAG was expressed on the cell surface at similar levels in the control and in discs overexpressing dUBPY (Figure 5A, B, E–E″, and F–F″). After a 3-h chase at 25°C, DFz2-FLAG was mostly degraded (Figure 5C) and barely detected on the cell surface (Figure 5G) in the control disc, although a small portion still remained intracellularly (see Figure 7A–A″). However, at the same time point, dUBPY overexpression by sd-Gal4 increased the amount of DFz2-FLAG in the wing pouch (Figure 5D). Quantification of the fluorescence intensity showed a nearly five-fold elevation in the DFz2 level on dUBPY overexpression. Co-staining with the F-actin marker phalloidin showed that the accumulated DFz2-FLAG mainly localized to the plasma membrane (Figure 5H–H″, I, and I′, arrows). The elevated surface DFz2 expression leads to higher cell responsiveness to Wg, as shown by the result showing that the expression area of a Wg target, Dll, is expanded more distally from the Wg-expressing dorso-ventral border in the dUBPY-overexpressing wing disc (Figure 5J and K). This expansion was also clearly shown by using decapentaplegic (dpp)-Gal4, which induced additional expression of dUBPY only near the anterior–posterior border (Figure 5L–M″). The level of Arm protein was also elevated in regions in which dUBPY was ectopically expressed (Figure 5N–O″). In contrast, expression of dpp-lacZ and Spalt (Sal), the targets for Hedgehog and Dpp, respectively, was not affected by dUBPY overexpression (Figure 5P–S).
Figure 5.
dUBPY regulates cell surface DFz2 level and Wg signalling in the wing disc. (A–H″) Staining of control (A, C, E–E″, G–G″) and dUBPY-overexpressing (B, D, F–F″, H–H″) DFz2-FLAG wing discs at the end of heat shock induction of DFz2-FLAG (A, B, E–E″, F–F″) and after a 3-h chase at 25°C (C, D, G–G″, H–H″) with anti-FLAG antibody (A–H) and phalloidin (E′–H′). E″–H″ are merged images. All the dUBPY-overexpressing discs examined (∼30) exhibited the same phenotype. (I, I′) High-magnification images of (H) and (H″). Arrows indicate the plasma membrane (H–H″, I, I′). (J, K) Anti-Dll staining of control (J) and dUBPY-overexpressing (K) wing discs. (L–O″) Anti-Dll (L, M–M″) and anti-Arm (N, O–O″) staining of control (L, N) and dpp-Gal4-driven dUBPY-overexpressing (M–M″, O–O″) wing discs. dUBPY-overexpressing discs were co-stained with anti-dUBPY antibody (M′, O′). M″ and O″ are merged images. Arrows indicate the positions of the dorso-ventral border (L–O). (P–S) Activity staining of β-gal derived from dpp-lacZ (P, Q) and anti-Sal (R, S) staining in control (P, R) and dUBPY-overexpressing (Q, S) wing discs. Bars, 100 μm (A–D, J, K, P–S); 10 μm (E″–H″, I′, L, M″, N, O″).
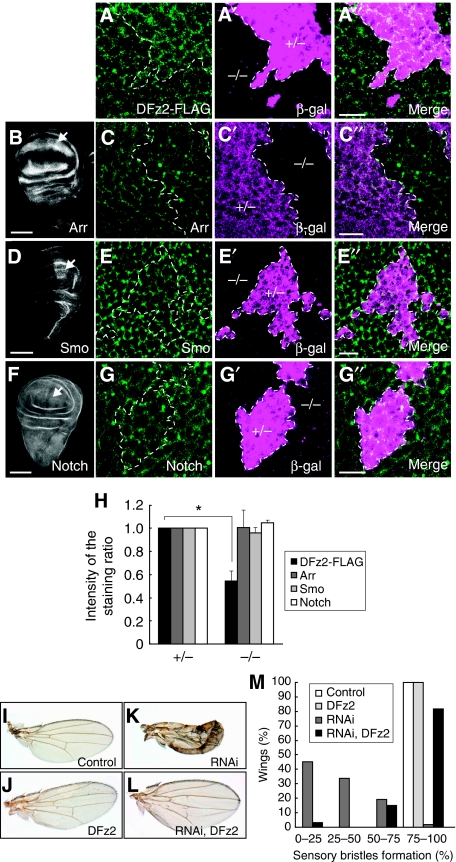
We next examined the cell surface level of DFz2 in dUBPY knockout clones in the wing disc, which enabled a side-by-side comparison of normal and dUBPY-deficient cells in a single disc. After a 2-h chase after heat shock induction of DFz2-FLAG, a lower level of plasma membrane staining with anti-FLAG antibody was observed in the dUBPY knockout disc cells than in wild-type cells (Figure 6A–A″ and H), further confirming that UBPY elevates the surface level of Fz. To test whether the regulation by UBPY is selective for Fz, we examined the surface levels of other plasma membrane proteins including the Wg co-receptor Arr, the Hedgehog co-receptor Smoothened (Smo), and Notch, as well as Flamingo (Fmi), β-integrin, and DE-cadherin in the dUBPY knockout disc. Anti-Arr, anti-Smo, and anti-Notch staining patterns in the wild-type disc were consistent with the established expression patterns of these receptors (Figure 6B, D, and F). Immunostaining of the discs harbouring dUBPY knockout clones showed that unlike DFz2, the surface levels of these receptors are indistinguishable between the control and dUBPY knockout cells (Figure 6C–C″, E–E″, G–G″, and H). Z sections of the wing discs also provided similar staining patterns for Arr, Smo, and Notch between the control and knockout cells (Supplementary Figure S5). Similarly, the surface levels of Fmi, β-integrin, and DE-cadherin were also unaffected by knockout of dUBPY in the wing disc (Supplementary Figure S6). We further examined the surface levels of Notch and Arr in the dUBPY-overexpressing wing disc and found that their levels were also unaffected by dUBPY overexpression (Supplementary Figure S7). These results suggested that aberrant dUBPY expression does not affect surface receptor levels non-selectively.
Figure 6.
dUBPY is a selective regulator for DFz2 in the wing disc. (A–A″) Anti-FLAG staining (A) of a dUBPY knockout clone, which is negative for β-gal (A′, −/−), in the DFz2-FLAG wing pouch after a 2-h chase of heat shock-induced DFz2-FLAG. (B–G″) Anti-Arr (B, C), anti-Smo (D, E), and anti-Notch (F, G) staining of the wild-type wing discs (B, D, F) and dUBPY knockout clones (C, E, G), which are negative for β-gal (C′, E′, G′, −/−), in the wing pouch. A″, C″, E″, and G″ are merged images. Arrows in B, D, and F indicate regions that were analysed in the dUBPY knockout discs in C–C″, E–E″, and G–G″. Bars, 10 μm (A″, C″, E″, G″); 100 μm (B, D, F). (H) Anti-FLAG (DFz2), anti-Arr, anti-Smo, and anti-Notch fluorescence intensity in experiments in A–G″ was quantified in the control and dUBPY knockout areas. Relative intensity in the knockout area (−/−) to that in the control area (+/−) is shown (mean±s.d., n=12 for each staining, *P<0.02, t-test). (I–L) Control (I), DFz2-overexpressing (J), dUBPY RNAi (K), and dUBPY RNAi/DFz2-overexpressing (L) wings in adult flies. (M) Quantification of sensory bristle formation in wings in experiments in I–L. Percentage of wings with a different degree of sensory bristle formation is shown (n=33–62).
To confirm that the reduced surface level of DFz2 is responsible for the phenotype of the dUBPY knockdown wing, we overexpressed DFz2-FLAG in the dUBPY knockdown wing disc. When DFz2 was co-expressed, the wing defect caused by dUBPY RNAi was largely rescued (Figure 6I–M). We, therefore, suggest that Fz is a bona fide target of dUBPY in the Drosophila wing disc.
dUBPY regulates endocytic Fz trafficking in Drosophila
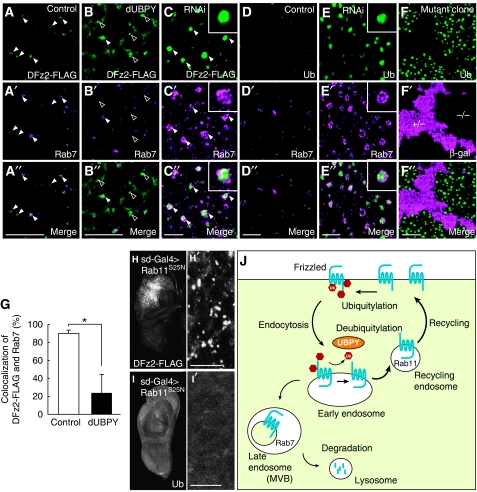
We finally addressed a mechanistic basis for the UBPY-regulated surface level of Fz in vivo by examining intracellular DFz2 trafficking in the dUBPY overexpressing and knockdown wing discs. As shown above, heat shock-induced DFz2-FLAG was mostly degraded in the control disc after a 3-h chase at 25°C (Figure 5C and G). When focused intracellularly, however, DFz2-FLAG was detected on cytoplasmic puncta that were positive for a late endosome marker Rab7 (Jordens et al, 2005) (Figure 7A–A″, closed arrowheads). Approximately 90% of DFz2-positive puncta were positive for Rab7 (Figure 7G). Although DFz2-positive puncta were similarly observed in the dUBPY overexpressing wing disc, only ∼20% of them were positive for Rab7 (Figure 7B–B″, closed versus open arrowheads; Figure 7G), suggesting that DFz2, when deubiquitylated by ectopically expressed dUBPY, is excluded from lysosomal trafficking through the late endosome. We speculate that the DFz2-positive/Rab7-negative puncta in dUBPY-overexpressing cells represent the early or recycling endosomes. In contrast, dUBPY knockdown caused enlargement of the Rab7-positive endosomes in which DFz2 highly accumulated (Figure 7C–C″). The enlarged late endosomes probably reflect accelerated membrane trafficking from the early to the late endosome as indicated earlier (Seto and Bellen, 2006) (see Discussion).
Figure 7.
dUBPY regulates endocytic DFz2 trafficking in the wing disc. (A–E″) Control (A–A″, D–D″), dUBPY-overexpressing (B–B″), and dUBPY RNAi (C–C″, E–E″) wing discs expressing DFz2-FLAG (A–C″) or not (D–E″) were stained with anti-FLAG (A–C) or FK2 (D, E) antibody together with anti-Rab7 (A′–E′). Closed and open arrowheads indicate Rab7-positive and Rab7-negative endosomes, respectively (A–C″). Insets in C–C″ and E–E″ show high-magnification images of typical Rab7-positive endosomes. (F–F″) FK2 staining (F) of a dUBPY knockout clone in the wing disc, which is negative for β-gal (F′, −/−). A″–F″ are merged images. (G) Control and dUBPY-overexpressing wing discs expressing DFz2-FLAG were double-stained with anti-FLAG and anti-Rab7 antibodies as in A–A″ and B–B″. Percentage of FLAG/Rab7-double-positive endosomes among total FLAG-positive endosomes was then determined (mean±s.d., n [field of view]=5–7, *P<0.01, t-test). (H–I′) Anti-FLAG (H, H′) and FK2 (I, I′) staining of DFz2-FLAG wing discs co-expressing Rab11S25N in the wing pouch. H′ and I′ show high-magnification images of the wing pouch regions in H and I. Bars, 10 μm. (J) A model for the regulation of the cell surface level of Fz.
As accumulated Fz4 was highly ubiquitylated in mammalian cells expressing UBPYC748A (Figures 3B and 4B), we examined whether the Rab7 endosomes harbouring DFz2-FLAG contain ubiquitylated proteins in the dUBPY knockdown wing disc. Immunofluorescence showed that the aberrant Rab7-positive endosomes were strongly stained with a monoclonal anti-Ub antibody FK2, which recognizes Ub-protein conjugates, but not free mono-Ub (Fujimuro and Yokosawa, 2005) (Figure 7E–E″). This was also observed in the dUBPY knockout cells (Figure 7F–F″). Although Rab7 localized to the limiting membrane of aberrant endosomes, DFz2-FLAG and Ub-protein conjugates were detected in the lumen of the organelles (Figure 7C–C″ and E–E″, insets), suggesting that these proteins were incorporated into the lumenal vesicles of the late endosome/MVB. Along with the fact that expression of the Wg-target neuralized was lost by knockdown of dUBPY (Figure 1G), DFz2-FLAG accumulated in the lumen of the MVB would no longer evoke Wg signalling. Accumulation of ubiquitylated proteins in aberrant endosomes has been reported in UBPY knockdown mammalian cells (Mizuno et al, 2006; Row et al, 2006), suggesting that dUBPY similarly participates in the lysosomal sorting of ubiquitylated cargo proteins in Drosophila.
To show that deubiquitylated DFz2 is potentially recycled back to the cell surface from the endosome, we expressed Rab11S25N, a GDP-locked Rab11 mutant that blocks recycling of endocytosed proteins to the plasma membrane (Emery et al, 2005; Marois et al, 2006), in the wing disc. Sd-Gal4-induced ectopic expression of Rab11S25N caused intracellular accumulation of DFz2-FLAG in the wing pouch (Figure 7H and H′, compare with the control discs in Figure 5C and G). In contrast to the endosomal co-localization of DFz2 and ubiquitylated proteins in the dUBPY knockdown disc (Figure 7C–C″ and E–E″), the DFz2-positive compartments in Rab11S25N-expressing cells were devoid of ubiquitylated proteins (Figure 7I and I′). These results suggested that endocytosed Fz, when deubiquitylated, is sorted for the recycling pathway back to the cell surface. Collectively, we propose that dUBPY up-regulates the cell surface Fz level in the wing disc by suppressing its lysosomal trafficking and promoting recycling to the plasma membrane (Figure 7J).
Discussion
It is well known that the target cell response to morphogens is controlled at the ligand level by the formation of the morphogen gradient (Kornberg and Guha, 2007). In this study, we show that regulation at the receptor level also has an essential function in the cellular response to morphogens.
This study showed that Fz undergoes ubiquitylation, which is likely to be multiple monoubiquitylation, as well as deubiquitylation catalysed by UBPY, in mammalian cells. We also showed that Fz degradation is delayed by lysosomal, but not proteasomal inhibition and that degradation of non-ubiquitylatable Fz mutant is inhibited. In addition, Fz accumulated in the endocytic compartment when dUBPY was inhibited in Drosophila. As monoubiquitylation is a tag shown to target plasma membrane proteins such as RTKs to the lysosome (Haglund and Dikic, 2005), we conclude that ubiquitylation serves as a lysosomal sorting signal for Fz.
The functions of ubiquitylation-mediated lysosomal degradation, however, seemed to be different between RTKs and Fz. Ubiquitylation and lysosomal degradation of RTKs are induced by ligand stimulation and promote the down-regulation of activated receptors (Gruenberg and Stenmark, 2004; Saksena et al, 2007). Binding of UBPY to EGFR is also ligand dependent (Mizuno et al, 2005). In contrast, the levels of ubiquitylation and UBPY binding of Fz4, as well as the rate of Fz4 degradation, were unchanged by stimulating cells with Wnt3a. These results suggest that ubiquitylation of Fz regulates its steady-state level at the cell surface to control cellular responsiveness to Wnt, rather than its ligand-induced down-regulation to terminate signalling.
Ubiquitylation has essential functions in Wg/Wnt signalling. In the absence of Wg/Wnt stimulation, β-catenin, a central player in the canonical pathway, undergoes constitutive polyubiquitylation and proteasomal degradation. Wg/Wnt transduces signals to the nucleus by suppressing the Ub-dependent degradation of β-catenin (MacDonald et al, 2009). Recently, Trabid, a deubiquitylating enzyme of the OTU (ovarian tumour protease) family, was shown to deconjugate Lys63-linked poly-Ub chains from adenomatous polyposis coli (APC) protein and facilitate the canonical pathway, although the function of Lys63-linked polyubiquitylation in Wg/Wnt signalling is still unclear (Tran et al, 2008). This study shows a third function for ubiquitylation in Wg/Wnt signalling: to control the surface level of Fz receptors.
The present findings indicate that the cell surface level of Fz is positively regulated by UBPY activity. In mammalian and Drosophila cells, cell surface Fz was increased by elevation of UBPY activity and decreased by UBPY inhibition. As ubiquitylation targets Fz for lysosomal degradation, deubiquitylation of Fz by UBPY would inhibit its lysosomal trafficking, causing Fz to be recycled back to the cell surface. Recycling of Fz was confirmed by the finding that DFz2, which was likely to be deubiquitylated, accumulated intracellularly when the recycling pathway was blocked by a dominant-negative Rab11 mutant in Drosophila. Moreover, we showed that overexpression of dUBPY in Drosophila wing disc cells causes a decrease in the Fz transported to the Rab7-positive late endosome. Taken together, we conclude that a balance of ubiquitylation and deubiquitylation determines the amount of Fz on the cell surface by controlling how much Fz is transported to the lysosome for degradation or recycled back to the plasma membrane from the endosome.
Expression of dominant-negative UBPYC784A caused a delay in Fz4 degradation in mammalian cells, which seems contradictory to our model. However, it has already been shown that inhibition of UBPY using RNAi or dominant-negative UBPYC748A causes the accumulation of ubiquitylated proteins in enlarged aberrant endosomes (Mizuno et al, 2006; Row et al, 2006). Consistently, knockdown of dUBPY in the Drosophila wing disc was observed to cause the accumulation of DFz2 and ubiquitylated proteins in the lumen of the morphologically aberrant late endosome. Therefore, we speculate that the delayed degradation of ubiquitylated Fz4 in UBPYC748A-expressing cells represents its intracellular accumulation, which was secondarily caused by the aberration of the late endosome. Interestingly, overexpression of Hrs, a component of ESCRT-0 that promotes lysosomal trafficking of ubiquitylated cargoes, has also been reported to cause aberrant morphology of the late endosome in the Drosophila wing disc (Seto and Bellen, 2006). This study proposes that accelerated endocytic trafficking by Hrs overexpression leads to aberrant late endosomal morphology. Therefore, the similar aberration of the late endosome in UBPY-deficient Drosophila cells probably reflects accelerated early-to-late endosomal trafficking of ubiquitylated proteins including Fz.
Experiments using anti-Ub antibody FK2 showed that the level of total cellular ubiquitylation is elevated in the Drosophila wing disc by knockdown of dUBPY and decreased by overexpression of wild-type dUBPY (Supplementary Figure S8). In addition to showing the effectiveness of our RNAi system in depleting dUBPY activity, these results suggest that DFz2 is not the only substrate for dUBPY. In contrast to DFz2, however, cell surface levels of other membrane proteins examined were unaffected in the dUBPY knockout or overexpressing disc cells, excluding the possibility that the altered DFz2 level in these cells is due to a general defect in endocytosis from or recycling to the plasma membrane. Therefore, the regulation by dUBPY probably has some selectivity to a restricted group of proteins including Fz.
Controlling the cell surface receptor level for extracellular ligands, such as growth factors and morphogens, is a critical regulatory process for normal development. Obviously, insufficient receptor levels cannot trigger a full cellular response to the ligands. However, excess of receptors also leads to abnormal situations. In Drosophila, forced expression of DFz2 has been shown to cause defects in wing patterning (Cadigan et al, 1998). This study shows, in vivo and in vitro, that the reduction of cell surface Fz by UBPY inhibition suppresses the cellular responsiveness to Wg/Wnt in the canonical pathway, whereas the elevation of Fz by UBPY gain-of-function enhances Wg/Wnt signalling. Accordingly, we conclude that the level of Fz ubiquitylation controls the level of canonical Wg/Wnt signals generated in target cells. Identifying the Ub ligase for Fz, and elucidating how the activities of UBPY and the Ub ligase are controlled, are important to fully understand the regulatory mechanism.
Finally, dysregulation of Wnt signalling is implicated in various human diseases (Logan and Nusse, 2004; Clevers, 2006), and up-regulation of Fz has been reported in human cancer (Lu et al, 2004; Bengochea et al, 2008). In chronic lymphocytic leukaemia (CLL), overexpression of mRNAs for several Wnt ligands and Fz receptors is involved in prolonged survival of cancer cells (Lu et al, 2004). We found that the mRNA expression of UBPY and its functionally related proteins is frequently elevated in CLL (Supplementary Figure S9). In CLL cells, therefore, elevated UBPY activity might facilitate higher levels of Wnt signalling by suppressing Fz degradation. An imbalance between Fz ubiquitylation and deubiquitylation could thus lead not only to developmental defects, but also to human cancer.
Materials and methods
dUBPY knockdown, knockout, and overexpressing flies
The cDNA for dUBPY (CG5798) was obtained from the Drosophila Genomics Resource Center (DGRC, Bloomington, IN), cloned into the expression vector pUAST-R57 (for dsRNAs) or pUAST-new MCS (for wild-type cDNA), and microinjected into Drosophila oocytes by standard procedures. The following transgenic strains were generated: (1) two UAS-dUBPY RNAi strains expressing dsRNAs targeting the dUBPY sequences from +74 to +573 and from +1386 to +1885 in the open reading frame, and (2) the UAS-dUBPY strain expressing wild-type dUBPY cDNA, under the control of the Gal4/UAS system.
The dUBPY knockout flies were generated using ends-out technology (Gong and Golic, 2004). The targeting vector was designed to replace the entire dUBPY gene on the chromosome with the white gene from the vector. Approximately 3 kb DNA fragments corresponding to 5′-upstream and 3′-downstream regions of the dUBPY gene were amplified from genomic DNA by PCR, and inserted into the white gene-flanking polylinker sites on the pW35 vector (obtained from DGRC). Most of the flies used for gene targeting were obtained from the Bloomington Drosophila Stock Center (BDSC, Bloomington, IN) and targeting crosses were performed as described (Gong and Golic, 2004). dUBPY knockout flies were partially rescued by a slight expression of dUBPY using UAS-dUBPY without Gal4 driver (UAS-dUBPY/dUBPY K.O.). Clone analysis was achieved by using hs-FLP; ubi-lacZ M FRT82B/TMB6 and hs-FLP; FRT82B dUBPY K.O./TM6B flies.
Fly stocks
Other fly strains used in this study are listed in Supplementary data.
Immunofluorescence and X-Gal staining
For immunofluorescence, wing discs dissected from Drosophila third instar larvae were fixed in 4% paraformaldehyde in PBS, incubated in 0.15% bovine serum albumin, and 0.2% Triton X-100 in PBS, and sequentially stained with primary and secondary antibodies listed in Supplementary data. Staining of extracellular Wg and cell surface Notch was performed as described earlier (Strigini and Cohen, 2000). Staining of cell surface FLAG-Fz4 in mammalian cells was performed by incubating living cells with mouse anti-FLAG antibody at 4°C for 1 h. Cells were then fixed, permeabilized, blocked, and incubated with biotinylated anti-mouse IgG antibody and rabbit anti-HA antibody. Subsequently, they were stained with Alexa488-conjugated streptavidin (Invitrogen, Carlsbad, CA) and Cy3-conjugated anti-rabbit IgG antibody. F-actin and DNA were stained with rhodamine-labelled phalloidin (Invitrogen) and TO-PRO-3 iodide (642/661) (Invitrogen), respectively. Fluorescence images were captured with a laser-scanning confocal microscope (FV500, OLYMPUS, Tokyo, Japan), and the fluorescence intensity was quantified using Photoshop (Adobe Systems, San Jose, CA). Fluorescence intensities in mammalian cells were normalized to the circumference of individual cells.
For X-Gal staining, wing discs from Drosophila third instar larvae were fixed with 1% glutaraldehyde in PBS for 10 min at room temperature, washed with 0.1% Triton X-100 in PBS, and incubated in 0.2% X-Gal, 7.2 mM Na2HPO4, 2.8 mM NaH2PO4, 150 mM NaCl, 1 mM MgCl2, 3 mM K3[Fe(CN)6], and 3 mM K4[Fe(CN)6] for 15–60 min at 37°C.
Mammalian expression constructs
Mammalian expression vectors used in this study are listed in Supplementary data.
Cells and DNA transfection
DNA transfection into HEK293T, HeLa, NIH3T3, and COS-7 cells was performed using the FuGENE6 (Roche Diagnostics, Indianapolis, IN) and Lipofectamine 2000 (Invitrogen) transfection reagents for 48 h. The siRNA expression vectors were transfected twice at 48 h intervals. Where indicated, cells were treated with cycloheximide (10 μg/ml, Sigma-Aldrich, St Louis, MO), bafilomycin A1 (0.2 μM, Sigma-Aldrich), or MG132 (0.1 μM, Merck, Whitehouse Station, NJ). L cells stably expressing Wnt3a (L-Wnt3a) and control cells (L-neo) were provided by Dr Shinji Takada (Kyoto University, Kyoto, Japan) (Shibamoto et al, 1998), and Wnt3a-conditioned medium was used at 1/5–1/2 strength for stimulation of cells.
Cell lysate preparation
Cell lysates were prepared by solubilizing cells in 100 mM Tris–HCl, pH 7.4, 100 mM NaCl, 50 mM NaF, 0.5% Nonidet P-40, 1 mM EDTA, and protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan) and collecting the supernatants after centrifugation. For detection of Fz4 ubiquitylation, cells were lysed in hot SDS lysis buffer (1% SDS, 50 mM NaF, and 1 mM EDTA) for 1 min at 100°C. After five-fold dilution with dilution buffer (25 mM Tris–HCl, pH 7.5, 1.25% Triton X-100, 125 mM NaCl, and 50 mM NaF), supernatants were collected as cell lysates. For subcellular fractionation, cells were scraped into 10 mM Tris–HCl, pH 7.4, 1 mM EDTA, and protease inhibitor cocktail, and homogenized using a glass homogenizer. After removing nuclei by brief centrifugation, homogenates were further centrifuged at 55 000 g for 1 h, and the supernatants were collected as cytoplasmic fractions. Biotinylation of cell surface proteins was performed using EZ-Link Sulfo-NHS-Biotin (Thermo Scientific, Rockford, IL).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed using standard procedures. Antibodies used are listed in Supplementary data. To precipitate biotin-labelled FLAG-Fz4, biotinylated cell surface proteins were first precipitated with streptavidin beads (GE Healthcare, Piscataway, NJ) and eluted with hot SDS lysis buffer. After a five-fold dilution with dilution buffer, the eluates were precipitated with anti-FLAG antibody. Blots were detected using the SuperSignal West Pico Chemiluminescent Substrate Reagent (Thermo Scientific). Band intensity in the immunoblot membranes was quantified using the luminescent image analyser LAS1000 PLUS (FUJIFILM, Tokyo, Japan).
TOP-FLASH reporter assay
Cells were transfected with super 8 × TOP-FLASH or 8 × FOP-FLASH firefly luciferase, together with phRL-SV40 renilla luciferase and UBPY or Fz4 constructs. The cells were treated with control or Wnt3a-conditioned medium overnight, and luciferase activity in the cell lysates was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Relative firefly luciferase units were normalized to the renilla reading.
In vitro deubiquitylation assay
In vitro deubiquitylation assay was performed as described earlier (Mizuno et al, 2007). Briefly, recombinant FLAG-UBPY proteins were immunoprecipitated from transfected COS-7 cells with anti-FLAG antibody and eluted with the FLAG peptide. Ubiquitylated HA-Fz4 was immunoprecipitated from transfected HeLa cells in a 6-cm culture dish and incubated with immunopurified UBPY (0.6 μg) for 1 h. Reaction products were subjected to electrophoresis and detected by immunoblotting.
Supplementary Material
Acknowledgments
We thank Drs HJ Bellen, SB Carroll, S Cohen, S DiNardo, I Duncan, JA Knoblich, Y Minami, A Nakamura, M Nishida, R Nusse, S Takada, and J-P Vincent, as well as the Drosophila Genomics Resource Center, Bloomington Drosophila Stock Center, and Developmental Studies Hybridoma Bank for reagents. This work was supported by Grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to SG (No. 60280575) and to MK (No. 19570178).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P (2008) Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer 99: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R (2006) A critical role for endocytosis in Wnt signaling. BMC Cell Biol 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R (1998) Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell 93: 767–777 [DOI] [PubMed] [Google Scholar]
- Campuzano S, Modolell J (1992) Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet 8: 202–208 [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ (2003) Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301: 1391–1394 [DOI] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A (1994) The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120: 621–636 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S (1997) Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124: 3241–3251 [DOI] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik E, Mayer B, Wirtz-Peitz F, Gaitan GM, Knoblich AJ (2005) Asymmetric Rab11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell 122: 763–773 [DOI] [PubMed] [Google Scholar]
- Fujimuro M, Yokosawa H (2005) Production of antipolyubiquitin monoclonal antibodies and their use for characterization and isolation of polyubiquitinated proteins. Methods Enzymol 399: 75–86 [DOI] [PubMed] [Google Scholar]
- Gagliardi M, Piddini E, Vincent J-P (2008) Endocytosis: a positive or a negative influence on Wnt signalling? Traffic 9: 1–9 [DOI] [PubMed] [Google Scholar]
- Gong WJ, Golic KG (2004) Genomic deletions of the Drosophila melanogaster Hsp70 genes. Genetics 168: 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H (2004) The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5: 317–323 [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24: 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I, Marsman M, Kuijl C, Neefjes J (2005) Rab proteins, connecting transport and vesicle fusion. Traffic 6: 1070–1077 [DOI] [PubMed] [Google Scholar]
- Kato M, Miyazawa K, Kitamura N (2000) A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J Biol Chem 275: 37481–37487 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H (2007) Regulation of Wnt signalling by receptor-mediated endocytosis. J Biochem 141: 443–451 [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB (1996) Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382: 133–138 [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M (2005) Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol 21: 155–176 [DOI] [PubMed] [Google Scholar]
- Komada M (2008) Controlling receptor downregulation by ubiquitination and deubiquitination. Curr Drug Discov Technol 5: 78–84 [DOI] [PubMed] [Google Scholar]
- Kornberg TB, Guha A (2007) Understanding morphogen gradients: a problem of dispersion and containment. Curr Opin Genet Dev 17: 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA (2004) Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 101: 3118–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois E, Mahmoud A, Eaton S (2006) The endocytic pathway and formation of the Wingless morphogen gradient. Development 133: 307–317 [DOI] [PubMed] [Google Scholar]
- Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M (2005) Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell 16: 5163–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno E, Kitamura N, Komada M (2007) 14-3-3-dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp Cell Res 313: 3624–3634 [DOI] [PubMed] [Google Scholar]
- Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M (2006) A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic 7: 1017–1031 [DOI] [PubMed] [Google Scholar]
- Naviglio S, Mattecucci C, Matoskova B, Nagase T, Nomura N, Di Fiore PP, Draetta GF (1998) UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J 17: 3241–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H, Feller S, Lewitzky M, Horak I, Knobeloch KP (2007) Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol 27: 5029–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102: 349–362 [DOI] [PubMed] [Google Scholar]
- Piddini E, Marshall F, Dubois L, Hirst E, Vincent J-P (2005) Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development 132: 5479–5489 [DOI] [PubMed] [Google Scholar]
- Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S (2006) Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol 293: 268–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row PE, Liu H, Hayes S, Welchman R, Charalabous P, Hofmann K, Clague MJ, Sanderson CM, Urbe S (2007) The MIT domain of UBPY constitutes a CHMP binding and endosomal localization signal required for efficient epidermal growth factor receptor degradation. J Biol Chem 282: 30929–30937 [DOI] [PubMed] [Google Scholar]
- Row PE, Prior IA, McCullough J, Clague MJ, Urbe S (2006) The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem 281: 12618–12624 [DOI] [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr SD (2007) ESCRTing proteins in the endocytic pathway. Trends Biochem Sci 32: 561–573 [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V (2007) The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci 28: 518–525 [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ (2006) Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol 173: 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S (1998) Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3: 659–670 [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM (2000) Wingless gradient formation in the Drosophila wing. Curr Biol 10: 293–300 [DOI] [PubMed] [Google Scholar]
- Tran H, Hamada F, Schwarz-Romond T, Bienz M (2008) Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev 22: 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL (2000) The C-terminal cytoplasmic Lys-Thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/β-catenin signalling. EMBO J 19: 4944–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A (2006) Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell 11: 213–223 [DOI] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T (2007) Association of dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a Wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.