Abstract
Skin cancers are the most commonly diagnosed cancers. Understanding what are the factors contributing to skin tumour development can be instrumental to identify preventive therapies. The myeloid differentiation primary response gene (MyD)88, the downstream adaptor protein of most Toll-like receptors (TLR), has been shown to be involved in several mouse tumourigenesis models. We show here that TLR4, but not TLR2 or TLR9, is upstream of MyD88 in skin tumourigenesis. TLR4 triggering is not dependent on lipopolysaccharide associated to skin-colonizing bacteria, but on the high mobility group box-1 protein (HMGB1), an endogenous ligand of TLR4. HMGB1 is released by necrotic keratinocytes and is required for the recruitment of inflammatory cells and for the initiation of inflammation. The expression of TLR4 on both bone marrow-derived and radioresistant cells is necessary for carcinogenesis. Consistently, a human tissue microarray analysis showed that melanoma and colon cancer display an over-expression of TLR4 and its downstream adaptor protein MyD88 within tumours. Together, our results suggest that the initial release of HMGB1 triggers a TLR4-dependent inflammatory response that leads to tumour development.
Keywords: HMGB1, inflammation, skin, TLR4, tumourigenesis
Introduction
Toll-like receptors (TLRs) are a class of pattern recognition receptors that are important sensors of pathogen invasion. To date, 13 mammalian members of the TLR family have been described that respond to different microbe-associated molecular patterns, including viral or bacterial nucleic acids, lipopolysaccharide (LPS) or lipoteichoic acids and flagellin (Miyake, 2007). However, TLRs can also bind endogenously generated ligands such as heat shock proteins (Ohashi et al, 2000; Vabulas et al, 2002; Roelofs et al, 2006), surfactant protein A18 (Guillot et al, 2002), extracellular matrix components (Rakoff-Nahoum and Medzhitov, 2009) and high mobility group box-1 (HMGB1) (Park et al, 2004; Apetoh et al, 2007). All TLRs except TLR3 signal through an adaptor protein called MyD88 that is responsible for initiating a cascade of events leading to NF-kB activation (Kawai and Akira, 2007).
Mice deficient in MyD88 are protected against the development of spontaneous intestinal tumours (Rakoff-Nahoum and Medzhitov, 2007) as well as chemically induced skin (Swann et al, 2008), intestinal (Rakoff-Nahoum and Medzhitov, 2007) and liver cancers (Naugler et al, 2007). Evidence that TLRs participate to cancer development comes from a study by Fukata et al (2007) showing that colitis-associated cancer is dependent on TLR4, as mice deficient for TLR4 display reduced risk to develop intestinal cancers. The intestine is colonized by trillions of commensal bacteria that participate to the digestive function (Ley et al, 2006) and could provide TLR4 ligands, but a contribution of endogenous TLR4 ligands in tumourigenesis remains an unresolved question. A possible function of endogenous ligands of TLRs in tumour development comes from expression association studies; for instance, HMGB1 has been shown to be over-expressed in several human neoplasms including breast, colorectal, lung, pancreatic, liver cancers and melanoma (Lotze et al, 2007). HMGB1 is either passively released by injured or necrotic cells (Scaffidi et al, 2002) or actively secreted by inflammatory cells, including neutrophils, monocyte/macrophages and dendritic cells (reviewed in van Beijnum et al, 2008). It can function as a damage-associated molecular pattern and is released extracellularly as a signal of tissue damage. HMGB1 has been shown in vitro to signal through several receptors, including the receptor for advanced glycation end products (RAGE) (Hori et al, 1995), TLR2, TLR4 (Yu et al, 2006) and TLR9 in conjunction with CpG-DNA (Ivanov et al, 2007). HMGB1 probably activates several different receptors in vivo, perhaps with different modes (Bianchi, 2009). HMGB1 administration in mice deficient for TLR2, TLR4 or RAGE showed that in TLR4 or RAGE knock-out (KO) mice, there was a drastically reduced production of inflammatory cytokines, whereas TLR2 KO mice displayed even increased inflammatory cytokine production (van Zoelen et al, 2009). This suggests that HMGB1 binding to TLR4, but not TLR2 may be involved in the induction of inflammatory processes in vivo. As inflammation has been linked to cancer development (Balkwill and Mantovani, 2001; Coussens and Werb, 2002; Beachy et al, 2004; Clevers, 2004; Balkwill et al, 2005; Robinson and Coussens, 2005; Karin et al, 2006), we analysed whether TLR4 and its endogenous ligand HMGB1 could be linked to inflammatory events and tumour development in the skin.
In this manuscript, we used the well-established model of two-step (croton oil (CO)/7,12-dimethylbenz(a)anthracene (DMBA)) chemically induced skin carcinogenesis. We analysed the contribution of different TLRs that are upstream of MyD88 in skin tumourigenesis. We show that TLR4, but not TLR2 or TLR9, is involved in tumour development. We identified HMGB1 and not skin-colonizing bacteria-derived LPS as the trigger of TLR4 that is responsible for the recruitment of immune cells and the generation of a pro-tumourigenic inflammatory response. Finally, TLR4 expression by both bone marrow-derived and radioresistant cells is required for tumour development.
Results
TLR4 has an important function in mouse skin tumourigenesis through an MyD88-dependent pathway
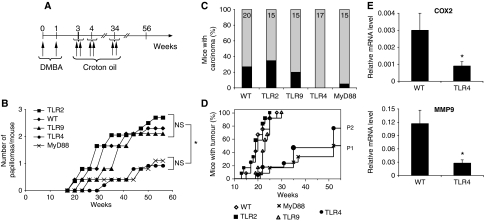
To analyse skin tumour development, we treated TLR4 KO and C57BL/6 wild-type (WT) mice with DMBA once a week for 2 weeks and then with CO twice a week for 34 weeks (Figure 1A). Mice were then followed up to 56 weeks for tumour development. We decided to use CO because it was shown to induce the formation of melanomas, as well as squamous cell carcinomas (Takizawa et al, 1985). However, we could detect primarily papillomas and squamous cell carcinomas (Supplementary Figure 1) and only two melanomas in 20 WT mice. TLR4 KO mice showed resistance to tumour induction (Figure 1; Supplementary Figure 1A); the number of papillomas (Figure 1B) and the percentage of mice with carcinomas (Figure 1C) were significantly lower in TLR4 KO than WT mice. Furthermore, the time of appearance of tumours after initiation was significantly different (P<0.05) between TLR4 KO and WT mice as shown in Figure 1B. More than 95% of WT mice appeared to have tumours, papilloma or carcinoma after 25 weeks, whereas >30% of TLR4 KO mice remained tumour free till the end of the experiment (Figure 1D). Similarly to TLR4 KO mice, MyD88 KO mice showed a significant resistance to tumour development (Figure 1B–D). Thus, we concluded that TLR4 is having an important function in tumour induction possibly through the ‘MyD88-dependent' signalling pathway. We also found a higher expression of COX2 and MMP9 in the papillomas of WT than TLR4-KO mice (Figure 1E). COX2 has been shown to have a major function in skin carcinogenesis, as its inhibition can reduce the occurrence of UV-induced skin tumours (Fischer, 2002), whereas MMP9 is associated with the invasiveness and motility of tumour cells as well as with the initiation and maintenance of growth of primary and metastatic tumours (Chambers and Matrisian, 1997).
Figure 1.
TLR4 and MyD88 KO mice are resistant to chemical-induced skin carcinogenesis. (A) Schematic representation of carcinogenesis protocol. Mice were followed up to a year and mice bearing a tumour >15 mm or sick were killed. (B) Average number of papillomas per mouse after DMBA/CO two-stage chemical carcinogenesis in function of time (weeks). *P<0.05 between WT, TLR2 KO and TLR9 KO versus TLR4 and MyD88 KO after 52 weeks. (C) Graph displaying percentage of mice carrying at least one carcinoma (in black) in the different genetic backgrounds; 15 mice (MyD88, TLR9, TLR2), 17 mice (TLR4) and 20 mice (WT) were used. The experiment was repeated twice using the same numerosity of mice with similar results. (D) Tumour incidence, showing the percentage of tumour-bearing mice over time. P1 (<0.01) represents statistical significance value between TLR4 and WT and P2 (<0.01) represents statistical significance value between MyD88 and WT. (E) Real-time PCR analysis for COX2 and MMP9 in the size-matched papillomas from WT and TLR4 KO mice. Data represents mRNA levels expressed relative to ribosomal protein L32. Values represent means±s.d. of the mean from at least five papillomas from each genotype. *P<0.05. The experiment was repeated twice with similar results.
Skin tumourigenesis is not dependent on TLR2 and TLR9
As MyD88 is a common adaptor molecule for all of the TLRs except TLR3, it was important to assess if other TLRs participated to tumourigenesis. We analysed tumour induction in TLR2 and TLR9 KO mice with the same two-stage skin carcinogenesis protocol. Interestingly, we found that in TLR2 and TLR9 mutant mice, tumour latency and tumour burden were not statistically different from those of control animals (Figure 1B–D; Supplementary Figure 1A). This data suggests that tumour induction is primarily dependent on TLR4/MyD88 and not on TLR2 and TLR9.
Environmental TLR4 is not involved in the growth of the established tumours
Next, we evaluated whether TLR4 expression on non-tumour cells was required to sustain the growth of established tumours. We dissected established primary carcinomas from WT mice, minced them into pieces of 1 mm3 and transplanted them into both WT and TLR4 KO mice in both flanks. After 3–4 weeks, we found that transplanted tumours started growing in both types of mice in a similar manner (Supplementary Figure 2A). Furthermore, we also injected 105 B16 melanoma cells in TLR4, TLR9, MyD88 KO and WT mice and found that in all of the cases tumour growth was similar (Supplementary Figure 2B). Injection of B16 cells into TLR2 KO mice resulted in a reduction of tumour growth (Supplementary Figure 2B). The reason for this delay is unknown, but one could speculate that it may be due to a reduction in T regulatory cells, whose generation and function is dependent on TLR2 (Sutmuller et al, 2006). Altogether, these results suggest that once the tumour is established, the presence or absence of environmental TLR4 does not affect tumour growth.
TLR4 is required for skin inflammation-mediated tumourigenesis
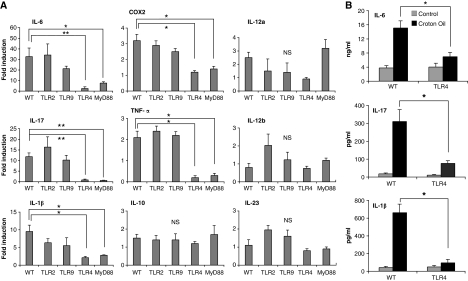
Many reports have shown a correlation between inflammation and cancer. Having shown that TLR4 and MyD88 KO mice displayed reduced skin carcinogenesis, we analysed the induction of an inflammatory reaction in the skins of treated mice. TLR2, 4, 9, MyD88 KO and WT mice were treated topically with CO one or multiple times as used in the carcinogenesis protocol. Skins were collected for RNA extraction 12 h after treatment. We found an increased mRNA level of inflammatory mediators, such as IL-6, IL-17, TNF-α, COX2 and IL-1β, in WT mice in comparison with TLR4 KO or MyD88 KO mice (Figure 2A). The results are expressed as fold induction of CO-treated versus acetone-treated mice in each group. When later time points after treatment were analysed (24, 48 h, 1 and 2 week), only the expression of TNF-α was sustained (Supplementary Figure 3A), whereas the expression of the other inflammatory cytokines was transient, but could be induced after additional treatments (data not shown). In contrast, TLR2 KO and TLR9 KO mice had a similar expression of cytokines such as WT mice (Figure 2A). For other mediators (IL-1α, IL-10, IL-12, IL-23), we did not detect any significant difference in the expression of genes in WT and TLR KO mice (Figure 2A). We confirmed at protein level an increase in IL-6, IL-17 and IL-1β expression in the skin of WT, but not of TLR4 KO mice (Figure 2B). 12-O-tetradecanoylphorbol-13-acetate (TPA) is another tumour promoter that has been frequently used along with DMBA for skin carcinogenesis studies. Hence, we wanted to confirm the function of TLR4 in skin inflammation-mediated tumourigenesis using TPA as tumour promoter. We painted the skin of WT or TLR4 KO mice with TPA and collected it for qPCR analysis. Again we found a reduced expression of inflammatory cytokines such as IL-6, IL-17, COX2, IL-12a, TNF-α, IL-1β in TLR4 KO mice (Supplementary Figure 3B). Overall, these results suggest that mice lacking TLR4 and MyD88 have reduced skin inflammation in response to tumour promoters.
Figure 2.
TLR4 is important for CO-induced skin inflammation. The skin from WT and different KO mice was taken after 12 or 24 h of CO or acetone treatment and analysed for gene expression or production of different cytokines. (A) Real-time PCR analysis of different inflammatory mediators on mRNA isolated from skin extracts after 12 h of CO treatment. The results are expressed as fold induction over acetone-treated skins in each group after normalization to L32 mRNA. (B) Production of inflammatory cytokines IL-6, IL-17 and IL1-β in the skins as measured by ELISA after 24 h of CO or acetone treatment. Values represent means±s.d. of the mean from at least three skin samples from each genotype and each experiment was repeated at least twice. *P<0.05, **P<0.01, NS, not significant.
TLR4 is required for the recruitment of inflammatory cells
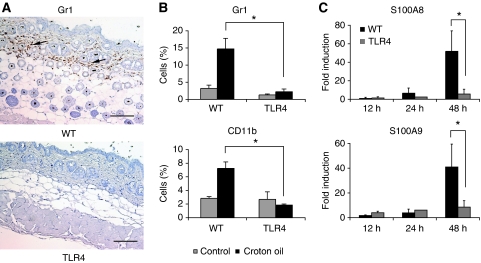
Immune cells recruited at the site of insult are the principal mediators of the inflammatory response. Consistent with the data on inflammatory mediators, after CO treatment we found an increased recruitment of granulocytes (Gr1+; Figure 3A and B; Supplementary Figure 3C) and monocytes/phagocytes (CD11b+; Figure 3B; Supplementary Figure 3C) in the skin of WT mice compared with TLR4 KO mice, as shown by immunohistochemistry (IHC) (Figure 3A) or FACS analysis on cells isolated from the skin (Figure 3B). No difference was observed in the frequency of dendritic cells (data not shown). The recruitment of inflammatory cells started at 24 h and was sustained at 48 h (Supplementary Figure 3C). This recruitment was likely dependent on the release of chemokines as we detected a time-dependent production of KC (CXCL1) and MIP-2 in WT, but not in TLR4 KO mice (Supplementary Figure 4). Of interest, there were different kinetics of chemokine production suggesting the recruitment of different inflammatory cells during the progression of inflammation. Interestingly, we found that at 48 h, there was also an increase in the expression of S100A8 and S100A9 proteins in the skins of WT, but not TLR4 KO mice, treated with CO (Figure 3C). S100A8 and S100A9 are the most abundant cytoplasmic proteins of neutrophils and monocytes, and strongly contribute to inflammation (Roth et al, 2003). This data indicate that in the absence of TLR4 also the recruitment of inflammatory cells is impaired.
Figure 3.
Recruitment of inflammatory cells in the skin after croton oil treatment. The skin from WT and TLR4 KO mice was collected 24 h after single treatment with CO and analysed for immune infiltrates. (A) Representative immunohistochemistry showing Gr1 staining of granulocytes in the skin of WT and TLR4 KO mice. Arrows show immune cell infiltrates. Scale bar, 100 μm. (B) FACS analysis showing the recruitment of granulocytes (Gr1) and monocytes/phagocytes (CD11b) in the skin. Values represent means±s.d. of the mean from at least three skin samples from each genotype and each experiment was repeated at least twice. *P<0.05, NS, not significant. (C) Real-time PCR analysis for S100A8 and S100A9 in the skins from WT and TLR4 KO mice 12, 24 and 48 h from CO treatment. Data represents fold induction of mRNA levels relative to acetone-treated animals after normalization to ribosomal protein L32. Values represent means±s.d. of the mean from at least five skins from each genotype. *P<0.05. The experiment was repeated twice with similar results.
Both radioresistant and BM-derived cells are required for tumour development
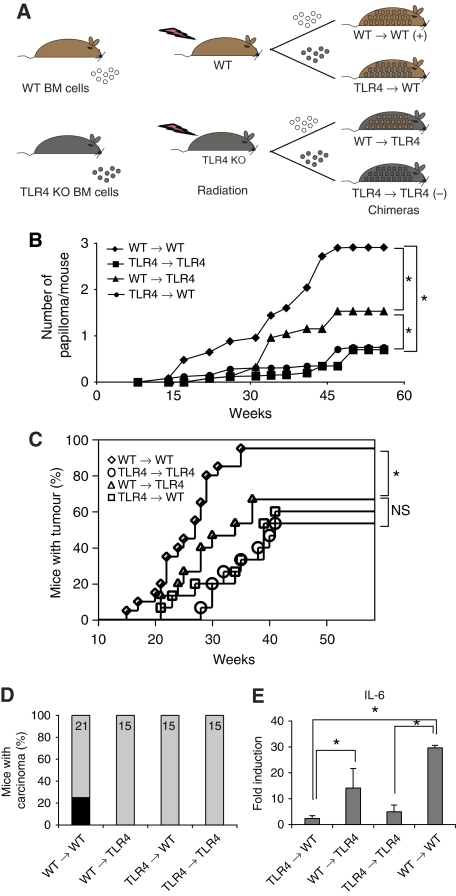
We then analysed the origin of the tumour-promoting cells. We generated BM chimeras by injecting TLR4 KO or WT BM cells into lethally irradiated WT or TLR4 KO mice (Figure 4A). Effective BM reconstitution was assessed by FACS analysis using CD45.1/CD45.2 markers of leukocyte populations. After reconstitution, we used the skin carcinogenesis protocol to induce tumours in chimeric mice. Interestingly, we found that TLR4 expression in BM-derived cells was required for papilloma development. The tumour burden and latency were significantly affected when BM cells were TLR4 deficient (Figure 4B). Chimeric mice of TLR4 WT BM into TLR4 KO recipients displayed papilloma formation at an intermediate level as compared with positive control chimeras (WT → WT) (Figure 4B and C). In contrast, carcinoma development required TLR4 expression both on BM-derived and radioresistant cells as none of the mice in the chimeric groups except the positive control group (WT → WT) developed carcinomas (Figure 4D). When we analysed the expression of inflammatory cytokines in response to CO, and in particular of IL-6, which is involved in tumourigenesis (Ancrile et al, 2007). We found that IL-6 was expressed primarily in the skins of WT → WT chimeras and at intermediate levels in WT → TLR4 KO chimeric skins, whereas it was minimally expressed in the other chimeric combinations (Figure 4E). We also found that TLR4 expression on inflammatory cells was required for their recruitment into the skin, independently of TLR4 expression of recipient mice (Supplementary Figure 5). Indeed, we found that inflammatory cells were recruited in the inflamed site only when the donor BM was WT for TLR4 (Supplementary Figure 5).
Figure 4.
Radioresistant and BM-derived cells both contribute to skin tumourigenesis. (A) Schematic representation of skin carcinogenesis protocol for chimeras. Mice were lethally irradiated (R=9.5 Gy), and 18 h later, injected i.v. with 2 × 106 BM cells. Eight weeks later, mice were treated after the carcinogenesis protocol as shown in Figure 1A. (B) Average number of papillomas per mouse in different chimeras group is shown over time. (C) Tumour incidence, showing percentage of tumour-bearing mice over time. (D) Graph displaying percentage of mice carrying at least one carcinoma (in black) in the different genetic backgrounds. N=21 for WT → WT and N=15 for the other groups. One experiment was carried out. (E) Fold induction of IL-6 gene expression in the skin from chimeras taken 12 h after CO treatment and normalized to L32 mRNA. Values represent means±s.d. of the mean from five mice per each group and the experiment was repeated three times. *P<0.05. NS, not significant.
Carcinogen-induced inflammation is dependent on HMGB1, but not on skin bacteria
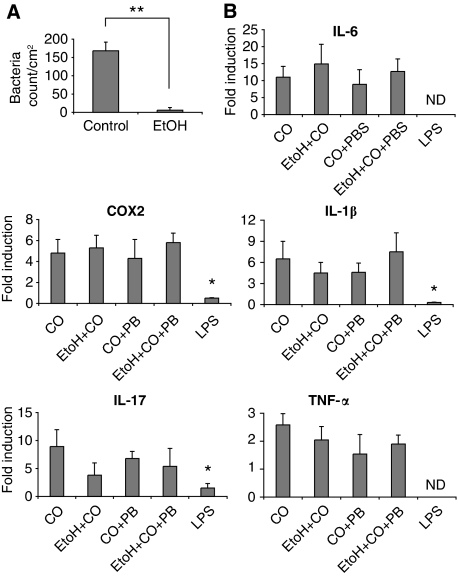
One of the most common exogenous ligand for TLR4 is LPS, the major component of the outer membrane of Gram-negative bacteria, which can contaminate several reagent preparations. We first ruled out the presence of endotoxin contamination in CO by limulus amebocyte lysate assay (not shown). Another explanation for the observed TLR4-dependent inflammation is possible: during CO or TPA application, skin-colonizing bacteria may breach the epithelial barrier and trigger TLR4 with their associated LPS. To test this possibility, we sterilized the shaved skin of WT mice with antiseptic or 90% ethanol 2 h before treating with CO. The effective elimination of colonizing bacteria was confirmed by plating out any remaining skin-associated bacteria (Figure 5A). Treatment with 90% ethanol had no effect on the release of inflammatory mediators after CO application except for IL-17 expression, whose reduction, however, did not reach a statistically significant difference (Figure 5B). This suggests that bacteria are not major contributors to CO-induced inflammation. To exclude an involvement of bacteria-released LPS after ethanol treatment to the induction of inflammatory cytokines, we treated the mice with polymyxin B during CO treatment with or without 90% ethanol. Polymyxin B is an LPS-binding antibiotic used to inactivate potential LPS contaminations. We found no effect of polymyxin B on CO-induced expression of inflammatory cytokines with or without ethanol (Figure 5B). Topically applied LPS diluted in acetone did not cause upregulation of genes of inflammatory cytokines (Figure 5B). This suggests that bacteria-associated LPS does not have a function in skin inflammation associated with tumourigenesis. Indeed, when LPS was coadministered with CO (Supplementary Figure 6A), a protective rather than a worsening effect was observed both in terms of number of papillomas per mouse (Supplementary Figure 6B) and percentage of mice that remained tumour free (Supplementary Figure 6C). The reason for the protective effect remains to be elucidated. Taken together, these data show that LPS does not have a function in skin tumourigenesis.
Figure 5.
Carcinogen-induced inflammation is not dependent on skin bacteria or LPS. (A) Total number of bacteria/cm2 of the back skin in mice treated or not (control) with ethanol (EtOH 90%). One representative experiment of three is shown. (B) Real-time PCR analysis of mRNA isolated from skin extracts showing expression of inflammatory cytokine genes in the group of WT mice treated or not with antiseptics along with croton oil in combination or not with polymyxin B (PB). As a control, mice were treated topically with LPS in acetone. *P<0.05, **P<0.01, ND, not determined.
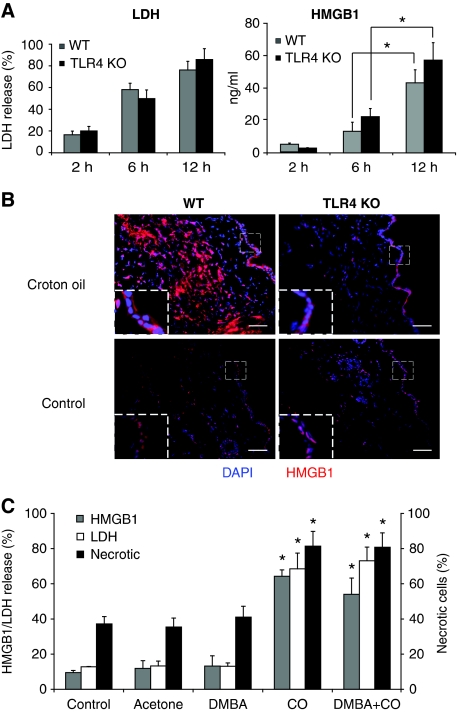
Having excluded LPS as the major mediator of TLR4-dependent skin inflammation, we sought for endogenous ligands of TLR4. We focused our attention on HMGB1 because of its association with tumourigenesis (Lotze et al, 2007). Both necrotic and inflammatory cells can be a source of HMGB1 during inflammation. Mice were treated with either DMBA, CO (Supplementary Figure 7A) or TPA (Figure 6A), and after 2, 6 or 12 h, a skin punchout was removed and cultured to reach a total of 24 h from TPA or CO treatment. The medium was then collected and the release of HMGB1 and LDH was measured by ELISA. We found that in the skins treated with TPA for 6 or 12 h, the release of HMGB1 and LDH was independent of the expression of TLR4 (Figure 6A). It is unlikely that this effect was due to a necrotic event induced by the removal of the skins from the animals as skins removed 2 h after CO treatment and left in culture even longer than the skins treated for 6 or 12 h with CO released very little HMGB1 (Figure 6A; Supplementary Figure 7A). Some release of HMGB1 was observed also in response to DMBA alone, but it was minimal as compared with that induced by CO (Supplementary Figure 7A). The correspondence between HMGB1 and LDH release suggests that CO treatment induces necrosis of skin cells, which release HMGB1. Consistently, when we stained the skins for HMGB1 by immunofluorescence, we found that HMGB1 was cytoplasmic in the superficial epidermal layer in both TLR4 KO and WT animals after CO treatment, whereas it was nuclear in control acetone-treated skins (Figure 6B). The cytoplasmic location of HMGB1 is associated with cell stress, and often precedes necrosis. The cells staining intensely for HMGB1 in the skins of WT, but not TLR4 KO mice, are inflammatory cells, consistent with the inability of TLR4 KO cells to respond to HMGB1.
Figure 6.
Croton oil or TPA treatment induces release of HMGB1, LDH and necrosis of keratinocytes. (A) Release of LDH (left panel) and HMGB1 (right panel) in skin culture supernatants. The skins were removed at the indicated time points after single treatment with TPA and cultured in medium to reach 24 h from the initial TPA treatment. Values represent means±s.d. of the mean from at least three mice from each group and the experiment was repeated at least twice. (B) Immunofluorescence staining of HMGB1 (red) after 18 h croton oil treatment of WT (left, top) and TLR4 KO mice (right, top) in comparison with the control skin from respective mice (bottom). Scale bar=250 μm. Insets show higher magnification of the areas reported in the dashed line boxes ( × 2.5). Nuclei are stained with dapi (blue). Images are representative of at least five mice from each genotype. One representative image is shown per mouse. One experiment of three is shown. (C) Primary mouse keratinocytes were isolated and kept in culture as described in Materials and methods section. A total of 1.5 × 105 cells were treated with DMBA (1 μM) or croton oil (0.5 mg/ml) and cell supernatant was collected after 16 h. Cells were collected for FACS analysis 4 h after treatment with DMBA or croton oil and stained with Annexin V and propium iodide. Supernatant was analysed for the release of LDH and HMGB1. Lysis buffer from a cytotoxicity assay kit was used as a positive control to measure total LDH. The graph shows the percentage of HMGB1 and LDH release (left y axis), and the percentage of necrotic cells (right y axis) compared with cells treated with lysis buffer (positive control) from the same experiment. *P<0.05 between CO or DMBA+CO groups versus control, acetone (Ac) or DMBA groups.
Our observations were reproduced in vitro: primary differentiated keratinocytes treated with CO underwent necrosis (as attested by double staining with propidium iodide and Annexin V), and released HMGB1 and LDH (Figure 6C; Supplementary Figure 7B). In addition, supernatants from CO-treated keratinocytes cultures were capable to induce the release of TNF-α by bone marrow-derived dendritic cells (BM-DCs) from WT and not TLR4 KO mice (Supplementary Figure 8). This was not due to the CO left over from the keratinocytes culture as CO was unable to induce inflammatory cytokines when applied directly on the DCs (Supplementary Figure 8).
HMGB1 is required to initiate inflammation and to recruit inflammatory cells
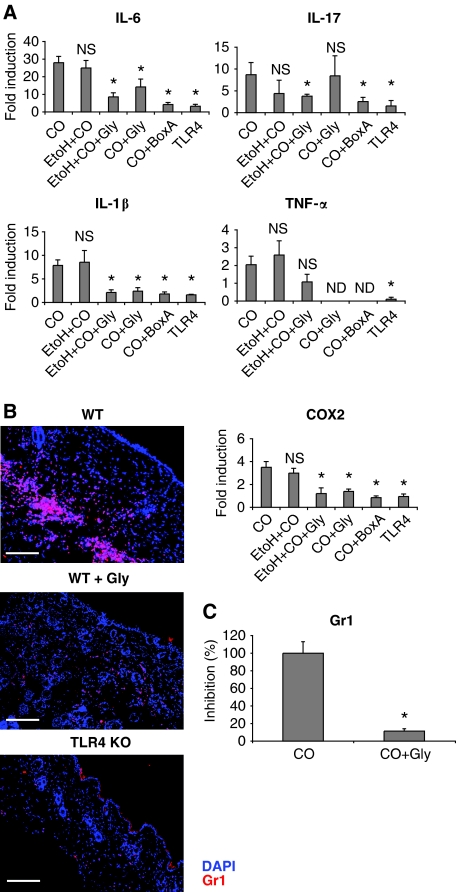
We then analysed whether HMGB1 was responsible for CO- or TPA-induced inflammation. Two drugs targeting HMGB1 have been used consistently: glycyrrhizin, a natural triterpene with anti-inflammatory properties (Mollica et al, 2007; Sitia et al, 2007; Curtin et al, 2009), and Box-A, a fragment of HMGB1 acting as a competitive antagonist (Yang et al, 2004; Sitia et al, 2007; Andrassy et al, 2008; Muhammad et al, 2008). HMGB1 was neutralized using glycyrrhizin or Box-A in combination or not with 90% ethanol. We found a drastic reduction of inflammatory cytokine expression when using either of the two inhibitors of HMGB1 both in WT mice (Figure 7A) and in TLR2 KO mice (Supplementary Figure 9). Inhibition of HMGB1 also resulted in a reduced recruitment of neutrophils in the skin of WT mice (Figure 7B and C). Together, these results suggest that HMGB1, which is released independently of TLR4 expression after CO treatment, is the endogenous ligand for TLR4 that induces both the upregulation of inflammatory cytokine expression and the recruitment of inflammatory cells.
Figure 7.
Blocking of HMGB1 in the skin reduces inflammatory cytokine production and inflammatory cell recruitment. The skin from WT mice was taken after 12 h for gene expression and 24 h for recruitment of inflammatory cells. (A) Real-time PCR analysis of mRNA isolated from skin extracts showing expression of inflammatory cytokine genes in the group of WT mice treated or not with ethanol (EtOH 90%) or HMGB1 inhibitors (glycyrrhizin or Box-A) along with CO. Values represent means±s.d. of the mean from at least three mice from each group and the experiment was repeated at least twice. P<0.05 (*) represents statistical significant difference between croton oil-treated group and the other groups. NS, not significant, ND, not determined. (B) Representative images of immunofluorescence staining for Gr1 (red) showing reduced infiltration of granulocytes in the skin of WT mice treated with HMGB1 inhibitor glycyrrhizin (middle). Nuclei staining: DAPI, blue. The results are representative of at least three independent experiments. Scale bar, 200 μm. (C) Quantification of Gr1-positive cells in the skin from WT mice treated or not with the HMGB1 inhibitor glycyrrhizin (panel B). P<0.05 (*) represents statistical significant difference between croton oil-treated group and CO + glycyrrhizin.
Tissue microarray analysis
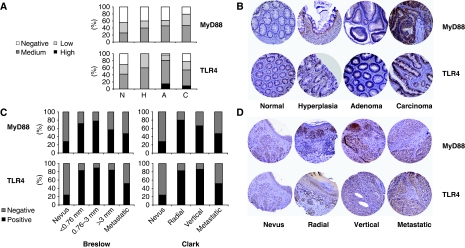
As HMGB1 is over-expressed in several tumours (Lotze et al, 2007), we analysed whether also MyD88 and TLR4 were upregulated in cancer patients. We carried out a tissue microarray screening on colon and melanoma patients in different stages of tumour progression from benign to malignant. For both pathologies, we found a marked over-expression of TLR4 and MyD88 in tumours (Figure 8). This over-expression was observed both on tumour cells and on the infiltrating leukocytes (Figure 8D). Interestingly, there was a strong correlation between TLR4 and MyD88 over-expression in the same patients (Supplementary Figure 10A), suggesting that these are not independent events. We also observed an upregulation in MyD88 expression in ovarian and prostate cancer (Supplementary Figure 10B), but not in other pathologies such as breast, lung and bladder cancers (not shown).
Figure 8.
TLR4 and MyD88 expression in the progression of colon and melanoma tumours. (A, B) Tissue microarray analysis showing the expression of MyD88 (top) and TLR4 (bottom) during colon tumour progression in normal (N), hyperplasia (H), adenoma (A) and carcinoma (C) tissue samples. (A) Bar graphs showing marker expression (colour code as in the legend); (B) representative immunohistochemical images of MyD88 (top) and TLR4 (bottom) staining. (C) Tissue microarray analysis showing the expression of MyD88 (top) and TLR4 (bottom) during melanoma tumour progression as staged using Breslow (left) or Clark parameters (right). Breslow parameter refers to the depth of melanoma invasion and Clark refers to the anatomical invasion of the melanoma in the skin. (D) Representative immunohistochemistry images of MyD88 (top) and TLR4 (bottom) staining in samples staged using the Clark parameter. A melanoma-specific microarray composed of 34 benign lesions (nevi), 135 melanoma and 61 metastatic melanoma and a colon-specific microarray composed of 39 normal colon epithelium, 5 hyperplasia, 23 adenoma and 104 carcinoma were used. From each sample were arrayed two representative areas.
Discussion
The involvement of chronic inflammation in tumour development has been clearly shown in several animal models of spontaneous or chemically induced carcinogenesis (Balkwill and Mantovani, 2001; Coussens and Werb, 2002; Beachy et al, 2004; Clevers, 2004; Balkwill et al, 2005; Robinson and Coussens, 2005; Karin et al, 2006). We describe here that chemically induced skin carcinogenesis is dependent on the expression of MyD88 and TLR4, but not TLR2 or TLR9. As MyD88 is the downstream adaptor also of IL-1/IL-18 receptors, we cannot exclude a possible involvement of these receptors in a ‘sterile' inflammation as recently suggested (Chen et al, 2007). A recent report has shown that TLR4 is protective against DMBA-induced skin tumours (Yusuf et al, 2008). However, one-step chemical carcinogenesis based only on continuous DMBA administration does not depend on inflammation as tumour promotion (Slaga, 1983). Further, topical application of DMBA leads to contact hypersensitivity, which can protect the animals against the carcinogenic activities of the compound (Klemme et al, 1987). Hence, to our knowledge, we are the first to show that TLR4 is involved in tumour development in a two-step inflammation-induced skin tumourigenesis model.
TLR4 primarily recognizes LPS, which is a major component of the Gram-negative outer membrane; however, we found that LPS associated to skin-colonizing bacteria was not involved in CO triggered inflammation, as antiseptics that completely eliminated bacteria from the skin had no effect on CO-induced inflammation and application of LPS concomitant with CO had a protective rather than a worsening effect on disease progression. Hence, we sought for an endogenous ligand of TLR4. RAGE-deficient mice display reduced brain (Taguchi et al, 2000) and skin (Gebhardt et al, 2008) tumours. As HMGB1 can bind both RAGE and TLR4, we analysed a possible contribution of HMGB1 in the TLR4-dependent inflammation. We found that HMGB1 was promptly released in the skins of CO-, but not DMBA-treated animals. HMGB1 and LDH release were closely correlated, which indicates a common origin of these two molecules from cells dying necrotically; cells dying by apoptosis release minute amounts of HMGB1 in comparison with LDH (Scaffidi et al, 2002). It is likely that in vivo the target cells of CO treatment are keratinocytes as they underwent necrosis, and released HMGB1 and LDH after CO treatment in vitro. This initial release of HMGB1 was independent of TLR4 and was required for leukocyte recruitment and inflammatory cytokine production. However, to be recruited, inflammatory cells had to express TLR4. Indeed, in chimeric mice, only TLR4-positive BM-derived cells were recruited in the skin in response to CO, independently of TLR4 expression in recipient skin cells. This may suggest a direct effect of HMGB1 on inflammatory cell migration through TLR4 triggering, maybe through Src activation and focal adhesion formation (Palumbo et al, 2009). HMGB1 has been shown to bind also TLR2 in vitro; however, we could not detect any reduction in tumourigenesis in TLR2 KO mice. There could be two explanations for this observation, the first that in vivo HMGB1 binds only to TLR4 as recently suggested (Yu et al, 2006; van Zoelen et al, 2009), and the second that HMGB1 binding to TLR2 may be structurally and mechanistically different. In fact, TLR2 was recently shown to be a receptor for nucleosome-bound HMGB1, and not for free HMGB1 (Urbonaviciute et al, 2008). HMGB1 can also bind CpG-DNA and signal through TLR9 (Ivanov et al, 2007). However, TLR9 KO mice were similarly susceptible to tumour development as WT mice. Bacteria that are the major source of CpG-DNA do not seem to induce local inflammation in the skin suggesting that TLR9 signalling may not occur. HMGB1 and HMGB2 have also been shown to mediate the nucleic acid-dependent activation of TLR3 and TLR7 (Yanai et al, 2009). Our results indicate that TLR4 is sufficient to induce tumour development, but do not rule out the possibility that TLR3 or TLR7 may also be involved.
Inflammatory cytokines, including IL-6, TNF-α, IL-17 and COX2, that are associated with several inflammation-induced cancers (Moore et al, 1999; Fosslien, 2000; Numasaki et al, 2003; Ancrile et al, 2007; Naugler et al, 2007; Grivennikov and Karin, 2008; Grivennikov et al, 2009) were expressed before an overt recruitment of inflammatory cells (12 h after CO treatment, whereas immune cells were recruited only at 24 h). This suggests that HMGB1 triggers TLR4-positive resident cells for an initial inflammatory response that then culminates with the recruitment of inflammatory cells. These resident cells, however, are not likely to be radioresistant, as chimeric mice in which TLR4 KO BM cells were transplanted into WT recipients did not show production of inflammatory cytokines. This suggests that keratinocytes release HMGB1 that then acts on resident bone marrow-derived cells. Indeed, culture supernatants of CO-treated keratinocytes could induce BM-DCs to release inflammatory cytokines in a TLR4-dependent manner. At 48 h, consistent with the presence of inflammatory cells, we observed an increase of S100A8 and S100A9, proteins that are strong mediators of inflammation (Roth et al, 2003). These molecules have been recently shown to interact with TLR4 (Vogl et al, 2007). Given the late expression of these proteins and the finding that specific inhibition of HMGB1 was sufficient to inhibit cytokine release and recruitment of leukocytes, it is unlikely that S100A8 and S100A9 may participate to the initiation of the inflammatory response. However, it is possible that they may contribute to tumour development through the amplification of the inflammatory response.
Another important observation of our study is that while papilloma formation is dependent on TLR4 expression on BM-derived cells, carcinoma development is a result of TLR4 engagement on both immune cells and redioresistant cells. This suggests that sensors of inflammation are needed both on immune and on host cells for cancer formation. Indeed, we found an over-expression of TLR4 and MyD88 both on tumour cells and on the infiltrating leukocytes in melanoma patients as compared with benign lesions. Thus, we can hypothesize that CO induces an initial TLR4-independent tissue damage event leading to HMGB1 release (Supplementary Figure 11A). This is supported by the observation that concomitant to HMGB1 release, we observed LDH release in response to CO treatment. LDH is a marker of necrotic cell death. This HMGB1 acts on resident TLR4 proficient cells by inducing an initial wave of inflammatory cytokines and is involved in the recruitment of inflammatory cells. The inflammatory cell recruitment is dependent directly on HMGB1 through TLR4 triggering. Hence, we show that inflammation-dependent skin tumourigenesis is a multifactorial process that is dependent on a damage response mediated by HMGB1 and on TLR4 expression on BM-derived cells for the recruitment of inflammatory cells and papilloma development, and on radioresistant cells for progression to carcinoma (Supplementary Figure 11B).
Materials and methods
Mice
C57BL/6 and C57BL/6 Ly5.1+ mice were purchased from Harlan and Charles River Laboratories, respectively. TLR2-deficient mice, TLR4-deficient mice, TLR9-deficient mice and MyD88-deficient mice were obtained from S Akira (Osaka University, Osaka, Japan). All animals were on pure C57BL/6 background and were maintained in microinsulator cages in a specific pathogen-free animal facility at the IFOM-IEO CAMPUS, Milan, Italy. All experiments were performed in accordance with the guidelines established in the Principles of Laboratory Animal Care (directive 86/609/EEC) and approved by the Italian Ministry of Health.
Reagents
The 7,12-dimethylbenz[a]anthracene (DMBA), CO, TPA were purchased from Sigma-Aldrich. Fluorescent-conjugated anti-CD11b, anti-CD11c, anti-GR1, anti-CD45.1, anti-CD45.2 were purchased from BD-pharmingen. Recombinant Box-A was provided by HMGBiotech (Milan, Italy); it was produced and purified as described earlier. Glycyrrhizin was obtained from Minophagen Pharmaceutical, Tokyo, Japan.
Skin tumourigenesis
Tumourigenesis was induced with two-stage skin carcinogenesis protocol (Takizawa et al, 1985) with slight modifications. In short, 4 weeks old female WT, TLRs KO and MyD88 KO mice (all on C57BL/6 background) were shaved and painted topically with 400 nM of DMBA in 0.2 ml of acetone. Two weeks later, 200 mg of CO in 0.1 ml of acetone was applied twice a week on the skin for 34 weeks. The occurrence of papillomas or carcinomas was recorded each week starting at 8 weeks after CO promotion. Growths that were >1 mm in diameter and that persisted for at least 2 weeks were defined as tumours and recorded. For acute treatment, mice were treated with a single dose of CO or TPA (10 nM in 0.1 ml of acetone), and 12 or 24 h after the last treatment, skin was taken for RNA analysis or IHC.
Primary keratinocytes culture
Murine epidermal keratinocytes were obtained from 2-day-old C57/BL6 mice and cultured as described earlier (Caldelari et al, 2000). Cells were seeded at a concentration of 150 × 103 cells per well on collagen I-coated 24-wells plate in defined keratinocyte serum-free medium (Gibco) supplemented with 10 ng/ml of EGF (R&D system) and 10−10 M of cholera toxin (Sigma). Differentiation of keratinocytes was induced by adding 1 mM of CaCl2 in the culture medium for 3 days. Cells were starved for 6 h in keratinocyte serum-free medium without the addition of growth factors before stimulation. Cells were stimulated with DMBA (1 μM), CO (0.5 mg/ml) or an equivalent volume of acetone as control, overnight at 37°C. Supernatants were collected and used to activate murine dendritic cells.
Isolation of BMDCs
Total bone marrow cells were flushed from femurs with DPBS. Two million cells/dish were plated in 10 ml of 30% R1 using 100 mm petri dishes. A total of 10 ml of fresh medium was added after 3 days and half of the medium was changed after every 2 days. Cells were replated after day 8 and used for the experiment. BMDCs were activated either with supernatants from keratinocytes pre-treated with DMBA and CO or with DMBA and CO directly. The supernatant was collected after 20 h for ELISA.
Tumour transplant
WT mice that reached the carcinoma size of 1 cm were killed and the tumour was excised. Tumour was dipped once into ethanol 70% and then washed twice with PBS. Sterile scalpels were used to mince tumour into pieces of 1 mm3 and 3–4 pieces of those minced tumours were transplanted subcutaneously into both flanks of WT and TLR4 KO mice (Reiners et al, 1997). Tumour growth was recorded every week and the mice that were found infected at the area of transplantation were killed and excluded from the study.
Quantitative RT–PCR analysis
Total RNA was extracted from mouse skin or papilloma by using Trizol reagent and purified through Qiagen columns; 1 μg of total RNA was reverse transcribed using random primers in a total volume of 20 μl at 42°C for 60 min. Quantitative real-time PCR (RT–PCR) was performed using Syber Green assays. The expression of different cytokines and chemokines, for example IL-6, IL-17, IL-10, IL-12a, IL-12b, COX2, MMP9, was normalized with ribosomal protein L32 (RPL32). All PCR experiments were performed in triplicate, and standard deviations were calculated and displayed as error bars. The primer sequences are listed in Supplementary Table 1.
Flow cytometry
The skin was incubated at 37°C with medium containing 1% collagenase and 5 U/ml DNase enzyme (DNase I, BioLabs) in RPMI supplemented with 2% foetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, at 37°C in 5% CO2 incubator for 1 h. Intermittently, skin was scraped with 21G needle every 10–20 min to release the cells. After washing the samples with PBS, skin cells were collected by passing the digested fragments through 70 μm cell filter. Cells were stained with fluorochrome-conjugated monoclonal antibodies for CD11b, CD11c, Gr-1.
Immunohistochemical analysis
The formalin-fixed biopsies were embedded in paraffin, and the snap frozen biopsies were placed in Tissue-Tek OCT (Sakura). Paraffin-embedded sections were sliced at 5 μm thickness, deparaffinized and heated in 0.25 mM EDTA (pH 8) for 50 min at 65°C. After blocking, the sections were incubated with Gr-1 antibody at 4°C overnight. Sections were incubated with secondary antibody directly conjugated with HRP and counterstained with haematoxylin. For tissue microarray studies, immunohistochemical staining was performed 2 h at room temperature with primary antibodies (anti-TLR4, anti-MYD88 diluted in Dako diluent) followed by detection with the DAKO EnVision Plus system and, for melanoma-specific TMA, VECTOR VIP (SK-4600, Peroxidase substrate kit, Vector laboratories, Inc. Burlingame, Ca 94010), as described earlier (Nicassio et al, 2005). A semiquantitative score was used to assess SOS1pY expression: 0, no staining; 1, weak; 2, moderate; 3, high staining. For statistical analysis purposes, two risk groups were then defined according to the given scores: Low-Neg class (intensity scores lower or equal to 1) and Mod-High Class (intensity scores higher than 1).
Immunofluorescence analyses
Excised skin samples were kept in PLP buffer (37.5% 0.1 M periodate—37.5% 0.2 M L-lysine—25% of 4% paraformaldehyde) overnight at +4°C. Samples were washed twice with PBS and dehydrated in 20% sucrose solution for at least 4 h at +4°C. Samples were embedded in Tissue-Tek OCT and 5 μm thick sections were stained with rabbit anti-HMGB1 (Abcam) or rat anti-GR1 after blocking with 1% bovine serum albumin in 0.1 M Tris–Cl. Samples were incubated with appropriate Alexa-fluor-conjugated secondary antibodies (Invitrogen). Images were obtained with a microscope (Biosystems BX-71, Olympus) and analysed using ImageJ software.
Bone marrow chimeras
Mice were lethally irradiated with 10 Gy of radiation and BM cells from CD45.1/CD45.2 congenic mice were purified using Ficoll reagent. Two million BM mononuclear cells were injected intravenously into the irradiated mice 18 h after irradiation to form chimeras. These mice were kept in isolators and provided neomycin-containing water until 4 weeks. Bone marrow reconstitution was confirmed by FACS analysis on the blood samples using CD45.1/CD45.2 markers of leukocyte populations. Skin tumourigenesis protocol was performed 8 weeks after bone marrow transfer.
Injection of Box-A or glycyrrhizin
Recombinant Box-A (2 mg/mouse) in acetone and glycyrrhizin (15 mg/mouse) were administered topically and intraperitoneally, 2 h before treatment with CO. Skin was taken 12 h after CO treatment for RNA analysis.
ELISA
WT and TLR4 KO mice were treated with CO or TPA and skin was taken and put in DMEM medium with 5% penicillin/streptomycin, 10% nonessential amino acids. Skin supernatant was taken 24 h after CO treatment and analysed for different cytokines with ELISA. LDH and HMGB1 release were determined using a cytotoxicity assay (Promega) and an ELISA kit (Shino-test Corporation), respectively.
Tissue microarray
For the large-scale screening study, formalin-fixed and paraffin-embedded tumour and normal (when available) specimens were provided by the Pathology Departments of Ospedale Maggiore (Novara), Presidio Ospedaliero (Vimercate) and Ospedale Sacco (Milano). Samples arrayed included breast, colorectal, lung, prostate, larynx, kidney carcinomas and lymphoma, sarcoma and melanoma.
Two tissue microarrays, one mainly composed of tumour and the other with corresponding normal tissues, were specifically designed for the screening and prepared as earlier described with minor modification (Kononen et al, 1998); 2-μm sections of each TMA were cut, mounted on glass slides and processed for IHC.
The in-depth analysis was designed by two melanoma-specific microarrays composed of 34 benign lesions (nevi), 135 melanoma and 61 metastatic melanoma provided by the Pathology Departments of Ospedale S. Paolo (HSP, Milano) and European Institute for Oncology (IEO, Milano) and a colon-specific microarray composed of 39 normal colon epithelium, 5 hyperplasia, 23 adenoma, 104 carcinoma and 1 metastatic carcinoma provided by the Presidio Ospedaliero (Vimercate) and European Institute for Oncology (IEO, Milano). From each sample were arrayed two representative areas.
Statistical analysis
For tissue microarray, association between the clinico-pathologic features of the tumours and gene expression was evaluated by the ANOVA and Student's t-test. The recognized prognostic factors including in analysis were Breslow index, Clark status, pathological stage, TNM staging system and histotype. Student's paired t-test was used to determine the statistical significance of the data. Significance was defined as *P<0.05; **P<0.01; ***P<0.001 (two-tailed test and two-sample equal variance parameters). All statistical calculations were performed by JMP 5.1 software (SAS Cary).
Supplementary Material
Acknowledgments
This work is supported by grants from the Italian Association for Cancer Research (AIRC), the Association for International Cancer Research (AICR) and the Italian Ministry of Health (Ricerca finalizzata).
Footnotes
The authors declare that they have no conflict of interest. However, ME Bianchi is founder and part owner of HMGBiotech, a company that provides goods and services related to HMGB proteins.
References
- Ancrile B, Lim KH, Counter CM (2007) Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev 21: 1714–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger S, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A (2008) High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117: 3216–3226 [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L (2007) The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 220: 47–59 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7: 211–217 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357: 539–545 [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM (2004) Mending and malignancy. Nature 431: 402. [DOI] [PubMed] [Google Scholar]
- Bianchi ME (2009) HMGB1 loves company. J Leukoc Biol 86: 573–576 [DOI] [PubMed] [Google Scholar]
- Caldelari R, Suter MM, Baumann D, De Bruin A, Muller E (2000) Long-term culture of murine epidermal keratinocytes. J Invest Dermatol 114: 1064–1065 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260–1270 [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13: 851–856 [DOI] [PubMed] [Google Scholar]
- Clevers H (2004) At the crossroads of inflammation and cancer. Cell 118: 671–674 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG (2009) HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 6: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SM (2002) Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol 21: 183–191 [PubMed] [Google Scholar]
- Fosslien E (2000) Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci 37: 431–502 [DOI] [PubMed] [Google Scholar]
- Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT (2007) Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133: 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P (2008) RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 205: 275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15: 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin M (2008) Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell 13: 7–9 [DOI] [PubMed] [Google Scholar]
- Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M (2002) Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168: 5989–5992 [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, Morser J, Stern D, Schmidt AM (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270: 25752–25761 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110: 1970–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V (2006) Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124: 823–835 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2007) TLR signaling. Semin Immunol 19: 24–32 [DOI] [PubMed] [Google Scholar]
- Klemme JC, Mukhtar H, Elmets CA (1987) Induction of contact hypersensitivity to dimethylbenz(a)anthracene and benzo(a)pyrene in C3H/HeN mice. Cancer Res 47: 6074–6078 [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844–847 [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848 [DOI] [PubMed] [Google Scholar]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T (2007) The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev 220: 60–81 [DOI] [PubMed] [Google Scholar]
- Miyake K (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19: 3–10 [DOI] [PubMed] [Google Scholar]
- Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14: 431–441 [DOI] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F (1999) Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med 5: 828–831 [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M (2008) The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28: 12023–12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317: 121–124 [DOI] [PubMed] [Google Scholar]
- Nicassio F, Bianchi F, Capra M, Vecchi M, Confalonieri S, Bianchi M, Pajalunga D, Crescenzi M, Bonapace IM, Di Fiore PP (2005) A cancer-specific transcriptional signature in human neoplasia. J Clin Invest 115: 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT (2003) Interleukin-17 promotes angiogenesis and tumor growth. Blood 101: 2620–2627 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol 164: 558–561 [DOI] [PubMed] [Google Scholar]
- Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, Bianchi ME (2009) Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol 86: 617–623 [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of TLR2 and TLR4 in cellular activation by high mobility group box 1 protein (HMGB1). J Biol Chem 279: 7370–7377 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R (2007) Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317: 124–127 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R (2009) Toll-like receptors and cancer. Nat Rev Cancer 9: 57–63 [DOI] [PubMed] [Google Scholar]
- Reiners JJ Jr, Singh KP, Yoon HL, Conti CJ (1997) Transplantation analyses of the immunogenicity of epidermal tumors generated in murine skin two-stage carcinogenesis protocols. Mol Carcinog 20: 48–57 [DOI] [PubMed] [Google Scholar]
- Robinson SC, Coussens LM (2005) Soluble mediators of inflammation during tumor development. Adv Cancer Res 93: 159–187 [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176: 7021–7027 [DOI] [PubMed] [Google Scholar]
- Roth J, Vogl T, Sorg C, Sunderkotter C (2003) Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 24: 155–158 [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195 [DOI] [PubMed] [Google Scholar]
- Sitia G, Iannacone M, Muller S, Bianchi ME, Guidotti LG (2007) Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol 81: 100–107 [DOI] [PubMed] [Google Scholar]
- Slaga TJ (1983) Overview of tumor promotion in animals. Environ Health Perspect 50: 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ (2006) Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 116: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ (2008) Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA 105: 652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM (2000) Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405: 354–360 [DOI] [PubMed] [Google Scholar]
- Takizawa H, Sato S, Kitajima H, Konishi S, Iwata K, Hayashi Y (1985) Mouse skin melanoma induced in two stage chemical carcinogenesis with 7,12-dimethylbenz[a]anthracene and croton oil. Carcinogenesis 6: 921–923 [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 295: 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112 [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Buurman WA, Griffioen AW (2008) Convergence and amplification of Toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11: 91–99 [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T (2009) Role of Toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31: 280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13: 1042–1049 [DOI] [PubMed] [Google Scholar]
- Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462: 99–103 [DOI] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101: 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H (2006) HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock 26: 174–179 [DOI] [PubMed] [Google Scholar]
- Yusuf N, Nasti TH, Long JA, Naseemuddin M, Lucas AP, Xu H, Elmets CA (2008) Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res 68: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.