Abstract
In contrast to the well-established efficacy of preventive vaccines, the effectiveness of therapeutic vaccines remains limited. To develop effective vaccination regimens against cancer, we have analyzed the effect of effector and memory CD8+ T cells on the ability of dendritic cells to mediate the immunologic and antitumor effects of vaccination. We show that in contrast to effector CD8+ T cells that kill antigen-carrying dendritic cells, IFNγ-producing memory CD8+ T cells act as “helper” cells, supporting the ability of dendritic cells to produce interleukin-12 (IL-12) p70. Promoting the interaction of tumor antigen-carrying dendritic cells with memory-type “heterologous” (tumor-irrelevant) CD8+ T cells strongly enhances the IL-12p70-dependent immunogenic and therapeutic effects of vaccination in the animals bearing established tumors. Our data show that the suppressive and helper functions of CD8+ T cells are differentially expressed at different phases of CD8+ T-cell responses. Selective performance of helper functions by memory (in contrast to effector) CD8+ T cells helps to explain the phenomenon of immune memory and facilitates the design of effective therapeutic vaccines against cancer and chronic infections.
Introduction
Preventive vaccines, usually composed of “priming” and “booster” doses, have proved effective in controlling multiple infectious diseases, but the efficacy of current therapeutic vaccines remains low (1–4). Successful induction of immune memory is considered to be essential for vaccine effectiveness, but the exact pathways of development of memory CD8+ T cells and the features of their biology allowing them to mediate protection on secondary antigen challenge remain unclear (5–8). Two long-recognized but poorly understood phenomena in vaccine biology include the importance of delayed application of booster doses of vaccines to achieve effective secondary T-cell expansion (9) and the paradoxical contraction of the CD8+ T-cell pool when the second antigenic exposure occurs too soon (9, 10).
CD8+ T cells are key to our ability to control intracellular infections and cancer. They act as CTLs (11) that not only eliminate the infected or transformed cells but also carry out regulatory functions, being capable of either suppressing (12–15) or supporting (16–19) immune responses. Whereas the mechanism of the suppressive activity of CD8+ T cells is far from clear, it has been shown that perforin- and granzyme-dependent elimination of antigen-carrying dendritic cells by antigen-specific CD8+ T cells (13–15, 20, 21) can act as a suppressive mechanism, providing a self-limiting character to CTL responses (13–15) and restricting the efficacy of vaccination (13–15, 20). In contrast to the long-known ability of CD8+ T cells to inhibit immune responses (12), it only recently became apparent that CD8+ T cells can also activate dendritic cells (16–19, 22) and support type 1 immunity (16–19). Such “helper” function of CD8+ T cells depends on their ability to produce IFNγ and to promote the dendritic cell production of interleukin-12 (IL-12) p70 (18, 19), the key factor supporting T helper 1 and CTL responses (23).
The relationship between the suppressor versus helper functions of CD8+ T cells remains unclear. Previous reports showed that the suppressive effects resulting from the elimination of antigen-carrying dendritic cells by CD8+ T cells are mediated by the effector, but not memory, cells (13, 14). However, the possibility that it is the memory CD8+ T cells that selectively play the reciprocal helper role has never been explored.
Here, using the model of therapeutic vaccination with tumor-loaded dendritic cells carrying additional tumor-unrelated (“heterologous”) antigens, which allowed us to uncouple the regulatory functions of CD8+ T cells from their effector activity, we show that the suppressor versus helper functions represent sequential phases of CD8+ T-cell responses. Our data show that promoting the interaction of tumor antigen–carrying dendritic cells with memory-type CD8+ T cells specific for tumor-unrelated antigens promotes the therapeutic activity of vaccination against the established tumors that are resistant to standard vaccines.
Materials and Methods
Mice
Six- to 8-week-old female C57BL/6, C57BL/6Tg (TcraTcrb)1100Mjb (OT-1), and C57BL/6-IL12tm1Jm (IL-12p40 knockout) mice, and perforin-deficient (C57BL/6-Prf1tm1Sdz/J) female mice, purchased from The Jackson Laboratory, were maintained in microisolator cages and used for all experiments at 8 to 10 weeks of age. All experimental procedures were approved by our Institutional Animal Care and Use Committee.
Cell lines, cell isolation, and culture
MC38 adenocarcinoma was provided by Dr. D.L. Bartlett (University of Pittsburgh, Pittsburgh, PA; originally from Dr. SA Rosenberg, National Cancer Institute). EL4 and EG7 [ovalbumin (OVA)-expressing EL4] cell lines were purchased from American Type Culture Collection. Spleen CD4+ and CD8+ T cells have been negatively selected using StemSep isolation columns (Stem Cell Technologies) with 90% to 95% purity. In some in vitro experiments (Fig. 1B), we carried out additional anti-LyC6–mediated removal (24) of preactivated OT-1 cells or flow-sorted CD44−CD62L+ (naïve) and CD44+CD62L− (memory) cells. All cells were maintained in RPMI 1640 with 10% heat-inactivated fetal bovine serum (Invitrogen), glutamine, streptomycin, and penicillin (Invitrogen).
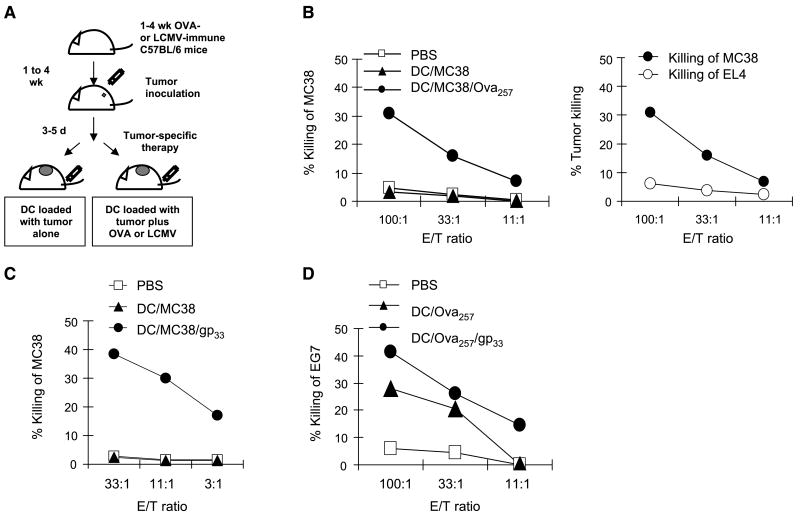
Figure 1.
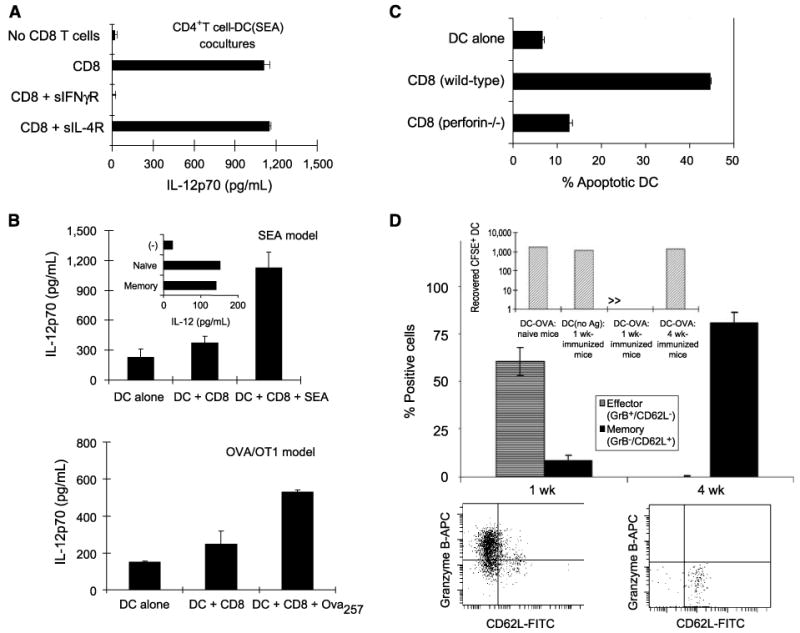
Dendritic cell–activating versus dendritic cell killing activity of resting and effector CD8+ T cells. A, CD8+ T cells support IL-12p70 induction in dendritic cell (DC)-CD4+ T-cell cocultures. SEA-coated (18, 26) dendritic cells (25) were coincubated with syngeneic CD4+ T cells in the absence or presence of (IFNγ-producing, not shown) spleen-isolated CD8+ T cells. Soluble IL-4R or IFNγR were used to selectively neutralize IFNγ or IL-4, two cytokines with IL-12–enhancing activities (18, 27, 30). Columns, mean from one experiment of three that yielded similar results; bars, SD. B, interaction with CD8+ T cells primes dendritic cells for high IL-12p70 production. Top, dendritic cells were cocultured for 48 h with CD8+ T cells from wild-type B6 mice, either in the absence or presence of SEA, before washing and stimulation with CD40L (18, 28). Addition of SEA alone (no T cells) had no or marginal effect (ref. 18 and data not shown). Inset, equivalent effectiveness of naïve and memory CD8+ T cells. Bottom, dendritic cells were cocultured with H-2Kb–restricted OVA257–264–specific CD8+ T cells, freshly isolated from spleens of OT-1 mice, in the presence of OVA257–264 peptide before washing and CD40L stimulation. C, effector T cells kill dendritic cells in vitro. SEA-loaded dendritic cells were cocultured with preactivated (granzyme Bhigh/CD62Llow; data not shown) effector CD8+ T cells from wild-type or perforin-deficient mice. Similar results were obtained in one additional experiment. D, predominance of OVA257–264/H-2Kb–specific effector versus memory T cells in 1 wk– versus 4 wk–immunized C57BL/6 mice. Note the predominance of tetramer-positive granzyme B+/CD62L− effector cells in 1 wk–immunized mice, as opposed to selective presence of granzyme B−/CD62L+ memory cells in the spleens of 4 wk–immunized mice (n = 3 mice per group; bottom, representative data from individual animals). The frequencies of tetramer-positive CD8+ T cells in 1 wk– and 4 wk–immunized mice were 4.3% (±1.5) and 0.7% (±0.3), respectively. Data from one of two experiments that yielded similar results. Inset, selective elimination of OVA257–264–carrying carboxyfluorescein diacetate succinimidyl ester–labeled dendritic cells in 1 wk–immunized, but not 4 wk–immunized, mice. Naïve mice and 1 wk–preimmunized mice receiving sham-loaded dendritic cells served as control groups. Mice (three per group) were injected with 106 dendritic cells and draining lymph nodes (13, 14) were removed after 16 h.
Dendritic cells
Bone marrow–derived dendritic cells were generated in cultures supplemented with granulocyte macrophage colony-stimulating factor and IL-4 (both 1,000 units/mL; Schering-Plough), as described (25). On days 6 to 7, CD11c+ dendritic cells were isolated using antimouse CD11c-coated magnetic beads (Miltenyi Biotech). Dendritic cells expressed CD11c, CD40, CD80, CD86, and MHC I and II (ref. 25 and data not shown).
Induction of IL-12p70
Dendritic cells (2 × 104/0.2 mL per well) loaded with SEA (CD4+ and CD8+ T-cell–activating superantigen; refs. 18, 26, 27; 1 ng/mL) were cocultured for 48 h with CD4+ T helper cells (105 per well) in the absence or presence of CD8+ T cells (105 per well). When indicated, soluble (neutralizing) IFNγ receptor or soluble IL-4 receptor was added (10 μg/mL; R&D Systems). Alternatively, antigen-free, OVA257–264–loaded, or SEA-loaded dendritic cells were first cocultured for 48 h with CD8+ T cells (0.75 × 105 or 3 × 105, respectively, from the spleens of OT-1 or wild-type mice), harvested, washed, counted, and stimulated (at 2 × 104/0.2 mL) with 5 × 104 CD40L-transfected J558 cells (18, 28) for 24 h. IL-12p70 concentrations were determined by ELISA (Endogen).
In vitro dendritic cell killing
CD8+ T cells from wild-type or perforin-deficient mice were stimulated with SEA-loaded dendritic cells and IL-2 (20 units/mL; Chiron Corp.) for 6 days. The resulting granzyme Bhigh/CD62Llow (not shown) effector cells were coincubated with dendritic cells (3 h; 5:1 ratio) in the presence or absence of SEA. Induction of apoptosis in CD11c+ dendritic cell was assessed by staining for surface CD11c, followed by intracellular staining for active caspase-3 (C92-605, BD PharMingen).
Dendritic cell elimination in vivo
Dendritic cells (OVA257–264 or PBS loaded; 1 × 106) were labeled with carboxyfluorescein diacetate succinimidyl ester (1 μmol/L for 10 min at 37°C), washed thrice, and injected into the footpads. After 24 h, single-cell suspensions from popliteal lymph nodes were prepared and analyzed by flow cytometry. Total node cellularity was counted in a hemocytometer.
Induction of lymphocytic choriomeningitis virus– or OVA-specific immune responses
Lymphocytic choriomeningitis virus (LCMV) glycoprotein peptide (LCMVgp33–41; KAVYNFATC), the dominant H2-Db/Kb-restricted epitope of LCMV, and dominant H-2Kb–restricted OVA epitope, OVA257–264 (SIINFEKL), were synthesized by the University of Pittsburgh Peptide Synthesis Facility. Peptide-loaded dendritic cells were washed twice and injected s.c. (3 × 105 in 0.2 mL of PBS) twice with 1-week interval. The presence of effector and memory CD8+ T cells in the spleens and lymph nodes of vaccinated animals was determined by three-color flow cytometry after staining of isolated CD8+ T cells with CD62L (MEL-14, BD PharMingen), granzyme B (GB12l, CalTag), and tetramer (iTAg, Beckman-Coulter).
Tumor vaccines
Dendritic cells were loaded overnight with MC38 tumor cells lysates (freeze-thawed thrice, centrifuged, and supernatant collected), at three tumor cell equivalents to one dendritic cell, in the presence of lipopolysaccharide. Dendritic cells were resuspended in RPMI 1640 and loaded with OVA257–264, LCMVgp33–41, or PBS. For preparation of the EG7 vaccine, dendritic cells were loaded with OVA257–264 (alone or with LCMVgp33–41). All vaccines were washed twice and suspended in PBS.
Tumor therapy models
Wild-type C57BL/6 mice (7–12 per group, including 2 animals per group for CTL assays), naïve or carrying week 1 or week 4 immune responses against LCMV or OVA, were inoculated s.c. into the right flank (day 0) with high numbers of tumor cells (3 × 105 MC38 or 3 × 106 EG7) to induce rapid tumor growth that was only marginally sensitive to standard therapeutic vaccines (see Results). The mice were vaccinated s.c. (3 × 105 dendritic cells, on the distant site on same flank) on day 3, or on days 5, 9, and 11, as indicated. Tumors were measured by vernier calipers every 3 to 4 days. Data are reported as the mean ± SE of tumor area (product of the largest perpendicular diameters).
CTL activity
Ten days after vaccination, splenocytes were harvested from two tumor-bearing mice per group. They were restimulated in vitro (1 × 106 per well) with 1 × 105 γ-irradiated (10,000 rad) MC38 or EG7 cells in the presence of 30 IU/mL recombinant human IL-2 in 24-well culture plates. Lymphocytes were harvested after 5 days and used in 5-h 51Cr release assays against MC38 and EG7 targets, with EL4 cells used as nonspecific controls.
Statistical analysis
Data collected (day 4 until the last day of tumor measurement) were natural log transformed and used to fit a parametric mixed linear model that included animals as random effects with treatment group and day of measurement as fixed effects. If either group differences or group by time interactions were significant at P < 0.05, the analysis was applied to the last day of tumor area measurement. Data were natural log transformed when appropriate and a one-way parametric ANOVA was used as an omnibus test of differences. Unless tests were significant at α = 0.05, no further testing of specific contrasts was conducted. Otherwise, individual pairwise comparisons were conducted with the t test. All tests were two tailed.
Results
Dendritic cell activating and dendritic cell killing activities of resting versus effector CD8+ T cells
To analyze the ability of mouse CD8+ T cells to affect dendritic cell functions in vitro, we used the previously established models of the SEA-driven (18, 26) or chicken OVA-driven (24, 29) stimulation of T cells from wild-type C57BL/6 mice or T-cell receptor–transgenic (29) OT-1 mice, respectively. These models allowed us to promote the interaction of dendritic cells with high numbers of CD8+ T cells without the need of prior T-cell activation and clonal expansion. In accordance with our data showing that the superior ability of human CD8+ T cells to produce IFNγ at early stages of activation allows them to costimulate IL-12p70 production by dendritic cells interacting with CD4+ T cells (18), freshly isolated mouse CD8+ T cells strongly supported IL-12p70 induction in cocultures of SEA-loaded bone marrow–derived dendritic cells (25) with autologous CD4+ T cells (Fig. 1A). They also primed the SEA- or OVA257–264–loaded dendritic cells for high IL-12p70 production during subsequent interaction with CD40L-transfected J558 cells, used as CD4+ T-cell surrogates (refs. 18, 28; Fig. 1B). Similar to the human system, neutralization of IFNγ, but not IL-4 (another IL-12–enhancing cytokine; refs. 27, 30), abolished the IL-12–enhancing activity of CD8+ T cells (Fig. 1A). As expected (18), not only the simultaneous interaction of dendritic cells with CD8+ T cells and CD4+ T cells (Fig. 1A) but also their sequential interaction with CD8+ T cells followed by CD40L stimulation (Fig. 1B) resulted in the augmented IL-12p70 production, with naïve and memory CD8+ T cells being similarly effective (Fig. 1B, top, inset). These data verified that, similar to human CD8+ T cells, mouse CD8+ T cells can act as helper cells, supporting IL-12 production by dendritic cells.
In contrast to resting T cells, 6-day preactivated effector CD8+ T cells efficiently killed antigen-carrying dendritic cells (Fig. 1C). In accordance with the in vivo shown key role of perforin in CTL-dependent dendritic cell elimination (14), CD8+ T cells from perforin-deficient mice were defective in their ability to kill dendritic cells (Fig. 1C).
Taken together, these data verified that CD8+ T cells can act both as activators and killers of antigen-carrying dendritic cells, but indicated that these two functions are done at different stages of CD8+ T-cell activation. They suggested that noncytotoxic naïve or memory CD8+ T cells versus the effector cells with CTL function may play opposite functions regulating the course of immune responses. To establish an in vivo model that would allow us to test in wild-type mice the regulatory effect of either the cytotoxic (effector) or noncytotoxic (memory) CD8+ T cells, we preimmunized wild-type C57BL/6 mice with OVA257–264 for 1 or 4 weeks. As expected, in 1 week–immunized animals, we observed high numbers of tetramer-positive CD8+ T cells with CD62Llow/granzyme Bhigh effector phenotype. In sharp contrast, CD8+ T cells that were obtained 4 weeks after immunization showed a selective presence of CD62Lhigh/granzyme Blow memory-type T cells (Fig. 1D). Similar to previous reports (13, 14, 20), we observed that the animals harboring CD62Llow/granzyme Bhigh effector CD8+ T cells, induced by preimmunization with OVA257–264 1 week earlier, rapidly eliminated antigen-loaded dendritic cells (Fig. 1D, inset), whereas OVA-loaded dendritic cells could be readily recovered from the vaccine-draining lymph nodes of naïve mice or mice harboring 4-week-old memory-type CD62Lhigh/granzyme Blow T-cell responses.
Memory CD8+ T cells support the dendritic cell–mediated induction of tumor-specific CTL responses
To test the possibility that antigen-specific CD8+ T cells at different stages of activation indeed play different regulatory roles, we have used a model of therapeutic vaccination with tumor-loaded dendritic cells carrying additional, tumor-unrelated, MHC class I–restricted epitopes of OVA257–264 or LCMVgp33–41 (Figs. 2 and 3). We analyzed the effect of including tumor-unrelated heterologous class I–restricted peptide epitopes into dendritic cell–based vaccines (tumor-loaded dendritic cells) on the induction of CTL responses and the therapeutic activity of vaccination against established tumors (Fig. 2A). In such models, the heterologous helper epitopes were only present in cancer vaccines and were not expressed by the tumor itself. Therefore, any helper or suppressor effect of (OVA257–264 or LCMVgp33–41 specific) CD8+ T cells on the development of the immune responses against MC38 or EG7 tumors could be analyzed in isolation from a possible indirect modulatory effect of CD8+ T cells, mediated by tumor antigens and other tumor-derived factors differentially released from the CTL-targeted tumor tissues.
Figure 2.
Memory CD8+ T cells support the dendritic cell–mediated induction of tumor-specific CTL responses. A, schema of the experimental protocol. B to D, heterologous memory-type CD8+ T cells support the induction of tumor-specific CTLs by dendritic cell–based cancer vaccines. B, OVA257–264–specific CD8+ T cells support the induction of CTLs specific for MC38 adenocarcinoma. Mice carrying memory-type OVA257–264–specific CD8+ T-cell responses and inoculated with MC38 tumor (day 0) were treated s.c. (day 3) with dendritic cells loaded with MC38 lysate alone or with OVA257–264 as tumor-unrelated heterologous helper epitope. Left, induction of CTL activity in the spleens of the differentially treated mice. Right, comparison of CTL activity of the splenocytes from the dendritic cell/MC38 lysate/LCMVgp33–41–treated mice against the vaccine-relevant (MC38) and irrelevant (EL4) targets. Data from one of two experiments that yielded similar results. C, memory-type LCMVgp33–41–specific CD8+ T cells support the induction of MC38 adenocarcinoma–specific CTLs. Tumor-bearing mice with memory-type LCMVgp33–41–specific CD8+ T-cell responses were injected s.c. with dendritic cells loaded with MC38 tumor lysate, alone or with LCMVgp33–41 as a heterologous helper epitope. Data from one of three independent experiments that all yielded similar results. D, LCMV-specific CD8+ T cells support the induction of CTLs against OVA257–264–expressing EG7 lymphoma. Tumor-bearing mice with memory-type LCMVgp33–41–specific CD8+ T-cell responses were injected s.c. with dendritic cells loaded with OVA257–264 peptide, as the EG7 tumor-relevant antigen, either alone or with the LCMVgp33–41 peptide, as a tumor-unrelated heterologous helper epitope. Similar results were obtained in an additional experiment.
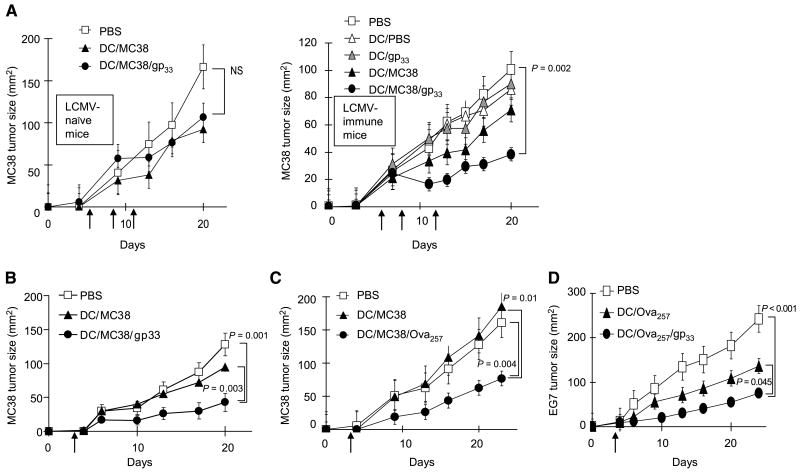
Figure 3.
Heterologous CD8+ T cells support the therapeutic activity of cancer vaccines. A, memory-type LCMVgp33–41–specific CD8+ T cells promote the therapeutic effects of vaccination against day 5 established MC38 tumors. MC38-bearing C57BL/6 mice (n = 5 per group), either naïve (left) or carrying memory-type (week 4) LCMVgp33–41–specific CD8+ T-cell responses (right), were inoculated with MC38 tumors on day 0 and were treated on days 5, 9, and 11 after tumor inoculation. B to D, memory-type CD8+ T cells specific for tumor-unrelated antigens enhance the therapeutic effects of cancer vaccines (single vaccination model). Day 3 tumor-bearing mice (B, n = 10 per group; C and D, n = 5 per group) with memory-type responses against tumor-unrelated heterologous helper antigens were injected s.c. with PBS as a negative control (□), dendritic cells loaded with tumor antigen alone (MC38 tumor lysate or OVA257–264 in the EG7 model, ▲), or the relevant tumor antigen plus a tumor-irrelevant heterologous helper epitope (●). B, memory-type LCMVgp33–41–specific CD8+ T cells support the therapeutic activity of vaccination against MC38 adenocarcinoma. C, memory-type OVA257–264–specific CD8+ T cells support the therapeutic activity of vaccination against MC38 adenocarcinoma. D, memory-type LCMVgp33–41–specific CD8+ T cells support the therapeutic activity of vaccination against OVA257–264–expressing EG7 lymphoma. Points, mean from one of two separate experiments in each model; bars, SE. The differences between the treatment groups were evaluated by ANOVA. NS, nonsignificant differences (P > 0.05).
To model a clinically relevant situation where significant numbers of resting T cells can be used to interact with cancer vaccine in wild-type animals, we have used wild-type C57BL/6 mice harboring memory-type CD8+ T cells against defined MHC class I–restricted epitopes of OVA or LCMV. For the same purpose, we have administered the vaccines to the animals bearing day 3 to 5 established tumors, the time point where, in analogy to the clinically applied therapeutic cancer vaccines (13, 14), standard vaccinations with tumor-loaded dendritic cells were only marginally effective.
As shown in Fig. 2B, the inclusion of OVA257–264 epitope in cancer vaccines composed of autologous dendritic cells loaded with the relatively poorly immunogenic MC38 tumor lysate supported the generation of MC38-specific CTL responses in wild-type C57BL/6 animals. Similar data were also obtained in a model of wild-type mice harboring memory responses against LCMVgp33–41, a dominant epitope of a natural mouse pathogen, where the inclusion of LCMVgp33–41 peptide as a “heterologous helper epitope” strongly enhanced the induction of CTLs against established MC38 tumors (Fig. 2C).
The vaccines including the LCMVgp33–41 “CD8 helper” epitope not only showed strongly elevated CTL-inducing function against the poorly immunogenic MC38 adenocarcinoma but also further enhanced the CTL responses against the highly immunogenic (OVA-expressing) EG7 lymphoma, induced by OVA257–264–loaded dendritic cells (Fig. 2D). The ability of the CD8+ T cells specific for the same individual epitope (OVA257–264) to both provide CD8 helper signals (Fig. 2B) and benefit from such signals (Fig. 2D) indicates that CD8+ T-cell help is not restricted to responses against some unique antigens (e.g., responses to “strong” immunogens facilitating the responses to “weak” immunogens) but that any naïve CD8+ T cells that can first receive the CD8 help and later, after becoming memory cells themselves, can provide helper signals to other cells.
Heterologous CD8+ T-cell help supports the therapeutic effects of cancer vaccines
To test whether helper signals from memory CD8+ T cells can enhance the therapeutic activity of vaccination against established tumors, we compared the therapeutic activity of the dendritic cells loaded with tumor-relevant antigens, alone or with LCMVgp33–41 peptide, as therapeutic vaccines against the established (day 3–5) MC38 and EG7 tumors in wild-type C57BL/6 mice, either naïve or carrying memory-type LCMV-specific CD8+ T cells. As shown in Fig. 3A, in such therapeutic settings, dendritic cells loaded with tumor material alone had only marginal effect on the growth of established MC38 tumors. In LCMV-naïve mice, this outcome was not improved by the inclusion of the LCMVgp33–41 helper epitope in the vaccines (Fig. 3A, left).
However, in mice harboring memory-type responses against the MHC class I–restricted LCMVgp33–41 epitope, the vaccination with dendritic cells loaded with MC38 tumor lysate and LCMVgp33–41, the “heterologous CD8 helper” peptide, resulted in a distinct therapeutic effect against day 5 established tumors that were resistant to treatment with standard dendritic cell–based vaccines (Fig. 3A, right). These beneficial effects of heterologous CD8 help could not be mimicked by the vaccination with dendritic cells loaded with LCMVgp33–41 alone, showing that the reduction in tumor growth is not due to nonspecific immunostimulatory effects of activation of high numbers of LCMV-specific CD8+ T cells present in these animals.
Similar enhancement of the therapeutic efficacy of vaccination has been observed in three additional models when using LCMVgp33–41 or OVA257–264 to enhance the antitumor effects of a single (rather than triple) dose of vaccine against the 3-day-old MC38 tumors (Fig. 3B and C) or when using LCMVgp33–41 to boost the antitumor effects of vaccination against significantly more immunogenic EG7 lymphoma (Fig. 3D). In accordance with the data on the induction of tumor-specific CTLs (Fig. 2), the results of these functional tests of antitumor activity showed that OVA257–264–specific CD8+ T cells at their naïve stage can benefit from helper signals delivered by memory T cells (in the current experiments, specific for LCMVgp33–41), but at the memory stage they can themselves act as a source of CD8+ T-cell helper signals, verifying that the ability to receive and provide CD8 helper signals is a general feature of CD8+ T cells.
Critical role of dendritic cells in mediating CD8 help
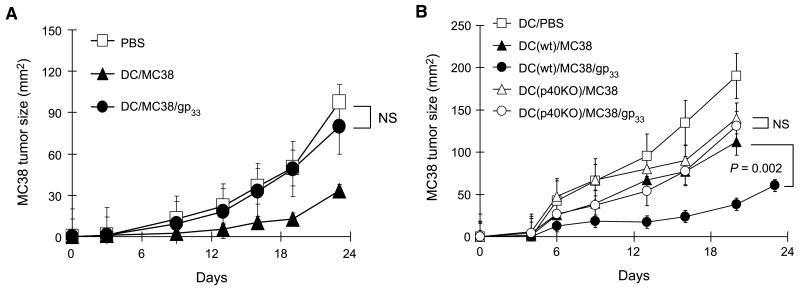
Whereas the inclusion of LCMVgp33–41 (or OVA257–264) peptides strongly enhanced the immunologic and antitumor effects of vaccination in the animals harboring memory-type CD8+ T cells specific for such heterologous helper epitopes (Fig. 3), in accordance with the dendritic cell killing activity of effector CD8+ T cells (13, 14, 21), these positive effects were eliminated in the animals preimmunized with LCMV at 1 week before tumor inoculation (Fig. 4A). In further support of the central role of dendritic cells and the dendritic cell–produced cytokines, the heterologous help from memory-type CD8+ T cells could not be mediated by IL-12/IL-23–deficient dendritic cells generated from the bone marrow of p40-knockout animals (Fig. 4B).
Figure 4.
Critical role of dendritic cells in mediating CD8 help for anticancer immunity. A, effector CD8+ T cells do not deliver heterologous CD8 help. Three days after MC38 tumor inoculation, C57BL/6 mice (n = 5) with 1-week-old LCMV-specific responses received a single s.c. injection of MC38 tumor lysate with or without LCMVgp33–41 peptide loaded on dendritic cells from wild-type mice and monitored for the kinetics of tumor growth. B, IL-12p40–deficient dendritic cells (unable to produce IL-12p70 and IL-23) do not mediate heterologous CD8 help. Three days after MC38 tumor inoculation, wild-type C57BL/6 mice (n = 5) with 4-week-old LCMV-specific CD8+ T-cell responses were treated with the indicated vaccines. Mice received single s.c. injections of dendritic cells (generated from wild-type or IL-12p40–knockout mice) loaded with MC38 tumor lysate alone or with LCMVgp33–41 peptide. Points, mean from two independent experiments in each model; bars, SE.
Discussion
The current data show that, in contrast to effector CD8+ T cells that kill dendritic cells and suppress antigen-specific immune responses (13–15, 20, 21), memory CD8+ T cells can act as de facto helper cells, supporting the dendritic cell–mediated immunologic and antitumor effects of cancer vaccines.
Our observations that OVA257–264–specific CD8+ T-cell responses can both benefit from the “helper signals” delivered by memory cells (e.g., from LCMV-specific CD8+ T cells) and provide such signals directly show that CD8+ T cells of a single specificity can first benefit from the help delivered by memory T cells and, once expanded and having reached memory stage, they can themselves act as helper cells. The ability of memory CD8+ T cells to support the dendritic cell–mediated activation of additional naïve CD8+ T cells suggests that the phenomenon of immune memory, in addition to qualitative changes in the activation requirements of memory cells (5–8), may also involve a different pattern of interaction of CD8+ T cells with antigen-carrying dendritic cells.
Our findings help to explain the requirement for a delayed administration of booster doses of preventive vaccines to achieve the optimal expansion of pathogen-specific CD8+ T cells and the optimal vaccine effectiveness (9, 10). Interestingly, recent evidence indicates that priming done under noninflammatory conditions allows for effective administration of booster vaccines substantially sooner than when priming with live pathogen (9). However, it remains to be tested whether such noninflammatory priming conditions result in impaired induction of the effector (and thus suppressive) functions of the pathogen-specific CD8+ T cells.
The existence of distinct suppressor and helper stages of CD8+ T-cell activation may help to explain the generally poor efficacy of therapeutic vaccinations of cancer-bearing patients that show predominance of terminally differentiated effector cells (31–33). It also helps to explain the high efficacy of prime-boosting vaccination strategies (8) when the first and second doses of vaccine are delivered using antigenically distinct vectors.
The mechanism of helper function of memory CD8+ T cells in the settings of established cancer is a subject of our current follow-up analyses. Whereas our data show the key role of antigen-carrying dendritic cells in this respect, it is unclear whether the heterologous memory CD8+ T cells just hyperactivate dendritic cells, allowing them to provide immunostimulatory signals to naïve/resting tumor-specific CD8+ T cells before their destruction by effector cells, or memory CD8+ T cells can also protect antigen-carrying dendritic cells from CTL killing. It has been recently proposed that the CD4+ T-cell help for CTL responses is essential mainly for the secondary expansion of CD8+ T cells rather than for their effective priming (10). These observations raise the possibility that the protection of dendritic cells from CTL killing (21, 34) may be as important as the originally proposed dendritic cell activation or “licensing” (35–37) in the overall mechanism of the CD4+ T-cell help.
The current data directly implicate the possibility of enhancing the effectiveness of therapeutic vaccination of cancer patients by incorporating tumor-unrelated heterologous epitopes that promote the interaction of the vaccine-carrying antigen-presenting cells with naturally occurring or artificially induced memory-type T cells. Whereas in the current studies we either have used (in vitro) high numbers of resting CD8+ T cells from TCR-transgenic animals or have involved (in vivo) the memory CD8+ T cells induced by a preimmunization of wild-type animals, the most obvious source of heterologous CD8 help in cancer patients are the memory-type CD8+ T cells induced naturally by past infections or prior vaccinations against “childhood diseases” or such pathogens as influenza or hepatitis.
Our data showing that the helper functions are a selective feature of memory CD8+ T cells, but not effector cells, indicate the existence of a novel mechanism contributing to the phenomenon of CD8+ T-cell memory. Our observations help to explain the benefits of delayed application of booster doses of preventive vaccines and the high efficacy of prime-boost vaccination strategies, facilitating the development of effective strategies of therapeutic vaccination of patients with cancer and chronic infections.
Acknowledgments
Grant support: NIH grants 1RO1CA095128 and 1PO1CA101944.
We thank Dr. Franca Ronchese for critical comments on this manuscript.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreckenberger C, Kaufmann AM. Vaccination strategies for the treatment and prevention of cervical cancer. Curr Opin Oncol. 2004;16:485–91. doi: 10.1097/00001622-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Spearman P. Current progress in the development of HIV vaccines. Curr Pharm Des. 2006;12:1147–67. doi: 10.2174/138161206776055859. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18:201–5. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 6.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 7.Tough DF. Deciphering the relationship between central and effector memory CD8+ T cells. Trends Immunol. 2003;24:404–7. doi: 10.1016/s1471-4906(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 8.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–56. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 10.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 11.Hirschhorn K, Bach F, Kolodny RL, Firschein IL, Hashem N. Immune response and mitosis of human peripheral blood lymphocytes in vitro. Science. 1963;142:1185–7. doi: 10.1126/science.142.3596.1185. [DOI] [PubMed] [Google Scholar]
- 12.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108:586–90. [PubMed] [Google Scholar]
- 13.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–52. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 16.Gurunathan S, Stobie L, Prussin C, et al. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J Immunol. 2000;165:915–24. doi: 10.4049/jimmunol.165.2.915. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Norbury CC, Greenwood R, Bennink JR, Yewdell JW, Frelinger JA. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J Immunol. 2001;167:1283–9. doi: 10.4049/jimmunol.167.3.1283. [DOI] [PubMed] [Google Scholar]
- 18.Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–83. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counterregulation. J Immunol. 2002;168:216–23. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
- 20.Ronchese F, Hermans IF. Killing of dendritic cells: a life cut short or a purposeful death? J Exp Med. 2001;194:F23–6. doi: 10.1084/jem.194.5.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medema JP, Schuurhuis DH, Rea D, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–67. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruedl C, Kopf M, Bachmann MF. CD8(+) T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189:1875–84. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 24.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 25.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–57. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 26.Fraser JD. T-cell recognition of superantigens. Res Immunol. 1993;144:173–4. doi: 10.1016/0923-2494(93)80112-c. [DOI] [PubMed] [Google Scholar]
- 27.Kalinski P, Smits HH, Schuitemaker JH, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–81. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 28.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carbone FR, Sterry SJ, Butler J, Rodda S, Moore MW. T cell receptor α-chain pairing determines the specificity of residue 262 within the Kb-restricted, ovalbumin257-264 determinant. Int Immunol. 1992;4:861–7. doi: 10.1093/intimm/4.8.861. [DOI] [PubMed] [Google Scholar]
- 30.Hochrein H, O'Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–33. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenzato G, Pezzutto A, Agostini C, Albertin M, Gasparotto G. T-lymphocyte subpopulations in chronic lymphocytic leukemia: a quantitative and functional study. Cancer. 1981;48:2191–7. doi: 10.1002/1097-0142(19811115)48:10<2191::aid-cncr2820481013>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maczek C, Berger TG, Schuler-Thurner B, et al. Differences in phenotype and function between spontaneously occurring melan-A-, tyrosinase- and influenza matrix peptide-specific CTL in HLA-A*0201 melanoma patients. Int J Cancer. 2005;115:450–5. doi: 10.1002/ijc.20901. [DOI] [PubMed] [Google Scholar]
- 34.Mueller SN, Jones CM, Stock AT, Suter M, Heath WR, Carbone FR. CD4+ T cells can protect APC from CTL-mediated elimination. J Immunol. 2006;176:7379–84. doi: 10.4049/jimmunol.176.12.7379. [DOI] [PubMed] [Google Scholar]
- 35.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 36.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 37.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]