Abstract
A droplet-based micro-total-analysis system involving biosensor performance enhancement by integrated surface-acoustic-wave (SAW) microstreaming is shown. The bioreactor consists of an encapsulated droplet with a biosensor on its periphery, with in situ streaming induced by SAW. This paper highlights the characterization by particle image tracking of the speed distribution inside the droplet. The analyte-biosensor interaction is then evaluated by finite element simulation with different streaming conditions. Calculation of the biosensing enhancement shows an optimum in the biosensor response. These results confirm that the evaluation of the Damköhler and Peclet numbers is of primary importance when designing biosensors enhanced by streaming.
It has been pointed out that biosensing performances can be limited by the diffusion of the analytes near the sensing surface.1 In the case of low Peclet number hydrodynamic flows, typical of microfluidic systems, molecule displacements are mainly governed by diffusive effects that affect time scales and sensitivity. To overcome this problem, the enhancement of biosensor performance by electrothermal stirring within microchannels was first reported by Meinhart et al.2 Other authors3, 4 numerically studied the analyte transport as a function of the position of a nanowire-based sensor inside a microchannel, stressing on the fact that the challenge for nanobiosensors is not the sensor itself but the fluidic system that delivers the sample. Addressing this problem, Squires et al.5 developed a simple model applicable to biosensors embedded in microchannels. However, the presented model is limited to the case of a steady flow. The use of surface-acoustic waves (SAWs) for stirring in biomicrofluidic and chemical systems is becoming a popular investigation field,6, 7, 8, 9 especially to overcome problems linked to steady flows by enhancing the liquid∕surface interaction.1, 10, 11 The main challenges that need to be addressed when using SAW-induced stirring are the complexity of the flow and its poor reproducibility. However, some technical solutions were proposed to yield a simplified microstreaming. Yeo et al. presented a centrifugation system based on SAW that produces the rotation of the liquid in a droplet in a reproducible way by playing on the configuration of the transducers and reflectors,12 and presented a comprehensive experimental study of the three-dimensional (3D) flow that causes particle concentration in SAW-stirred droplets,13 revealing the presence of an azimuthal secondary flow in addition to the main vortexlike circular flow present in acoustically stirred droplets. The efficiency of SAW stirring in microdroplets to favorably cope with mass transport issues was finally shown by Galopin et al.,14 but the effect of the stirring on the analyte∕biosensor interaction was not studied. It is expected to overcome mass transport limitations by bringing fresh analytes from the bulk solution to the sensing surface.
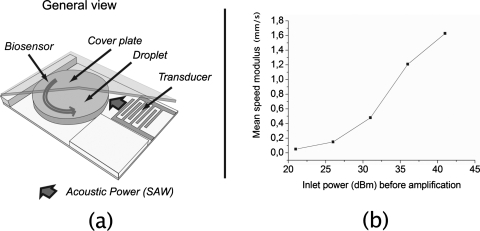
The studied system, described in Fig. 1, consists of a microliter droplet microchamber squeezed between a hydrophobic piezoelectric substrate and a hydrophobic glass cover. Rayleigh SAWs are generated using interdigitated transducers (interdigital spacing of 50 μm) laid on an X-cut LiNbO3 substrate.1, 15, 16 The hydrophobicity of the substrate and the cover are obtained by grafting octadecyltrichlorosilane (OTS) self-assembled monolayers (contact angle of 108° and hysteresis of 9°). To do so, the surface is first hydroxylized using oxygen plasma (150 W, 100 mT, and 30 sccm3 O2) during 1 min and then immersed for 3 h into a 1 mM OTS solution with n-hexane as a solvent.
Figure 1.
(a) General view of the considered system. (b) Mean value of the measured speeds within the droplet as a function of the inlet power before amplification.
When Rayleigh waves are radiated toward one-half of the microchamber, a vortex is created in the liquid around an axis orthogonal to the substrate due to the momentum transfer between the solid and the liquid. This wave is generated under the Rayleigh angle into the liquid.
Speed cartographies of the flow induced in the droplet are realized using the particle image tracking technique for different SAW generation powers. To do so, instantaneous images of the flow are taken with a high-speed video camera at 200 frames∕s and an aperture time of 500 μs on a 0.25 μl droplet containing 1 μm diameter fluorescent particles. Figure 1 shows the mean speed measured in the droplet as a function of the inlet power. The great dependence of the induced mean speed with the SAW power enables a large range of flow speeds in the stirred droplet. Moreover, the flow was visualized with a low depth of field objective. It was found to be circular and two dimensional (2D) in a large thickness range of the droplet.
The binding of analytes to immobilized ligands on a biosensor is a two step process, including the mass transport of the analyte to the surface, followed by a complexation step,
| (1) |
with km as the constant rate for mass transport from and to the sensor, and ka and kd as the constant rates of association and dissociation of the complex.
At the biosensor surface, the reaction kinetics consumes analytes but their transport is limited by diffusive effects. In this case, the Damköhler number brings valuable information by comparing these two effects. Calling the characteristic time of reaction and diffusion, respectively, τC and τM, the mixing time in diffusion regime can be approximated by τM≈h2∕D with D as the diffusion coefficient and h a characteristic length of the microchannel. Calling RT the ligand concentration on the surface in mole∕m2, the Damköhler number (Da) can be written as
| (2) |
Depending on the type of reaction, the calculation of Da helps determine if a specific biointeraction will benefit from a mass SAW-based microstreaming. If the Damköhler number is low, the reaction is slow compared to mass transport and the reaction will not significantly benefit from microstirring. For example, the hybridization of 19 base single stranded DNA in a microfluidic system with a characteristic length of 500 μm is characterized by a Damköhler number of 0.07 and is therefore not significantly influenced by mass transport. On the contrary, the binding of biotin to immobilized streptavidin is characterized by a Da number of approximately 104. In this case, the stirring solution will significantly improve the reaction rate.
COMSOL numerical simulations were carried out to study the efficiency of the SAW stirring in the case of a droplet-based microbioreactor with a diameter of 1 mm. Assuming a 2D flow, the simulated model takes into account the convective and diffusive effects in the analyte-carrying fluid and the binding kinetics on the biosensor surface. This approach was thoroughly developed by Meinhart et al.2
On the biosensor surface, the following equations are solved:
| (3) |
| (4) |
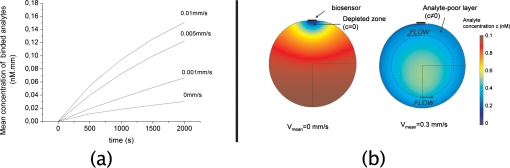
with c as the local concentration of analytes in the droplet and B as the surface concentration of bound analytes on the biosensor surface. Simulation results show that a depleted zone is formed near the biosensor in the case of an interaction without stirring. This zone is characterized by a low concentration of analytes and results from the trapping of analytes on the biosensor surface, thus creating a concentration gradient on the vicinity of the biosensor. When stirring is applied, the geometry of the depleted zone is modified, as it is pushed in the direction of the flow. The geometry of the depleted zone then depends on many parameters, among which the diffusion coefficient D, the speed distribution of the flow (not only near the biosensor but also in the whole microfluidic system), and the reaction kinetics on the biosensor. In our case, which is assimilated to a simple circular flow, the depleted zone reaches a permanent state consisting of an analyte-poor layer situated in the exterior perimeter of the stirred droplet. The diffusion of analytes is then limited again by diffusion from the inner part of the droplet toward its exterior perimeter (see Fig. 2).
Figure 2.
(a) Mean concentration of bound analytes vs time for different mean flow speeds. (b) The obtained concentration profiles with and without circular stirring, t=10 000 s.
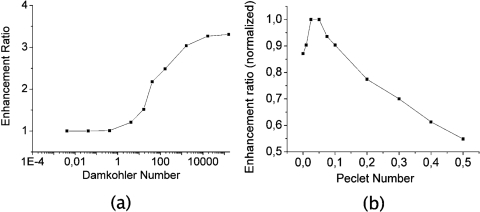
The initial analyte and receptor concentrations are, respectively, 0.1 nM in the solution and 3.3×10−3 nM m on the biosensor surface, the diffusion coefficient is D=10−11 m2 s−1, and the reaction constants are ka=106 M−1 s−1 and kd=10−3 s−1. Simulations show that the mean concentration of bound analytes highly increases with the flow speed, improving the efficiency of the biosensing device. To evaluate the benefits of in situ microstreaming with SAW, the same simulations were conducted for Da numbers ranging from 104 to 108 M−1∕s, by ranging the diffusion coefficient from 4×10−12 to 4×10−9 m2∕s, and the association coefficient ka from 104 to 108 M−1∕s. The enhancement factor of analyte capture, defined as the ratio of the binding rate with streaming B and the binding rate without streaming B0, is plotted in Fig. 3 for different values of Da. Calculations are done in the case of a mean flow speed of 0.5 mm∕s.
Figure 3.
(a) Enhancement factor (defined as the ratio between binding rate with streaming B and binding rate without streaming B0) for different Damkhöler numbers and (b) normalized enhancement factor for different Peclet numbers.
One can notice the saturation of the enhancement factor curve for large value of Da to the value of 3.5 for high Da. This can be explained by the fact that for large ka∕Da ratios, the analytes, which normally require penetration in the depleted zone by diffusion, do not have time to interact with the biosensor when they pass in the vicinity of its surface. The efficiency of the streaming is then reduced for large values of Da. In the case of our specific flow configuration, the enhancement factor reaches 3.2 for the interaction of streptavidin on immobilized biotin (Da=103).
The reported simulation results can be compared to an experimental value obtained using the droplet-based surface plasmon resonance sensor streamed in situ using SAW reported by Yeo et al.12 By monitoring the streptavidin∕biotin binding interaction on an activated gold slide, they showed that SAW stirring brings an improvement factor of more than 2. This difference can be accounted to the high complexity of the induced 3D flow, which was modeled in a simple manner in our calculations.
Other factors must be taken into account when optimizing the improvement factor, such as the flow velocity and the characteristic length of the mixing. To do so, the Peclet number allows the comparison of the convective and diffusive effects.17 For δC a typical variation in concentration on the distance h, the Peclet number is given by
| (5) |
A significantly high Peclet number causes a decrease in biosensing efficiency as the analytes do not have enough time to interact with the biosensing surface by diffusion through the analyte-poor layer. On the contrary, the case of a low Peclet number corresponds to the diffusion-limited problem. Therefore, for each Damköhler number, there is a Peclet number optimizing this factor. To illustrate this fact, Fig. 3b shows the calculation of the enhancement factor as a function of the Peclet number for a given Da.
In this paper, we showed that surface loading of typical analytes on a droplet-based biosensor can be highly increased by SAW microstirring. The system permits the enhancement of the biosensing performances by the continuous renewal of the analyte-carrying fluid near the sensing surface. Thanks to mean flow speeds measured up to 1800 μm∕s, the SAW microstreaming can be beneficial to the biosensing of a large range of analyte∕ligand interactions. In addition to the biosensing performance improvement, such a method can be easily integrated in micro-micro-total-analysis systems, which makes it a convenient tool for liquid handling in future biochips.
References
- Shilton R., Tan M. K., Yeo L., and Friend J. R., J. Appl. Phys. 104, 014910 (2008). 10.1063/1.2951467 [DOI] [Google Scholar]
- Sigurdson M., Wang D., and Meinhart C. D., Lab Chip 5, 1366 (2005). 10.1039/b508224b [DOI] [PubMed] [Google Scholar]
- Kim D. and Zheng X., Nano Lett. 8, 3233 (2008). 10.1021/nl801559m [DOI] [PubMed] [Google Scholar]
- Kim J., Junkin M., Kim D., Kwon S., Shin Y., Wong P., and Gale B., Microfluid. Nanofluid. 7, 149 (2009). 10.1007/s10404-009-0431-8 [DOI] [Google Scholar]
- Squires Tm., Messinger Rj., and Manalis Sr., Nat. Biotechnol. 26, 417 (2008). 10.1038/nbt1388 [DOI] [PubMed] [Google Scholar]
- Li H., Friend J., Yeo L., Dasvarma A., and Traianedes K., Biomicrofluidics 3, 034102 (2009). 10.1063/1.3194282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixforth A., Strobl C., Gauer Ch., Toegl A., Scriba J., and Guttenberg Z., Anal. Bioanal. Chem. 379, 982 (2004). 10.1007/s00216-004-2693-z [DOI] [PubMed] [Google Scholar]
- Renaudin A., Sozanski J. P., Verbeke B., Zhang V., Tabourier P., and Druon C., Sens. Actuators B 138, 374 (2009). 10.1016/j.snb.2009.02.031 [DOI] [Google Scholar]
- Kulkarni K., Friend J., Yeoh L., and Perlmutter P., Lab Chip 9, 754 (2009). 10.1039/b819217k [DOI] [PubMed] [Google Scholar]
- Renaudin A., Galopin E., Thomy V., Druon C., and Zoueshtiagh F.,, Phys. Fluids 19, 091111 (2007). 10.1063/1.2775412 [DOI] [Google Scholar]
- Dubois P., Marchand G., Fouillet Y., Berthier J., Douki T., Hassine F., Gmouh S., and Vaultier M., Anal. Chem. 78, 4909 (2006). 10.1021/ac060481q [DOI] [PubMed] [Google Scholar]
- Yeo L. Y., Lastochkin D., Wang S. -C., and Chang H. -C., Phys. Rev. Lett. 92, 133902 (2004). 10.1103/PhysRevLett.92.133902 [DOI] [PubMed] [Google Scholar]
- Raghavan R. V., Friend J. R., and Yeo L. Y., Microfluid. Nanofluid. 8, 73 (2010). 10.1007/s10404-009-0452-3 [DOI] [Google Scholar]
- Galopin E., Beaugeois M., Pinchemel B., Camart J. -C., Bouazaoui M., and Thomy V., Biosens. Bioelectron. 23, 746 (2007). 10.1016/j.bios.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Yeo L. and Friend J. R., Biomicrofluidics 3, 012002 (2009). 10.1063/1.3056040 [DOI] [Google Scholar]
- Shilton R., Tan M. K., and Friend J. R., J. Appl. Phys. 104, 012002 (2009). [Google Scholar]
- Bird R. B., Stewart W. E., and Lightfoot E. N., Transport Phenomena, 2nd ed. (Wiley, New York, 2002). [Google Scholar]