Abstract
Separation of colorectal cancer cells from other biological materials is important for stool-based diagnosis of colorectal cancer. In this paper, we use conventional dielectrophoresis in a microfluidic chip to manipulate and isolate HCT116 colorectal cancer cells. It is noticed that at a particular alternating current frequency band, the HCT116 cells are clearly deflected to a side channel from the main channel after the electric activation of an electrode pair. This motion caused by negative dielectrophoresis can be used to simply and rapidly separate cancer cells from other cells. In this manuscript, we report the chip design, flow conditions, dielectrophoretic spectrum of the cancer cells, and the enrichment factor of the colorectal cancer cells from other cells.
INTRODUCTION
In the United States, colorectal cancer is the second most common cause of cancer death, with approximately 130 000 new cases and 55 000 deaths∕year.1, 2 Often, if the preinvasive form of the disease can be detected early, it can be cured and cancer spread can be prevented. Separation of colorectal cancer cells from other biological materials is very important to improving the accuracy and cost effectiveness of cancer diagnosis. Although there are some cell separation methods, microfluidic-based cell separation could be a new alternative to efficiently isolate colorectal cancer cells with high specificity at low cost.
Compact microfluidic systems that can be used to manipulate and separate biological particles are of wide interest in biodefense and clinical diagnostic applications, and have attracted much attention recently.3, 4, 5 It is a critical component in biochemistry, molecular biology, and synthesis protocols.6 Microfluidic systems are expected to have major impacts on biomedical research, clinical diagnosis,7 point of care, food pathogen screening,8 environmental testing, and other endeavors by providing automated, portable solutions to a wide range of fluid based problems.9, 10, 11
There are several methods to manipulate biological and nonbiological particles in a microfluidic platform. These include, for instance, optophoresis,12 magnetic,13 acoustics,14 and dielectrophoresis (DEP).15 Among them, DEP has emerged as a promising method for a variety of engineering applications involving manipulation of micro- and nanoparticles.16, 17, 18, 19 The advantages it can offer include label-free detection, easy operation, and high specificity. DEP has been used for the separation of prepared mixtures of micro-organisms, mammalian cells,20 and natural biological samples.21 Compared to devices that use other electrokinetic approaches to move particles, such as electrophoresis or electro-osmosis, DEP systems operate using a low alternating current (ac) voltage instead of high direct current (dc) voltage and can easily be combined with electronic detection technologies (e.g., resistive and∕or capacitive sensing) to generate a fully electronic laboratory on a chip.22, 23
DEP is a phenomenon in which a force is exerted on a dielectric particle when it is subjected to a nonuniform ac electric field. DEP force does not require the particle to be charged. This is due to the fact that when an electric field is applied to systems consisting of particles suspended in a liquid, a dipole moment is induced on the particles due to the electrical polarizations at the interface between the particle and the suspending liquid.24, 25 If the field is nonuniform, the particles experience a translational force (DEP force) of magnitude and polarity, depending not only on the electrical properties of the particles and the medium but also on the magnitude and frequency of the applied electric field. The polarizability of living cells depends strongly on their composition, morphology, and phenotype and is also highly dependent on the frequency of the applied electrical field.26, 27 This means that for a given particle type and suspending medium, the particle can experience, at a certain ac frequency applied to the electrodes, a translational force directed toward regions of high electric field strength (this phenomenon is called positive DEP, i.e., pDEP). Alternatively, by simply changing the frequency, they may experience a force that will direct the particle away from the high electric field strength regions (this phenomenon is called negative DEP, i.e., nDEP).22
DEP has been demonstrated for manipulation of biological particles, such as cells,21, 28 bacteria,29, 30 viruses,31, 32, 33, 34 yeast (S. cerevisiae),35, 36 and even breast cancer cells.16, 17 The applicability of this technology to colorectal cancer cell separation has, to our knowledge, never been reported.
In this paper, we report a DEP-based cell sorter capable of continuous cancer cell isolation from other cells. The present device is developed in a microfluidic-based format, which would facilitate ease of integration with current and next-generation laboratory-on-a-chip system37, 38 and biodetection platforms.
THEORY AND METHOD
Figure 1 gives a schematic representation of DEP forces acting on two different kinds of particles in a nonuniform electric field. The red particle is more polarizable than the surrounding medium and is attracted toward the strong field at the center of the electrode, while the blue particle of low polarizability on the right is directed away from the strong field region.
Figure 1.
DEP force acting on two different particles in a wedge with nonuniform electric field. The blue particles experience nDEP and move away from the tip of the electrodes, whereas the red particles experience pDEP and move toward the tip of the electrodes; there the electric field is the highest.
Charges in the particle itself are not necessary for the effect of DEP to occur. This is due to the fact that when an electric field is applied to a system consisting of particles suspended in a liquid, a dipole moment is induced due to the electrical polarizations at the interface between the particle and the suspending liquid.
For a homogeneous sphere of radius a, the time-averaged DEP force in an ac electric field can be expressed as39
| (1) |
where εm is the surrounding media dielectric constant and K is the Clausius–Mossotti factor. is the root mean square value of the electric field. The Clausius–Mossotti factor K can be expressed in terms of complex permittivities,
| (2) |
| (3) |
where ε is the dielectric constant, σ denotes the electrical conductivity, ω is the field frequency, and j is the imaginary number. The subscript p refers to the particles suspended in a medium and m represents the medium.
Indicated by these equations, the DEP force mainly depends on a sphere radius a, its complex dielectric constant , surrounding medium dielectric constant , and the electric field . Based on this, we could predict, when we apply a constant electric field (constant , ω) on a constant media (with a constant ), that the particles with different a or in this medium will experience different DEP forces at the same time. Consequently, selective separation can be achieved by applying an additional force, such as gravity or hydrodynamic force by fluid flow.
Based on the aforementioned concept, we have designed a DEP colorectal cancer cell separation system, as shown in Fig. 2. In this design, there are two electrodes at the bottom surfaces of the microchannel. The electrode pair is in parallel and has a 45° angle to the streamwise direction in the main channel. ac electric signals are applied to the two electrodes having a phase shift of 180°. At one side of the main channel, near the electrode gap, a side channel is placed for carrying the migratory target particles that experience a larger DEP force for separation from the other particles in the main channel. The operational principle is as follows. As the target particles approach the gap between the electrode pair, they experience a strong negative DEP, which repels them against the hydrodynamic force in the direction 135° to the flow direction in the main channel. The hydrodynamic force is obtained from the Stokes equation as40
| (4) |
Figure 2.
Schematic of the prototype design of the DEP sorter. Two electrodes are placed on the bottom surfaces of the microchannel. While the colorectal cells are pushed to the side channel by the sum force of hydrodynamic force and DEP force as they flow to the upstream of the electrodes pair (high electric field area), other bioparticles keep their motion to the end of the main channel due to the hydrodynamic force.
The net force of DEP (fDEP) and hydrodynamic force (fh1) push the target particles (i.e., colorectal cells) to the side channel, while other particles (i.e., bioparticles 2 and 3 here) will keep their motion to the end of the main channel by the hydrodynamic force (fh2 and fh3).
MICROFABRICATION OF THE MICROFLUIDIC SORTER
A brief structure description of the microfluidic DEP sorter is shown in Fig. 3. The separation chip was made using plastic lamination based microfabrication.41 The benefits of using lamination techniques to build complex, three dimensional devices include the use of a variety of inexpensive, high quality, and biocompatible materials that easily accommodate the incorporation of other planar structures.42 Two transparent acrylic plastic substrates of 24×20×1.25 mm3 were used as top (cap) and bottom layers, respectively. One inlet and two outlet wells (one for the main channel and the other for the side channel) are drilled on the top layer. The diameter of all the wells is 1∕16 in. Main and side channels are formed on a middle layer, with the same height of 40 μm but different widths of 1 mm and 120 μm, respectively. A pair of indium tin oxide (ITO) thin film electrodes is placed at the bottom of the channel with a gap of 30 μm. The transparent ITO is 50 μm in thickness and consists of indium (III) oxide (In2O3), tin (IV) oxide (SnO2), typically 90% In2O3, and 10% SnO2 by weight. We use pressure sensitive adhesive to bind these layers together. The chip is optically transparent to ensure the visualization of the cell motion.
Figure 3.
Schematic of layered DEP sorter. Inlet and two outlet wells (one for the main channel and the other for the side channel) are drilled on the top layer. Main and side channels are formed on a middle layer. A pair of ITO thin film electrodes is placed at the bottom of the channel with a gap; these layers are bonded together by pressure sensitive adhesive.
Figure 4 shows the actual microfluidic DEP sorter we have fabricated. The sorter lies beside a standard AA battery on the test bed of a microscope. Tubings are used to connect the inlet of the chip to a syringe pump and the other two outlets to reservoirs, respectively. Two cuprum electrodes have been used to connect the ITO electrodes inside the DEP sorter for applying electric signal on both of them.
Figure 4.
Cancer cell separation chip lies beside a standard AA battery on the test bed of a microscope. Tubing is used to connect the inlet of the chip to a syringe pump and the other two outlets to reservoirs, respectively.
EXPERIMENTAL SETUP
Materials
The human colorectal cancer cell line HTC116 was used for the experiments. For the clinical application, usually it is more challenging to separate the colon cancer cells from normal epithelial cells. It is also important to be able to separate cancer cells from bacteria to purify the target sample. Furthermore, DEP force depends on particle size and its dielectric property, and separation of the same size, but different dielectric properties could often be more challenging. For these reasons, we tested our system by mixing HTC116 colon cancer cells with Human Embryonic Kidney 293 cells (HEK 293) and Escherichia coli (E. coli) bacterium. Both HCT 116 cells and HEK 293 cells have almost the same diameter of about 20 μm. It is difficult to discriminate between the HCT 116 cells and HEK 293 cells under microscope. To be able to distinguish cells with visualization under the microscope, we labeled the kidney cells with dye Hoechst 33342. The E. coli cells are typically rod shaped and are about 2 μm long and 0.5 μm in diameter, and are much smaller than HCT 116 cells. Then these three kinds of cells were mixed in a phosphate-buffered saline (PBS) buffer (P0195-PBS Solution, TEKnova, Inc.) and the medium’s conductivity was 790 μs∕cm.
Setup
A schematic of the setup used for the DEP separation is shown in Fig. 5. The microchip was placed on the test bed of an inverted epifluorescent microscope (Olympus-IX70). A function generator (Tektronix, Model AFG3102) was used to supply ac electrical signal to these two ITO electrodes. A 10× objective lens (numerical aperture of 0.25) was used for the imaging. The light was captured by a high-resolution charge coupled device (CCD) camera (SensiCam-QE, Cooke Corp.) with a 0.6× adapter. Harvard PHD 2000 syringe pump was used to deliver the sample to the chip.
Figure 5.
DEP sorter and experimental setup. Microchip was placed on the test bed of an inverted epifluorescent microscope. A function generator was used to supply ac electrical signal to these two ITO electrodes. A 10× objective lens was used for the imaging. The light was captured by a high-resolution CCD camera. A syringe pump was used to deliver the sample to the chip.
As shown in Fig. 5, there are two ITO electrodes at the bottom of the microchannel. The two electrodes are parallel and the angle between the flow direction in the main channel (horizontal line) and the electrode is 45°. The electric signals applied to the electrodes have a phase shift of 180°. A side channel is placed near the electrode gap for separating and isolating the cancer cells that experience a larger nDEP force. The main and side channels of the chip are designed to have a flow rate ratio of 90%–10%. This ensures that most cells will flow through the main channel and only target cells (HCT 116) will be mostly isolated and collected into the side channel through DEP force.
RESULT AND DISCUSSION
The separation process is described as below. As cancer cells flow along the main channel and approach the gap between the electrodes pair, they experience a strong negative DEP force. On the one hand, the hydrodynamic force drives the cells in the flow direction of the main channel. On the other hand, the nDEP force repels the cell in the direction perpendicular to the electrodes in the gap. The effective net force of the nDEP and hydrodynamic force pushes the responsive cancer cells to the side channel. Without electrode activation, 90% of the cancer cells move downstream of the main channel and the other 10% flow through the side channel due to hydrodynamic splitting. Similarly, without electrode activation, 90% of normal HEK293 cells move downstream of the main channel and the other 10% flow through the side channel. However, when the electrodes are electrically activated, the cancer cells will be pushed only to the side channel, whereas the other cells keep their motion in the main channel. Thus, isolation of the cancer cells from other cells can be realized.
DEP spectrum
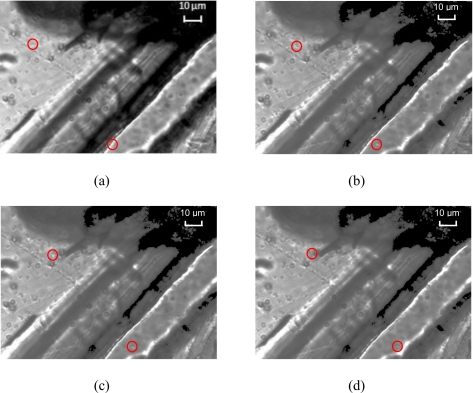
Before the isolation of the cancer cells from others in the DEP sorter, we need to know whether the colorectal cancer cell has a negative DEP. To answer this question, we first measured the DEP spectrum using a wedge microfluidic chip, as shown in Fig. 6.
Figure 6.
Motion of the colorectal cancer cells under nDEP force in a microfluidic chip with electrodes in wedge configuration for DEP spectrum measurement. (a) Two cells located near the electrodes tips are marked by red circles; [(b)–(d)] driven by the nDEP force, the two cells start moving away from the tips at different positions.
Since DEP spectrum is independent of the geometry of the electrodes and flow condition, a wedge chip was designed to measure the spectrum of the HCT 116 cells. The wedge was formed by two gold electrodes with thickness of 230 μm. The distance between their edges is 40 μm. With the sharp edges of the two gold electrodes, we can achieve a nonuniform electric field required for the DEP. The HCT 116 cell solution will first be loaded into the wedge chip. The solution should be exactly the same as the one that will be used in the DEP sorter for cell separation. Since the solution in this chip is almost at rest, the effect of hydrodynamic force on the cells due to the pressure driven flow is neglected. Hence, DEP force could become the major force on these cells, and could be easily observed and characterized, if it exists. For nDEP, the cells will move away from the tips, whereas for the pDEP, the cells will move toward the tips. The faster the motion, the stronger the DEP force. With this wedge DEP test microfluidic chip, a very small amount of sample (about 5 μl) is sufficient for measuring the DEP spectrum.
Figure 6 shows the actual motion of the cells under the nDEP force, in the wedge microfluidic chip after electric activation of the electrodes. The time interval between consequence images, the ac frequency, and voltage are 1 s, 100 kHz, and 5 Vp.-p., respectively. It shows clearly that when two sinusoidal electric signals with a phase shift of 180° are applied to the electrodes, the cells begin moving away from the two sharp tips, where the electric field intensity is the strongest. This means that these cells experience negative DEP force, which leads them moving from the high electric field area to the low electric field region. The faster the motion, the stronger the nDEP force. Thus the DEP force can be estimated by measuring the cell flow velocity and the DEP spectrum can be obtained by measuring the velocity of the cell motion in the initial stage after the electric activation at various frequencies. Figure 6 shows that cells were moving for relatively a long distance in a short period, i.e., 3 s in this case. In the light of the measured DEP spectrum, we could select the frequency to achieve either the nDEP or pDEP force conveniently for the cell separation experiment. The HCT 116 cells’ DEP spectrum is displayed in Fig. 7 for the given medium. Figure 7 shows that in the frequency band of 1 Hz–6MHz and 31–75 MHz, respectively, the cancer cells experience a nDEP force, whereas in the frequency range of 6–31 MHz, the cells display a pDEP force. The motion of the cancer cells is sensitive to the ac frequency. The cancer cells experience the approximately maximum nDEP at the frequency of 100 kHz, which was then selected for the DEP sorter to isolate the cancer cell.
Figure 7.
DEP spectrum of the HCT116 cells. The cancer cells experience a nDEP force in the frequency band of 1 Hz–6 MHz and 31–75 MHz, respectively, whereas in the frequency range of 6–31 MHz they display a pDEP force.
Visualization of the DEP separation
Figure 8 shows a visualization of the separation of HCT116 from the HEK 293 and E. coli cells due to DEP force in the microfluidic chip, with and without the electrical activation. The fluid flows from the inlet at the left side to the outlet at the right side in the main channel. In Fig. 8, the flow rate is 0.1 μl∕min. In Fig. 8a, approximately 90% of the colorectal cells flow through the gap between the two electrodes in the main channel without ac electrode activation. However, with the electric activation of the electrodes at voltage of 16 Vp.-p. and frequency of 100 kHz, almost all colorectal cells migrate to the side channel under the nDEP force, as shown in Fig. 8b, whereas the HEK 293 cells and E. coli remain in the same motion through the gap as if there was no ac activation. Figure 8 demonstrates that DEP has high specificity for cell separation and the HCT116 cancer cells can be separated from the HEK 293 and E. coli cells. Thus, DEP can provide an opportunity for cancer cell isolation from other cells.
Figure 8.
Experimental demonstration of the DEP deflection. (a) Without ac activation, most cells (colorectal cells, kidney cells and E. coli) flow through the electrode gap along the main channel. (b) With ac activation, almost all colorectal cells were repelled to the side channel due to negative DEP force, whereas the HEK 293 cells and E. coli retain the same motion through the gap as if there was no ac activation. The dark spheres are the HCT 116 cells and the white spheres are the HEK 293 cells. E. coli cells are too small to be seen in these images.
Due to their extremely small size compared to HCT 116 cells and HEK 293 cells, the E. coli cells cannot be seen in Fig. 8. Therefore, high magnification lens are needed to make the observation of the E. coli cell’s behavior in the separation process unambiguous. The E. coli cells can be observed under 24× magnification, as shown in Fig. 9, where the exposure time is 0.3 s and the time step between the two sequences is 1.5 s.
Figure 9.
Experimental demonstration of the negligible DEP force on E. coli (no deflection) under 24× magnification. This is a zoom-in view for the field marked in Fig. 8a.
As shown in Fig. 9, the small E. coli cells flow along with the labeled HEK 293 cells. After the electric activation, the bacteria keep moving along the flow direction in the main channel with HEK 293 cells. This indicates that there is either no nDEP force or the DEP force is too small to deflect the E. coli to the side channel. This result matches our prediction that E. coli has negligible DEP force compared to cancer cells for separation that is based on cell size since according to Eq. 1, DEP force is proportional to cube of the particle’s radius and E. coli is much smaller than the cancer cell. This result also shows that with large differentiation in particle or cell’s radius a, size based DEP separation could also be leveraged for separation. This is one of the traditional methods for DEP selectivity.
Enrichment factor
Flow rate effect
To quantify the presented DEP sorter performance for the cancer cell separation, we measured the cell enrichment factor, which is defined as
| (5) |
where A is the total cells blocked right before the electrode gap by DEP force, B denotes the total collected cells that flow into the side channel, and C is the total cells flowed through the gap in the main channel. The enrichment factor is calculated based on movie obtained from the visualization. The measured enrichment factor as a function of the sample flow rate at constant voltage of 16 Vp.-p. is shown in Fig. 10.
Figure 10.
Enrichment factor as a function of the sample flow rate.
It is noted that the enrichment factor decreases with the flow rate since the hydrodynamic force experienced by the cancer cells increases with the increased flow rate. The total force that the cells experience is the sum of the vector forces of DEP and hydrodynamics. The hydrodynamic force mainly pushes the cells to flow along the main channel. At high flow rate, due to the large hydrodynamic force, it is very difficult to deflect the cells to the side channel. Thus, hydrodynamic and DEP forces are competitive. The higher the flow rate, the lower the enrichment factor. When the flow rate is less than 0.1 μl∕min, the enrichment factor is 98%, indicating that the DEP force is much larger than the hydrodynamic forces. However, when the flow rate increases to 0.2 μl∕min, the hydrodynamic force overwhelms DEP with resultant minimal deflection of cancer cells.
Voltage effect
The effect of applied voltage on the DEP separation is shown in Fig. 11, which indicates that the enrichment factor increases with increased electric field (or equivalently voltage). The flow rate in Fig. 11 was maintained constant at 0.1 μl∕min.
Figure 11.
Enrichment factor as a function of applied voltage.
As we can see from Eq. 1, DEP force increases with the increased gradient of the square of the electric field (or equivalently voltage). Consequently, enrichment factor is rapidly increased with the increase in the voltage within the test range of the voltage. Figure 11 indicates that increasing the voltage could be an easy way to enhance the DEP enrichment factor.
Purification
One of the objectives of the DEP separation is to purify the cancer cells for sample preparation in clinical diagnostics. To quantify the DEP purification of the colon cancer cells, we estimated the concentration of the colon cancer cell on the side channel with and without the electrical activation. To simplify the calculation, we only focus on the HCT116 and HEK 293 cells since E. coli is too small to see clearly. The result is given in Fig. 12, where the concentration of the HCT 116 cells is 57% before the electric activation. However, with the electric activation on the DEP sorter, the concentration of HCT 116 cell was increased to 95% in the side channel. Another way to quantify the purification is the ratio of cell number of HCT116 cell to that of HEK 293 cell. As shown in Fig. 12, the ratios in the side channel with and without the electric activation are 17.4 and 1.3, respectively, i.e., approximately increase in 13 folds. With such a simple device, Fig. 12 demonstrates the capability of the DEP sorter to separate and purify the cancer cells.
Figure 12.
Quantitative comparison of HCT116 cell purification at the entrance of the side channel. (a) HCT 116 cell concentration without electric field, (b) HCT 116 cell concentration with electric field, (c) cell number ratio of HCT 116/HEK 293 without electric field, and (d) cell number ratio of HCT 116/HEK 293 with electric field.
In this work, DEP principle is applied to the isolate the HCT116 colorectal cancer cell from HEK293 kidney cell and E. coli bacteria based on cell dielectric property, e.g., permittivity, conductivity, and size. A given cell may have a specific dielectric property that is different from that of other cells. Such a difference may be leveraged for DEP separation. Since the structure is very simple, it should be easy to be integrated and applied to other system as well, as long as the electrodes can be built in the channel. However, in clinical application, the separation of the colorectal cancer cells from other cancer cells or epithelial cells could be required and could be more difficult; as shown in Fig. 10, the enrichment factor decreases with an increase in flow rate; furthermore, in practice, often high throughput is required, which in turn, demands high flow rate. Fortunately, as shown in Fig. 11, the enrichment factor increases rapidly with the voltage. Therefore, both multiple parallel channels and higher voltage could be used in high throughput to ensure high enrichment factor so that sufficient amount of interested cells can be isolated and collected for assays downstream. Another method is to add another electrode pair on the top side of the channel, opposite to the electrode pair on the bottom side. In this case, the electric field could be higher to increase the enrichment factor.
CONCLUSION
We have designed and fabricated a microfluidic cell sorter based on conventional DEP and successfully applied it to manipulate and isolate the colorectal cancer HCT116 cells from the mixture with human embryonic kidney 293 (HEK 293) and E. coli cells. The sorter is microfabricated using plastic lamination technology. DEP spectrum of the colorectal cancer cell has been measured. The effect of parameters such as flow rate and applied voltage has been investigated as well. The present work indicates that colorectal cancer cells can be separated and isolated from other cells by DEP. Therefore, DEP could provide a new opportunity for clinical cancer diagnosis.
ACKNOWLEDGMENTS
This work has been financially supported by the NSF RII funding (Grant No. EPS-0447660). Help from W. J. Zhang and Xiaoming He for labeling the cell is appreciated.
References
- Nakamura R. M., Grody W. W., Wu J. T., and Nagle R. B., Cancer Diagnostics Current and Future Trends (Humana, Totowa, NJ, 2004). [Google Scholar]
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., and Thun M. J., Ca-Cancer J. Clin. 58, 71 (2008). [DOI] [PubMed] [Google Scholar]
- Cheng I. F., Chang H. -C., Hou D., and Chang H. -C., Biomicrofluidics 1, 021503 (2007). 10.1063/1.2723669 [DOI] [Google Scholar]
- Fergenson D. P., Pitesky M. E., Tobias H. J., Steele P. T., Czerwieniec G. A., Russell S. C., Lebrilla C. B., Horn J. M., Coffee K. R., Srivastava A., Pillai S. P., Shih M. -T. P., Hall H. L., Ramponi A. J., Chang J. T., Langlois R. G., Estacio P. L., Hadley R. T., Frank M., and Gard E. E., Anal. Chem. 76, 373 (2004). 10.1021/ac034467e [DOI] [PubMed] [Google Scholar]
- Chang H. -C. and Yossifon G., Biomicrofluidics 3, 012001 (2009). 10.1063/1.3056045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. E., Gagnon Z., and Chang H. -C., Biomicrofluidics 1, 044102 (2007). 10.1063/1.2818767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S., Mahon A. R., Gordon J., Nowak C., Sengupta S., Powell T. H. Q., Feder J., Lodge D. M., and Chang H. -C., Biomicrofluidics 3, 022407 (2009). 10.1063/1.3127142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmel S., Guilbault G. G., and O'Sullivan C. K., Biosens. Bioelectron. 18, 881 (2003). 10.1016/S0956-5663(02)00214-2 [DOI] [PubMed] [Google Scholar]
- Figeys D. Proteomics 2, 373 (2002). [DOI] [PubMed] [Google Scholar]
- Sato K., Tokeshi M., Kimura H., and Kitamori T., Anal. Chem. 73, 1213 (2001). 10.1021/ac000991z [DOI] [PubMed] [Google Scholar]
- Easley C. J., Karlinsey J. M., Bienvenue J. M., Legendre L. A., Roper M. G., Feldman S. H., Hughes M. A., Hewlett E. L., Merkel T. J., Ferrance J. P., and Landers J. P., Proc. Natl. Acad. Sci. U.S.A. 103, 19272 (2006). 10.1073/pnas.0604663103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchin V. V., IEEE J. Sel. Top. Quantum Electron. 13, 1621 (2007). 10.1109/JSTQE.2007.911313 [DOI] [Google Scholar]
- McCloskey K. E., Chalmers J. J., and Zborowski M., Anal. Chem. 75, 6868 (2003). 10.1021/ac034315j [DOI] [PubMed] [Google Scholar]
- Ryll T., Dutina G., Reyes A., Gunson J., Krummen L., and Etcheverry T., Biotechnol. Bioeng. 69, 440 (2000). [DOI] [PubMed] [Google Scholar]
- Doh I. and Cho Y. -H., Sens. Actuators, A 121, 59 (2005). 10.1016/j.sna.2005.01.030 [DOI] [Google Scholar]
- Huang Y., Yang J., Wang X. -B., Becker F. F., and Gascoyne P. R. C., Journal of Hematotherapy & Stem Cell Research 8, 481 (1999). 10.1089/152581699319939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker F. F., Wang X. B., Huang Y., Pethig R., Vykoukal J., and Gascoyne P. R., Proc. Natl. Acad. Sci. U.S.A. 92, 860 (1995). 10.1073/pnas.92.3.860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Huang Y., Wang X. -B., Becker F. F., and Gascoyne P. R. C., Anal. Chem. 71, 911 (1999). 10.1021/ac981250p [DOI] [PubMed] [Google Scholar]
- Wang J., Pumera M., Chatrathi M. P., Escarpa A., Konrad R., Griebel A., Doner W., and Loe H., Electrophoresis 23, 596 (2002). [DOI] [PubMed] [Google Scholar]
- Gascoyne P. R. C., Huang Y., Pethig R., Vykoukal J., and Becker F. F., Meas. Sci. Technol. 3, 439 (1992). 10.1088/0957-0233/3/5/001 [DOI] [Google Scholar]
- Talary M., Mills K., Hoy T., Burnett A., and Pethig R., Med. Biol. Eng. Comput. 33, 235 (1995). 10.1007/BF02523050 [DOI] [PubMed] [Google Scholar]
- Gawad S., Schild L., and Renaud P. H., Lab Chip 1, 76 (2001). 10.1039/b103933b [DOI] [PubMed] [Google Scholar]
- Ferguson J. A., Boles T. C., Adams C. P., and Walt D. R., Nat. Biotechnol. 14, 1681 (1996). 10.1038/nbt1296-1681 [DOI] [PubMed] [Google Scholar]
- Fiedler S., Shirley S. G., Schnelle T., and Fuhr G., Anal. Chem. 70, 1909 (1998). 10.1021/ac971063b [DOI] [PubMed] [Google Scholar]
- Jones T. B., Electromechanics of Particles (Cambridge University Press, Cambridge, 1995). [Google Scholar]
- Pethig R. and Kell D. B., Phys. Med. Biol. 32, 933 (1987). 10.1088/0031-9155/32/8/001 [DOI] [PubMed] [Google Scholar]
- Sukhorukov V., Arnold W., and Zimmermann U., J. Membr. Biol. 132, 27 (1993). 10.1007/BF00233049 [DOI] [PubMed] [Google Scholar]
- Kua C. H., Lam Y. C., Rodriguez I., Yang C., and Youcef-Toumi K., Anal. Chem. 80, 5454 (2008). 10.1021/ac800947e [DOI] [PubMed] [Google Scholar]
- Pethig R. and Markx G. H., Trends Biotechnol. 15, 426 (1997). 10.1016/S0167-7799(97)01096-2 [DOI] [PubMed] [Google Scholar]
- Markx G. H., Dyda P. A., and Pethig R., J. Biotechnol. 51, 175 (1996). 10.1016/0168-1656(96)01617-3 [DOI] [PubMed] [Google Scholar]
- Hughes M. P., Electrophoresis 23, 2569 (2002). [DOI] [PubMed] [Google Scholar]
- Schnelle T., Müller T., Gradl G., Shirley S. G., and Fuhr G., Electrophoresis 21, 66 (2000). [DOI] [PubMed] [Google Scholar]
- Schnelle T., Müler T., Hagedorn R., Voigt A., and Fuhr G., Biochim. Biophys. Acta 1428, 99 (1999). [DOI] [PubMed] [Google Scholar]
- Hughes M. P., Morgan H., Rixon F. J., Burt J. P. H., and Pethig R., Biochim. Biophys. Acta 1425, 119 (1998). [DOI] [PubMed] [Google Scholar]
- Crane J. S. and Pohl H. A., J. Electrochem. Soc. 115, 584 (1968). 10.1149/1.2411345 [DOI] [Google Scholar]
- Kadaksham J., Singh P., and Aubry N., Electrophoresis 26, 3738 (2005). 10.1002/elps.200500133 [DOI] [PubMed] [Google Scholar]
- Medoro G., Manaresi N., Leonardi A., Altomare L., Tartagni M., and Guerrieri R., IEEE Sens. J 3, 317 (2003). 10.1109/JSEN.2003.814648 [DOI] [PubMed] [Google Scholar]
- Manaresi N., Romani A., Medoro G., Altomare L., Leonardi A., Tartagni M., and Guerrieri R., IEEE J. Solid-State Circuits 38, 2297 (2003). 10.1109/JSSC.2003.819171 [DOI] [Google Scholar]
- Ramos A., Morgan H., Green N. G., and Castellanos A., J. Phys. D: Appl. Phys. 31, 2338 (1998). 10.1088/0022-3727/31/18/021 [DOI] [Google Scholar]
- Deen W. M., Analysis of Transport Phenomena (Oxford University Press, New York, 1998). [Google Scholar]
- Yang R., Feeback D. L., and Wang W., Sens. Actuators, A 118, 259 (2005). 10.1016/j.sna.2004.09.001 [DOI] [Google Scholar]
- Levine T. C. L. M., “Combining Additive and Subtractive Techniques in the Design and Fabrication of Microfluidic Devices,” presented at the 2007 NSTI Nanotechnology Conference and Trade Show, 3 385 (2007).