Abstract
A long-standing concept in vision science has held that a single photoreceptor expresses a single type of opsin, the protein component of visual pigment. However, the number of examples in the literature of photoreceptors from vertebrates and invertebrates that break this rule is increasing. Here, we describe a newly discovered Limulus opsin, Limulus opsin5, which is significantly different from previously characterized Limulus opsins, opsins1 and 2. We show that opsin5 is co-expressed with opsins1 and 2 in Limulus lateral and ventral eye photoreceptors and provide the first evidence that the expression of co-expressed opsins can be differentially regulated. We show that the relative levels of opsin5 and opsin1 and 2 in the rhabdom change with a diurnal rhythm and that their relative levels are also influenced by the animal's central circadian clock. An analysis of the sequence of opsin5 suggests it is sensitive to visible light (400–700 nm) but that its spectral properties may be different from that of opsins1 and 2. Changes in the relative levels of these opsins may underlie some of the dramatic day–night changes in Limulus photoreceptor function and may produce a diurnal change in their spectral sensitivity.
Keywords: opsin, opsin co-expression, Limulus, photoreceptor, circadian rhythm
INTRODUCTION
A long-standing concept in vision science has held that a single photoreceptor expresses a single type of opsin, the protein component of visual pigment. However, the number of examples in the literature of photoreceptors from vertebrates and invertebrates that break this rule is increasing. For example, cones of rodent retinas and the photoreceptors of the lizard parietal eye each express two opsins with different spectral sensitivities (Röhlich et al., 1994; Applebury et al., 2000; Lukats et al., 2002; Lukats et al., 2005; Su et al., 2006), and chick photoreceptors express melanopsin in addition to their rod and cone opsins (Baily and Cassone, 2005). Direct and strong suggestive evidence for opsin co-expression in invertebrate rhabdomeral photoreceptors comes from studies of all three major groups of arthropods: insects (Kitamoto et al., 1998; Gao et al., 2000; Mazzoni et al., 2008), crustaceans (Sakamoto et al., 1996; Frank et al., 2009; Porter et al., 2009) and chelicerates (DeVoe, 1972).
The functional significance of opsin co-expression is clear in only a few instances. If the co-expressed opsins have different spectral sensitivities, the spectral sensitivity of the photoreceptor is thought to be broadened. This has been demonstrated for some photoreceptors in the butterfly Papilio xuthus (Arikawa et al., 2003). In the case of the lizard parietal eye, the co-expressed opsins are coupled to different transduction cascades, such that activation of the short wavelength sensitive photopigment produces a photoreceptor hyperpolarization while activation of the medium wavelength sensitive pigment produces a depolarization (Su et al., 2006). But in most instances, the functional significance of opsin co-expression is not yet understood, especially when the opsins are thought to have similar spectral properties, as in the photoreceptors of the crab Hemigrapsus sanguineus (Sakamoto et al., 1996).
Limulus polyphemus Linnaeus 1758, a chelicerate arthropod known for the dramatic structural and functional changes that occur in its lateral compound eyes in response to diurnal light and signals from a central circadian clock (reviewed in Battelle, 2002), expresses at least two very similar visible-light-sensitive opsins in its photoreceptors, opsin1 (Ops1) and opsin2 (Ops2) (Smith et al., 1993). These opsins differ from one another at only four amino acid residues not known to alter opsin spectral tuning; therefore, their spectral sensitivities are probably identical. Although our original studies using northern blots suggested that Ops1 and Ops2 were differentially expressed in the lateral eye (LE) and median eye (ME), respectively (Smith et al., 1993), subsequent ribonuclease protection assays revealed the presence of Ops1 and Ops2 transcripts in both LEs and ventral eyes (VEs) (Dalal et al., 2003). The similarities between the Ops1 and Ops2 transcripts and the resulting proteins have prevented us from determining conclusively whether both are expressed in the same photoreceptors.
By sequencing Limulus genomic DNA, we discovered two additional Limulus opsin genes (Ops3 and Ops4) that are identical to Ops1 in their coding regions but different in the lengths and sequences of their introns (Dalal et al., 2003). Thus, Limulus contains at least four genes encoding identical or nearly identical opsins. Limulus opsin genes that encode identical or nearly identical proteins are probably the result of recent gene duplications, as has been suggested for other arthropods (Kashiyama et al., 2009; Sakamoto et al., 1996; Oakley and Huber, 2004) (wFleaBase, http://wfleabase.org), but their functional relevance is not yet clear. For example, it is not yet clear whether all Limulus Ops1-like genes are expressed.
In the present study, we describe a newly discovered Limulus opsin, Ops5, with a predicted amino acid sequence that is substantially different from those of Ops1 and Ops2. Ops5, like Ops1 and Ops2, is predicted to form a functional, visible-light-sensitive opsin; however, Ops5 and Ops1 and 2 cluster in different phylogenetic clades, indicating that their spectral sensitivities may be different. Using specific antibodies, we show here that Ops5 is co-expressed with Ops1 and/or 2 in LE and VE photoreceptors and that the concentrations of these co-expressed opsins in the rhabdom are regulated differently by diurnal light and the animal's central circadian clock such that their relative levels at the rhabdom change from day to night. Finally, we show that the abundance of Ops5 in LEs and VEs, relative to Ops1 and 2, is sufficiently high to contribute significantly in the animal's photoresponse. Changes in the relative levels of Ops5 and Ops1 and 2 in the rhabdom may underlie some of the dramatic diurnal and circadian changes observed in the functions of Limulus photoreceptors and may produce a diurnal change in the spectral properties of the eye.
MATERIALS AND METHODS
Animals
Adult animals were collected from the Indian River near Melbourne, FL, USA and housed at the Whitney Laboratory in natural, continuously flowing seawater maintained at temperatures between 18°C and 20°C and a depth of about 24 cm. The aquarium room is equipped with a skylight so animals were exposed to only natural illumination.
In some experiments, we examined the effects of the circadian clock on relative levels of Ops5 and Ops1 and 2 at the rhabdomeres of LE photoreceptors. Photoreceptor cells and other cells in LEs receive synaptic input from the animal's central circadian clock via a bilateral group of clock-driven central neurons that project to the LEs through the lateral optic nerves (Barlow et al., 1977; Calman and Battelle, 1991). Therefore, a LE can be deprived of this input by cutting the lateral optic nerve. In the present study, the lateral optic nerve projecting to one LE of a group of animals was severed as described previously (Battelle et al., 2000b) at least 10 days before the experiment while the lateral optic nerve to the other LE was left intact.
Reagents
Unless otherwise specified, reagents were from Sigma-Aldrich (St Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA).
Cloning Limulus opsin5
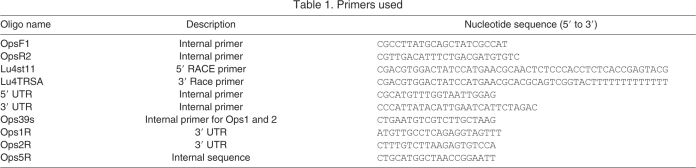
A 701-bp opsin-like fragment with a sequence substantially different from that of Limulus Ops1 and Ops2 (Smith et al., 1993) was first identified in an expressed sequence tag (EST) collection prepared as described previously (Matz, 2003) from the entire central nervous system (CNS) of juvenile Limulus. Forward (OpsF1) and reverse (OpsR2) primers (Table 1) based on this initial sequence were used to screen various cDNA libraries and other EST collections from Limulus. An anticipated 250-bp product with a sequence identical to the new opsin was obtained from a LE cDNA library (Smith et al., 1993), VE and adult brain cDNA libraries (Chen et al., 1999) and VE and adult CNS EST collections. Using the VE EST collection as template, the 250-bp sequence was extended with a RACE (rapid amplification of cDNA ends) strategy, using the 5′ and 3′ adaptor primers Lu4st11 and Lu4 TRSA (Matz, 2003) (Table 1). The entire open reading frame of this new opsin, opsin5 (Ops5), was then amplified from the VE EST collection using primers specific for sequences within the 5′and 3′untranslated regions (Table 1). The resulting 1417-bp piece included the start and stop codons. Three separate full-length clones were sequenced in the forward and reverse directions to obtain a consensus sequence. All PCR and RACE reactions were performed using the LATaq polymerase system (Takara, Madison, WI, USA) and Eppendorf Mastercycler (Hauppague, NY, USA).
Table 1.
Primers used
Phylogenetic analysis
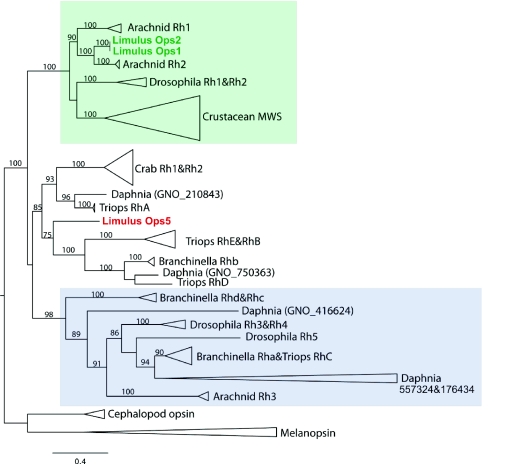
The Limulus Ops1 (GenBank Accession No. L03781), Ops2 (L03782) and Ops5 (FJ791252) amino acid sequences were combined with representative arthropod opsin sequences from wFleaBase and GenBank (see Fig. 2 legend for accession numbers). Amino acid sequences were aligned using MAFFT v.6.717 (Katoh et al., 2002; Katoh and Toh, 2008), resulting in a final alignment of 364 positions. To root the tree, a selection of cephalopod opsin (X07797, AF000947) and melanopsin (NM_013887) sequences was used. The best-fit model of protein model evolution (LG+I+G+F) was determined using ProtTest v.2.4 (Abascal et al., 2005), and an amino acid maximum likelihood tree was reconstructed using PhyML (Guindon and Gascuel, 2003; Guindon et al., 2005). Branch support values were estimated from 100 PhyML bootstrap replicates as bootstrap proportions (BPs). BP values greater than or equal to 70% were considered strong support for a clade (Hillis and Bull, 1993).
Fig. 2.
Phylogenetic tree of arthropod opsins. The tree was constructed using a maximum likelihood analysis of amino acid sequences. Numbers on branches represent bootstrap proportions from 100 replicates; only bootstrap values above 70% are indicated. For clarity, major clades have been collapsed. The sequence of interest, Limulus polyphemus opsin5, is in red. Medium-long wavelength sensitive opsins are highlighted in green; UV-short wavelength sensitive opsins are highlighted in blue. Sequences represented on the tree are as follows: Arachnid Rh1 – AB251846, AB251849; Limulus polyphemus opsins1 and 2 – L03781, L03782; Arachnid Rh2 – AB251847, AB251850; Drosophila Rh1 & Rh2 – M12896, K02315; Crustacean MWS – GQ221739, DQ852590, DQ852586, GQ221732, DQ646869, S53494; Crab Rh1 & Rh2 – GQ228846, GQ228847, D50583, D50584, EF110527; Daphnia pulex (sequences from wFleaBase) – GNO_210843, GNO_750363, GNO_416624, GNO_557324, GNO_176434, GNO_366144; Triops RhA – AB293433, AB293428; Triops Rhe & RhB – AB293434, AB293429, AB293432; Branchinella Rhb – AB298794, AB298792; Triops RhD – AB293431; Limulus polyphemus opsin5 – FJ791252; Branchinella Rhd & Rhc – AB293438, AB293437; Drosophila Rh3 & Rh4 – M17718, NM_057353; Drosophila Rh5 – DMU80667; Branchinella Rha – AB293436; Triops RhC – AB293435, AB293430; Arachnid Rh3 – AB251851, AB251848; cephalopod opsin (outgroups) – X07797, AF000947; melanopsin (outgroups) – NM_013887.
Assaying for Ops5 transcripts in median eye
RNA was isolated (RNAeasy; Qiagen, Valencia, CA, USA) from MEs freshly dissected from light-adapted animals and reverse transcribed (Superscript III; Invitrogen, Carlsbad, CA, USA). Using PCR, we probed the resulting cDNA for the Ops5 transcript with primers OpsF1 and Ops5R, the Ops2 transcript with primers Ops39s and Ops2R, and the Ops1 transcript with primers Ops39s and Ops1R (Table 1). As a positive control, the same primers were used to probe for Ops5, Ops1 and Ops2 in reverse-transcribed RNA prepared from the LEs of light-adapted animals.
Antibody production
cDNA encoding the C-terminus of Ops5 (K340→N392) was subcloned into pET 28a (Novagen, EMD Chemicals, Gibbstown, NJ, USA) at the HindIII and NdeI restriction sites. The sequence of the cloned Ops5 cDNA fragment was verified by sequencing the entire insert in both directions; then the pET 28a plasmid containing the Ops5 cDNA fragment was transformed into Escherichia coli (Rosetta; Novagen, EMD Chemicals). Expression produced a partially insoluble polypeptide that was extracted in 6 mol l–1 urea, enriched by standard Ni+ chelation choromatography (His-Bind™ resin; Novagen, EMD Chemicals) in urea and dialyzed against phosphate-buffered saline (PBS). A battery of hybridoma cell lines producing monoclonal antibodies specific for Ops5 was prepared by fusing SP2/0 mouse myeloma cells with splenocytes from an immunized SJL mouse (Adamus et al., 1988; Adamus et al., 1991). All experiments described in this study used monoclonal antibody #31 directed against Ops5 (mAbOps5) and a polyclonal antibody directed against Ops1 (pAbOps1) (Battelle et al., 2001).
Tissue homogenization and preparation of membrane for western blots
Membranes from LEs and ventral photoreceptors were prepared as described previously (Battelle et al., 2001). LEs from daytime, light-adapted animals were homogenized in ambient room light; LEs from night-time dark-adapted animals were homogenized under infrared illumination.
Western blotting and immunostaining western blots
Proteins solubilized in SDS sample buffer (Laemmli, 1970) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) through 12.5 or 15% gels, transferred onto nitrocellulose (Nitro ME; Micron Separations, Westborough, MA, USA) or PVDF (Immobilon P; Millipore, Bedford, MA, USA) membranes with standard protocols and fixed using Fast Green. The membranes were then rinsed, blocked with 3% BSA and incubated with the primary antibody followed by an appropriate horseradish peroxidase-bound secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:30,000 or 1:50,000. The secondary antibody was visualized using a chemiluminescence detection kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The concentrations of the primary antibodies used are indicated in the figure legends.
Absorbing mAbOps5 with antigen
A 1:375 dilution of mAbOps5 was incubated with about 100 μg of antigen bound to nitrocellulose as described previously (Battelle et al., 2001). A second aliquot of the same diluted antibody was incubated with a similarly sized strip of blocked nitrocellulose to which no Ops5 antigen had been bound. Absorbed and control mAbOps5 were diluted to 1:500 and applied to western blots of equal aliquots of SDS solubilized membranes from LE and VE or diluted 1:1000 and applied to frozen sections of LE.
Tissue fixation and immunostaining
LEs were dissected from the animal, fixed in ice-cold methanolic formaldehyde for 6–16 h (Battelle et al., 2001), then rehydrated in a graded series of methanol in water. Eyes from light-adapted animals were fixed in the light. If eyes were to be dissected from animals during the night in the dark, animals were transferred to an aquarium in an experimental dark room about an hour before sunset, and all subsequent manipulations of night-time animals and eyes were performed under infrared illumination.
Frozen sections of LEs were prepared and immunostained as described previously (Battelle et al., 2001). Ventral photoreceptors were dissected from animals in a block of tissue that included the brain and ventral eye end organ. This tissue was fixed and rehydrated as described for the LE. After rehydration, the brain was cut away from the rest of the tissue, and the ventral eye end-organ and distal segments of ventral optic nerves were dissected away from the ventral cuticle. To obtain ME retinas for immunocytochemistry, a piece of carapace to which the ME retinas were attached was cut from the animal; then the retinas were treated in one of three ways: (1) fixed immediately while attached to the carapace and dissected from the carapace after rehydration; (2) dissected from the carapace in the light and then fixed immediately; or (3) dissected from the carapace and incubated in an organ culture medium (OCM) for 60 h at 15°C in the dark and fixed in the dark. The OCM was modified from that described previously (Bayer and Barlow, 1978). It contained Medium 199, 10% heat-inactivated horse serum, 25 mmol l–1 d-glucose and the following final concentrations of salts and buffers (in mmol l–1): NaCl, 470; KCl, 12.6; CaCl2, 10; MgSO4, 27.9; NaCO3, 1.3; Hepes, 0.58; TES, 0.66. Its osmolarity was 1050 mOsm l–1, which matches the osmolarity of the seawater in our tanks.
Image collection and analysis
Immunostained frozen sections were viewed with a confocal microscope (Leica SP2; Leica Microsystems, Mannheim, Germany). Double-labeled fluorescent images were acquired with sequential scans to avoid bleed-through between collecting channels. Samples to be compared were analyzed during a single session with identical magnification, laser power and gain settings. Gain settings were established by prescreening sections to assure that all signals would be below saturation and then rigorously held constant.
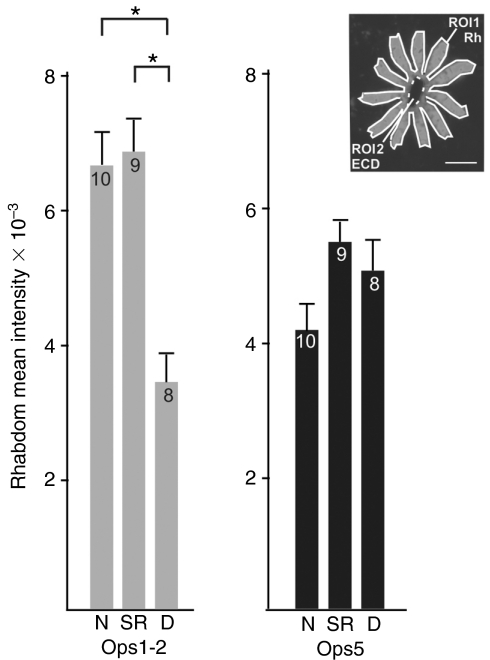
The average intensity of Ops-immunoreactivity (ir) associated with the rhabdom was determined as follows using Leica LCS software. With the polygon tool, the perimeter of the rhabdomeres, as defined by Ops1-ir, in each ommatidium analyzed was outlined to create a region of interest (ROI1). The area at the center of the ommatidium, which is occupied by the eccentric cell dendrite, was also outlined to create a second region of interest (ROI2). Then the total intensity of ROI1 minus the total intensity of ROI2 was divided by the total area of ROI1 minus the total area of ROI2. Typically, eight separate ommatidia from an individual eye were analyzed to determine the average intensity per μm2 of rhabdomeral Ops-ir for that eye (see Fig. 9 for an example).
Fig. 9.
Quantification of rhabdomeral Ops1-2-immunoreactivity (Ops1-2-ir) and Ops5-ir in lateral eye (LE) photoreceptors fixed under natural illumination during the night (N), between 18 and 20 h after sunrise, at sunrise (SR), and during the day (D), between 9 and 10 h after sunrise. The mean intensity of rhabdomeral Ops-ir in an ommatidium was determined by measuring the total intensity within ROI1 minus ROI2 divided by the area of ROI1 minus ROI2 (see insert). The mean intensity for each eye was determined by averaging data from at least eight separate ommatidia. Data are pooled from three separate experiments done during April, July and August in which LEs from 2–4 animals were analyzed in each experiment. Data are expressed as the mean intensity of rhabdomeral Ops-ir × 10–3 ± s.e.m. for the number of animals indicated in the columns. The significance of differences among time points was tested using a one-way ANOVA followed by a t-test. Significant differences are indicated with parentheses and an asterisk (P<0.001). The mean intensity of rhabdomeral Ops1-2-ir during the day is about 50% of that observed during the night and at sunrise. The mean intensity of rhabdomeral Ops5-ir does not change significantly from day to night.
Determining relative molar concentrations of Ops5 and Ops1 in membrane preparations of LEs
We first determined the protein concentrations of aliquots of the heterologously expressed C-terminals of Ops1 and Ops5, the antigens against which our opsin antibodies were raised. The antigens were separated by SDS-PAGE together with known amounts of a standard protein (trypsin inhibitor). The gel was stained with Coomassie Blue R-250 and afterwards scanned (hp Scanjet 2200C; Hewlett-Packard Development Co., Palo Alto, CA, USA) to obtain a digitized image. The intensities of stained bands were quantified using Image QuantTL™ (GE Healthcare, Piscataway, NJ, USA), and the staining intensities of the antigens were compared with that of a standard curve generated with the protein standard. Known amounts of the Ops1 and Ops5 antigens were then separated by SDS-PAGE on 12.5 or 15% gels together with different volumes of SDS solubilized membranes from LEs or VEs. The separated proteins were blotted to PVDF, immunostained for Ops1 and Ops5 as described above, and immunoreactivity was visualized with chemiluminescence as described above. Scanned, digitized images of the immunostained antigen standards and the Ops1- and Ops5-immunoreactive bands from the membrane preparations were quantified with Image QuantTL™ and compared.
RESULTS
Comparison between Limulus Ops5 and Limulus Ops1 and 2
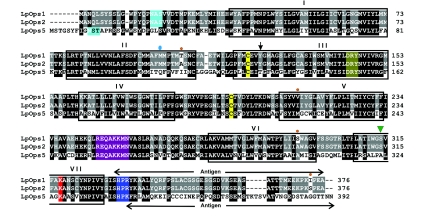
The predicted amino acid sequences of Ops1 and 2 are 99% identical to one another, with only four amino acid differences between them (Smith et al., 1993) [updated by Dalal et al. (Dalal et al., 2003)]. By contrast, the predicted amino acid sequence of Ops5 is only 45% identical to Ops1 and 2. Yet, the sequence of Ops5 contains features that are characteristic of rhabdomeral opsins (Fig. 1) (Arendt et al., 2004; Gartner and Towner, 1995). These include: seven predicted transmembrane domains, a predicted glycosylation site in its N-terminus (NST12) (http://www.cbs.dtu.dk/services/NetNGlyc), two conserved cysteine residues (C129/C206) that form a disulfide bond, a conserved lysine (K327) in transmembrane helix VII that is critical for the Schiff base binding of the chromophore, and a serine/threonine rich C-terminal tail. It also contains the (E/D)RY motif at the cytoplasmic end of helix III, which is conserved in G-protein-coupled receptors, an amino acid triplet (HPR/K343) characteristic of rhabdomeral opsins that activate the Gαq GTP binding protein, and a string of eight amino acids in cytoplasmic loop 3, which is highly conserved among arthropod opsins (R255 to N262) (Porter et al., 2007).
Fig. 1.
Alignment of Limulus opsin5 (LpOps5) with Limulus opsin1 and 2 (LpOps1 and LpOps2). Positions of the transmembrane domains (in boxes) are estimated from an alignment with bovine rhodopsin (Palczewski et al., 2000). Amino acids highlighted in black are identical or conserved in all three predicted sequences; amino acids highlighted in gray are identical in two of the three predicted sequences. The following amino acids and sequences are also highlighted: in cyan, predicted glycosylation sites; in bright yellow, the two Cys (C) residues conserved in all opsins; dark yellow, the (E/D)RY motif conserved in G-protein-coupled receptors at the cytoplasmic end of helix III; in purple, a highly conserved sequence in arthropod opsins; in red, the conserved Lys (K) residue that is critical for Schiff base formation with the chromophore; in blue, a triplet of amino acids characteristic of rhabdomeral opsins that couple to Gαq. Also indicated are: the four amino acid differences between LpOps1 and 2 (orange dots); the site equivalent to Gly90 in bovine rhodopsin that is responsible for determining UV sensitivity in invertebrate opsins (blue oval); the position equivalent to the Schiff base counter ion in vertebrate opsins (arrow); the site corresponding to Ala292 in bovine rhodopsin at which a serine-to-alanine substitution in long wavelength sensitive Drosophila rhodopsin causes a blue shift in spectral sensitivity (green inverted triangle); the C-terminal tail sequences of LpOps1 and LpOps5 used as antigens to produce the antibodies applied in this study (elongated arrows).
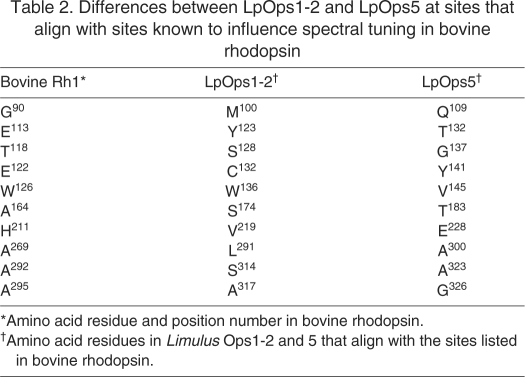
Ops5, like Ops1 and 2, is probably sensitive to visible light (400–700 nm) because it lacks a lysine at the site equivalent to glutamic acid-90 in bovine rhodopsin, which is characteristic of UV-sensitive invertebrate opsins (Salcedo et al., 2003). However, Ops5 differs from Ops1 and 2 at a number of sites known to affect spectral tuning in vertebrate opsins (Table 2) (Takahashi and Ebrey, 2003). Of particular interest is the serine-to-alanine substitution at the site corresponding to alanine-292 in bovine rhodopsin. In Drosophila, this substitution red-shifts the spectral sensitivity of Rh1 and blue-shifts the spectral sensitivity of Rh6 (Salcedo et al., 2009). The presence of a threonine in Ops5 at the site equivalent to the Schiff base counter ion in bovine rhodopsin is particularly unusual (Fig. 1). In most other visible-light-sensitive invertebrate opsins sequenced to date, including Limulus Ops1 and 2, this site is occupied by a tyrosine; in UV-sensitive invertebrate opsins it is a phenylalanine (Terakita et al., 2004).
Table 2.
Differences between LpOps1-2 and LpOps5 at sites that align with sites known to influence spectral tuning in bovine rhodopsin
The difference between Ops5 and Ops1 and 2 is emphasized further by the observation that Ops5 does not cluster with Ops1 and 2 and other chelicerate long-wavelength opsins in phylogenetic analyses. Rather it clusters with a group of crustacean opsins from branchiopods and crabs about which little is known (Fig. 2).
Distribution of the Ops5 transcript
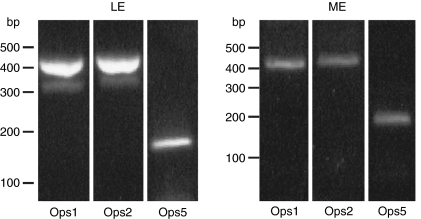
The Ops5 transcript was detected in VE, LE and brain cDNA libraries but not in our ME cDNA library (Smith et al., 1993). The Ops5 cDNA in the brain libraries probably originated from ventral photoreceptors that typically cluster near the brain at the proximal ends of the ventral optic nerves. To test further whether Ops5 is expressed in ME, freshly isolated ME RNA was reverse transcribed and amplified with PCR using primers specific for Ops1, Ops2 and Ops5 (see Materials and methods and Table 1). Freshly isolated and reverse-transcribed LE RNA was amplified in parallel with the same primers. All three opsin transcripts were amplified from reverse-transcribed ME and LE RNA (Fig. 3). Water controls were blank. Although we detected Ops5, Ops1 and Ops2 transcripts in ME cDNA, we failed to detect any of these opsin proteins in the ME. We assayed for the opsins by immunostaining western blots of ME membranes and frozen sections of MEs (supplementary material Fig. S1) using the same antibodies described below that consistently immunostained Ops5, Ops1 and Ops2 on western blots and frozen sections of LEs and ventral photoreceptors. Our failure to detect these opsin proteins in the ME remains a puzzle.
Fig. 3.
Ethidium bromide fluorescent images of PCR products amplified from reverse-transcribed RNA isolated from lateral eyes (LE) and median eyes (ME). The PCR primers were specific for Ops1, Ops2 and Ops5. See the text and Table 1 for details. The sizes of the products are indicated as base pairs (bp) at the left of each set of lanes. All three transcripts were detected in the LE and ME.
Characterization of antibodies generated against Ops5 and Ops1
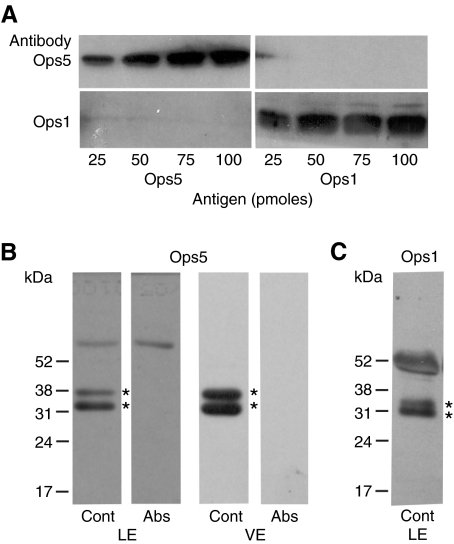
The specificities of mAbOps5 and pAbOps1 (Battelle et al., 2001) were tested first using the heterologously expressed C-terminal opsin polypeptides against which they were raised. We found that each antibody immunostained only its own antigen (Fig. 4A). However, preliminary studies revealed that pAbOps1 also immunostained a synthetic peptide with the sequence of the C-terminal of Ops2. This is not surprising since there is only one amino acid difference between Ops1 and 2 in their C-terminal regions. Thus, pAbOps1 immunostains both Ops1 and 2 but not Ops5, whereas mAbOps5 is specific for Ops5.
Fig. 4.
Chemiluminescent images of western blots. (A) Ops5 and Ops1 antigens immunostained with antibodies generated against these antigens. Increasing amounts (25–100 pmoles) of the heterologously expressed C-terminal tails of Ops5 and Ops1 were separated by SDS-PAGE on duplicate 15% gels and blotted to PVDF. The blot in the upper panels was immunostained with monoclonal antibody #31 generated against the Ops5 antigen (mAbOps5, 1:350 dilution); the blot in the lower panel was immunostained with a polyclonal antibody generated against the Ops1 antigen (pAbOps1, 1:2000 dilution). Horseradish peroxidase (HRP)-conjugated secondary antibodies were used at a dilution of 1:30,000. Each opsin antibody is specific for the antigen against which it was generated. (B) Lateral eye (LE) and ventral eye (VE) membranes immunostained with mAbOps5 (1:500) that had been preincubated with a strip of nitrocellulose to which ∼100 μg of Ops5 antigen had been blotted (Abs) or a similarly sized strip of nitrocellulose to which no protein had been blotted (Cont). The same volume of membrane extract was loaded onto lanes incubated with the absorbed and control antibodies. The secondary antibody was diluted as in A. mAbOps5 specifically immunostains a doublet in both LE and VE membrane preparations, as indicated by asterisks. Both bands of the doublet were eliminated by preincubating mAbOps5 with Ops5 antigen. (C) LE membranes immunostained with pAbOps1 (1:1000). The secondary antibody was diluted as in A. Ops1-immunoreactivity also migrates as a doublet under these electrophoretic conditions, as indicated by asterisks. The locations of the molecular mass markers are indicated on the left of each set of western blots. The heavily stained band seen at ∼52 kDa in C is haemocyanin, which stains nonspecifically with almost all antibodies.
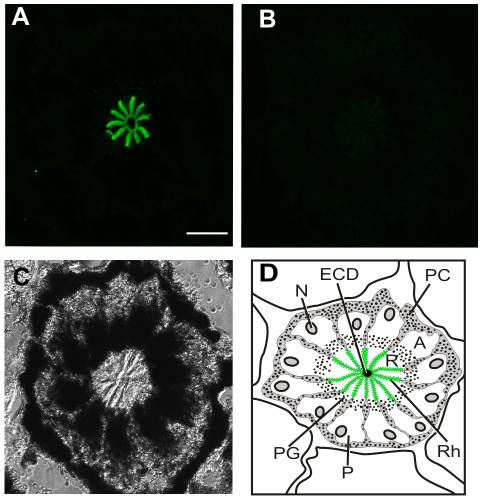
On western blots of LE and ventral photoreceptor membranes that were separated by SDS-PAGE on 7% gels, both mAbOps5 and pAbOps1-2 immunostained a broad protein band. However, on western blots of membranes separated through the 12.5% and 15% gels used in the present study, both Ops5- and Ops1-2-ir appeared as doublets (Fig. 4B,C). Preincubating mAbOps5 with its antigen eliminated the staining of both Ops5-immunoreactive bands (Fig. 4B), suggesting that both bands are opsin. Preincubating mAbOps5 with its antigen also eliminated all Ops5-ir observed on fixed frozen sections of LE (Fig. 5) and ventral photoreceptors (not shown).
Fig. 5.
(A,B) Images of single optical sections of ommatidia from frozen sections of the same lateral eye (LE) fixed 20 h after sunrise in the dark. A and B were collected with identical confocal settings. (A) Ommatidium from a section immunostained with mAbOps5 (1:1000) that had been preincubated with a strip of nitrocellulose without antigen. Strong Ops5-immunoreactivity (Ops5-ir) is detected over the rays of the rhabdom. (B) Ommatidium from a section immunostained with mAbOps5 (1:1000) that had been preincubated with a strip of nitrocellulose to which ∼100 μg of antigen had been blotted. No Ops5-ir is detected. (C) Transmitted light image of the ommatidium shown in B. (D) Diagram of a cross section through one LE ommatidium, showing 12 photoreceptor cell bodies (P) with their arhabdomeral (A) and rhabdomeral (R) segments, rhabdom (Rh, in green), nucleus (N) and pigment granules (PG). The photoreceptors are surrounded by pigment cells (PC). In the center of the ommatidium is the dendrite of the eccentric cells (ECD), which is electrically coupled to the photoreceptors. Scale bar, 25 μm.
The reason for the appearance of the ops-immunoreactive doublets following separations through high-percentage SDS gels is not clear. Different levels of opsin phosphorylation are not the cause because incubating ventral photoreceptor membrane extracts with alkaline phosphatase (Battelle et al., 2000a) did not eliminate the doublet. Absorption control experiments with pAbOps1-2 on western blots and tissue sections were described previously (Battelle et al., 2001).
Distribution of Ops-5- and Ops1-2-ir in LE and VE photoreceptors
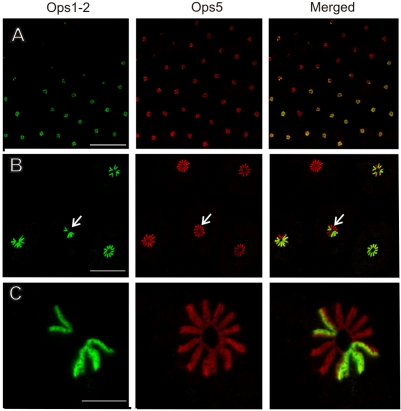
All photoreceptors examined so far in LEs and VEs were immunoreactive for Ops5, and all ventral photoreceptors double-labeled for Ops5 and Ops1-2. Most LE photoreceptors (retinular cells) also double-labeled for Ops5 and Ops1-2 but, surprisingly, some showed no detectable Ops1-2-ir. The upper left portion of Fig. 6A shows a region of one LE retina in which an unusually large number of retinular cells appear to express only Ops5. Fig. 6B emphasizes that an individual ommatidium can be heterogeneous with respect to the opsins expressed in its retinular cells. That is, while all retinular cells in an ommatidium express Ops5, all, some or none may express Ops1-2. Note that in the ommatidium shown in Fig. 6C, only half of four rhabdomeral rays are double-labeled for Ops1-2 and 5. This is because each retinular cell contributes microvilli to only half the total width of each ray. We have not yet determined the frequency or distribution of retinular cells that appear to express Ops5 only.
Fig. 6.
Ops1-2-immunoreactivity (Ops1-2-ir) and Ops5-ir in a frozen section of a lateral eye (LE) fixed 20 h after sunrise in the dark. Shown are images of a single optical section obtained with sequential scans of each fluorophore and their merged images. Ops1-2 (green); Ops5 (red). (A) A field of ommatidia in which all retinular cells are immunoreactive for Ops5 but only some are immunoreactive for Ops1-2. Many retinular cells in the upper left lack Ops1-2-ir. Scale bar, 300 μm. (B) Ommatidia from the field shown in A. In one ommatidium (lower right), all rhabdomeres are double labeled for Ops5 and Ops1-2; in the remaining three ommatidia, only some rhabdomeres are double labeled. The arrow points to the ommatidium shown in C. Scale bar, 100 μm. (C) All 11 rhabdomeral rays show Ops5-ir. One complete ray and half of four other rays are double labeled for Ops5 and Ops 1-2. Scale bar, 20 μm.
Diurnal changes in relative levels of rhabdomeral Ops1-2- and Ops5-ir
The photoreceptors of animals living in diurnal environments typically ‘shed’ some of their photosensitive membranes each day. In Limulus, shedding involves the internalization of rhabdomeral membranes by two different mechanisms: transient rhabdom shedding (TRS), a synchronous process that is triggered by the dim light of dawn after being primed during the night by signals from a central circadian clock, and light-driven shedding (LDS), a continuous process that does not require clock input but rather bright, prolonged light. LDS is a clathrin-mediated endocytosis that involves arrestin as an adaptor protein (Sacunas et al., 2002).
It had been assumed that these shedding mechanisms reduce opsin levels in Limulus rhabdomeral membranes during the day and contribute to the daytime down-regulation of photoreceptor sensitivity. To test directly whether rhabdomeral opsin levels are lower during the day than during the night under natural illumination and whether Ops1-2 and 5 are regulated similarly, we compared relative levels of rhabdomeral Ops1-2 and 5 in VEs fixed during the day (10 h after sunrise) and during the night (18–20 h after sunrise) and LEs fixed at sunrise, during the day and during the night.
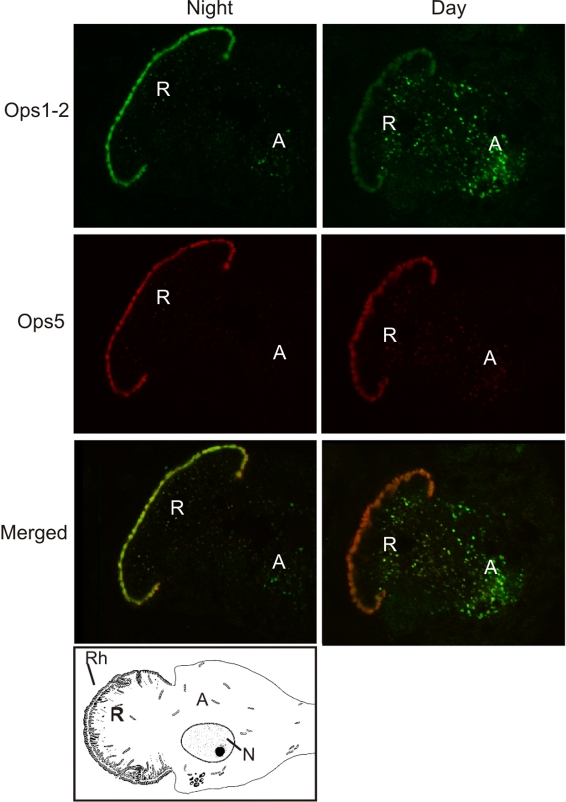
In ventral photoreceptors, rhabdomeral Ops1-2-ir was consistently lower during the day compared with the night, while extra-rhabdomeral Ops1-2 membranous debris in the rhabdomeral – and arhabdomeral – lobes, the product of rhabdom shedding, was consistently more abundant during the day. Surprisingly, rhabdomeral Ops5-ir did not appear to be lower during the day compared with the night, even though extra-rhabdomeral Ops5-ir membranes were more abundant during the day (Fig. 7).
Fig. 7.
Ops1-2-immunoreactivity (Ops1-2-ir) and Ops5-ir in the R- and A-lobes of ventral photoreceptors. Ventral eye end organs of animals maintained under natural illumination were fixed during the night, between 18 and 20 h after sunrise, and during the day (D), ∼10 h after sunrise. Frozen sections of the end organs from two night-time and two daytime animals were immunostained at the same time, and six photoreceptors for each animal were imaged in a single session with identical confocal settings. Shown are the sequential scans and merged images of single optical sections of photoreceptors representative of those typically observed in night-time and daytime animals. A simplified diagram of a ventral photoreceptor cell body is located below the fluorescent images. Rhabdomeral Ops1-2-ir is lower in daytime compared with night-time photoreceptors while the amount of Ops1-2-ir debris in the R- and A-lobes is greater during the day compared with the night. Rhabdomeral Ops5-ir appears to change little between night and day, although the amount of Ops5-ir debris observed in the R- and A-lobes is also higher during the day. Rh, rhabdom; R, rhabdomeral lobe; A, arhabdomeral lobe; N, nucleus.
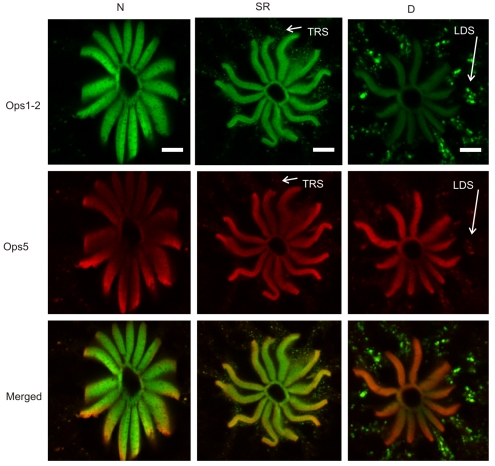
Similar results were obtained with LE photoreceptors (Fig. 8). In retinular cells fixed at sunrise, levels of rhabdomeral Ops1-2- and Ops-5-ir were similar to those in night-time eyes. This was a surprise because extra-rhabdomeral Ops1-2- and Ops5-ir debris was detected in these eyes, indicating that TRS was already underway. However, in retinular cells fixed 10 h after sunrise, the level of rhabdomeral Ops1-2-ir was consistently and dramatically lower than that observed in night-time eyes, and a large amount of intensely Ops1-2-immunoreactive extra-rhabdomeral membranous debris, the product of LDS (Sacunas et al., 2002), was detected in the rhabdomeral-segment and proximal region of the arhabdomeral-segment. As in the VE, the level of rhabdomeral Ops5-ir in retinular cells did not appear to fall during the day, even though extra-rhabdomeral Ops5-ir debris was evident. As a result, the ratio of rhabdomeral Ops5- to Ops1-2-ir appears dramatically higher in daytime compared with night-time LEs.
Fig. 8.
Ops1-2-immunoreactivity (Ops1-2-ir) and Ops5-ir in the R-segment and proximal A-segment of retinular cells in frozen sections of LEs fixed at different times of the day under natural illumination: during the night (N), between 18 and 20 h after sunrise, at sunrise (SR) and during the day (D), at ∼10 h after sunrise. Shown for each time point are images of sequential scans of a single optical section and their merged images (Ops1-2, green; Ops5, red). Sections were immunostained at the same time, and images were collected in a single session using identical confocal settings. At night, Ops1-2- and Ops5-ir are highly localized to the rays of the rhabdom, with little Ops-ir debris in the R-lobe or proximal A-lobe. At sunrise, levels of rhabdomeral Ops1-2- and Ops5-ir are similar to those seen during the night, but extra-rhabdomeral Ops1-2-ir and, to a lesser extent, Ops5-ir membranous debris, produced by transient rhabdom shedding (TRS), is detected in the R-segment and proximal A-segment. Later during the day (D), rhabdomeral Ops1-2-ir appears reduced compared with that observed in night-time and sunrise eyes, while intensely Ops1-2-ir extra-rhabdomeral membranous debris, produced by light-driven shedding (LDS), is detected in the R- and A-segments. By contrast, rhabdomeral Ops5-ir does not appear reduced in daytime eyes compared with eyes fixed during the night and at sunrise, although some extra-rhabdomeral Ops5-ir membranous debris is detected. At least some Ops5-ir co-localizes with Ops1-2-ir debris that is known to be in endosomes destined for degradation. Scale bars, 10 μm.
These qualitative observations were confirmed by quantifying the average intensity of rhabdomeral Ops1-2- and 5-ir in ommatidia of LEs fixed at different times during the day (Fig. 9). The mean level of rhabdomeral Ops1-2-ir in eyes fixed at sunrise is not significantly different from that in night-time eyes; however, in eyes fixed at 8–10 h after sunrise, it is about half the night-time level. By contrast, there is no significant day/night difference in the mean level of rhabdomeral Ops5-ir. It should be emphasized that the intensity of opsin-ir over the rhabdom is divided by rhabdom area; therefore, we are measuring changes in the concentration or packing density of opsin molecules in a given area of membrane. Total opsin in the rhabdom clearly depends on total rhabdom area. In Limulus maintained under natural illumination, rhabdom area fluctuates within the same range during the day and the night (Chamberlain and Barlow, 1984).
Influence of signals from the central circadian clock on rhabdomeral Ops1-2- and Ops5-ir
A central clock in Limulus activates efferent neurons that project from the brain to the eyes through the lateral optic nerves. This clock-driven efferent input, referred to here as clock input, becomes active about 45 min before sunset, remains active throughout the night and is inactive during the day (Barlow 1983; Pieprzyk et al., 2003). During the night, clock input has dramatic and diverse effects on LE structure, function and biochemistry (reviewed in Battelle, 2002) and, as mentioned above, is required to prime TRS that occurs at first light. We tested here whether eliminating clock input to the LE influences night-time levels of rhabdomeral Ops1-2- and Ops5-ir.
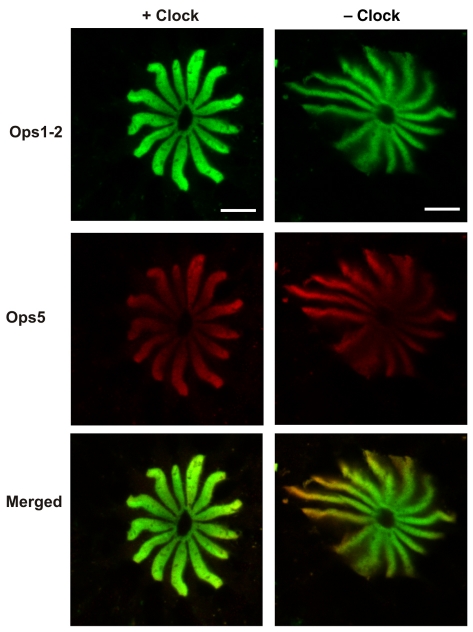
Clock input to one LE of each 12 animals was eliminated by cutting one lateral optic nerve. At 20 h after sunrise both LEs were dissected in the dark from animals that had been placed in the dark about an hour before sunset. The eyes were fixed in the dark, and frozen sections from the retinas were immunostained for Ops1-2 and Ops5. Confocal images suggested the rhabdomeral Ops5 to Ops1-2 ratio was higher in eyes with cut optic nerves (– Clock) compared with eyes with intact optic nerves (+ Clock) (Fig. 10). Quantification of rhabdomeral Ops1-2- and Ops5-ir in the + Clock and – Clock eyes confirmed a significant, 36% reduction in the level of Ops1-2-ir in the eyes without clock input (P>0.05, t-test, N=12) while there was no significant change in the level of rhabdomeral Ops5-ir.
Fig. 10.
Ops1-2-immunoreactivity (Ops1-2-ir) and Ops5-ir in the R-segments and proximal A-segments of photoreceptors in frozen sections of lateral eyes (LEs) from a single animal fixed at night in the dark about 20 h after sunrise. The optic nerve to one of the LEs was cut to eliminate clock input (– Clock) at least 10 days before the experiment. The optic nerve to the other LE remained intact (+ Clock). Shown are images of sequential scans obtained from a single optical section and their merged images (Ops1-2, green; Ops5, red). Sections were immunostained at the same time, and images were collected in a single session using identical confocal settings. At night, Ops1-2 and Ops5 are highly localized to the rays of the rhabdom with little opsin-ir debris in the R-lobe or proximal A-lobe. This and other images suggest the ratio of rhabdomeral Ops5-ir to Ops1-2-ir is higher in LEs with cut lateral optic nerves (– Clock). Scale bars, 10 μm.
What is the molar ratio of Ops5 to Ops1-2 in rhabdomeral membranes?
While the immunocytochemical results described above indicate that the ratio of Ops5 to Ops1-2 in the rhabdomeres of retinular cells and ventral photoreceptors changes in response to diurnal light and is influenced by signals from an internal circadian clock, they provide no information about the relative molar ratio of Ops5 and Ops1-2 in the rhabdom. To quantify this ratio, we used western blots prepared from night-time, dark-adapted LEs. Different volumes of SDS-solubilized membranes from night-time LEs were separated by SDS-PAGE together with known amounts of Ops1 or Ops5 antigens and blotted to PVDF. Duplicate blots were immunostained for Ops1-2 or Ops5. Immunoreactivity was visualized with chemiluminescence, and digitized images of the chemiluminescent signals were quantified. The intensities of the Ops1-2 and 5 immunoreactive bands from the membranes were then compared with that of the antigen standards. A sample assay is shown in Fig. 11.
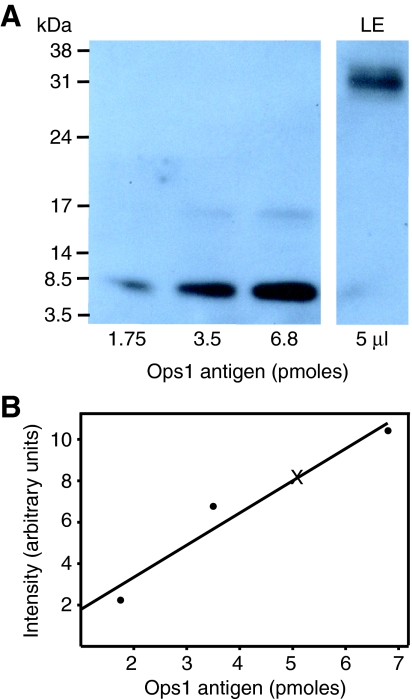
Fig. 11.
Sample quantification of opsin in photoreceptor membranes. (A) Chemiluminescent image of Ops1-2-immunoreactivity (Ops1-2-ir) obtained with known amounts of Ops1 antigen and a known volume (5 μl) of lateral eye (LE) membranes from a night-time eye. (B) Standard curve obtained by quantifying the immunoreactive intensity of the antigen standards shown in A (R2=0.99). The intensity of Ops1-2-ir in 5 μl of the LE membrane preparation is indicated on the curve (X).
The use of night-time dark-adapted LEs, 18–20 h after sunrise, for these assays is important. It is not possible to purify rhabdomeral membranes away from other membranes in LEs; therefore, preparations of LE membranes include extra-rhabdomeral membranes as well as rhabdomeral membranes. In daytime light-adapted eyes, much Ops-ir is associated with extra-rhabdomeral membranes. However, at 18–20 h after sunrise, almost all of the Ops-ir in retinular cells is in the rhabdom (Fig. 8) (Sacunas et al., 2002). Therefore, assays of membranes from night-time eyes should provide the best estimate of the relative levels of rhabdomeral Ops5 and Ops1-2. Assays of night-time LEs showed that the mean molar level of Ops5 in membranes was 20±2.6% (N=6) of Ops1-2. Since our immunocytochemical results show a 50% night-to-day fall in Ops1-ir at the rhabdom with no significant change in rhabdomeral Ops5-ir, we estimate the daytime molar level of Ops5 is about 40% of Ops1-2.
The molar level of Ops5 relative to Ops1-2 at the rhabdom may be even higher under some conditions. For example, in two separate preparations of membranes from highly light-adapted VE photoreceptor cell bodies, with each preparation containing cells from four animals, the molar levels of Ops5 were 130% and 140% of Ops1-2. These values may still underestimate the ratio of Ops5 to Ops1-2 at the rhabdom since light-adapted ventral photoreceptors contain abundant, mostly Ops1-2-immunoreactive debris (Fig. 7).
DISCUSSION
In this study, we characterize a newly identified Limulus opsin (Ops5) that differs significantly from opsins previously cloned from this animal (Ops1 and Ops2). Although, like Ops1 and 2, Ops5 is predicted to form a visual pigment sensitive to visible light, in a phylogenetic analysis it does not cluster with Ops1 and 2. We show that Ops5 is co-expressed with Ops1-2 in both lateral and ventral eye photoreceptors and present evidence that the spectral sensitivities of Ops5 and Ops1-2 may be different. Most interestingly, we found that the rhabdomeral levels of Ops5 and Ops1-2 are regulated differently and that their relative levels at the rhabdoms change in response to diurnal light and are influenced by the animal's circadian clock. To the best of our knowledge, this is the first clear example that rhabdomeral levels of co-expressed opsins can be differentially regulated by time of day or a circadian clock. Finally, we present evidence that the molar concentration of Ops5 in the rhabdom is sufficiently high relative to Ops1-2 to play a significant role in photoreceptor function, especially during the day. The differences between Ops5 and Ops1-2 in primary sequence and regulation suggest they function differently. Changes in their relative levels at the rhabdom may underlie some of the diurnal and circadian changes observed in the LE and perhaps change the spectral properties of the eyes from day to night.
Distribution of Ops5 and Ops1-2
Ops1, 2 and 5 transcripts were clearly detected in the LE, VE and ME (Fig. 3) and Ops1-2 and 5 proteins are clearly present in the rhabdomeres of LEs and VEs (Figs 5, 6, 7). Our failure to detect Ops1-2 or 5 proteins in ME rhabdomeres by either western blots or immunocytochemistry remains a puzzle (supplementary material Fig. S1) since about 30% of the photoreceptors in the ME are sensitive to visible light (Nolte and Brown, 1972). It is possible that, although transcripts for Ops1, 2 and 5 are present in these photoreceptors, the proteins are not highly expressed. Another possible explanation is that these photoreceptors contain another visible-light-sensitive opsin that has not yet been identified.
All VE photoreceptors examined so far co-express Ops1-2 and 5, as do most retinular cells. All retinular cells appear to express Ops5 but not all express Ops1-2. In regions of some LEs, photoreceptors expressing Ops5 but not Ops1-2 were relatively frequent (Fig. 6) but, overall, these cells were detected infrequently, and it is not yet clear whether they have a particular distribution. A systematic search for these cells will be required to determine their frequency and distribution. It is also not yet clear whether these cells express Ops5 only or whether they express one or more additional opsins that have not yet been identified.
Relationship between Limulus opsins and other arthropod opsins
Opsins with similar spectral properties often cluster together in phylogenetic groupings (Porter et al., 2007; Briscoe and Chittka, 2001); therefore, phylogenetic analyses can be useful for predicting the spectral properties of newly described opsins. For example, Limulus long wavelength sensitive Ops1 (Knox et al., 2003) clusters most closely with Rh2 spider opsins within a large clade consisting of medium and long wavelength sensitive opsins from other arthropods. Ops5 clusters with a group of crustacean opsins that form a sister group to the UV and short wavelength sensitive opsins but about which little else is known. This clade was thought to have arisen after the Pancrustacea–Chelicerata split (Group 4) (Kashiyama et al., 2009), but the addition of a Limulus opsin to this group indicates that it arose before the split. The only spectral information about opsins in this group is that the eyes of the brachyuran crab, Hemigrapsus sanguineus, which express two distinct opsin transcripts, have a maximum sensitivity of about 480 nm, as recorded with electroretinograms (Sakamoto et al., 1996).
It should be noted that the UV-sensitive opsin present in many Limulus ME photoreceptors (Nolte and Brown, 1972) has not yet been characterized at the molecular level.
Do Ops5 and Ops1-2 have different spectral or functional properties?
The differences noted between the amino acid sequences of Ops5 and Ops1 and 2 in Table 1 lead to the hypothesis that their spectral sensitivities are different. However, biochemical and electrophysiological studies of Limulus ventral photoreceptors and retinular cells give no indication of the presence of more than one visible-light-sensitive photopigment. Spectral sensitivity curves show a single peak that is not considered unusually broad (Hubbard and Wald, 1960; Nolte and Brown, 1970). Therefore, it was concluded that all visible-light-sensitive photoreceptors in Limulus express the same photopigment with a maximum sensitivity of about 520–525 nm. Based on the results of our current study, all of these biochemical and electrophysiological spectral sensitivity assays must have been made on preparations containing a mixture of Ops1, 2 and 5 and thus could be interpreted to mean that all three opsins have similar spectral properties.
Direct measurements show that the maximum sensitivity of Ops1 expressed in Drosophila photoreceptors is 513 nm, which is somewhat blue-shifted from the maximum sensitivity of Limulus visible-light-sensitive photoreceptors. This could be because Drosophila and Limulus utilize different chromophores (Smith et al., 1992; Vogt and Kirschfeld, 1984). Alternatively, the spectral sensitivities measured from Limulus photoreceptors could be the average maximum sensitivity of opsins with somewhat different spectral properties.
Some retinular cells express Ops5 in the absence of Ops1-2 (Fig. 6). If the spectral properties of Ops5 are significantly different from Ops1-2, it is reasonable to expect that a spectrally distinct class of retinular cells would have been discovered during the extensive electrophysiological studies of these cells. However, most electrophysiological studies describe a single spectral class of retinular cells; only one report describes two classes (Wasserman, 1969). Since retinular cells expressing Ops5 in the absence of Ops1-2 are far less abundant than those that co-express these opsins, they may not have been routinely detected in electrophysiological studies. Alternatively, as discussed above, cells expressing Ops5 in the absence of Ops1-2 may express another, as yet unidentified, opsin with spectral properties similar to those of Ops1-2. Clearly, the spectral properties of Ops5 will not be resolved unambiguously until they are measured in the absence of other opsins.
Even if Ops1-2 and 5 have similar spectral sensitivities, they may differ in other important aspects of their biochemistry that would significantly alter photoreceptor function, such as the efficacy or kinetics with which they activate the downstream phototransduction cascade and/or become inactivated.
Differential regulation of rhabdomeral levels of Ops5 and Ops1-2 and its possible effects
Our results demonstrate clear differences in the ways rhabdomeral concentrations of Ops5 and Ops1-2 are regulated by light and the circadian clock. In summary, we found that diurnal light and signals from the circadian clock strongly influence rhabdomeral concentrations of Ops1-2 but not Ops5.
Diurnal changes
Our results provide the first direct evidence that daytime rhabdom shedding reduces the concentration of Ops1-2 at the rhabdom. This light-dependent fall in rhabdomeral Ops1-2 to a concentration about half that observed during the night could contribute to the light-dependent portion of the daytime reduction in LE sensitivity observed in animals maintained in diurnal light (Pieprzyk et al., 2003). About half the daytime reduction in LE sensitivity occurs in the absence of light and can be attributed to endogenous structural changes in LE ommatidia that are regulated by the circadian clock (Barlow et al., 1980; Chamberlain and Barlow, 1987; Kier and Chamberlain, 1990). The other half requires light and probably involves the combined effects of physiological light adaptation, clock-driven structural changes in the ommatidia that are amplified by light (Chamberlain and Barlow, 1987) and the reduction in rhabdomeral Ops1-2 levels described here.
Since the rhabdomeral Ops5 concentration does not change from night to day, there is a significant increase in the level of Ops5 relative to Ops1-2 in the rhabdom during the day. If Ops5 were only a trace opsin in photoreceptors, this finding might have little physiological relevance. Ops1-2 is clearly more abundant than Ops5 in the rhabdom during the day and night; however, we found that the concentration of rhabdomeral Ops5 is sufficiently high to impact the photoresponse. At night, there is approximately one Ops5 molecule in the rhabdom for every five Ops1-2; during the day, this ratio increases to approximately two Ops5 molecules for every five Ops1-2. The functional significance of the diurnal change in the ratio of these two opsins in the rhabdom is not yet clear but, as described above in our discussions of differences between Ops5 and Ops1-2, changes in their ratios could influence photoreceptor spectral and response properties and other aspects of photoreceptor function.
Circadian changes
Our results also show, for the first time, that the circadian clock can influence opsin levels in the rhabdomeres of retinular cells. Specifically, we show that in eyes exposed to diurnal light, input from the circadian clock is required for rhabdomeral Ops1-2 to achieve its normal more elevated night-time concentration. The reduced night-time Ops1-2 levels observed in rhabdomeres of LEs exposed to diurnal light in the absence of night-time clock input may contribute to the reduced LE sensitivity observed in such eyes at night compared with control LEs that were exposed to diurnal light and received normal night-time clock input (Pieprzyk et al., 2003).
Unanswered questions
Our current findings raise a number of important new and unanswered questions. For example, is Ops5 an active photopigment? This question must be addressed directly in future studies, but our observation that Ops5 co-localizes, at least in part, with Ops1-2 in debris shed via LDS (Fig. 8) provides indirect evidence that it is. LDS is a clathrin-mediated endocytosis involving arrestin as an adaptor protein (Sacunas et al., 2002), and arrestin binding and clathrin-mediated endocytosis are hallmarks of activated G-protein-coupled receptors.
If Ops5 participates in phototransduction, of what functional significance is its co-expression with Ops1-2 in Limulus photoreceptors and what are the functional consequences of the observed diurnal change in the ratio of rhabdomeral Ops5 to Ops1-2? Can the influence of the clock on the night-time ratio of rhabdomeral Ops5 to Ops1-2 explain any of the clock-regulated changes in photoreceptor function? If Ops5 and Ops1-2 have different spectral properties, as some aspects of their sequences suggest, their changing ratios in the rhabdom could change the spectral properties of the eyes from day to night. The spectral properties of LE retinular cells have been studied extensively, as discussed above, but no reported studies have addressed the possibility of a diurnal spectral shift or the influence of the circadian clock on spectral sensitivity.
The efficacy and kinetics of other aspects of the photoresponse could also be regulated differently by Ops5 and Ops1-2 and change the response properties of the photoreceptors. Some response properties of retinular cells change from day to night under the influence of circadian clock input. For example, when clock input becomes active, retinular cell gain (response amplitude per photon absorbed) increases, noise (spontaneous activity in the dark) decreases (Kaplan and Barlow, 1980; Barlow et al., 1987) and the duration of the elemental photoresponse increases (Kaplan et al., 1990). It is tempting to speculate that a night-time increase in rhabdomeral levels of Ops1-2 relative to Ops5 contributes to some of these functional changes. However, it must be pointed out that the physiological studies were performed on animals maintained in the dark for as much as 48 h, and the relative levels of Ops1-2 and 5 in the rhabdom under these conditions is not yet known. Furthermore, clock-driven changes in photoreceptor gain and noise begin quickly, within seconds or minutes, after the onset of clock input to the eyes (Barlow et al., 1987), and it is not yet known how quickly rhabdomeral opsin levels begin to change in response to clock input. An understanding of the relationship between clock-regulated changes in photoreceptor physiology and relative levels of rhabdomeral Ops5 and Ops1-2 must await more extensive comparisons of the functional properties of Ops1-2 and 5 and the dynamics of clock- and light-regulated Ops5 and Ops1-2 shedding and renewal. It is also possible that Ops5 does not participate in the light response. This finding would raise even more questions regarding its function in the rhabdom.
What mechanisms control the differential expression levels of Ops1-2 and 5 in the rhabdom? The concentration of opsins in rhabdomeres is regulated by two distinct processes – shedding and renewal – and, as described above, shedding in Limulus photoreceptors occurs by two distinct processes: TRS and LDS. We do not yet know whether the daytime fall in rhabdomeral Ops1-2 concentration results from TRS or LDS or both, but two observations suggest that rhabdomeral Ops1-2 levels are inversely related to light intensity. LDS of Ops1-2 is observed best in eyes fixed midafternoon during the summer months (Sacunas et al., 2002) when light is brightest, and the ratio of Ops1-2 to 5 is very low in ventral photoreceptors exposed to very bright light. On the other hand, light also upregulates Ops1-2 transcription levels, an early step in opsin renewal (Dalal et al., 2003), raising the possibility that LDS is balanced by renewal. Still unknown are when during the day new Ops1-2 protein is translated and inserted into the membrane and to what extent shed opsin protein is reinserted into the rhabdom.
Ops5 appears to be shed by the same mechanisms as Ops1-2, as extra-rhabdomeral Ops5-ir membranes are detected in LEs fixed at sunrise and in the afternoon, and at least some co-localizes with shed Ops1-2 debris that is targeted for degradation (Sacunas et al., 2002). But less Ops5 appears to be shed. The intensity of Ops 1-2-ir in extra-rhabdomeral debris becomes much more intense than that observed over the rhabdom while the intensity of Ops5-ir extra-rhabdomeral debris is rarely as intense as rhabdomeral Ops5-ir. In order for the rhabdomeral concentration of Ops5 to remain stable throughout the day and night, Ops5 shedding and renewal must be balanced. This raises the question of the relative roles of light and the circadian clock in regulating Ops5 shedding and renewal.
Our results show that input from the animal's central circadian clock to LE retinular cells is required for rhabdomeral Ops1-2 to achieve its normal, more elevated, night-time level. A number of different processes could contribute to this and each could be influenced by the clock. Among these is an increase in Ops1-2 transcript levels. However, previous studies showed that Ops1-2 transcript levels are not influenced by the clock (Dalal et al., 2003). This suggests that the clock influences processes downstream of transcription such as translation and/or the transport of new opsin-containing membranes to the rhabdom. In contrast to Ops1-2, rhabdomeral Ops5 levels appear to remain stable in the absence of clock input. This observation underscores that mechanisms regulating rhabdomeral levels for Ops5 and Ops1-2 must be different.
Addressing the questions posed above should extend our understanding of the functional relevance of opsin co-expression, the functions of a clade of opsins about which relatively little is currently known, and extend our knowledge of the manner and mechanisms through which light and the circadian clock interact to regulate photoreceptor function.
Supplementary Material
Acknowledgments
We thank Leonid Moroz and his collaborators at the Whitney Laboratory for preparing and sequencing the EST collection from juvenile Limulus CNS and for preparing the VE EST collection; Steven Britt, University of Colorado at Denver, for helpful conversations on the spectral tuning of invertebrate opsins; W. Clay Smith for helpful comments on the manuscript; Lynn Milstead for assistance with preparing figures; and Leanne Adams for technical assistance.
This work was supported by the following grants: the NSF – IOS-0517272 to B.-A. Battelle, REU-0648969 to the Whitney Laboratory, and IOS-0721608 to T. W. Cronin; the NIH – EY08571 to the Department of Ophthalmology, University of Florida School of Medicine, and S10RR14638 to the Whitney Laboratory; Research to Prevent Blindness Inc. – to the Department of Ophthalmology, University of Florida School of Medicine; and the Air Force Office of Scientific Research – FA9550-09-1-0149 to T. W. Cronin. A.L., R.G. and E.G. were REU research trainees. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/15/2589/DC1
- bp
- base pair
- BP
- bootstrap proportion
- CNS
- central nervous system
- EST
- expressed sequence tag
- ir
- immunoreactivity
- LDS
- light-driven shedding
- LE
- lateral eye
- mAbOps5
- mouse monoclonal antibody directed against the C-terminus of opsin5
- ME
- median eye
- Ops
- opsin
- OCM
- organ culture medium
- pAbOps1
- rabbit polyclonal antibody directed against the C-terminus of opsin1
- PAGE
- polyacrylamide gel electrophoresis
- PCR
- polymerase chain reaction
- RACE
- rapid amplification of cDNA ends
- ROI
- region of interest
- TRS
- transient rhabdom shedding
- VE
- ventral eye
REFERENCES
- Abascal F., Zardoya R., Posada D. (2005). ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104-2105 [DOI] [PubMed] [Google Scholar]
- Adamus G., Arendt A., Zam Z. S., McDowell J. H., Hargrave P. A. (1988). Use of peptides to select for anti-rhodopsin antibodies with desired amino acid sequence specificities. Peptide Res. 1, 42-47 [PubMed] [Google Scholar]
- Adamus G., Zam Z. S., Arendt A., Palczewski K., McDowell J. H., Hargrave P. A. (1991). Anti-rhodopsin monoclonal-antibodies of defined specificity-characterization and application. Vis. Res. 31, 17-31 [DOI] [PubMed] [Google Scholar]
- Applebury M. L., Antoch M. P., Baxter L. C., Chun L. L. Y., Falk J. D., Farhangfar F., Kage K., Krzystolik M. G., Lyass L. A., Robbins J. T. (2000). The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27, 513-523 [DOI] [PubMed] [Google Scholar]
- Arendt D., Tessmar-Raible K., Snyman H., Dorresteijn A. W., Wittbrodt J. (2004). Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306, 869-871 [DOI] [PubMed] [Google Scholar]
- Arikawa K., Mizuno S., Kinoshita M., Stavenga D. (2003). Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of the butterfly Papilio xuthus. J. Neurosci. 23, 4527-4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. J., Cassone V. M. (2005). Melanopsin expression in the chick retina and pineal gland. Mol. Brain Res. 134, 345-348 [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Jr (1983). Circadian rhythms in the Limulus visual system. J. Neurosci. 3, 856-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Jr, Bolanowski S. J., Jr, Brachman M. L. (1977). Efferent optic nerve fibers mediate circadian rhythms in the Limulus eye. Science 197, 86-89 [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Jr, Chamberlain S., Levinson J. (1980). Limulus brain modulates the structure and function of the lateral eyes. Science 210, 1037-1039 [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Jr, Kaplan E., Renninger G. H., Saito T. (1987). Circadian rhythms in Limulus photoreceptors. I. Intracellular studies. J. Gen. Physiol. 89, 353-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle B. (2002). Circadian efferent input to Limulus eyes: anatomy, circuitry, and impact. Microsc. Res. Tech. 58, 345-355 [DOI] [PubMed] [Google Scholar]
- Battelle B.-A., Andrews A., Kempler K., Edwards S., Smith W. (2000a). Visual arrestin in Limulus is phosphorylated at multiple sites in the light and in the dark. Vis. Neurosci. 17, 813-822 [DOI] [PubMed] [Google Scholar]
- Battelle B.-A., Williams C. D., Schremser-Berlin J. L., Cacciatore C. (2000b). Regulation of arrestin mRNA levels in Limulus lateral eye: Separate and combined influences of circadian efferent input and light. Vis. Neurosci. 17, 217-227 [DOI] [PubMed] [Google Scholar]
- Battelle B., Dabdoub A., Malone M., Andrews A., Cacciatore C., Calman B., Smith W., Payne R. (2001). Immunocytochemical localization of opsin, visual arrestin, myosin III, and calmodulin in Limulus lateral eye retinular cells and ventral photoreceptors. J. Comp. Neurol. 435, 211-225 [DOI] [PubMed] [Google Scholar]
- Bayer D., Barlow R. B., Jr (1978). Limulus ventral eye. Physiological properties of photoreceptor cells in an organ culture medium. J. Gen. Physiol. 72, 539-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe A. D., Chittka L. (2001). The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471-510 [DOI] [PubMed] [Google Scholar]
- Calman B. G., Battelle B.-A. (1991). Central origin of the efferent neurons projecting to the eyes of Limulus-polyphemus. Vis. Neurosci. 6, 481-495 [DOI] [PubMed] [Google Scholar]
- Chamberlain S. C., Barlow R. B., Jr (1984). Transient membrane shedding in Limulus photoreceptors: control mechanisms under natural lighting. J. Neurosci. 4, 2792-2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S., Barlow R. (1987). Control of structural rhythms in the lateral eye of Limulus – interactions of natural lighting and circadian efferent activity. J. Neurosci. 7, 2135-2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. H., Ukhanova M., Thomas D., Afshar G., Tanda S., Battelle B.-A., Payne R. (1999). Molecular cloning of a putative cyclic nucleotide-gated ion channel cDNA from Limulus polyphemus. J. Neurochem. 72, 461-471 [DOI] [PubMed] [Google Scholar]
- Dalal J., Jinks R., Cacciatore C., Greenberg R., Battelle B.-A. (2003). Limulus opsins: diurnal regulation of expression. Vis. Neurosci. 20, 523-534 [DOI] [PubMed] [Google Scholar]
- De Voe R. D. (1972). Dual sensitivity of cells in wolf spider eyes at ultraviolet and visible wavelengths of light. J. Gen. Physiol. 59, 247-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T. M., Porter M., Cronin T. W. (2009). Spectral sensitivity, visual pigments and screening pigments in two life history stages of the ontogenetic migrator Gnathophausia ingens. J. Mar. Biol. Assoc. UK 89, 119-129 [Google Scholar]
- Gao N., Foster R. G., Hardie J. (2000). Two opsin genes from the vetch aphid, Megoura viciae. Insect Mol. Biol. 9, 197-202 [DOI] [PubMed] [Google Scholar]
- Gartner W., Towner P. (1995). Invertebrate visual pigments. Photochem. Photobiol. 62, 1-16 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696-704 [DOI] [PubMed] [Google Scholar]
- Guindon S., Lethiec F., Duroux P., Gascuel O. (2005). PHYML Online – a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 33, W557-W559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis D. M., Bull J. J. (1993). An empirical-test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 42, 182-192 [Google Scholar]
- Hubbard R., Wald G. (1960). Visual pigment of the horseshoe crab, Limulus-polyphemus. Nature 186, 213-215 [DOI] [PubMed] [Google Scholar]
- Kaplan E., Barlow R. B., Jr (1980). Circadian clock in Limulus brain increases response and decreases noise of retinal photoreceptors. Nature 286, 393-395 [DOI] [PubMed] [Google Scholar]
- Kaplan E., Barlow R. B., Renninger G., Purpura K. (1990). Circadian rhythms in Limulus photoreceptors. II. Quantum bumps. J. Gen. Physiol. 96, 665-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiyama K., Seki T., Numata H., Goto S. G. (2009). Molecular characterization of visual pigments in Branchiopoda and the evolution of opsins in Arthropoda. Mol. Biol. Evol. 26, 299-311 [DOI] [PubMed] [Google Scholar]
- Katoh K., Toh H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 9, 286-298 [DOI] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059-3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier C. K., Chamberlain S. C. (1990). Dual controls of screening pigment movement in photoreceptors of the Limulus lateral eye: Circadian efferent input and light. Vis. Neurosci. 4, 237-255 [DOI] [PubMed] [Google Scholar]
- Kitamoto J., Sakamoto K., Ozaki K., Mishina Y., Arikawa K. (1998). Two visual pigments in a single photoreceptor cell: identification and histological localization of three mRNAs encoding visual pigment opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 201, 1255-1261 [DOI] [PubMed] [Google Scholar]
- Knox B., Salcedo E., Mathiesz K., Schaefer J., Chou W., Chadwell L., Smith W., Britt S., Barlow R. (2003). Heterologous expression of Limulus rhodopsin. J. Biol. Chem. 278, 40493-40502 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227, 680-685 [DOI] [PubMed] [Google Scholar]
- Lukats A., Dkhissi-Benyahya O., Szepessy Z., Rohlich P., Vigh B., Bennett N. C., Cooper H. M., Szel A. (2002). Visual pigment coexpression in all cones of two rodents, the Siberian hamster, and the pouched mouse. Invest. Ophthalmol. Vis. Sci. 43, 2468-2473 [PubMed] [Google Scholar]
- Lukats A., Szabo A., Rohlich P., Vigh B., Szel A. (2005). Photopigment coexpression in mammals: comparative and developmental aspects. Histol. Histopathol. 20, 551-574 [DOI] [PubMed] [Google Scholar]
- Matz M. V. (2003). Amplification of representative cDNA pools from microscopic amounts of animal tissue. Methods Mol. Biol. 221, 103-116 [DOI] [PubMed] [Google Scholar]
- Mazzoni E. O., Celik A., Mathias F. W. C., Vasiliauskas D., Johnston R. J., Cook T. A., Pichaud F., Desplan C. (2008). Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 6, 825-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. (1970). Spectral sensitivities of single receptor cells in lateral, median, and ventral eyes of normal and white-eyed Limulus. J. Gen. Physiol. 55, 787-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. (1972). Electrophysiological properties of cells in median ocellus of Limulus. J. Gen. Physiol. 59, 167-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley T. H., Huber D. R. (2004). Differential expression of duplicated opsin genes in two eye types of ostracod crustaceans. J. Mol. Evol. 59, 239-249 [DOI] [PubMed] [Google Scholar]
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., et al. (2000). Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739-745 [DOI] [PubMed] [Google Scholar]
- Pieprzyk A., Weiner W., Chamberlain S. (2003). Mechanisms controlling the sensitivity of the Limulus lateral eye in natural lighting. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 189, 643-653 [DOI] [PubMed] [Google Scholar]
- Porter M., Cronin T., McClellan D., Crandall K. (2007). Molecular characterization of crustacean visual pigments and the evolution of pancrustacean opsins. Mol. Biol. Evol. 24, 253-268 [DOI] [PubMed] [Google Scholar]
- Porter M. L., Bok M. J., Robinson P. R., Cronin T. W. (2009). Molecular diversity of visual pigments in Stomatopoda (Crustacea). Vis. Neurosci. 26, 255-265 [DOI] [PubMed] [Google Scholar]
- Rohlich P., Vanveen T., Szel A. (1994). Two different visual pigments in one retinal cone cell. Neuron 13, 1159-1166 [DOI] [PubMed] [Google Scholar]
- Sacunas R., Papuga M., Malone M., Pearson A. J., Marjanovic M., Stroope D., Weiner W., Chamberlain S., Battelle B. (2002). Multiple mechanisms of rhabdom shedding in the lateral eye of Limulus polyphemus. J. Comp. Neurol. 449, 26-42 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Hisatomi O., Tokunaga F., Eguchi E. (1996). Two opsins from the compound eye of the crab Hemigrapsus sanguineus. J. Exp. Biol. 199, 441-450 [DOI] [PubMed] [Google Scholar]
- Salcedo E., Zheng L. J., Phistry M., Bagg E. E., Britt S. G. (2003). Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 23, 10873-10878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E., Farrell D. M., Zheng L. J., Phistry M., Bagg E. E., Britt S. G. (2009). The green-absorbing Drosophila Rh6 visual pigment contains a blue-shifting amino acid substitution that is conserved in vertebrates. J. Biol. Chem. 284, 5717-5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C., Friedman M. A., Goldsmith T. H. (1992). Retinoids in the lateral eye of Limulus-evidence for a retinal photoisomerase. Vis. Neurosci. 8, 329-336 [DOI] [PubMed] [Google Scholar]
- Smith W. C., Price D. A., Greenberg R. M., Battelle B.-A. (1993). Opsins from the lateral eyes and ocelli of the horseshoe-crab, Limulus-polyphemus. Proc. Natl. Acad. Sci. USA 90, 6150-6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. Y., Luo D. G., Terakita A., Shichida Y., Liao H. W., Kazmi M. A., Sakmar T. P., Yau K. W. (2006). Parietal-eye phototransduction components and their potential evolutionary implications. Science 311, 1617-1621 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ebrey T. G. (2003). Molecular basis of spectral tuning in the newt short wavelength sensitive visual pigment. Biochemistry 42, 6025-6034 [DOI] [PubMed] [Google Scholar]
- Terakita A., Koyanagi M., Tsukamoto H., Yamashita T., Miyata T., Shichida Y. (2004). Counterion displacement in the molecular evolution of the rhodopsin family. Nat. Struct. Mol. Biol. 11, 284-289 [DOI] [PubMed] [Google Scholar]
- Vogt K., Kirschfeld K. (1984). Chemical identity of the chromophores of fly visual pigment. Naturwissenschaften 71, 211-213 [Google Scholar]
- Wasserman G. S. (1969). Limulus receptor action spectra. Vis. Res. 9, 611-620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.