Abstract
The functional and possible adaptive significance of non-avian reptiles' dual aortic arch system and the ability of all non-avian reptiles to perform central vascular cardiac shunts have been of great interest to comparative physiologists. The unique cardiac anatomy of crocodilians – a four-chambered heart with the dual aortic arch system – allows for only right-to-left (R–L; pulmonary bypass) cardiac shunt and for surgical elimination of this shunt. Surgical removal of the R–L shunt, by occluding the left aorta (LAo) upstream and downstream of the foramen of Panizza, results in a crocodilian with an obligatory, avian/mammalian central circulation. In this study, R–L cardiac shunt was eliminated in age-matched, female American alligators (Alligator mississippiensis; 5–7 months of age). We tested the hypothesis that surgical elimination of R–L cardiac shunt would impair growth (a readily measured proxy for fitness) compared with sham-operated, age-matched controls, especially in animals subjected to exhaustive exercise. While regular exercise caused a decrease in size (snout-to-vent length, head length and body mass), elimination of the capacity for R–L cardiac shunt did not greatly reduce animal growth, despite a chronic ventricular enlargement in surgically altered juvenile alligators. We speculate that, despite being slightly smaller, alligators with an occluded LAo would have reached sexual maturity in the same breeding season as control alligators. This study suggests that crocodilian R–L cardiac shunt does not provide an adaptive advantage for juvenile alligator growth and supports the logic that cardiac shunts persist in crocodilians because they have not been selected against.
Keywords: cardiac hypertrophy, crocodilian, exercise, fitness, growth, reptile, cardiac shunt
INTRODUCTION
All crocodilians have complete anatomical separation between the right and left ventricles, similar to birds and mammals, but retain the dual aortic arch system, a feature common to all non-avian reptiles. In crocodilians, the left aortic arch (LAo) emerges from the right ventricle alongside the pulmonary artery, and the right aortic arch (RAo) emerges from the left ventricle (Webb, 1979). The two arches are connected via the foramen of Panizza (FoP) (Panizza, 1833) downstream of their aortic valves and via an anastomosis in the abdominal cavity. Downstream of the anastomosis, the right aorta becomes the dorsal aorta, and the LAo becomes the coeliac artery, which gives rise to smaller arteries that supply most of the blood flow to the gastrointestinal tract. The cardiac anatomy of crocodilians allows for a ‘pulmonary bypass’ or ‘right-to-left’ cardiac shunt [R–L, i.e. ‘right side of the heart's circulation to the left side of the heart's circulation’ (Hicks, 1998)]. During periods of increased pulmonary vascular resistance and/or reduced systemic vascular resistance, blood from the right ventricle can be ejected into the LAo (Jones and Shelton, 1993; Axelsson and Franklin, 2001). The mechanistic basis for the development of a R–L shunt has been thoroughly described in reptiles, but the functional and possible adaptive significance of the reptilian R–L cardiac shunt remains unclear, though much discussed (e.g. White, 1968; Grigg and Johansen, 1987; Jones, 1996; Hicks and Wang, 1996; Hicks, 2002; Wang and Hicks, 2008; Farmer et al., 2008; Eme et al., 2009a). Reptilian R–L shunt is hypothesised to be of adaptive significance in a number of ecological and physiological situations. For example, pulmonary bypass shunt may extend aerobic dive times (Grigg and Johansen, 1987), possibly through tissue hypometabolism (Hicks and Wang, 1999; Platzack and Hicks, 2001). This return of systemic venous blood back into the systemic arterial circulation has also been suggested to facilitate digestion by providing the stomach with a CO2-rich blood supply, thus aiding the formation of gastric acid (Jones and Shelton, 1993; Farmer et al., 2008). Finally, the development of a R–L shunt may speed recovery from an activity-induced metabolic acidosis by directing hypercapnic, acidic blood away from the lung and subsequently sequestering hydrogen ions into the stomach to restore blood bicarbonate levels (Farmer, 2000).
Although each of these hypothesised functions of R–L shunt is intuitively appealing, most lack convincing experimental evidence (Hicks and Wang, 1996; Wang and Hicks, 2008; Eme et al., 2009a) (but see Farmer et al., 2008). One experimental approach to investigate the functional significance of this blood flow pattern is to remove or reduce the capacity for R–L shunt and measure the resulting physiological changes in diving, metabolism, digestion or growth (Farmer et al., 2008; Wang and Hicks, 2008; Eme et al., 2009a). The anatomical separation of the atria and ventricles in the crocodilian heart allows for complete elimination of R–L cardiac shunt by surgical occlusion of the LAo upstream and downstream of the FoP (Fig. 1) (Farmer et al., 2008; Eme et al., 2009a). Therefore, the unique cardiac morphology of crocodilians allows for the creation of a chronic experimental model to address the functions and possible adaptive significance of crocodilian R–L shunt (Farmer et al., 2008; Eme et al., 2009a). If R–L cardiac shunt provides vital whole-animal physiological benefit(s) to crocodilians, surgical elimination of R–L cardiac shunt should cause a large detriment to whole-animal performance. Indeed, complete surgical occlusion of the LAo and elimination of R–L cardiac shunt significantly reduced the ability of alligators to produce gastric acid and demineralise bone in the stomach (Farmer et al., 2008), suggesting that elimination of crocodilian R–L shunt may adversely affect food digestion. If this reduction in stomach digestive capacity is important for growth, elimination of R–L shunt should strongly constrain growth of alligators and affect the time frame for achieving reproductive size. In addition, if the LAo serves to direct acidic blood to the gastrointestinal tract and contribute to recovery of acid–base status following intensive exercise (Farmer, 2000), then surgical occlusion of the LAo should prolong recovery from exercise, which in turn may increase total metabolic cost and negatively affect growth.
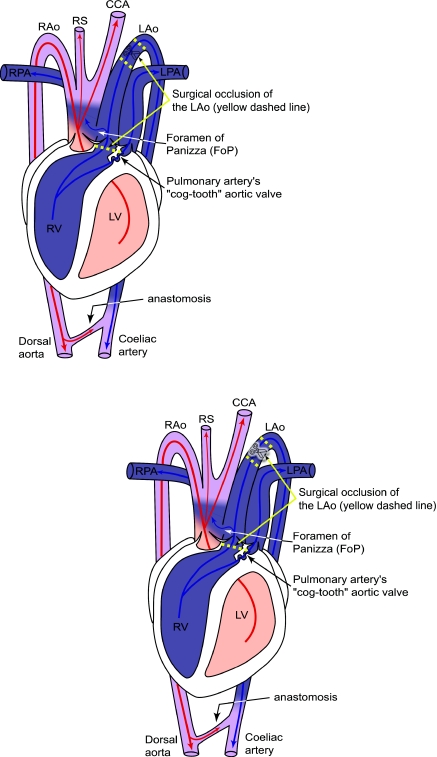
Fig. 1.
Schematic drawing of crocodilian central circulation and potential blood flow during right–left (R–L; pulmonary bypass) cardiac shunt, with blood flow from the left aorta (LAo) through the foramen of Panizza (FoP) into the right aortic arch (RAo). Yellow dashed lines indicate the two locations where we attempted to occlude the LAo in young alligators, in order to completely eliminate R–L cardiac shunt. We were completely successful (N=36) in surgically occluding and cutting the LAo, downstream of the great vessel truncus (superior yellow dashed lines with scissors symbol). However, we were only successful in occluding the LAo upstream of the FoP, with surgical needle and thread, for exactly two of every three surgical attempts (from N=36, N=24 successful surgeries or ‘S-LAo’). CCA, common carotid artery; LPA, left pulmonary artery; LV, left ventricle; RPA, right pulmonary artery; RS, right subclavian artery; RV, right ventricle. Adapted from Axelsson et al. (Axelsson et al., 1996).
The present study tested the hypotheses that R–L shunt is important for growth in the American alligator (Alligator mississippiensis; Daudin 1801), and that regular exercise (a potential metabolic stressor at the whole-animal level) can exacerbate the negative effect of R–L shunt elimination (Farmer, 2000). Using age-matched American alligators, we surgically eliminated R–L cardiac shunt in one group (Fig. 1) and performed sham surgeries in another (all animals hatched late August to early September 2005 and 5–7 months old at the time of surgery). From 9 to 24 months of age, we subjected subsets of sham-operated and experimental (LAo-occluded) alligators to regular exhaustive exercise (running on a treadmill or swimming in a swim flume) (Eme et al., 2009b) or no exercise (sedentary control). For all animals, we measured body mass, snout-to-vent length and head length from 7 to 24 months of age, and all animals were killed at 26–27 months of age for confirmation of surgical status and ventricular tissue harvest.
MATERIALS AND METHODS
Animal maintenance
Fertile American alligator eggs (N=70) were obtained from the Rockefeller Wildlife Refuge in Grand Chenier, LA, USA, and transported by air-freight to the University of California, Irvine (UCI). Eggs were potted in moist vermiculite and incubated at 30°C until hatching (August–September 2005), which ensured that all hatchlings were female (confirmed at the time of sacrifice by cloacal examination). All alligators were group housed in 1 m×2.5 m×1 m tanks (N=2–7 tanks) at 30°C with free access to water and heat lamps for basking. Alligators from either sham or experimental treatments (see below) were randomly distributed amongst tanks. In order to avoid density-dependent growth effects (Elsey et al., 1990), density was progressively decreased from hatching (∼16 alligators m–2) until they were sacrificed (∼4 alligators m–2), as alligators grew. In addition, animals were housed in tanks based on body mass (e.g. ‘larger’ animals with ‘larger’ animals) to avoid larger animals ‘out-competing’ smaller ones for food, so that all could feed ad libitum (see below). Adjustments for variable individual animal growth were made each month, with a subset of animals moved from one tank to another to match animals by size. Moving subsets of animals from tank to tank effectively served as a randomisation control in our experimental design. All animals were fed 2–3 times per week a matching, ad libitum diet of live goldfish or ground whole chicken (primarily ground whole chicken). Alligators were all likely sated following each feeding, as enough food was provided so that food remained in the tanks ∼3 h after it was presented. Chicken was ground with a meat grinder equipped with variable guards (M-12 FS, TorRey, Mexico City, Mexico), so that bone fragments were initially kept so small (<0.3 cm) as to be unlikely to cause digestive blockage in small hatchling and juvenile alligators (<50–300 g) and were then allowed to be larger (<0.7 cm) once alligators had reached an appropriate size (>300 g, ∼14 months old). Animals were fed 3 times per week from August 2005 to December 2006, then 2 or 3 times per week from December 2006 until prior to sacrifice. Only Sedentary (sham and experimental) animals were used in analyses in one of our previous studies (Eme et al., 2009a), and only Sham (sedentary, run and swim exercise) animals were used in analyses in another of our studies (Eme et al., 2009b). Approval for animal use in this study was given by the UCI Institutional Animal Care and Use Committee (protocol no. 1999-2123).
Chronic surgical occlusion of LAo
From January to March 2006, alligators were randomly divided into two groups, experimental surgery (N=36) and sham surgery (N=34). Surgery was performed as detailed previously (Eme et al., 2009a). Animals were fasted for 5–7 days prior to surgery. At the time of surgery (5–7 month old animals), the body mass (mean ± s.e.m.) of sham alligators was 65±2 g and that of experimental alligators was 67±2 g. Anesthesia was induced by placing an alligator in a sealed container with gauze soaked in isoflurane (Isoflo®; Abbott laboratories, North Chicago, IL, USA). The animal's trachea was intubated, and the lungs artificially ventilated using a SAR-830 Ventilator (CWE, Ardmore, PA, USA) downstream of a vaporiser providing 1–2% isoflurane (Foregger Fluomatic, Smithtown, NY, USA). The animal's ventral surface was scrubbed with Prepodyne (Iodine scrub; WestAgro, Kansas City, MO, USA) and 70% ethanol, and a 2 cm midline incision was made between the scales. The skin was blunt dissected away from the underlying musculature, and the pericardium and great vessels exposed by cutting through the musculature and 1 cm of the sternum. The pericardium was opened to expose the ventricles and proximal portion of the great vessels. For experimental animals, the LAo was isolated from surrounding tissue downstream of the great vessels, 6-0 silk suture was used to occlude the vessel at two points (Deknatel, Research Triangle Park, NC, USA), and the LAo was severed in between (Fig. 1). At the proximal exit of the LAo from the right ventricle, a single loop of 6-0 silk suture attached to a tapered Kalt 3 needle (UNIMED S.A., Lausanne, Switzerland) was wrapped around the LAo (in between the LAo and pulmonary artery) and back through the shared wall of the LAo and RAo. This LAo tie was intended to occlude the LAo upstream of the FoP, eliminating the FoP as a communication point between the LAo and RAo (Fig. 1). The pericardium was sewn shut with 6-0 silk suture, and the sternum, musculature and skin were sewn shut in succession with 3-0 silk suture (Ethicon, Somerville, NJ, USA). Following surgery, animals were artificially ventilated on room air until voluntary breathing resumed. Intramuscular injections of the antibiotic enrofloxacin (10 mg kg–1; Baytril; Bayer Corporation, Shawnee Mission, KS, USA) and the analgesic flunixin meglumine (5 mg kg–1; Flunixamine; Fort Dodge, Madison, NJ, USA) were given at the conclusion of surgery, and enrofloxacin for two additional post-operative days. For the sham group, the surgical procedure was identical to that of the experimental group, but the LAo was not isolated and no suture was wrapped around the proximal exit of the LAo from the right ventricle. Surgeries were performed on an approximately alternating schedule by surgical status (i.e. sham surgery, followed by experimental surgery, followed by sham surgery, and so on). For both groups, food was withheld for 5–7 days following surgery.
Growth measurements
From the age of 7 months to 24 months (‘day 0’, 25th March 2006 – ‘final day 558’, 4th October 2007), body mass (±0.1 g; Mettler PJ 12, Mettler-Toledo Inc., Columbus, OH, USA or MXX-5001, Denver Instrument Inc., Bohemia, NY, USA), snout-to-vent length (SVL; ± 1mm, metric ruler) and head length (HL; ± 0.1 mm; Mitutoyo digital calipers, Aurora, IL, USA) of all animals were measured every 2 weeks, but HL measurements were not begun until 21st April 2006. A single 2 week measurement was not taken (missing between measurement day 475 and 503; Fig. 2). SVL was defined as the distance from the tip of the snout to the anterior edge of the cloaca, and HL was defined as the distance from the tip of the snout to the posterodorsal edge of the occiput. We do not report total lengths of our alligators because tail tips were occasionally bitten off by tank mates. However, no alligator in this study lost more than a small (<3 cm) part of her tail, and aggression was not commonly observed within tanks.
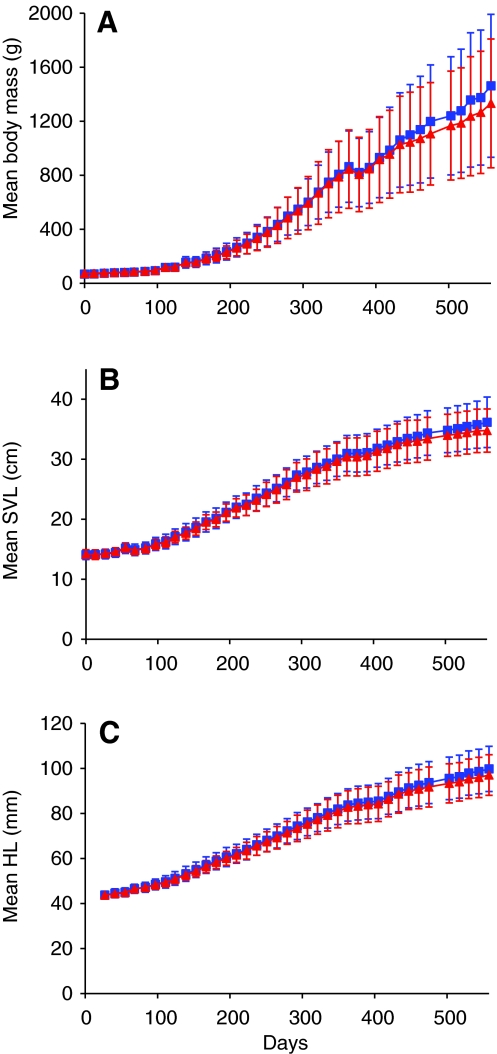
Fig. 2.
Longitudinal growth traces of (A) mean body mass (g), (B) mean snout-to-vent length (SVL, cm) and (C) mean head length (HL, mm) for age-matched Sham (N=34; blue squares) and S-LAo alligators (N=24; red triangles). Day 0 is 25th March 2006 for body mass and snout-to-vent length, and 21st April 2006 for head length, and the final measure is day 558, 4th October 2007. The reduction in growth at day 377 resulted from injection of all animals with a dye to track bone growth. Measurements were made every 2 weeks, except for one missed measurement (between day 475 and 503). Error bars are s.d.
All animals were given intramuscular injections of Calcein (25 mg kg–1) and Alizarin (75 mg kg–1) to label new bone formation for another study. The Alizarin injection, given on 23rd March 2007, induced vomiting and is likely to have caused a temporary drop in growth observed on day 377 (Fig. 2). We did not observe any adverse reactions to either of two Calcein injections.
Exercise training protocol
The development of a R–L cardiac shunt may speed recovery from metabolic acidosis (Farmer, 2000); therefore, we began exercise-training regimes to induce a metabolic stress in subgroups of our experimental alligators and possibly exacerbate the effect of LAo occlusion on experimental alligator growth (Farmer, 2000). At approximately 9 months of age (June 2006), alligators were randomly assigned to three exercise groups: Run (N=11 sham; N=12 experimental), Swim (N=12 sham; N=12 experimental) and Sedentary (N=11 sham; N=12 experimental). Animals in the Run and Swim groups were exercised to exhaustion on approximately each Monday, Wednesday and Friday and every other Sunday on a treadmill (1.0–2.0 km h–1) or in a swim flume (0.5–1.0 km h–1) until they were sacrificed in October to December 2007 (Eme et al., 2009b). Animals were encouraged to run or swim by tapping them on the tail by hand or with a pair of long forceps. Exercise bouts were kept at ∼5 min by incrementally increasing flume and treadmill speeds over the training period, as animals grew. Five minutes is a typical endurance time for 1–2 kg crocodilians exercising at ∼1–2.0 km h–1 (Farmer and Carrier, 2000; Munns et al., 2005; Owerkowicz and Baudinette, 2008). Endurance was defined as the time to exhaustion (to the nearest 15 s), at which point the animal failed to respond to repeated stimulation and was judged unable to maintain treadmill or flume speed. Animals in the Sedentary group were not exercised and were only handled once every 2 weeks for the purpose of size measurements.
Confirmation of chronic occlusion of LAo
In October to December 2007, alligators were killed, and alligators with an unoccluded FoP were excluded from growth analyses. The presence or absence of blood flow between the LAo and RAo through the FoP was determined using a H2-electrode technique (Clark et al., 1960; Hicks and Comeau, 1994; Malvin et al., 1995), as previously described (Eme et al., 2009a). Animals were fasted for between 2 and 6 days prior to experimentation and sacrifice. With the animal anesthetised (as above), the heart and the proximal portion of the great vessels were exposed with a longitudinal incision made through the length of the sternum and clavicle. The bare tip of an insulated platinum wire was inserted into the lumen of the right aortic arch underneath the right atrium by puncturing the vessel wall. A silver reference electrode was brought into contact with exposed muscle tissue. The silver reference electrode and platinum electrode were connected to DC amplifiers on a Beckman R610 polygraph system, and signals collected at 10–100 Hz using AcqKnowledge data-acquisition software and an A/D MP100 board (v 3.8.1; Biopac, Goleta, CA, USA). A 0.2 ml bolus of saline saturated with hydrogen gas was injected into the left ventricle (positive control) and subsequently into the right ventricle using a 27 gauge needle. If a voltage differential was detected following injection into the right ventricle, it was concluded that blood could flow from the LAo to the RAo, and the animal remained capable of generating a R–L cardiac shunt (Eme et al., 2009a).
This confirmation procedure identified three surgical groups of animals with different shunt capabilities: S-LAo (N=24), for ‘successful surgery’, were animals with a completely occluded LAo and FoP that were incapable of generating any R–L cardiac shunt; unsuccessful surgery (N=12) were animals with an occluded LAo downstream of the great vessel truncus that were still capable of limited R–L shunt through an unoccluded FoP; and Sham (N=34) were animals that underwent a sham surgery (Eme et al., 2009a). We eliminated the unsuccessful surgery group from any further analyses in the present study. The two ‘shunt’ groups were further divided among the following exercise groups, within each shunt group: S-LAo Run (N=8), S-LAo Swim (N=8) and S-LAo Sedentary (N=8); Sham Run (N=11), Sham Swim (N=12) and Sham Sedentary (N=11).
Tissue harvest
Subsequent to the H2-electrode technique described above, alligators were killed by removal of the heart (Eme et al., 2009a). The atria and great vessels were carefully dissected away from both ventricles. The combined ventricles were blotted dry with gauze, and wet mass measured on an analytical balance following harvesting (±0.001 g; Mettler AE 163, Mettler-Toledo Inc.).
Statistical analyses
Three size metrics, body mass (g), SVL (cm) and HL (mm), of all animals on 4th October 2007 (i.e. ‘final size’) were each compared with a separate two-way analysis of covariance (2-way ANCOVA), with the respective beginning size metric as the covariate and shunt capability (S-LAo and Sham) and exercise group (Run, Swim or Sedentary) as class factors. Least square means (LSM) were compared using pairwise comparisons with α=0.025. This represents the most modest Bonferroni correction (α=0.025), rather than the total number of pairwise comparisons (5) for interactions across all combined class factors (three exercise groups within two shunt groups), which would result in a much more stringent α=0.01.
Simple linear regression analysis (SLR) was used to model the relationship of final body mass (g; 4th October 2007) with beginning body mass (g; 25th March 2006) for each age-matched shunt group, with all exercise groups pooled into each shunt group: Sham (N=34) and S-LAo (N=24). One-way analysis of variance (1-way ANOVA) was used to determine the effect on final body mass of the interaction between shunt group and beginning body mass, pooling all exercise groups within each shunt group (α=0.05). A 1-way ANCOVA was also used to analyse the effect of beginning body mass on final body mass for the two shunt groups (Sham N=34 and S-LAo N=24, α=0.05).
SLR (α=0.05) was also used to model the dependence of mean body mass, SVL and HL on time (days during which measurements were taken) for each age-matched shunt group (with all exercise groups pooled into each shunt group). In the Discussion, SVL linear regressions are used to speculate on the potential for growth to reproductive size of each shunt group.
A 2-way ANOVA was used to analyse the interaction between shunt capability and exercise group on combined ventricular mass (α=0.05). A 1-way ANOVA was used to compare mass-specific combined ventricular mass (ventricle g kg–1) between all subgroups: S-LAo Run (N=8), S-LAo Swim (N=8), S-LAo Sedentary (N=8), Sham Run (N=11), Sham Swim (N=12) and Sham Sedentary (N=11). A Student–Newman–Keuls test separated ANOVA results into statistically distinct subsets (α=0.05). An ANOVA, instead of an ANCOVA, was used because vertebrate heart size scales isometrically with body size (e.g. Schmidt-Nielsen, 1984).
One Sham–Run alligator used previously (Eme et al., 2009b) was eliminated from the present study because of missing growth data points. Statistical analyses were performed using SAS Version 9.1 (SAS Institute, Inc., Cary, NC, USA) and JMP Version 8 (SAS Institute, Inc.).
RESULTS
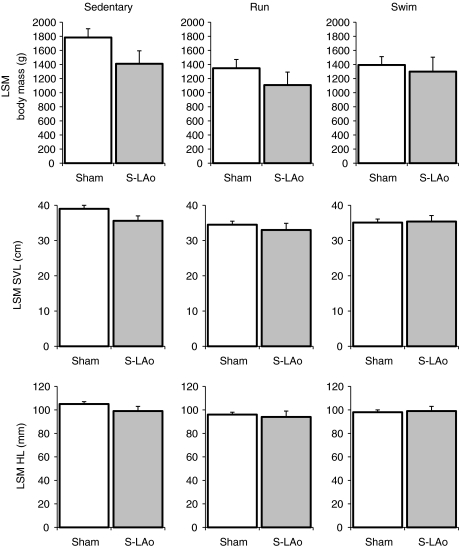
Animals with a completely occluded LAo (S-LAo) tended to be slightly smaller than Sham animals (Figs 2 and 3), and the LSM values generated by ANCOVA produced a larger difference for S-LAo versus Sham animal final sizes, relative to raw mean value comparisons (Table 1). However, the capacity to generate a R–L shunt (S-LAo or Sham) only had a significant effect on final body mass (2-way ANCOVA; F1,51=4.4; P=0.04) and not on final SVL (F1,51=3.0; P=0.09) or HL (F1,51=1.5; P=0.23). Within any exercise group, there were no significant differences between Sham and S-LAo final body mass or lengths (P>0.03; α=0.025). In addition, only the Sham Sedentary group was different from any other exercise group's body mass or lengths (Fig. 3; for pairwise comparisons, see supplementary material Tables S1–S3; α=0.025). Compared with sedentary alligators, chronic running or swimming exercise significantly reduced final body mass, SVL and HL (2-way ANCOVA; F2,51>3.4; P≤0.040).
Fig. 3.
Least square mean (LSM) values for body mass (g), SVL (cm) and HL (mm) from 2-way ANCOVA for Sedentary, Run and Swim alligators within each shunt group (S-LAo and Sham). Only the Sham sedentary group was different from any other exercise group, but it was not different from the S-LAo sedentary group. Refer to supplementary material Tables S1–S3 for pairwise comparisons of each subgroup. S-LAo Run N=8, S-LAo Swim N=8 and S-LAo Sedentary N=8; Sham Run N=11, Sham Swim N=12 and Sham Sedentary N=11. Error bars are s.e.m.
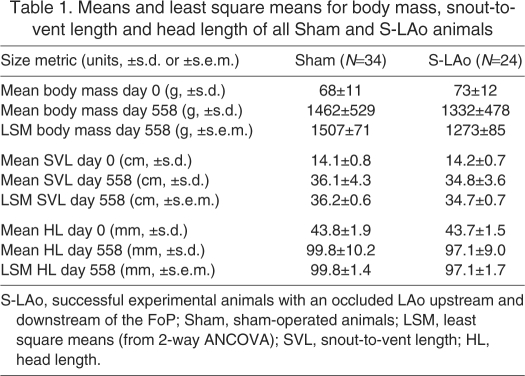
Table 1.
Means and least square means for body mass, snout-to-vent length and head length of all Sham and S-LAo animals
Alligator size at the beginning of growth measurements was the best predictor of final size. Initial body mass, SVL (25th March 2006) and HL (21st April 2006) had a significant effect on the respective size of the animals at the end of the study (4th October 2007; final day 558; 2-way ANCOVA; F>9.5; P<0.01). SLRs of final body mass on beginning body mass were significant for the Sham (N=34; F1,32=8.82, P<0.01) and S-LAo group (N=24; F1,22=16.68, P<0.001).
Growth rates of alligators estimated by SLRs of mean body mass, SVL and HL on days of measurement were each significant (R2>0.95, F1,38 or F1,36>818, P<0.001). For body mass, Sham animals (N=34) gained 2.70 g day–1 and S-LAo animals (N=24) gained 2.52 g day–1. For SVL, Sham animals gained 0.046 cm day–1 and S-LAo animals gained 0.044 cm day–1. For HL, Sham animals gained 0.116 mm day–1 and S-LAo animals gained 0.111 mm day–1 (see supplementary material Fig. S1 for graphical regressions and equations).
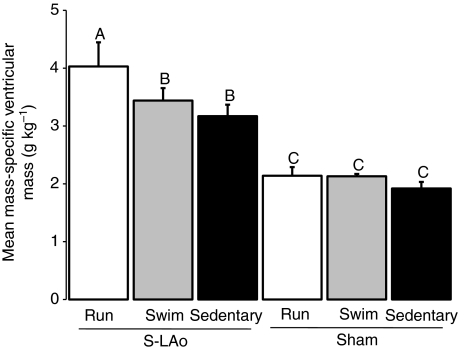
Exercise and occlusion of the LAo resulted in significant ventricular enlargement (1-way ANCOVA exercise×shunt capability interaction: F5,52=26.8, P<0.001). In S-LAo animals run on a treadmill, the ventricular mass (4.0±1.1 g kg–1; mean ± s.e.m.; Fig. 4) was 95% greater than the grand mean for all Sham exercise groups (2.1 g kg–1). In S-LAo animals swum in a flume, ventricular mass (3.4±0.6 g kg–1) was 66% higher than the grand mean of all Sham exercise groups (Fig. 4). Finally, in S-LAo sedentary animals, ventricular mass (3.4±0.6 g kg–1) was 54% higher than the grand mean of all Sham exercise groups (Fig. 4).
Fig. 4.
Mean values for mass-specific combined ventricular mass (g kg–1) for all subgroups. Uppercase letters above error bars indicate significant differences for each group, derived from Student–Newman–Keuls post-hoc test (α=0.05A,B,C) following 1-way ANOVA (F5,52=26.84, P<0.001). S-LAo Run N=8, S-LAo Swim N=8 and S-LAo Sedentary N=8; Sham Run N=11, Sham Swim N=12 and Sham Sedentary N=11. Error bars are s.e.m.
DISCUSSION
Surgical removal of R–L cardiac shunt does not greatly affect growth in alligators
Surgical removal of R–L cardiac shunt caused only minor reductions in the growth and size of alligators. Indeed, surgically altered alligators were not readily distinguishable from sham-operated alligators during the course of our study (J.E., J.G., T.O., J.M.B. and J.W.H., personal observations), with growth rates only ∼3–6% higher for the sham group (from linear regressions). Growth has been used as a proxy for fitness or to estimate fitness in a variety of organisms because growth can be considered an integrated measure of physiological function [e.g. insect (Lampert and Trubetskova, 1996); reptile (Sinervo and Adolph, 1989); mammal (Kraus et al., 2005); bird (Richner, 1989)]. Alligator growth can be highly variable, and dependent upon environment, life history and food availability (Coulson et al., 1973; Chabreck and Joanen, 1979; Jacobsen and Kushlan, 1989; Rootes et al., 1991; Elsey et al., 1992). For example, a population of wild, 1 year old alligators can weigh less than 0.5 kg, whereas artificially high temperatures and unlimited access to food can allow pen-reared, 1 year old alligators to weigh 7 kg (Coulson et al., 1973). In our laboratory population of alligators fed a diet of chicken and goldfish, mean body mass for all animals at 1 year of age was a relatively small ∼200 g, but mean body mass for all animals at 2 years of age was ∼1.3 kg. Reproductive maturity in crocodilians is based on size, not age (Rootes et al., 1991), and higher growth rates could increase fitness, with alligators reaching reproductive size more quickly. If R–L cardiac shunt conferred a strong adaptive advantage to crocodilians, one would expect a large and consistent decrement in growth to be apparent in all animals with their LAo occluded. No matter how data in this study are analysed, this is not the case.
Growth, R–L cardiac shunt and the potential impact on alligator reproductive fitness
Male and female alligators have been reported to reach sexual maturity at the same size, 1.83 m total length or approximately 0.915 m SVL (Rootes et al., 1991), though growth for female alligators can be reduced compared with that of male alligators, particularly approaching and following sexual maturity (Chabreck and Joanen, 1979). Fitness calculations would be impractical in the present study, as they require fecundity measures over multiple generations, and alligators can take >5 years to reach reproductive maturity. However, our data did show a trend towards smaller sizes for animals with the LAo occluded (S-LAo groups).
Given the final mean SVL and mean SVL growth rates in our groups (Table 1; 0.046 cm day–1 in Sham animals and 0.044 cm day–1 in S-LAo animals) and assuming constant linear growth, we speculate here on the average time to sexual maturity for animals in our S-LAo and Sham groups. In Sham animals, sexual maturity would have been reached 1204 days after 4th October 2007 (0.554 m ÷ 0.046 cm day–1), approximately January 20th 2011 at ∼5 years 5 months of age and 0.915 m SVL (body mass, 4.74 kg). S-LAo animals would have reached sexual maturity in 1289 days after 4th October 2007 (0.567 m ÷ 0.044 cm day–1), approximately April 15th 2011 at ∼5 years 8 months of age and 0.915 m SVL (body mass, 4.61 kg). Our estimates assume linear growth, which has been observed for the first ∼5 years of alligators' life (Chabreck and Joanen, 1979). In addition, our SVL growth rates of ∼1.3 cm per month are reasonable for female alligators beginning at ∼35 cm SVL (Chabreck and Joanen, 1979). This speculative analysis indicates that sexual maturity in our alligators would have occurred during the same breeding season (spring–summer 2011; alligators have a single, annual breeding season), suggesting minimal reproductive benefit of R–L cardiac shunt in alligators.
Chronic exercise and occlusion of the LAo, not removal of R–L shunt per se, moderately reduces alligator growth rate
Chronic exhaustive exercise (running or swimming) significantly reduced final body mass and length in alligators, compared with sedentary controls, demonstrating that increased relative metabolic expenditure can alter growth in alligators. Our exercise-trained alligators were fed ad libitum and readily ate during feeding sessions (J.E., J.G., T.O., J.M.B. and J.W.H., personal observations), and it is likely that exhaustive exercise increased catabolism in our exercise-trained alligators relative to sedentary controls. However, we did not find any effect of the ability to develop a R–L shunt on final sizes within exercise groups. This finding indicates that R–L cardiac shunt does not help crocodilians recover from exercise or a metabolic acidosis, and that regular exercise does not exacerbate any possible significance to surgical R–L shunt elimination in alligators (cf. Farmer, 2000).
Blood flow in the LAo has been shown to increase after a meal (Axelsson et al., 1991; Farmer et al., 2008), and surgical manipulation to occlude the LAo and eliminate R–L cardiac shunt in alligators reduces the rate of gastric acid secretion and bone demineralisation, which suggests that shunted, CO2-rich blood is important for digestion (Farmer et al., 2008). However, conversion of food matter into tissue building blocks depends not only on acid and enzyme secretion but also on nutrient absorption and assimilation. Although R–L shunt may significantly influence crocodilian digestion, it remains an open question whether the R–L shunt affects absorption and assimilation efficiencies. Studies of blood flow, specific dynamic action and assimilation efficiency in sham and surgically altered animals are required to answer this question. The present study indirectly tested the effects of R–L shunt on the alligators' capacity to convert food into body tissue, by examining the ultimate relevant indicator – growth. An overall 9% reduction in mean final body mass and 3–4% reduction in mean final lengths for S-LAo alligators suggests that controlled mixing of central systemic and pulmonary blood is not critical for healthy growth in crocodilians (Table 1). The reductions in body mass in the present study and in gastric acid secretion and bone demineralisation found previously (Farmer et al., 2008) may be due to a reduction in blood flow in the coeliac artery and not to a lack of pulmonary bypass shunt, per se. Other studies have shown that forward blood flow from the right ventricle to the LAo can be substantial and is probably a common event (e.g. Axelsson and Franklin, 2001; Axelsson et al., 1996; Farmer et al., 2008; Eme et al., 2009a). It is possible that blood flows during crocodilian activity or prandial states are only a byproduct of regulating vascular resistances, with the intermittent breathing pattern common to reptiles regularly causing an increase in pulmonary vascular resistance and a R–L shunt through the LAo. Correlations between blood flow in the LAo and a particular physiological state may not reflect a necessity of crocodilian physiology.
Exercise and LAo occlusion cause a synergistic increase in ventricular mass
This study demonstrates that ventricular mass of alligators synergistically increases in response to the combined stimuli of exhaustive exercise and surgical occlusion of the LAo (Eme et al., 2009a; Eme et al., 2009b). We have shown previously that surgical occlusion of the LAo causes a significant increase in ventricular mass in Sedentary alligators (when analysed compared only with Sham Sedentary alligators, without all other subgroups) (Eme et al., 2009a) and that exhaustive exercise significantly increases ventricular mass in the Sham alligators (when analysed without exercise subgroups) (Eme et al., 2009b). Occlusion of the LAo likely causes pathological, pressure overload hypertrophy of the right ventricle (Eme et al., 2009a) [see Faber et al. (Faber et al., 2006) and Leeuwenburgh et al. (Leeuwenburgh, 2008) for mammalian examples], whereas chronic exercise can increase alligator heart mass (Eme et al., 2009b), possibly leading to a greater stroke volume (e.g. Rowell, 1974; Scheuer and Tipton, 1977). These two stimuli – increased afterload and exercise (likely increased preload) – combined to cause a remarkable increase in ventricular mass for our S-LAo Run (95%) and S-LAo Swim alligators (66%), relative to Sham alligators (Fig. 4). Our S-LAo exercised alligators did not show any ill effects that could be associated with cardiac failure, such as reduced exercise capacity, peripheral edema, ascites, hepatomegaly or lethargy (J.E., unpublished observations). To our knowledge, no other animal has ever demonstrated such a remarkable cardiac enlargement while continuing to perform strenuous, regular exercise.
Is the crocodilian R–L cardiac shunt adaptive?
The physiological and environmental conditions producing crocodilian R–L cardiac shunt are well established (e.g. Grigg and Johansen, 1987; Jones and Shelton, 1993). With the exception of the study on crocodilian digestive ability (Farmer et al., 2008), there is limited experimental evidence that a truly four-chambered heart without the dual aortic arch system would be at all disadvantageous or advantageous to extant ectothermic crocodilians. Data reported in the present study, together with other recent studies (Wang and Hicks, 2008; Eme et al., 2009a), support the hypothesis that central vascular shunts and the dual aortic arch system are not an adaptive circulatory design (e.g. Foxon, 1955; Hicks and Wang, 1996; Hicks, 2002; Eme et al., 2009a). The ectothermic metabolic strategy of extant reptiles demands far less oxygen than the endothermic strategy of mammals and birds. Therefore, crocodilians may experience no detrimental or beneficial effects of shunting, despite the potential utility of the extra systemic aorta of non-avian reptiles (the LAo) in supplying blood to the gastrointestinal tract via the coeliac artery. Cardiac shunts could be primarily the result of regulating pulmonary and systemic vascular resistances, as parallel central circulatory designs of extant non-avian reptiles are adequate to meet their ectothermic metabolic demands. The present study supports the logic that central cardiac shunts persist in crocodilians because they are plesiomorphic characters that have not been selected against (Hicks, 2002). An adaptive argument for cardiac shunts presumes that altering blood gas levels would be strongly favored by natural selection in a manner that would affect overall animal survival and fitness. It is possible that shunts are important in physiological functions that have not been investigated thus far. To date, however, all experimental studies of aspects of physiology and behavior which influence evolutionary fitness of alligators – growth, resting metabolism, aerobic capacity and dive duration – have failed to demonstrate significant effects of shunt removal, and thus strengthen the argument that the R–L shunt may not be adaptive in crocodilians.
Supplementary Material
The authors sincerely thank Ruth Elsey (Rockefeller Wildlife Refuge, Louisiana Department of Wildlife and Fisheries) for access to alligators and alligator eggs, and for continuing support of our research. Special thanks go to Amanda Szucsik for incubating the eggs. Most importantly, we would like to thank the following students for their assistance with weighing, measuring and exercising the alligators: Krista Felbinger, Saemyi Moon, Jennifer Bautista, Lindsay Peltz, Dore Pei and Tim Cho. J.M.B. and T.O. were supported for part of this study by National Institutes of Health Training Grant 2T32AR047752 to the Multidisciplinary Exercise Science Programme at UCI. J.E. was supported for part of this study by NSF-GK-12 grant number DGE-0638751 to UCI. National Science Foundation Grant IOB 0445680 to J.W.H. provided funding for this research. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/15/2673/DC1
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- FoP
- foramen of Panizza
- HL
- head length
- LAo
- left aorta or left aortic arch
- LSM
- least square means generated by ANCOVA
- RAo
- right aorta or right aortic arch
- R–L cardiac shunt
- ‘right-to-left’ (i.e. ‘pulmonary bypass’) cardiac shunt
- S-LAo
- successful experimental animals with an occluded LAo upstream and downstream of the FoP
- SLR
- simple linear regression
- SVL
- snout-to-vent length
REFERENCES
- Axelsson M., Franklin C. E. (2001). The calibre of the foramen of Panizza in Crocodylus porosus is variable and under andrenergic control. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 171, 341-346 [DOI] [PubMed] [Google Scholar]
- Axelsson M., Fritsche R., Holmgren S., Grove D. J., Nilsson S. (1991). Gut blood flow in the estuartine crocodile, Crocodylus porosus. Acta Physiol. Scand. 142, 509-516 [DOI] [PubMed] [Google Scholar]
- Axelsson M., Franklin C. E., Lofman C. O., Nilsson S., Grigg G. (1996). Dynamic anatomical study of cardiac shunting in crocodiles using high-resolution angioscopy. J. Exp. Biol. 199, 359-365 [DOI] [PubMed] [Google Scholar]
- Chabreck R. H., Joanen T. (1979). Growth rates of American alligators in Louisiana. Herpetologica 35, 51-57 [Google Scholar]
- Clark L. C., Bargeron L. M., Lyons C., Bradley N., McArthur K. T. (1960). Detection of right-to-left shunts with an arterial potentiometric electrode. Circulation 23, 949-955 [DOI] [PubMed] [Google Scholar]
- Coulson T. D., Coulson R. A., Hernandez T. (1973). Some observations on growth of captive alligators. Zoologica 58, 47-52 [Google Scholar]
- Elsey R. M., Joanen T., McNease L., Lance V. (1990). Growth rate and plasma corticosterone levels in juvenile alligators maintained at different stocking densities. J. Exp. Zool. 255, 30-36 [Google Scholar]
- Elsey R. M., Joanen T., McNease L., Kinler N. (1992). Growth rates and body condition factors of Alligator mississippiensis in coastal Louisiana wetlands: a comparison of wild and farm-released juveniles. Comp. Biochem. Physiol. A Physiol. 103, 667-672 [Google Scholar]
- Eme J., Gwalthney J., Blank J. M., Owerkowicz T., Barron G., Hicks J. W. (2009a). Surgical removal of right-to-left cardiac shunt in the American alligator (Alligator mississippiensis) causes ventricular enlargement but does not alter apnoea or metabolism during diving. J. Exp. Biol. 212, 3553-3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eme J., Owerkowicz T., Gwalthney J., Blank J. M., Rourke B. C., Hicks J. W. (2009b). Exhaustive exercise training enhances aerobic capacity in American alligator (Alligator mississippiensis). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 179, 921-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M. J., Dalinghaus M., Lankhuizen I. M., Steendijk P., Hop W. C., Schoemaker R. G., Duncker D. J., Lamers J. M. J., Helbing W. A. (2006). Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am. J. Physiol. Heart Circ. Physiol. 291, H1580-H1586 [DOI] [PubMed] [Google Scholar]
- Farmer C. G. (2000). Evolution of the vertebrate cardiopulmonary system: new insights. Comp. Biochem. Physiol. B Biochem. Syst. Environ. Physiol. 126, 33 [Google Scholar]
- Farmer C. G., Carrier D. R. (2000). Respiration and gas exchange during recovery from exercise in the American alligator. Resp. Physiol. 120, 81-87 [DOI] [PubMed] [Google Scholar]
- Farmer C. G., Uriona T. J., Olsen D. B., Steenblick M., Sanders K. (2008). The right-to-left shunt of crocodilians serves digestion. Physiol. Biochem. Zool. 81, 125-137 [DOI] [PubMed] [Google Scholar]
- Foxon G. E. H. (1955). Problems of the double circulation in vertebrates. Biol. Rev. 30, 196-228 [Google Scholar]
- Grigg G. C., Johansen K. (1987). Cardiovascular dynamics in Crocodylus porosus breathing air and during voluntary aerobic dives. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 157, 381-392 [Google Scholar]
- Hicks J. W. (1998). Cardiac shunting in reptiles: mechanisms, regulation, and physiological function. In Biology of the Reptilia, Vol. 19. Morphology G: Visceral Organs (ed. Gans C., Gaunt A. S.), pp. 425-483 Ithaca, NY: SSAR Press; [Google Scholar]
- Hicks J. W. (2002). The physiological and evolutionary significance of cardiovascular shunting patterns in reptiles. News Physiol. Sci. 17, 241-245 [DOI] [PubMed] [Google Scholar]
- Hicks J. W., Comeau S. G. (1994). Vagal regulation of intracardiac shunting in the turtle, Pseudemys scripta. J. Exp. Biol. 186, 109-126 [DOI] [PubMed] [Google Scholar]
- Hicks J. W., Wang T. (1996). Functional role of cardiac shunts in reptiles. J. Exp. Zool. 275, 204-216 [Google Scholar]
- Hicks J. W., Wang T. (1999). Hypoxic hypometabolism in the anesthetized turtle, Trachemys scripta. Am. J. Physiol. 277, R18-R23 [DOI] [PubMed] [Google Scholar]
- Jacobsen T., Kushlan J. A. (1989). Growth dynamics in the American alligator (Alligator mississippiensis). J. Zool. 219, 309-328 [Google Scholar]
- Jones D. R. (1996). The crocodilian central circulation: reptilian or avian? Verh. Dtsch. Zool. Ges. 82, 209-218 [Google Scholar]
- Jones D. R., Shelton G. (1993). The physiology of the alligator heart-left aortic flow patterns and right-to-left shunts. J. Exp. Biol. 176, 247-269 [Google Scholar]
- Kraus C., Trillmich F., Kunkele J. (2005). Reproduction and growth in a precocial small mammal Cavia magna. J. Mammol. 86, 763-772 [Google Scholar]
- Lampert W., Trubetskova I. (1996). Juvenile growth rate as a measure of fitness in Daphnia. Func. Ecol. 10, 631-635 [Google Scholar]
- Leeuwenburgh B. P. J., Helbing W. A., Wenink A. C. G., Steendijk P., de Jong R., Dreef E. J., Gittenberger-de Groot A. C., Baan J., van der Laarse A. (2008). Chronic right ventricular pressure overload results in a hyperplastic rather than hypertrophic myocardial response. J. Anat. 212, 286-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvin F. M., Hicks J. W., Greene E. R. (1995). Central vascular flow patterns in the alligator Alligator mississippiensis. Am. J. Physiol. 269, R1133-R1139 [DOI] [PubMed] [Google Scholar]
- Munns S. L., Hartzler L. K., Bennett A. F., Hicks J. W. (2005). Terrestrial locomotion does not constrain venous return in the American alligator, Alligator mississippiensis. J. Exp. Biol. 208, 3331-3339 [DOI] [PubMed] [Google Scholar]
- Owerkowicz T., Baudinette R. V. (2008). Exercise training enhances aerobic capacity in juvenile estuarine crocodiles (Crocodylus porosus). Comp. Biochem. Physiol. A 150, 211-216 [DOI] [PubMed] [Google Scholar]
- Panizza B. (1833). Sulla struttura del cuore e sulla circolazione del angue del Crocodilus lucius. Biblioth. Ital. 70, 87-91 [Google Scholar]
- Platzack B. R., Hicks J. W. (2001). Reductions in systemic oxygen delivery induce a hypometabolic state in the turtle Trachemys scripta. Am. J. Physiol. 281, R1295-R1301 [DOI] [PubMed] [Google Scholar]
- Richner H. (1989). Habitat-specific growth and fitness in Carrion crows (Corvus corone corone). J. Anim. Ecol. 58, 427-440 [Google Scholar]
- Rootes W. L., Chabreck R. H., Wright V. L., Brown B. W., Hess T. J. (1991). Growth rates of American Alligators in estuarine and palustrine wetlands in Louisiana. Estuaries and Coasts 14, 489-494 [Google Scholar]
- Rowell L. B. (1974). Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 54, 75-159 [DOI] [PubMed] [Google Scholar]
- Scheuer J., Tipton C. M. (1977). Cardiovascular adaptations to physical training. Annu. Rev. Physiol. 39, 221-251 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. (1984). Scaling: Why is Animal Size so Important? Cambridge, UK: Cambridge University Press; [Google Scholar]
- Sinervo B., Adolph S. C. (1989). Thermal sensitivity of growth rate in hatchling Sceloporus lizards: environmental, behavioral and genetic aspects. Oecologica 78, 411-419 [DOI] [PubMed] [Google Scholar]
- Wang T., Hicks J. W. (2008). Changes in pulmonary blood flow do not affect gas exchange during intermittent ventilation in resting turtles. J. Exp. Biol. 211, 3759-3763 [DOI] [PubMed] [Google Scholar]
- Webb G. J. W. (1979). Comparative cardiac anatomy of the Reptilian. III. The heart of crocodilians and a hypothesis on the completion of the interventricular septum of crocodilians and birds. J. Morph. 161, 221-240 [DOI] [PubMed] [Google Scholar]
- White F. N. (1968). Functional anatomy of the heart of reptiles. Am. Zool. 8, 211-219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.