Abstract
The effects of in vivo freezing and glucose cryoprotectant on protein glycation were investigated in the wood frog, Rana sylvatica. Our studies revealed no difference in the fructoselysine content of blood plasma sampled from control, 27 h frozen and 18 h thawed wood frogs. Glycated hemoglobin (GHb) decreased slightly with 48 h freezing exposure and was below control levels after 7 d recovery, while glycated serum albumin was unchanged by 48 h freezing but did increase after 7 d of recovery. In vitro exposure of blood lysates to glucose revealed that the GHb production in wood frogs was similar to that of the rat but was lower than in leopard frogs. We conclude that wood frog hemoglobin was glycated in vitro; however, GHb production was not apparent during freezing and recovery when in vivo glucose is highly elevated. It is possible that wood frog blood proteins have different in vivo susceptibilities to glycation.
Keywords: freeze tolerance, protein glycation, glucose cryoprotectant, hemoglobin, serum albumin, fructoselysine, Rana pipiens
Glucose reacts chemically with protein amino groups to initiate a post-translational modification process known as nonenzymatic glycosylation or glycation [1]. The γ-amino groups of lysine (as well as the N-terminal amino acid residues) are the primary sites for glycation of most proteins. This process begins with the Amadori rearrangement of a reversible Schiff base adduct to form fructoselysine. This important intermediate can then degrade to a variety of carbonyl compounds that are much more reactive than the original sugar molecule. Amadori products undergo dehydration of the sugar moiety to form deoxyglucosones that in turn can lead to the production of advanced glycosylation end products or AGEs. A wide range of AGE products, including both adducts and cross-links involving lysine and arginine residues such as bis(lysyl)imidazolium cross-links, hydroimidazolones and monolysyl adducts [1, 3], have been detected and characterized in vivo. AGEs remain irreversibly bound to long-lived proteins and accumulate with age and at an accelerated rate during hyperglycemia in diabetes. The pathological effects of AGEs are due to their reaction with long-lived proteins. For example, the glycation of vascular collagen is especially damaging in diabetes [5] and is known to contribute to endothelial dysfunction, vascular thickening, loss of blood vessel elasticity, hypertension and kidney damage.
The minor hemoglobin species, A1c (HbA1c), is one of the best-studied examples of protein glycation [8]. HbA1c is generated by the addition of glucose to the NH2-terminal valine of the hemoglobin β-chain and accumulates slowly over the life of red blood cells. Because the amount of Amadori product that forms reflects the average blood glucose concentration during the lifespan of the red blood cell, the measurement of HbA1c and total glycated hemoglobin (GHb) is used clinically as an index of long-term (previous 8-10 weeks) blood glucose in patients with diabetes. Assay of glycated albumin, which has a shorter half-life, is used similarly for assessment of intermediate-term (previous 2-4 weeks) blood glucose. Measurements of glycated hemoglobin and albumin complement the acute measurement of blood glucose in the management of diabetes.
Freeze tolerant organisms accumulate high concentrations of low molecular weight carbohydrates that act as cryoprotectants to preserve the liquid state of the cytoplasm and maintain a minimum cell volume by limiting water loss into extracellular ice [10]. Many organisms utilize chemically or metabolically inert carbohydrate species; for example, polyhydric alcohols (e.g. glycerol, sorbitol) are the common cryoprotectants of freeze tolerant insects [10]. Unusually, the terrestrial wood frog (Rana sylvatica) relies on glucose for cryoprotection [10]. Ice formation at a peripheral site on the skin acts immediately through hormonal or neural stimulation of β-adrenergic receptors to initiate glycogenolysis in the liver. Thus, the production of glucose for cryoprotection is triggered within seconds when freezing begins and the sugar is rapidly distributed to all organs via the circulatory system. Glucose levels in blood and organs can rise to over 300 mM within a few hours. Moreover, when as much as two-thirds of total body water freezes as extracellular ice, the glucose concentration in remaining liquid spaces (e.g., cytoplasm, blood plasma) can be 1 M or more for the days or weeks that the frog is frozen. To use glucose as a cryoprotectant must require not only major adjustments to the metabolism of the frog (to override normal homeostatic controls on glucose levels) [7, 10] but also mechanisms the minimize AGE formation. In this study, we report on the glycation of hemoglobin (GHb) and plasma albumin in the blood of wood frogs over the freeze-thaw cycle.
Wood frogs, R. sylvatica, and leopard frogs, Rana pipiens, were collected in October close to Ottawa, Ontario. Wood frogs were mainly mature females (5-8 g) or juveniles (1-2 g). In the laboratory, frogs were washed in a tetracycline bath, and were then put in plastic boxes containing damp sphagnum moss and held at 5 °C until use. Freezing protocols were carried out as previously described [7]. Briefly, wood frogs were placed in a plastic box with damp paper toweling on the bottom. The box was placed in an incubator set at -4 °C and held for 1 h for cooling and ice-nucleation. The temperature was then raised and held at -2.5 °C for the duration of the freeze exposure; a thermistor suspended inside the box confirmed a stable -2.5 to -2.8 °C throughout the freeze. After freezing exposure, frogs were removed from the freezer and placed at room temperature for 1 h prior to sacrifice and blood sampling. For thawing, frozen wood frogs were removed and returned to a 5 °C fridge. Controls animals were maintained at 5 °C throughout. All frogs were sacrificed by double-pithing; a ventral incision exposed the body cavity, and blood was collected with a heparinized, glass micro-capillary pipette after clipping the aorta. Whole blood was placed in labeled microcentrifuge tubes and held on ice for immediate use. Whole blood from Wistar rats (Rattus norvegicus) was obtained by aortic puncture following euthanization and sacrifice of animals. All animal experimentation was approved by the Carleton University Animal Care Committee.
Whole blood from wood frogs was centrifuged (11,000 g; 5 min) to separate plasma from packed red blood cells. Plasma was removed to a second microcentrifuge tube, frozen in liquid nitrogen and stored at -80 °C until use. Following extraction with perchloric acid, plasma samples were analyzed spectrophotometrically for glucose using the hexokinase and glucose-6-phosphate dehydrogenase-coupled enzyme assay [9]. Hexokinase, glucose-6-phosphate dehydrogenase and other biochemicals were from Sigma Chemical Co. (St. Louis, MO).
For the analysis of fructoselysine (measured as the hydrolysis product furosine), sample processing, derivatization and GC/MS analyses of the trifluoroacetyl methyl ester were performed as described previously [3] using a Hewlett-Packard model 5890 gas chromatograph equipped with a model 7673A autosampler, model 5970 mass selective detector and a 30-m DB-5 capillary column (J & W Scientific, Folsom, CA).
Glycated hemoglobin and glycated albumin contents of the blood samples were measured using a Glyc-Affin GHb kit (Isolab Inc., Akron, OH). The kit utilizes a boronate affinity chromatography assay to separate hemolysates of red blood cells into two fractions prior to spectrophotometric quantification [4]. One fraction contains glycated proteins and the other contains non-glycated proteins. Hemoglobin content in the two fractions was measured by reading the absorbance of the two fractions at 415 nm. Serum albumin content of the two fractions was determined with the bromocresol green dye-binding method [6].
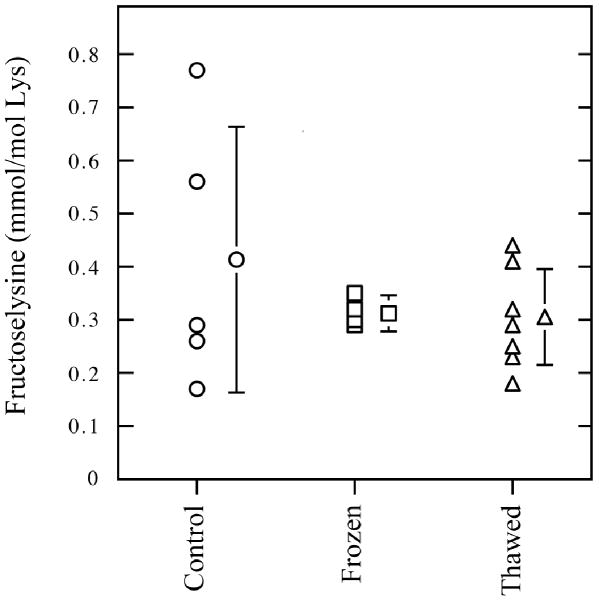
In the classical Maillard reaction scheme, the reducing sugar undergoes non-enzymatic reaction with a primary amine to form the Amadori product. With glucose as the reducing sugar, the Amadori product is Nε-(1-deoxy-D-fructos-1-yl)-lysine (fructoselysine, FL), a fructosamine [1]. Our initial investigations did not identify any significant changes in the FL content of wood frog blood after a freeze/thaw episode (Figure 1). This is in contrast to the massive increase in blood glucose levels measured in freezing and recovery states (Table 1). As shown in Figure 1, there was no difference in the FL content of blood plasma from control, frozen (27 h frozen + 1 h thawed) or thawed (24 h frozen + 18 h thawed) wood frogs. FL levels in these samples were approximately 0.3 mmol per mole lysine. Thus, the temporary high blood glucose concentration in the wood frog during the freeze/thaw cycle did not significantly affect the extent of protein glycation. It is difficult to directly compare the FL content of wood frog blood with previously published mammalian values. However, a recent study reported FL content in red blood cells, liver and brain of Sprague-Dawley rats as ∼1.5 mmol FL/mol lysine [2]. Skin collagen had the highest and skeletal muscle had the lowest FL contents in rats, 4.5 and 0.2 mmol FL/mol lysine, respectively. By contrast, in the streptozotocin-induced diabetic rat, the FL content increased to mirror changes in blood glucose. Blood glucose rose approximately 5-fold from control, non-diabetic values of 5 mM, and red blood cell FL content increased to approximately 10 mmol FL/mol lysine. In mammals, it is known that the in vivo accumulation of FL is highly dependent on both the rate of its formation and degradation and the rate of protein turnover. For long-lived extracellular proteins, the rate of formation of FL is dependent on the ambient glucose concentration and the availability of lysine residues for modification. However, for wood frogs, body temperature is presumably an equally important determinant to the rate of non-enzymatic glycation. Although the effects of temperature on the rate of protein glycation (Q10) have not been reported, the environmental conditions that promote cryoprotectant synthesis in the wood frog would also tend to retard the rate of in vivo protein glycation. Temperature-dependent Q10 effects on the Maillard reaction rate would tend to limit AGE production in wood frog blood and tissues.
Figure 1.
Fructoselysine levels as measured by GS/MS in blood plasma of control (○), frozen (□), and thawed (△) wood frogs. Control frogs were kept at 5 °C, frozen frogs were frozen at -2.5 °C for 27 h plus 1 h thaw time (to facilitate blood sampling), and thawed frogs were recovered at 5 °C for 18 h after a 24 h freeze. Data are means ± SEM, n = 5-7.
Table 1.
Effect of freeze-induced hyperglycemia on hemoglobin and serum albumin glycation in blood of R. sylvatica
| Glucose (μmol/mL) | % GHb | % Glycated Albumin | |
|---|---|---|---|
| Control, 5 °C | 4.2 ± 0.4 | 6.6 ± 0.2 | 21.0 ± 1.1 |
| 2 d Frozen, -2.5 °C | 198 ± 12 a | 5.3 ± 0.4 a | 21.8 ± 1.2 |
| 3 d Thawed, 5 °C | 164 ± 17 a | 5.7 ± 0.3 a | 22.4 ± 2.2 |
| 7 d Thawed, 5 °C | 10.9 ± 0.7 a,b | 4.6 ± 0.1 a,b | 25.9 ± 1.0 a,b |
Glycated hemoglobin (GHb) and glycated albumin were isolated using a Glyc-Affin GHb kit (Isolab Inc; Akron, OH). Hemoglobin was measured by absorbance at 415 nm. Serum albumin was quantified with the bromocresol green dye binding method. Data are means ± SEM, n = 6. Significantly different from the control (a) or 2 d frozen (b) values using ANOVA and post-hoc Student-Newman-Keuls test (two-tailed), p < 0.05
In vivo glycation of haemoglobin can be estimated by measuring total glycated haemoglobin (GHb) by boronate affinity chromatography. GHb measurements take into account glycation of lysyl residues on the α and β-chains whereas HbA1c measurements reflect a specific glycation event at the N-terminal valine of the β-chain [8]. While there is good conservation of the amino acid sequence of frog and human haemoglobin β-chains, we felt that it was appropriate to use GHb measurements in this study to assay total glycated haemoglobin in wood frog blood. As expected, blood glucose levels rose dramatically after a 48 h freeze (47-fold to 198 μmol/mL), and glucose levels were still high after 3 d and 7 d recovery periods, 39- and 2.6-fold greater than control, respectively. There was a 20% decrease in the amount of glycated hemoglobin species present after the 48 h freezing episode (Table 1). The amount of GHb also continued to decline during the recovery from freezing, with levels at 3 d and 7 d thawing that were significantly different from controls, possibly as a result of hemophoresis following thawing. In humans, GHb levels are approximately 6% of total Hb levels, and the GHb proportion has been shown to increase to nearly 10% in diabetic patients [8]. Thus, under control conditions, it appears that wood frog GHb levels approximate those found in humans. We also assayed the levels of glycated serum albumin in wood frog blood (Table 1). The percentage of albumin present in the glycated form was also significantly higher after 7 d recovery, increasing by approximately 5 % over control values. This increase occurred even with an observed elevation in total serum albumin values during recovery from 17.6 ± 0.2 mg/mL to 19.3 ± 0.2 mg/mL.
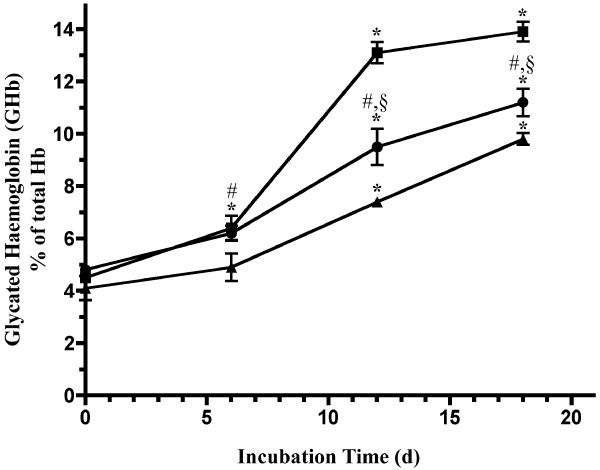
The effect of high glucose concentration on wood frog (R. sylvatica), leopard frog (R. pipiens) and rat hemoglobin was also studied in vitro (Figure 2). Samples of whole blood were mixed 1:1 (v:v) with lysis buffer, incubated in the presence of 0.4 M glucose for up to 18 days and then total glycated hemoglobin, GHb, was measured. Whole blood lysates of both frog species showed significant, though minor, increases in the amount of GHb after 6 days at 23 °C. Wood frog, leopard frog and rat blood all exhibited significant increases in GHb after 12 d and 18 d incubations; the leopard frog blood showed the greatest increase, closely followed by wood frog blood. The amount of GHb found in rat blood was significantly lower than that found in the two frog blood incubations. Our research indicates that wood frog hemoglobin appears to be glycated in vitro at a rate exceeding that of mammalian hemoglobin. However, the increased potential for GHb production in vitro is not apparent during natural freezing and recovery when glucose concentration is maintained at a level many-fold higher than that found in even the most severe human diabetic conditions. Thus, we hypothesize that low temperature may prevent the increase in FL and accumulation of GHb in wood frogs when cryoprotectant glucose rises to high concentrations during freezing exposure.
Figure 2.
A comparison of hemoglobin glycation in whole blood from wood frog, R. sylvatica (●), leopard frog, R. pipiens (■) and rat (▲) during in vitro incubation at 23 °C with glucose. Blood was collected by aortic punch. Whole blood was mixed 1:1 with lysis buffer (Glyc-Affin GHb kit Sample Preparation Buffer), and glucose was added to a final concentration of 0.4 M. The samples were mixed well, tightly capped, and then incubated at 23 °C for up to 18 d on a rotary mixer. After the incubation period, GHb content was immediately analyzed with the Glyc-Affin GHb kit. Data are means ± SEM, n = 3 separate trials. (*)- Indicates a significant difference from the corresponding intraspecies control value at 0 d by ANOVA and post-hoc Student-Newman-Keuls test (two-tailed), P< 0.05. (#) and (§) - Indicates significant differences between R. sylvatica and the corresponding rat or R. pipiens, respectively, interspecies value at the same incubation time.
Acknowledgments
This work was supported by the Canadian Diabetes Foundation (K.B.S.), the Canada Research Chairs program (K.B.S. & J.A.M.) and also by the United States National Institute of Diabetes and Digestive and Kidney Diseases (NIH grant DK-19971). We also acknowledge the assistance of J.M. Storey for critical commentary on the manuscript.
References
- 1.Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, Thorpe SR. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. [PubMed] [Google Scholar]
- 2.Brown SM, Smith DM, Alt N, Thorpe SR, Baynes JW. Tissue-specific variation in glycation of proteins in diabetes: evidence for a functional role of amadoriase enzymes. Ann N Y Acad Sci. 2006;1043:817–823. doi: 10.1196/annals.1333.094. [DOI] [PubMed] [Google Scholar]
- 3.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl) hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 4.Fluckiger R, Gallop PM. Measurement of nonenzymatic protein glycosylation. Meth Enzymol. 1984;106:77–87. doi: 10.1016/0076-6879(84)06009-2. [DOI] [PubMed] [Google Scholar]
- 5.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in the progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 6.Hill PG. The measurement of albumin in serum and plasma. Ann Clin Biochem. 1985;22:565–578. doi: 10.1177/000456328502200604. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald JA, Storey KB. Protein phosphatase responses during freezing and thawing in wood frogs: control of liver cryoprotectant metabolism. Cryo-Lett. 1999;20:297–306. [Google Scholar]
- 8.Rahbar S. The discovery of glycated hemoglobin: a major event in the study of nonenzymatic chemistry in biological systems. Ann N Y Acad Sci. 2005;1043:9–19. doi: 10.1196/annals.1333.002. [DOI] [PubMed] [Google Scholar]
- 9.Stein MW. D-glucose, determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Academic Press; New York, NY: 1963. pp. 117–123. [Google Scholar]
- 10.Storey KB, Storey JM. Natural freeze tolerance in ectothermic vertebrates. Annu Rev Physiol. 1992;54:619–637. doi: 10.1146/annurev.ph.54.030192.003155. [DOI] [PubMed] [Google Scholar]