Abstract
Bryonolic acid (BA) is a triterpenoid found in the Cucurbitaceae family of plants. Our interests in the immunomodulatory effects of this class of natural products led us to discover that BA induces a marked increase in the expression of a phase 2 response enzyme, heme oxygenase 1 (HO-1) in a dose dependent manner. This phenotype has translational implications in malarial disease progression, and consequently we developed a large scale isolation method for BA that will be enabling in terms of future in vitro and in vivo analysis. We have determined ideal growth conditions and time scale for maximizing BA content in the roots of Cucurbita pepo L., and analyzed BA production by HPLC. Large-scale extraction yielded 1.34% BA based on dry weight, allowing for the isolation of BA on a multi-gram scale.
Bryonolic acid (3β-hydroxy-D:C-friedoolean-8-en-29-oic acid, BA) is a naturally occurring triterpenoid that has been identified in multiple species of the Cucurbitaceae family and in dispersed species of other reported plant families (Meliaceae, Tetramelaceae, and Anisophylleaceae).1–3 It was first isolated in 1960 from roots of Bryonia dioica Jacq. (Cucurbitaceae), with an initial structure reporting a carboxylic acid moiety at C–19, and unsaturation at C–12/C–13.1 A revised structure was subsequently published assigning unsaturation to the C–8/C–9 B–C ring fusion and the carboxylic acid moiety at C–20 (Figure 1).4
Figure 1.
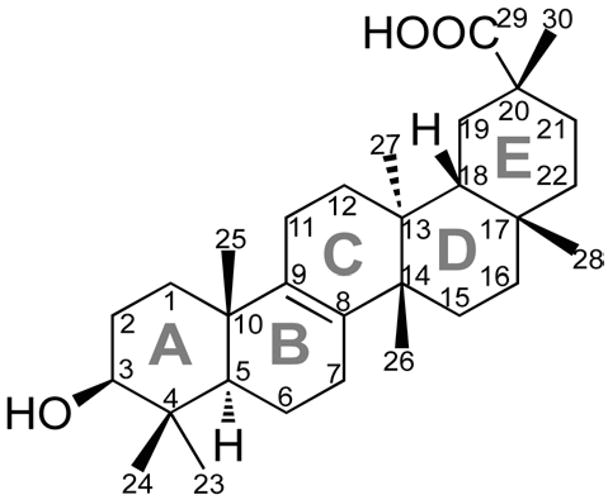
Structure and numbering for bryonolic acid.
Several biological activities have been reported for BA, including anti-allergic properties in rodents5 and a panel of cytotoxic and anti-tumor activities in various cancer cell lines.6–8 Despite these reports, there has never been an investigation into the molecular underpinnings of these phenotypes. Our laboratory has central interests in how triterpenoid natural products signal through the phase 2 response, an activity that is best represented by the semi-synthetic oleanane triterpenoids, which have been implicated in induction of the phase 2 response through the Nrf2:INrf2 (Keap1) signaling pathway.9,10 In accordance with these interests, we investigated whether the molecular basis of BA’s reported activities could be related to induction of expression through the phase 2 response.
Results and Discussion
The broad attention garnered by the oleanane triterpenoids is related to their potent anti-inflammatory activities which are mechanistically linked to the inhibition of expression of key inflammatory mediators, namely inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2).11,12 When we investigated BA in this context, we were surprised that the expression profiles were markedly different (as compared to the oleanane triterpenoids), in that iNOS and COX-2 expression levels were only moderately perturbed (data not shown). The most striking phenotype was a robust induction of heme oxygenase 1 (HO-1) levels. As seen in Figure 2, BA elicits robust HO-1 expression in RAW 264.7 treated cells in a dose dependent manner after a 24 h treatment. HO-1 expression is induced by 3.3 fold and 14 fold compared to LPS control in the presence of 50 μM and 100 μM BA, respectively. In comparison to untreated cells, treatment with 50 μM and 100 μM BA increases HO-1 by 13 fold and 55 fold, respectively.
Figure 2.
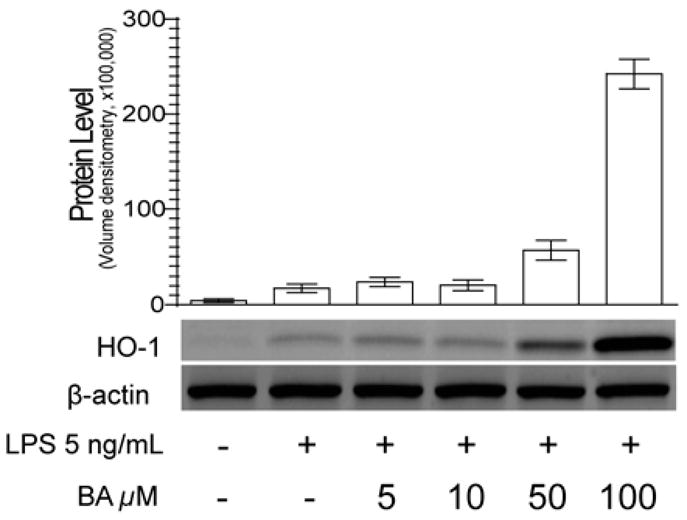
Western blot analysis and quantification of LPS induced RAW 264.7 cells treated with increasing concentrations of bryonolic acid for 24 h.
The implications of this observed phenotype have direct relevance to human disease. Plasmodium is a genus of parasites that cause malaria, resulting in more than 500 million infections and one million deaths per year.13 Recent studies have implicated HO-1 expression as a key therapeutic target in treating malaria. This connection has been rationalized by the enzymatic activity of HO-1 (converting heme to biliverdin),14–16 in conjunction with the clinical manifestations of malaria being linked to the hemolysis of red blood cells, and subsequent deposition of free heme to the vasculature.17,18 In addition to this rationalized connection, in vivo studies comparing wild type and Hmox−/− (Hmox is the gene encoding HO-1) mice have demonstrated that HO-1 expression protects against the development of the cerebral form of malaria in Plasmodium infected mice.14
To facilitate further studies of the in vitro and in vivo activity of BA, a protocol for robust isolation of BA is needed. To this end we have developed a reliable and scalable method for isolating gram quantities of BA from the roots of Cucurbita pepo L. (C. pepo L.), which was chosen based on literature precedence in addition to its ready commercial availability. Although BA has been isolated using alternate methods including callus cell culture,19,20 these methods are most appropriate for analytical scale isolation and biosynthetic studies. The key to our approach is a scalable method for obtaining biomass that is rich in BA content. Our initial step in defining our strategy was to determine if BA production was dispersed throughout C. pepo L. or limited to specific plant tissues. HPLC traces demonstrate that BA production is confined to the fine hairy root structure of C. pepo L. (Figure 3), which is consistent with previous BA isolation from the root (or radicle) portion of plants and seedlings in Cucurbitaceae.1,6,19–26
Figure 3.
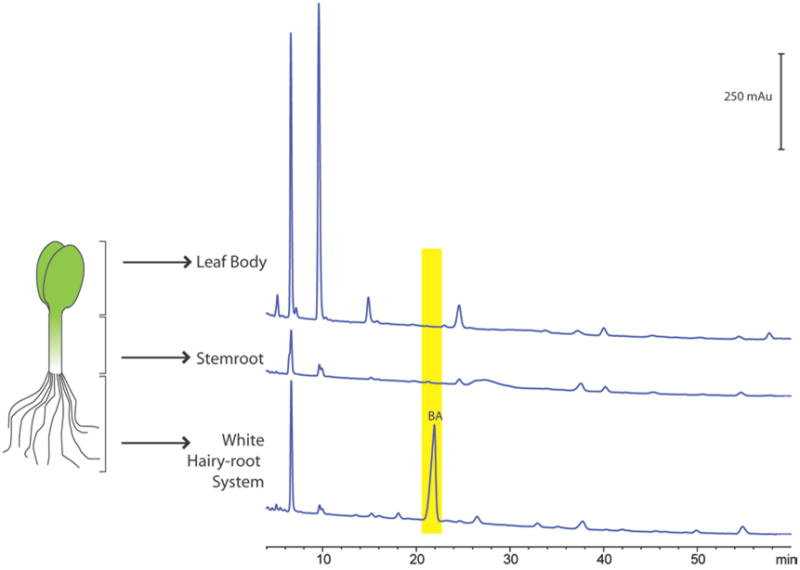
HPLC traces for extracts from the fine hairy root, stemroot, and dicotyledon leaf body of 14 day germinations. Bryonolic acid is detected only in the root portion of Cucurbita pepo L.
To address scalability in biomass accumulation we compared two germination methods which are standard in the field: moist blotting paper and peat-based growth media. In both cases, seeds and germinations of C. pepo L. were maintained in a medium that retained a moist environment, but did not contribute any level of nutrients to the germinations. For both approaches, roots were isolated every two days and evaluated for BA content by HPLC. For germinations grown in a peat-based media, root isolation was continued for 40 days. The timecourse and HPLC trace overlay show increasing production of BA from day 2 to day 16, peaking at 1.26 mg g−1 dry weight (Figure 4a, c). BA content subsequently decreased from day 16 to day 40, suggesting that BA presence in the roots is most prevalent during the early germination stage. Decreased BA content after day 16 may also be suggestive of further localization of BA to the fine hairs and extremities of the root system; these grew increasingly delicate with age and became more difficult to recover during the washing process.
Figure 4.
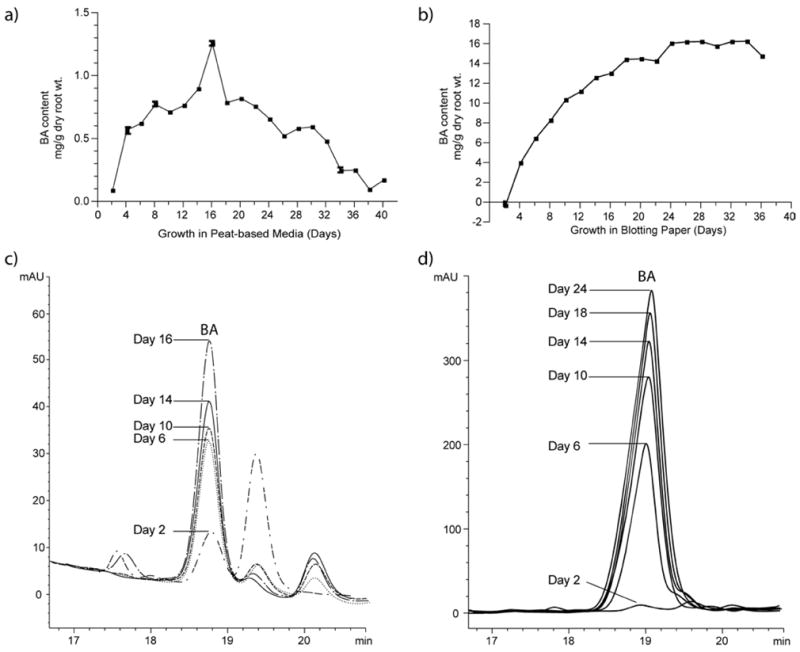
Bryonolic acid production in Cucurbita pepo L. roots under two growth conditions, peat-based media (a) and moist blotting paper (b). Bryonolic acid content is observed to increase from day 2 to day 16 in peat-based media and from day 2 to day 24 in moist blotting paper, as observed by growing HPLC peak areas (c and d). At its maximum, BA content in roots from moist blotting paper is roughly tenfold greater than that detected in roots from peat-based media, as is apparent in the respective scales.
In a parallel experiment, germinations were grown between moist blotting paper for 36 days. Germinations were not continued to day 40 as in the peat-based media method due to overgrowth and initial signs of morbidity by day 26. Under these growth conditions, BA content increased from day 2 to day 24 and plateaued thereafter, leveling at approximately 15 mg g−1 dry weight (Figure 4b). Presumably, BA content changed little after germinations no longer maintained vitality. The increased BA content can be observed by HPLC peak height on select days between day 2 and day 24 (Figure 4d), and reached a maximum of 16.1 mg g−1 dry weight.
Taken together, BA content in roots from moist blotting paper is roughly tenfold that detected in roots from peat-based media. Maximum and total BA production per unit mass in roots from blotting paper germinations far outweighed the total amount produced in the peat-based growth media (Figure 5a). On those days resulting in maximum BA content (day 16 for peat-based, day 24 for blotting paper) the magnitude of BA production in germinations grown in blotting paper is apparent in the HPLC trace overlays for these respective days (Figure 5b). In germinations grown in blotting paper, we do not observe a decrease in BA content following achievement of maximum content. This may be due to the capability of retaining 100% of root material when isolated from the blotting paper as opposed to the unavoidable root loss experienced when isolating root material from the peat-based germination media as discussed above.
Figure 5.
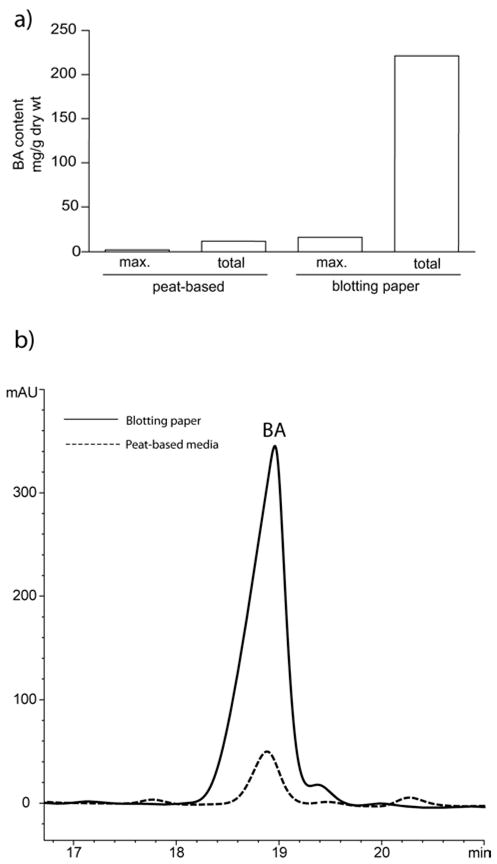
Comparison of maximum and total BA production in roots from peat-based media versus roots from moist blotting paper (a). Comparison of HPLC peak area for maximum BA production under both conditions (b).
To translate the above analytical scale observations to a large-scale isolation of BA we began scaling our blotting paper germinations of C. pepo L. Based on the data above, roots were collected from germinations grown between moist blotting paper for 18 days. At this time, germinations remained healthy and we anticipated that BA content was approaching its maximum (Figure 4b). Lyophilized and powdered roots were combined in a Soxhlet extractor, and subjected to a three day extraction using a solvent mixture of CHCl3 and MeOH to afford a BA rich extract. Subsequent column purification and recrystallization resulted in the isolation of 200 mg BA from 14.9 g dry roots (1.34%). The process proved to be further scalable, as the same procedure resulted in isolation of 949 mg BA from 80.5 g roots (1.18%). Our results indicate that the method is scalable and multiple gram quantities BA can be isolated proportionally from increased root masses.
Taken together, this is the first report to show that BA bioactivity is potentially due to induction of expression via the phase 2 response, as illustrated by the robust induction of HO-1 expression. This phenotype is of clear translational significance, and given the clear importance of malaria, it is axiomatic that future studies of malaria will necessarily include BA. Because plants from the Cucurbitaceae family are grown throughout the world and could serve as an abundant source of the natural product in developing countries where malaria is widespread, BA is likely to become the target of many future biological studies. With the purification strategy reported here, the therapeutic value of this compound can now be carefully explored.
Experimental Section
General Experimental Procedures
Melting points were measured on an Electrothermal melting point apparatus (Barnstead/Thermolyne) and are uncorrected. Optical rotation was determined using a Perkin-Elmer 241 polarimeter. NMR spectra were recorded on a Varian AS400 spectrometer operating at 400/100 MHz (1H/13C). Chemical shifts are reported in ppm using residual pyridine-d5 (δ 8.74 for 1H and 150.4 for 13C) as internal reference. HPLC separation was performed on an Agilent 1200 HPLC system and ZORBAX Eclipse XDB-C18 column (5 μm particle size, 80 Å pore size, 4.6 mm ID x 250 mm) fitted with a guard column. HPLC operation and data analysis was performed using Agilent ChemStation for LC 3D systems software, Rev. B.04.01.
Reagents and Chemicals
Cucurbita pepo L. seeds (Spineless Beauty hybrid) were purchased from Siegers Seed Co (Holland, MI). The peat-based medium, a mixture of peat, vermiculite, and perlite (Pro-Mix BX, Premier Horticulture), was purchased from a local distributor. All solvents were purchased from Fisher Scientific (Pittsburgh, PA). Pyridine-d5 was purchased from Cambridge Isotope Laboratories (Andover, MA). The leukemic mouse macrophage cells (RAW 264.7) were obtained as a gift from Dr. Michael Sporn from Dartmouth College. The DMEM medium and PBS were obtained from ATCC (Manassas, VA), and were supplemented with heat inactivated fetal bovine serum (FBS) with low endotoxin (≤0.06 EU/mL) from Thermo Fisher Scientific (Waltham, MA). The penicillin- streptomycin, RIPA buffer, Novex 4-20% Tris-Glycine gel, and 0.2 μm PVDF membrane were from Invitrogen (Carlsbad, CA). The cells were induced with LPS from Escherichia coli purchased from Sigma Aldrich (St. Louis, MO) and dissolved in PBS (ATCC). The Complete Protease Inhibitor cocktail tablet was purchased from Roche (Indianapolis, IN). The heme oxygenase 1 (HO-1) primary rabbit polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA) while the secondary donkey anti-rabbit IgG (H+L)-HRP was from Southern Biotech (Birmingham, AL). The ECL Plus™ Western Blot Detection Reagent was purchased from GE Biosciences (Piscataway, NJ).
Germination
Twenty planters (14 × 7 × 4 in3) were filled to 3.5 inches with moistened growing medium. Ninety-six seeds were planted under 1.5 inches moistened growing medium. Planters were watered from the bottom for 15 min. Planters were covered until seedlings began to break through the media (Day 4). Following sprouting, planters were watered from the bottom for 20 min daily until plants were strong enough to be watered from the top (Day 8). Plants were subsequently watered with 500 mL water every other day for the 40 day growth duration.
Forty seeds were folded between a stack of moist blotting paper. Stacks were paired in a 9 × 12 in2 zip-seal bag, partially unsealed to allow for airflow. Twenty bags were placed in a dark incubator at 25.0 ºC for 3 d. Upon germination, the bags were transported to a university greenhouse where the temperature ranged from 20–29 ºC over the course of the 40 d growth duration. The bags were rotated each day and kept moist as needed to maintain 100% humidity.
Germination Harvest Procedure
Roots from germinations grown in peat-based media were clipped from the stems and cleared of residual peat-mixture in a water bath. Roots were blotted dry and frozen at −80 ºC. Roots from germinations grown in moist blotting paper were clipped from stems and frozen at −80 ºC. Stems and leaves from 14 day-old germinations were also separated for verification of anatomical localization of BA.
Calibration for Bryonolic Acid Content
Eight standard BA solutions were made to give concentrations ranging from 25-400 μg/mL. Forty μL of each solution was injected into the HPLC in triplicate. A linear gradient elution was applied at a flow rate of 1.0 mL/min for 20 minutes from 85% to 100% MeCN in H2O. Both solvents contained 0.02% TFA (v/v). Elution was monitored at 205 nm. The calibration curve for BA content was constructed by plotting the average peak area as a function of the analyte concentration.
Analytical Extraction and HPLC Procedure
Roots, stems, and leaves were dried by lyophilization and ground to a fine powder. The powder (200 mg) was extracted in 10 mL MeOH at reflux for 3 h. The filtered extract was brought to a volume of 10 mL with fresh MeOH and analyzed by HPLC, using 40 μL injections repeated in triplicate. For root extracts and evaluation of BA content, a linear gradient elution was applied at a flow rate of 1.0 mL/min for 20 min from 85% to 100% MeCN in H2O. For root, stem, and leaf extracts and evaluation of anatomical localization of BA, a linear gradient was applied at a flow rate of 1.0 mL/min for 60 min from 85% to 100% MeCN in H2O. Both solvents contained 0.02% TFA (v/v). The system was run at ambient temperature. Elution was monitored at 205 nm. BA was quantified by referencing peak area to the linear calibration generated with standard BA solutions.
Preparative Extraction and Purification Approach
Powdered roots of C. pepo L. germinations (14.9 g lyophilized) were extracted for 3 d by Soxhlet using 700 mL 2:1 CHCl3-MeOH heated to reflux with stirring. The extract was immobilized on silica gel (1:1) and evaporated to dryness. The crude extract was subjected to a short (2.5 in) silica gel column (I.D. 1.5 in) and the column was eluted with 80:20:1 hexanes-EtOAc-HOAc followed by 50:50:1 hexanes-EtOAc-HOAc followed by 85:15:1 EtOAc-hexanes-HOAc to yield fractions containing BA and two impurities as observed by TLC. BA-containing fractions were combined and repeated recrystallization in 40:1 CHCl3-THF resulted in the isolation of 200 mg BA (1.34%). BA purification from 80.5 g root extraction was executed similarly, only requiring 5 days for extraction and a larger chromatography column (I.D. 2.5 in).
Bryonolic Acid: white powder (CHCl3-THF); mp 274–278 °C (discoloration prior to melting began at 246–248 °C); [α25 D] +18 (c 15, pyridine); 1H NMR (pyridine-d5, 400 MHz) δ 1.02 (3H, s), 1.06 (3H, s), 1.08 (3H, s), 1.11 (3H, s), 1.23 (3H, s), 1.30 (3H, s), 1.44 (3H, s), 2.50 (1H, m), 2.60 (1H, d, J = 13.6) 2.78 (1H, d J=15.6), 3.39 (1H, t, J = 8.0); 13C NMR (pyridine-d5, 100 MHz) δ 17.1 (CH3), 18.5 (CH3), 20.1(CH2), 20.7(CH3), 21.6 (CH2), 22.9(CH3), 26.0 (CH2), 28.5 (CH2), 29.1 (CH3), 29.2 (CH2), 31.0 (CH2), 31.2 (CH2), 31.7 (CH2), 31.8 (C), 32.0 (CH3), 33.9 (CH3), 35.6 (CH2), 36.0 (CH2), 38.0 (CH2), 38.2 (C), 38.3 (C), 39.9 (C), 41.1 (C), 42.7 (C), 45.7 (CH), 51.4 (CH), 78.5 (CH), 134.7 (C), 135.1 (C), 181.8 (C). NMR spectra are shown in supplemental data and are consistent with published spectroscopic data for BA.24,26–28 Treatment of the isolated compound with diazomethane yields the expected methyl ester by comparison with reported 13C NMR data.29
Biological Data of Bryonolic Acid in RAW 264.7 Cells
RAW 264.7 cells were grown in DMEM medium with 10% FBS and 100 U/mL penicillin-100 μg/mL streptomycin. Cells were cultured in a 37 °C incubator with 5% CO2. The cells were plated at 2x106 cells/well in 6-well plates and allowed to attach for 2 hours. Cells were then induced with 5 ng/mL LPS from E. coli and treated with BA dissolved in DMSO at increasing concentrations (5, 10, 50, 100 μM) for 24 h with a final concentration of 0.25% DMSO in the cells. Cells were lysed using RIPA lysing buffer with a Complete Protease Inhibitor tablet per 10 mL lysing buffer. The lysates were loaded on a Novex 4-20% Tris-Glycine gel, transferred into 0.2 μm PVDF membrane and blocked with 5% milk for 1 h at room temperature. The membrane was probed for HO-1 (1:1000 dilution) for 1 h at room temperature and with a secondary donkey anti-rabbit antibody (1:5000 dilution) under the same conditions as the primary antibody. For detection of the bands, the blot was incubated with ECL Plus™ Western Blot Detection Reagent for 5 min at room temperature and quantified using GE Healthcare Typhoon 9400 Imager with the Image Quant software for band intensity calculation.
Supplementary Material
Acknowledgments
The RAW 264.7 cell line was provided by the Dr. Michael Sporn from Dartmouth College. This work was supported by PHS grant R03 CA132168 to G.P.T and J.J.L, AACR INNOVATOR award to G.P.T., SPUR summer funding to J.E.C. from HHMI, and predoctoral fellowships CA134211 from the NIH in addition to a Silber Fellowship from the American Cancer Society to T.N.G.
Footnotes
Supporting Information Available. 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Biglino G. Ann Chim. 1959;49:782–92. [Google Scholar]
- 2.Sim KY, Lee HT. Phytochemistry. 1972;11:3341–3. [Google Scholar]
- 3.Dayal R, Dobhal PC. J Med Aromat Plant Sci. 2001;23:660–661. [Google Scholar]
- 4.Biglino G, Cattel L, Caputo O. Ricerca Scientifica. 1969;39:207–9. [PubMed] [Google Scholar]
- 5.Tanaka S, Uno C, Akimoto M, Tabata M, Honda C, Kamisako W. Planta Med. 1991;57:527–30. doi: 10.1055/s-2006-960199. [DOI] [PubMed] [Google Scholar]
- 6.Takeda T, Kondo T, Mizukami H, Ogihara Y. Chem Pharm Bull. 1994;42:730–2. doi: 10.1248/cpb.42.730. [DOI] [PubMed] [Google Scholar]
- 7.Kondo T, Inoue M, Mizukami H, Ogihara Y. Biol Pharm Bull. 1995;18:726–9. doi: 10.1248/bpb.18.726. [DOI] [PubMed] [Google Scholar]
- 8.Akihisa T, Tokuda H, Ichiishi E, Mukainaka T, Toriumi M, Ukiya M, Yasukawa K, Nishino H. Cancer Lett. 2001;173:9–14. doi: 10.1016/s0304-3835(01)00689-9. [DOI] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Proc Natl Acad Sci U S A. 2005;102:4584–9. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. Cancer Res. 2005;65:4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 11.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. Cancer Res. 1999;59:336–41. [PubMed] [Google Scholar]
- 12.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB. Cancer Res. 1998;58:717–23. [PubMed] [Google Scholar]
- 13.Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. J Clin Invest. 2008;118:1266–76. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Proc Natl Acad Sci U S A. 2009;106:15837–42. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP, Mota MM. Nat Med. 2007;13:703–10. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 16.Cuadrado A, Rojo AI. Curr Pharm Des. 2008;14:429–42. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira A, Balla J, Jeney V, Balla G, Soares MP. J Mol Med. 2008;86:1097–111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 18.Droge W. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 19.Kamisako W, Morimoto K, Makino I, Isoi K. Plant and Cell Physiol. 1984;25:1571–4. [Google Scholar]
- 20.Tabata M, Tanaka S, Cho HJ, Uno C, Shimakura J, Ito M, Kamisako W, Honda C. J Nat Prod. 1993;56:165–74. doi: 10.1021/np50092a001. [DOI] [PubMed] [Google Scholar]
- 21.Saltykova IA, Matyukhina LG, Shavva AG. Khim Prir Soedinenii. 1968;4:324. [Google Scholar]
- 22.Cho HJ, Tanaka S, Fukui H, Tabata M. Phytochemistry. 1992;31:3893–6. [Google Scholar]
- 23.Isaev MI. Chem Nat Compd. 1995;31:336–41. [Google Scholar]
- 24.Akiyama K, Hayashi H. Biosci, Biotechnol, Biochem. 2002;66:762–769. doi: 10.1271/bbb.66.762. [DOI] [PubMed] [Google Scholar]
- 25.Kongtun S, Jiratchariyakul W, Kummalue T, Tanariya P, Kunnachak S, Frahm August W. Planta med. 2009;75:839–42. doi: 10.1055/s-0029-1185455. [DOI] [PubMed] [Google Scholar]
- 26.Khallouki F, Hull WE, Owen RW. Food Chem Toxicol. 2009;47:2007–2012. doi: 10.1016/j.fct.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Honda C, Suwa K, Takeyama S, Kamisako W. Chem Pharm Bull. 2002;50:467–474. doi: 10.1248/cpb.50.467. [DOI] [PubMed] [Google Scholar]
- 28.Kamisako W, Suwa K, Honda C, Isoi K, Nakai H, Shiro M, Machida K. Magn Reson Chem. 1987;25:848–55. [Google Scholar]
- 29.Kamisako W, Suwa K, Morimoto K, Isoi K. Org Magn Reson. 1984;22:93–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


