Abstract
This study determined the potential for neo tissue formation and the role of STRO-1+ cells in immature versus mature articular cartilage. Cartilage explants from immature and mature bovine knee joints were cultured for up to 12 weeks and stained with Safranin O, for type II collagen and STRO-1. Bovine chondrocyte pellet cultures and murine knee joints at the age of 2 weeks and 3 months and surgically injured cartilage were analyzed for changes in STRO-1 expression patterns. Results show that immature explants contained more STRO-1+ cells than mature explants. After 8 weeks in culture, immature explants showed STRO-1+ cell proliferation and newly formed tissue, which contained glycosaminoglycan and type II collagen. Mature cartilage explants showed only minimal cell expansion and neo tissue formation. Pellet cultures with chondrocytes from immature cartilage showed increased glycosaminoglycan synthesis and STRO-1+ staining as compared to pellets from mature chondrocytes. The frequency of STRO-1+ cells in murine knee joints significantly declined with joint maturation. Following surgical injury, immature explants had higher potential for tissue repair than mature explants. In conclusion, these findings suggest that the high percentage of STRO-1+ cells in immature cartilage changes with joint maturation. STRO-1+ cells have the potential to form new cartilage spontaneously and after tissue injury.
Keywords: Tissue neogenesis, cartilage, stem cells, STRO-1, aging
INTRODUCTION
Adult mature articular cartilage has limited capacity for remodeling, while fetal or immature cartilage has potential to repair cartilage lesions [1]. Current efforts in tissue engineering are directed at generating hyaline cartilage by using mesenchymal stem cells (MSCs) [2]. These cells can be characterized by the expression of putative cell surface markers such as CD29, CD44, CD71, CD73, CD90, CD105, CD146, CD166, Notch-1, Sca-1 and STRO-1 [3–9]. STRO-1 was initially reported to identify stromal precursors in bone marrow [5, 10] and STRO-1+ cells were then shown to have multipotential for differentiation towards adipocytes, osteoblasts and chondrocytes, suggesting that STRO-1 is a useful marker for mesenchymal progenitor cells [11, 12]. MSCs are present in various tissues, including bone marrow and adipose tissues [5, 10, 13], articular cartilage and synovium [14–16]. The superficial zone of immature bovine, equine and human articular appears to be the primary location of progenitor cells [3, 17, 18]. However, little is known on the fate of progenitor cells during cartilage maturation and their role in spontaneous and induced tissue formation and repair. In this study, we demonstrate differences in neo cartilage formation in immature and mature cartilage explants, and this correlated with differences in the frequency of STRO-1+ cells.
MATERIALS AND METHODS
Cartilage procurement and culture
Articular cartilage was obtained from bovine knees (n=24; Animal Technologies, Inc., TX). The animals were in two different age groups, 6–9 months of age, with the growth plate still present and thus skeletally immature [1, 19], and animals >2 years of age which are skeletally mature. Eight millimeter diameter cores of full thickness explants were extracted from distal femoral condyles as described previously [20]. The cartilage explants were cultured in DMEM supplemented with 10% calf serum (CS) for the first 2 days and then in 2 % CS thereafter to remove the CS effect on chondrocyte viability [20]. Medium was changed every two or three days. Chondrocytes were isolated by collagenase digestion of immature and mature articular cartilage and cells pellets were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with ITS, 0.1 mM ascorbic acid 2-phosphate, 10−7 M dexamethasone (Sigma-Aldrich, St. Louis, MO), 1.25 mg/ml human serum albumin (Bayer, Leverkusen, Germany), and 10 ng/ml TGF-β1 (Peprotech, Rocky Hill, NJ) [21]. Pellets were harvested at 2 and 14 days after culture for analysis.
Murine knee joints
Wild type murine knee joints, from C57BL/6 mice at the age of 2 weeks and 3 months were examined by immunohistochemistry to determine the influence of cartilage maturation on STRO-1 expression. Knee joints were de-calcified and immunohistochemical analysis was performed.
Histology and immunohistochemistry
Cartilage explants, chondrocyte pellets and murine knee joints were fixed in zinc-buffered formalin (Z-Fix; Anatech, Battle Creek, MI) for 24 hours and embedded in paraffin. Paraffin embedded samples were sectioned at 4 µm, deparaffinized in a xylene substitute, PRO-PAR CLEARANT (Anatech, Battle Creek, MI), passed through an ethanol series and finally placed in water for rehydration. Sections were stained with safranin O/fast green [22]. For immunohistochemistry sections were washed with PBS, sections were blocked with 0.1% Tween 20 with 3% normal goat serum for 30 minutes at room temperature. Primary antibodies STRO-1 (Mouse IgM, R&D Systems, Minneapolis, MN at 1 µg/ml), anti-type II collagen (II-II6B3; Hybridoma Bank, University of Iowa at 2 µg/ml) and negative controls (normal mouse IgG and IgM; 1 µg/ml) were applied and incubated for 1 hour at room temperature. After washing with PBS, sections were incubated with biotinylated goat anti-mouse IgM for STRO-1 and IgG for type II collagen (Vector Laboratories, Burlingame, CA) as secondary antibody for 30 minutes. Sections were then incubated with HRP (Zymed) for 10 minutes at room temperature and stained with 3,3-diaminobenzidine (DAB; Vector Laboratories) substrate for 3–10 minutes.
Quantification and localization of signals throughout cartilage
STRO-1 localization throughout each cartilage zone was assessed systematically by counting positive and negative cells in a 50µm × 50µm grid (using a 40× field objective) starting from the cartilage surface to the deep zone. The cartilage zones were identified based on cell shape and organization. Briefly, superficial zone (SZ) cells predominate within the first 50 µm (top 10%) and the middle zone (MZ) represented next 40% of total tissue height. Deep zone (DZ) represented the remaining 50% [23]. Positive cells were counted in the original cartilage explants (n=12). This was repeated for a minimum of 5 times (in 50µm × 50µm grids) at the center of the explants, to avoid the tissue edges that may be affected by the harvesting procedure.
Cartilage injury model
Immature and mature bovine knee articular cartilage explants were harvested as above. The articular cartilage explants were surgically injured with a fresh scalpel (No.11) [24–26] by performing a vertical incision from the cartilage surface to the subchondral bone. The explants were cultured for up to 4 weeks and processed for safranin O staining to assess cartilage repair and via immunohistochemistry to determine changes in STRO-1 expression.
Statistical analysis
Statistically significant differences between two groups were determined with Student’s t-tests. The results are reported as mean ± S.D. P values less than 0.05 were considered significant.
RESULTS
Maintenance and formation of neo cartilage in immature and mature cartilage
Immature bovine cartilage explants did not show zonal cellular organization typical of mature cartilage, but a discrete surface zone with reduced safranin O staining and flattened horizontally aligned cells was identifiable (Fig.1A). During culture, the morphology of immature cartilage explants showed profound changes. After 2 weeks a new cell layer appeared on top of the surface (Fig. 1B). By 4 and 8 weeks of culture, the upper third of immature articular cartilage expanded (Fig.1C, D). In contrast, there was no apparent change in the shape of the mature explants (Fig. 1E–H). Immature cartilage explants maintained safranin O staining, while mature explants showed progressive loss of GAG and cells (Fig. 1D, H). New cells also appeared on top of the superficial zone (SZ) of mature cartilage by 8 weeks (Fig. 1H), but the number of cells was lower compared to immature cartilage.
Figure 1.
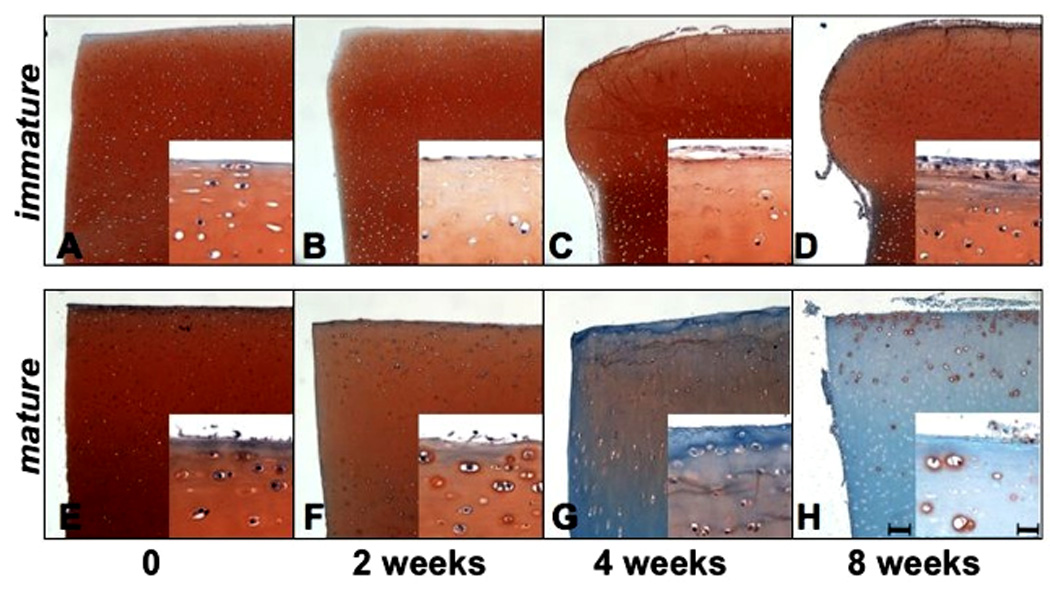
Immature (A–D) and mature (E–H) bovine articular cartilage explants were cultured for up to 8 weeks and stained with safranin O / fast green (n=24). The immature explants retained safranin O staining and expanded with increasing time in culture. The mature explants showed a time dependent loss of safranin O staining and no increase in tissue volume. Bar = 100µm in lower and 25µm in higher magnifications.
Expression of STRO-1+ cells during long-term culture
Immunohistochemistry for STRO-1 was performed with immature and mature bovine articular cartilage at tissue harvest and 8 weeks after culture in DMEM with 2% CS (Fig. 2A–H, n=12). The freshly harvested immature cartilage explants (day 0) contained STRO-1+ cells in the upper third of the samples (Fig. 2A, E). The newly formed tissue at 8 weeks contained many STRO-1+ cells, whereas only few positive cells were found in the original cartilage (Fig. 2B, F). Numbers of STRO-1+ cells in freshly isolated mature cartilage were lower compared to the immature samples (Fig. 2C, G) and diminished even further after 8 weeks in culture (Fig. 2D, H). STRO-1+ cells in original explants of immature and mature cartilage were measured in specific cartilage zones. Numbers of positive cells in superficial and mid zone were significantly higher in freshly harvested immature explants than after 8 weeks in tissue culture. The number of STRO-1+ cells was reduced and the typical zonal distribution was lost by 8 weeks of culture (I, **P< 0.01).
Figure 2.
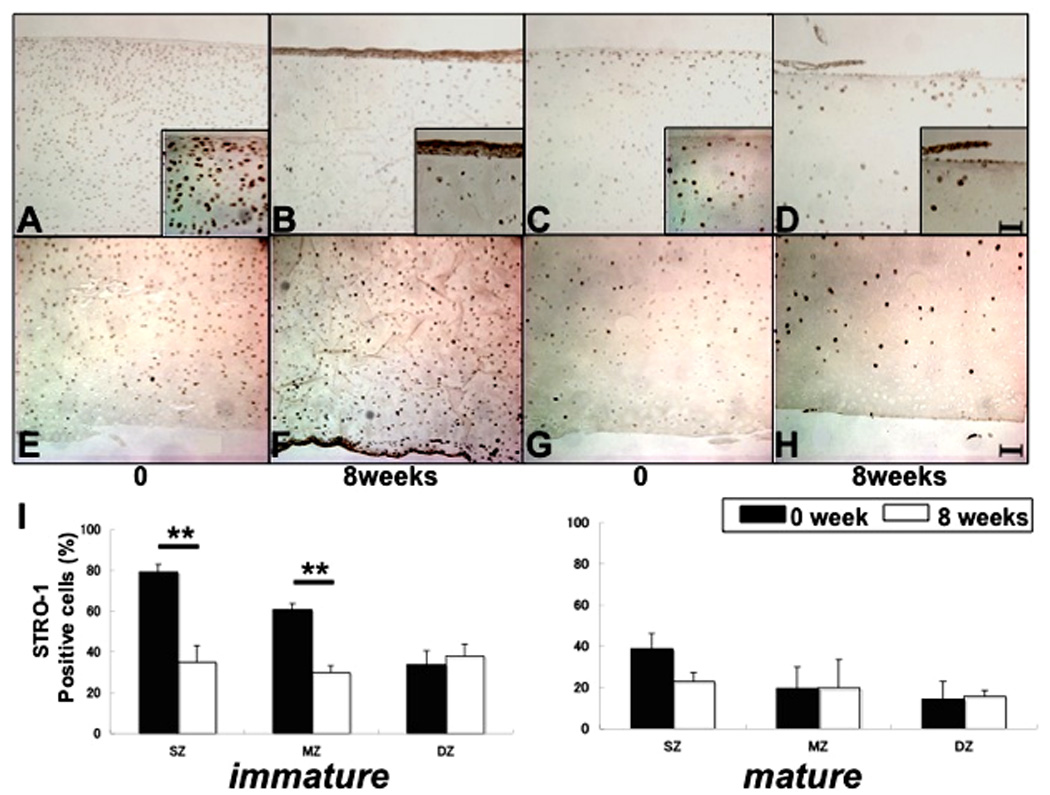
STRO-1 expression in immature and mature cartilage explants. Immunohistochemistry for STRO-1 was performed with immature (A,B;E,F) and mature (C,D;G,H) cartilage at 0 and 8 weeks culture (A–H, n=12). Upper images show superficial zone (SZ) to middle zone (MZ) (A–D), and lower images show mid zone (MZ) to deep zone (DZ) (E–H). Positive cells were counted in each zone (I, **P< 0.01). In fresh explants (0 weeks) immature explants contained significantly higher numbers of STRO-1+ cells in the SZ and MZ as compared to the DZ and the frequency of STRO-1+ cells was significantly higher in immature as compared to mature explants. More STRO-1+ cells were found in immature as compared to mature explants in the SZ and MZ. After 8 weeks in culture the frequency of STRO-1+ cells was reduced in the SZ and MZ of immature explants. Bar = 100µm in lower and 25µm in higher magnifications.
After 12 weeks of culture, nodules of newly formed tissue could be observed emanating from the SZ and the corner of cut edges of the immature explants (Fig. 3A–D). Many STRO-1+ cells were present in the neo tissue but not in the original tissue suggesting that this was the result of STRO-1+ cell proliferation (Fig 3E,H, I). Importantly, these nodules showed homogenous interterritorial and strong pericellular safranin O staining (Fig. 3F, J), and were positive for type II collagen expression. These findings demonstrate that immature cartilage explants have the potential for neo cartilage formation (Fig. 3G, K).
Figure 3.
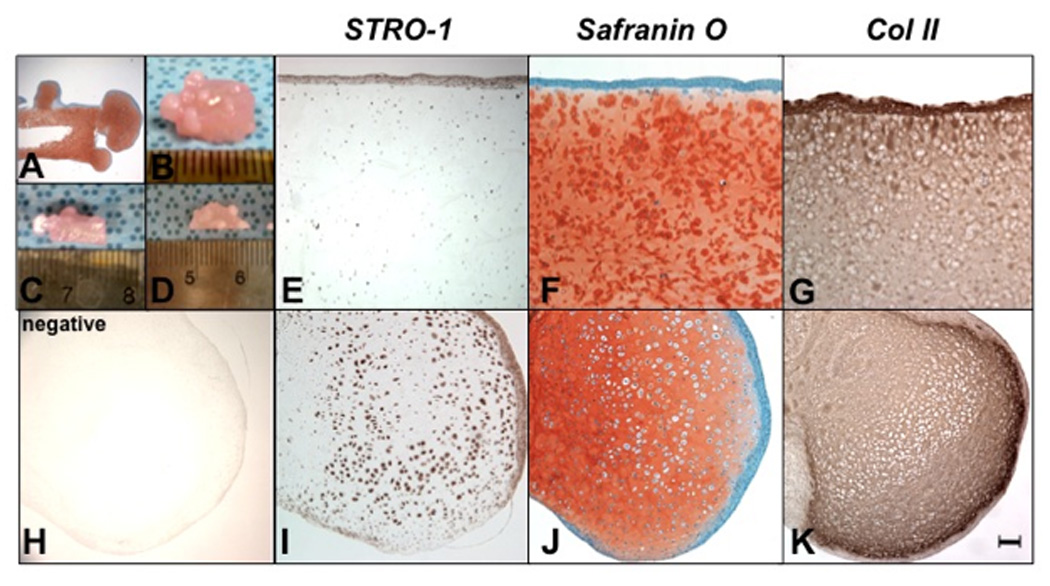
Neo tissue formation in cultured immature explants. The immature articular cartilage explants were cultured for 12 weeks (n=3) and sections were stained with safranin O (A, F, J), with STRO-1 (E, I) negative control (Mo IgM) for STRO-1 (H) and for type II collagen (Mo IgG) (G, K). STRO-1+ cells appeared around the explants (F) and in the newly formed tissue (J), which is positive for type II collagen and safranin O staining (F, J). The magnification is 20× (A) and Bar = 100µm (E–K).
STRO-1 expression in chondrocyte pellet and murine knee joints
STRO-1 expression and extracellular matrix formation were determined in three-dimensional pellet cultures with cells from immature and mature bovine cartilage (n=12). The pellets were stained with safranin O (Fig. 4A–D) and with antibody to STRO-1 (Fig. 4E–H). Pellets with immature chondrocytes synthesized more GAG than mature pellets after 2 weeks of culture as seen with safranin O staining. STRO-1 expression in pellets with cells from immature cartilage was stronger than in the pellets with cells from mature cartilage (Fig. 4E–H).
Figure 4.
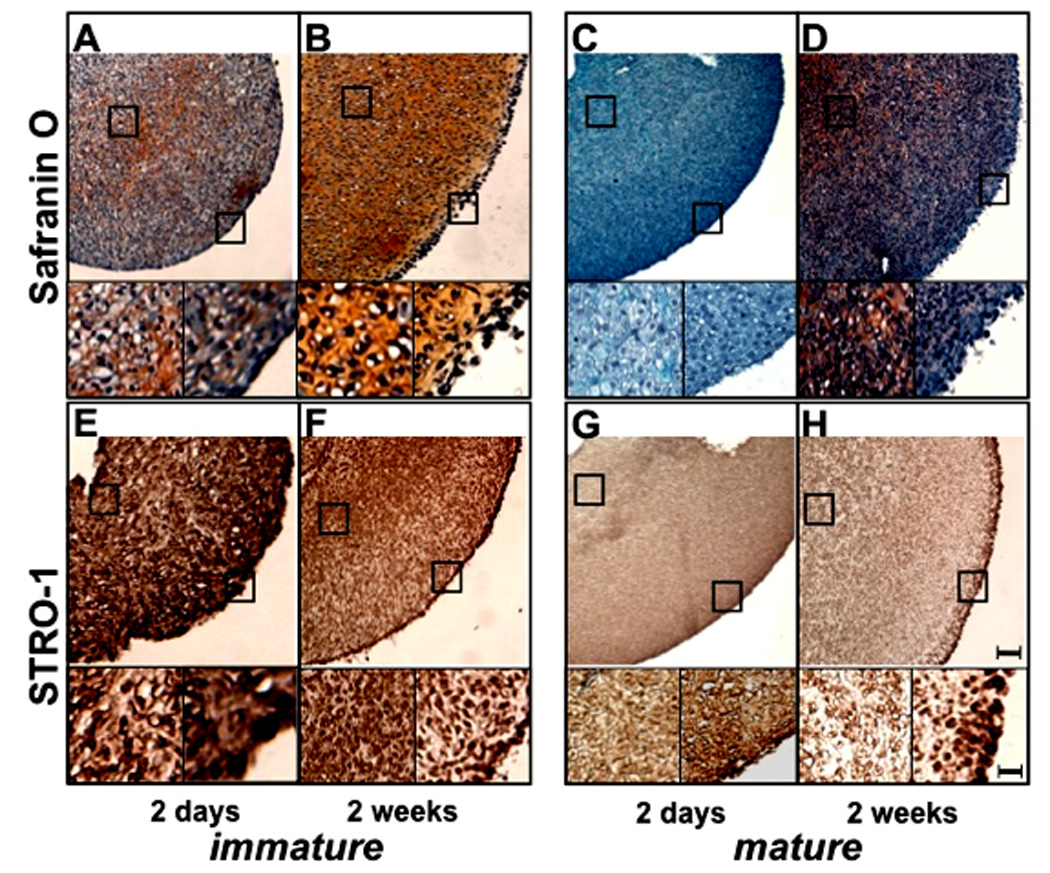
Pellet cultures with cells from immature and mature cartilage.
Pellet cultures were performed with cells from immature and mature bovine cartilage (n=12) and collected after 2 and 14 days and stained with safranin O (A–D) and STRO-1 (E–H). Pellets with immature chondrocytes showed stronger staining with safranin O and contained more STRO-1+ cells (B, F) as compared to pellets composed chondrocytes from mature cartilage (D, H). Bar = 100µm in large and 25µm in small images.
To address changes in STRO-1 during joint maturation, we analyzed murine knee joints (n=6). In knee cartilage of 2 weeks old mice, STRO-1+ cells were abundant in all layers of cartilage. This was significantly reduced as joint maturation occurred by the age of 3 months. At that time STRO-1+ cells were restricted to the SZ and MZ (Fig. 5C, D). The number of STRO-1+ cells in immature knee joints were significantly higher than in mature knee joints (Fig. 5E).
Figure 5.
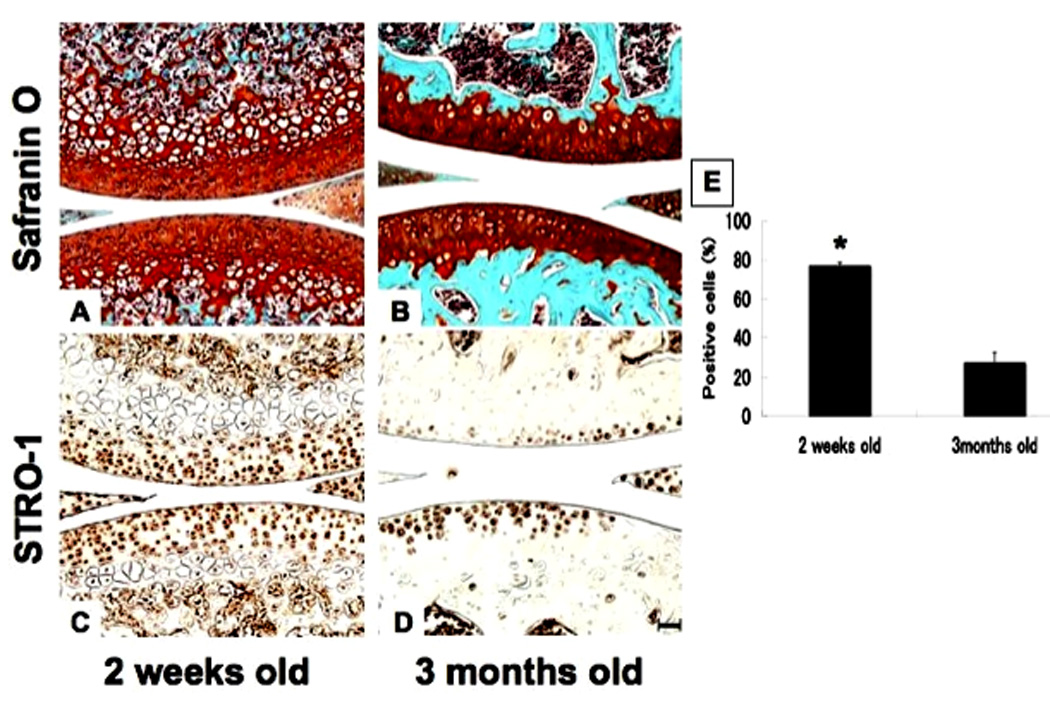
STRO-1 expression in immature and mature mouse knee joints. Knee joints from C57/BL6 mice at the age of 2 weeks (immature; A, C) and 3 months (mature; B, D) were processed for safranin O staining and immunohistochemistry for STRO-1 detection (n=6). Percent positive cells in all layer of cartilage were compared in immature and mature knee joints (E, *: P<0.01). STRO-1+ cells were significantly reduced in mature joints. Bar = 100µm.
Cartilage repair and STRO-1 expression after injury
Immature and mature bovine cartilage explants (n=8 each) were subjected to surgical injury and cultured for up to 4 weeks. Immature cartilage explants did not show a reduction in safranin O staining intensity and neo tissue filled the defect area after 4 weeks of culture (Fig. 6A,B). In contrast, mature cartilage explants showed loss of safranin O staining in the areas surrounding the defects and the defects were not filled with new tissue (Fig. 6C,D). The new matrix in the defect of immature explants had lower cell density as compared to the surrounding tissue, but the majority of the cells in the newly formed tissue were STRO-1+. The original tissue adjacent to the defect in immature explants also had a higher percentage of STRO-1+ cells as compared to mature explants (Fig. 6F, H).
Figure 6.
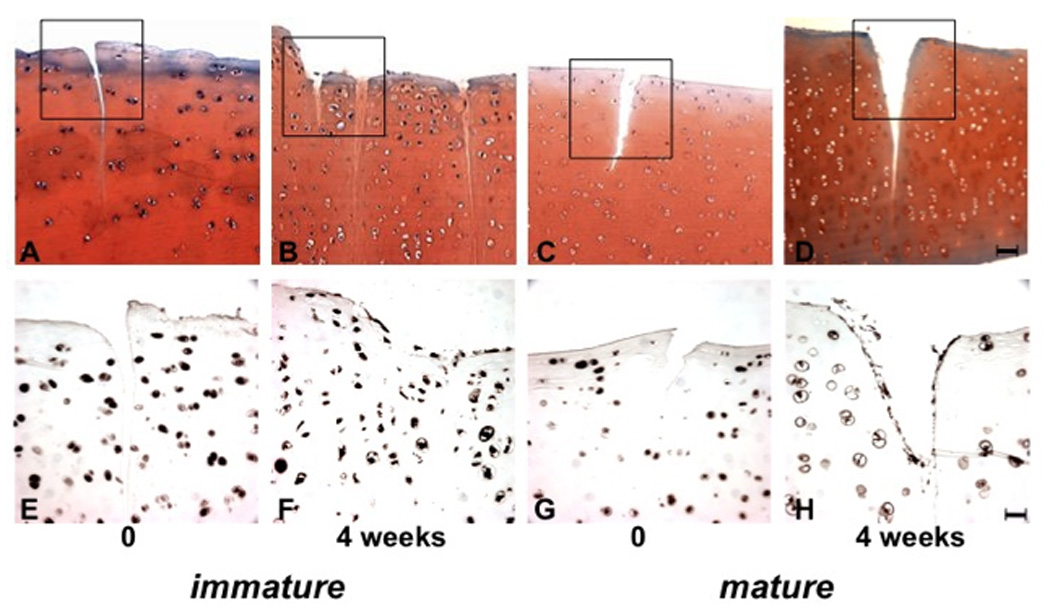
Tissue response to surgical injury. Immature and mature cartilage explants were subjected to surgical injury and processed immediately or after 4 weeks culture for safranin O staining (A–D) and STRO-1 immunohistochemistry (E-H, n=8). The cartilage defects in immature tissues were filled with new extracellular matrix, which was not seen in mature explants. Bar = 100µm (A–D) in safranin O and 25µm in STRO-1 staining (E–H).
DISCUSSION
The limited repair capacity of mature articular was already recognized in the 18th century [27]. Subsequent reports demonstrated that fetal and immature cartilage has the potential to respond to injury with the formation of a new cartilage-like tissue [1, 28]. Insight into mechanisms that account for these differences and that mediate responses in immature tissue have the potential to support approaches towards pharmacological stimulation of tissue repair and engineering. The present study utilized immature and mature bovine articular cartilage explant cultures as models of spontaneous tissue regeneration and we observed that i) cells in immature cartilage explants have higher potential for neo tissue formation, as compared with mature tissues; ii) mature cartilage possess a lower capacity to maintain tissue integrity in vitro with a progressive GAG loss over time in culture; iii) cells in the SZ and top of MZ appear to have the greatest potential to synthesize neo cartilage since the tissue neo genesis was observed especially on the top layer and corners of the cartilage explants and finally; iv) neo tissue formation initiated from a response of cells that express STRO-1, a marker of mesenchymal progenitor cells. From this data we conclude that this neo tissue formation was the result of a chondroprogenitor population located primarily in the upper cartilage layers and with greater frequency in immature tissue.
A previous study reported on the cellular origin of neocartilage formation and showed high levels of cell proliferation in SZ, DZ and wound edge [29]. Here we show similar new tissue formation in immature cartilage explants, however the localization is different, mainly at SZ and corners of explants. To characterize cells involved in neo tissue formation we focused on STRO-1, a widely used marker of mesenchymal progenitor cells [11, 12]. While several recent studies reported the presence of stem cells in articular cartilage, the expression of STRO-1 has not been reported and differences in frequency and localization of STRO-1+ cells during joint maturation were unknown. The present study demonstrates that immature articular cartilage from bovine and murine contains high numbers of STRO-1+ cells that are present in the upper third of tissue height and edge of the explants, or throughout all zones of cartilage as seen in immature murine joints. As articular cartilage matures and the discrete zones evolve the percentage of STRO-1+ cells decreases and these cells become more concentrated in the SZ. We also examined Notch-1 expression and it showed similar distribution as STRO-1 (data not shown). Several differences between immature and mature cartilage were observed in regards to STRO-1+ cells and new tissue formation during explant culture. New tissue spontaneously formed in immature cartilage and this contained large numbers of STRO-1+ cells, suggesting that these cells proliferate and are responsible for neo tissue production. The neo tissue was strongly positive for type II collagen and safranin O, indicating that the STRO-1+ cells in immature cartilage are committed to differentiation towards articular chondrocytes without the requirement for exogenous stimuli that promote this process. We also used a cartilage injury model to determine the response of STRO-1+ cells. Interestingly, in immature explants there was spontaneous filling of the surgical defect with new extracellular matrix and cells in this new tissue as well as in the areas around repaired lesions showed many STRO-1+ positive cells. However mature explants did not display any attempt to fill cartilage defects and showed weaker STRO-1 staining, adjacent to the defect. This suggests that STRO-1+ cells play a critical role in cartilage repair. Although more detailed investigations concerning the progenitor potential of STRO-1+ cells in articular cartilage is needed, STRO-1 might be considered a putative marker of cartilage self-repair capacity. If our hypothesis is proven correct, STRO-1+ cells may represent a suitable cell population for applications in cartilage repair and tissue engineering.
ACKNOWLEDGEMENTS
We thank Lilo Creighton for excellent technical support. The work was supported by NIH grants AG07996 and AG033409 (ML), AR050631 (HA) and by a Postdoctoral Fellowship from the Arthritis Foundation (SO).
REFERENCES
- 1.Vasara AI, Hyttinen MM, Pulliainen O, et al. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage. 2006;14:1066–1074. doi: 10.1016/j.joca.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 4.Saalbach A, Haustein UF, Anderegg U. A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882–888. doi: 10.1046/j.1523-1747.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 5.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 6.Majumdar MK, Thiede MA, Mosca JD, et al. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Barry FP, Boynton RE, Haynesworth S, et al. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 8.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Graves SE, Ohta S, et al. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 11.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 12.Neumann K, Dehne T, Endres M, et al. Chondrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral cortico-spongious bone. J Orthop Res. 2008 doi: 10.1002/jor.20635. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 14.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruger B, Giurea A, Wanivenhaus AH, et al. Endothelial precursor cells in the synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2004;50:2157–2166. doi: 10.1002/art.20506. [DOI] [PubMed] [Google Scholar]
- 16.Alsalameh S, Amin R, Gemba T, et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 17.Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Ustunel I, Ozenci AM, Sahin Z, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008;110:397–407. doi: 10.1016/j.acthis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Pulliainen O, Vasara AI, Hyttinen MM, et al. Poly-L-D-lactic acid scaffold in the repair of porcine knee cartilage lesions. Tissue Eng. 2007;13:1347–1355. doi: 10.1089/ten.2006.0347. [DOI] [PubMed] [Google Scholar]
- 20.Otsuki S, Brinson DC, Creighton L, et al. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58:1076–1085. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 21.Barbero A, Grogan S, Schafer D, et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Zemmyo M, Meharra EJ, Kuhn K, et al. Accelerated, aging-dependent development of osteoarthritis in alpha1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki S, Taniguchi N, Grogan SP, et al. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntley JS, Simpson AH, Hall AC. Use of non-degenerate human osteochondral tissue and confocal laser scanning microscopy for the study of chondrocyte death at cartilage surgery. Eur Cell Mater. 2005;9:13–22. doi: 10.22203/ecm.v009a03. [DOI] [PubMed] [Google Scholar]
- 25.Amin AK, Huntley JS, Bush PG, et al. Chondrocyte death in mechanically injured articular cartilage-the influence of extracellular calcium. J Orthop Res. 2008 doi: 10.1002/jor.20809. [DOI] [PubMed] [Google Scholar]
- 26.Huntley JS, McBirnie JM, Simpson AH, et al. Cutting-edge design to improve cell viability in osteochondral grafts. Osteoarthritis Cartilage. 2005;13:665–671. doi: 10.1016/j.joca.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Hunter W. Of the Structure and Diseases of Articulating Cartilages. Philosophical Trans of the Royal Society. 1743;42:514–521. [Google Scholar]
- 28.Yamamoto T, Wakitani S, Imoto K, et al. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthritis Cartilage. 2004;12:636–641. doi: 10.1016/j.joca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Bos PK, Kops N, Verhaar JA, et al. Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthritis Cartilage. 2008;16:204–211. doi: 10.1016/j.joca.2007.06.007. [DOI] [PubMed] [Google Scholar]


