Abstract
Background
The aim was to collate all myasthenia gravis (MG) epidemiological studies including AChR MG and MuSK MG specific studies. To synthesize data on incidence rate (IR), prevalence rate (PR) and mortality rate (MR) of the condition and investigate the influence of environmental and technical factors on any trends or variation observed.
Methods
Studies were identified using multiple sources and meta-analysis performed to calculate pooled estimates for IR, PR and MR.
Results
55 studies performed between 1950 and 2007 were included, representing 1.7 billion population-years. For All MG estimated pooled IR (eIR): 5.3 per million person-years (C.I.:4.4, 6.1), range: 1.7 to 21.3; estimated pooled PR: 77.7 per million persons (C.I.:64.0, 94.3), range 15 to 179; MR range 0.1 to 0.9 per millions person-years. AChR MG eIR: 7.3 (C.I.:5.5, 7.8), range: 4.3 to 18.0; MuSK MG IR range: 0.1 to 0.32. However marked variation persisted between populations studied with similar methodology and in similar areas.
Conclusions
We report marked variation in observed frequencies of MG. We show evidence of increasing frequency of MG with year of study and improved study quality. This probably reflects improved case ascertainment. But other factors must also influence disease onset resulting in the observed variation in IR across geographically and genetically similar populations.
Background
Myasthenia gravis (MG) is an archetypal autoimmune disorder in which muscle weakness occurs as a result of impairment of neuromuscular transmission. It is likely to occur as the result of a number of disease entities that result in an indistinguishable clinical picture [1]. There are paraneoplastic forms (thymoma-associated) and non-paraneoplastic forms and the disorder is immunologically heterogeneous - for example serum antibodies can be detected to muscle acetylcholine receptors (AChR-Ab) [2] or the muscle-specific receptor tyrosine kinase (MuSK-Ab) [3], but not both in the same patient. From case series and epidemiological studies, a bimodal distribution of MG IR has been frequently described suggesting a hormonal or environmental influence on disease onset. MG occurs in both sexes, at all ages and in all races [4].
A large number of MG epidemiological studies have been performed worldwide over the last 60 years with marked variability in observed incidence and prevalence of the disease. Systematic review of MG epidemiology has been carried out in the past by Phillips [5]; but 28 further studies have been performed since and this review predates the discovery of MuSK-Ab MG. In his review, Phillips commented upon apparent increasing IR and PR with time but without similar change in MR, and proposed that these trends were due to improved diagnosis and a changing natural history of disease related to better treatment.
The aim of this review was to summarize the findings of all population-based epidemiological studies of myasthenia gravis (MG) paying attention to serological subtype-specific studies and age- and sex- specific incidence. We sought to establish if there is a consensus in IR, PR and MR of MG worldwide and investigate any trends or differences in observed rates over time and in populations.
Methods
To identify all relevant studies, 4 medical databases (Pubmed, Medline, EMBASE, Cochrane library) and 2 proceedings databases (Zetoc, ISI proceedings) were searched using 21 search terms as keywords or MESH terms (Additional file 1 shows search strategy and terms used). English and non-English studies were included and non-English studies were translated when required. The bibliographies of all included studies were searched and the final list was discussed with experts in the field (Professor Angela Vincent, Neurosciences group, Oxford; Dr J McConville, Belfast). Good overlap between sources assured almost complete ascertainment of relevant studies (See additional file 1).
All population-based epidemiological studies of myasthenia gravis were included and case series without a defined denominator population were excluded. Inclusion criteria for MG cases in individual studies were broadly based on Osserman clinical criteria [6] with supportive evidence from anti-acetylchoinesterase responsiveness, neurophysiological and serological testing. There are no universally accepted and validated criteria for the diagnosis of MG therefore we accepted all cases as defined by the authors. The majority of studies considered all patients with a clinical diagnosis of MG together (henceforth referred to as All MG). A proportion of studies performed in the past 20 years examined serological MG subgroups separately: anti-acetylcholine receptor antibody positive MG (AChR-MG) and anti-muscle specific kinase antibody positive MG (MuSK-MG). There were no epidemiological studies on seronegative MG (SNMG).
Crude data (number of incident cases, number of prevalent cases, prevalent population, number of population years studied, and number of sources used for case ascertainment, inclusion and exclusion criteria, final-year of study and country of study) were extracted using a piloted questionnaire. IR, PR and MR and exact 95% confidence intervals (CI) based upon the Poisson distribution were calculated using STATA (Copyright 1996-2009 Stata Corp LP). Where the relevant crude data were available (9 studies), a standardised IR was calculated to WHO standard world population [7]. There was no significant difference between crude and standardised rates so crude rates are used throughout this review. Each included study was graded as 'High', 'Intermediate' and 'Low' quality according to number of ascertainment sources used and number of person years studied (details in additional file 1). Search strategy, study selection, data extraction and quality grading were performed by AC and independently conducted by JMcC with good agreement.
Meta-analysis was performed using the random effects model with 95% confidence intervals to the Poisson distribution. Heterogeneity was tested using Chi-squared test and measured using the I2 statistic [8]. A value of I2 < 25% was chosen to represent an appropriate level of homogeneity for calculation of a pooled estimate. Meta-regression analysis was used to investigate the association between rates and study characteristics such as year, latitude and quality of study. Publication bias was assessed using funnel plots.
Results
From 4030 electronic hits, 114 papers were read in full and 55 selected for inclusion in the review (Additional file 2: Included studies; Additional file 3: Excluded studies).
The 55 studies [4,9-62] included 8033 cases from 1.7 × 109 person years studied; 47 studies examined ALL MG, 9 studies examined AChR MG and 2 studies examined MuSK MG. One study provided rates on All MG, AChR MG and MuSK MG [54] and two provided rates for All MG and AChR MG within the same populations [50,55]. Additional file 2 lists all included studies, descriptive statistics and quality grading.
The time period studied ranged from 1950 to 2007. There was wide geographical distribution of studies, with representation of all continents except Australia (Figure 1). 34/52 studies have been performed in Europe with particular contribution from Scandinavian countries (9 studies), northern Italy (5 studies) and the UK (6 studies).
Figure 1.
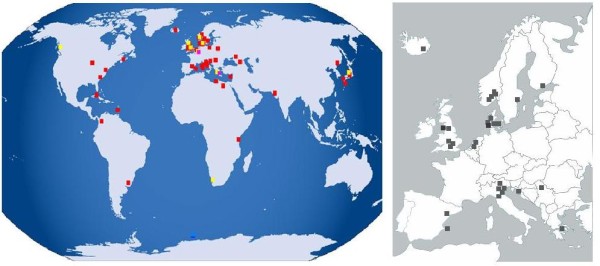
Geographical distribution of epidemiological studies in myasthenia gravis. Studies of all patients with autoimmune myasthenia gravis: RED. AChR MG specific studies: YELLOW. MuSK MG specific studies: PINK. Broad geographical distribution of included studies is shown with a preponderance of European studies.
Incidence rates
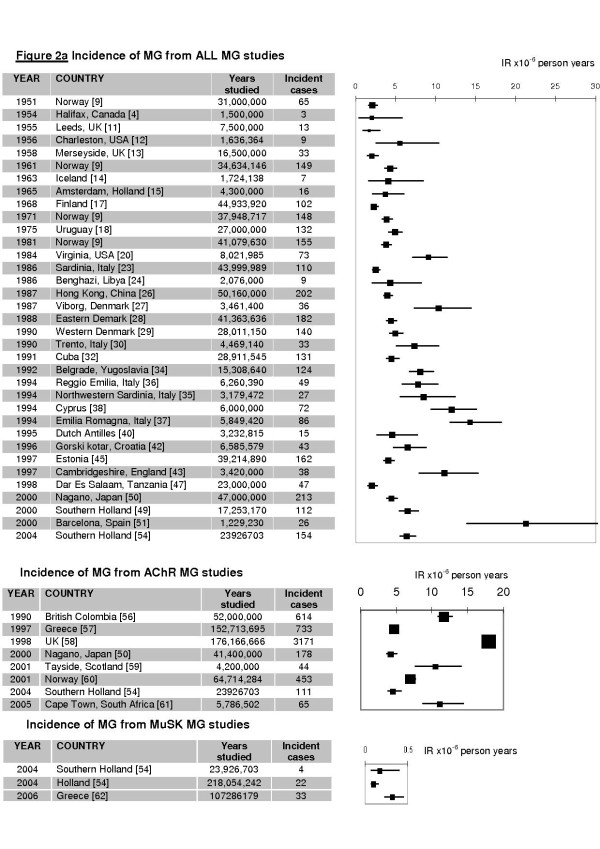
For the All MG group 35 studies examined incidence. IR ranged from 1.7 to 21.3 cases per million person-years (Figure 2a). The forest plot illustrates a marked variation in IR between studies, even when outliers are excluded [51]. Pooled estimate IR (eIR) was calculated as 5.3 per million person-years (C.I.: 4.41 - 6.12). However the marked heterogeneity across studies, I2 = 96% (C.I.: 95-98%) undermined the validity of the pooled estimate as a generalisable statistic.
Figure 2.
Incidence rates of myasthenia gravis. Incidence rate (IR) is shown in cases per million person years with 95% confidence intervals according to the Poisson distribution and weighting according to the random effects model. The variation between rates, or heterogeneity, leaves the estimated pooled IR difficult to interpret so it is better to summarise this data using ranges. All MG: estimated pooled IR (eIR) = 5.3 cases per million person years (C.I.: 4.41 - 6.1), range = 1.7 to 21.3; AChR MG: eIR = 7.3 (C.I.: 5.5, 7.8), range = 4.3 to 18.0; MuSK MG: range = 0.1-0.32.
Likewise, heterogeneity is seen in the AChR MG group (8 studies examined incidence, Figure 2b) with an I2 = 100%. AChR MG IR ranged from 4.3 to 18.0 per million person-years with a pooled AChR MG eIR of 7.3 per million person-years (C.I.: 5.5, 7.8).
Only 2 epidemiological studies have been performed to date on MuSK MG, in Holland [54] and Greece [62]. In Holland IR: 0.1 per million person-years (C.I.: 0.07, 0.15), and in Greece IR: 0.32 per million person-years (C.I.: 0.00, 1.32).
The observed heterogeneity might be explained by a number of factors; either biological or technical. We investigated how the effects of year of study, geographical area of study and study quality upon observed frequency.
Linear regression of IR against the final year of study suggests a significant correlation (Meta-regression p:0.0001, r2:0.12) equivalent to a 3% increase per year (Figure 3). In 1976 a version of the modern acetylcholine receptor antibody assay was first described [2]. There was no evidence of a reduction in heterogeneity when studies were grouped into those published before (I2 = 91%, C.I.:86, 94%) and after this date (I2 = 95%, C.I.:93, 96%). However, the average incidence rate after 1976 (eIR = 6.5 per million person-years, C.I.:5.3, 7.9) was significantly higher (t test: p = 0.0001) than before 1976 (eIR = 3.5 per million person-years, C.I.: 2.7, 4.4) corresponding to an approximate doubling (Rate ratio: 1.86, C.I.:1.22, 2.88).
Figure 3.
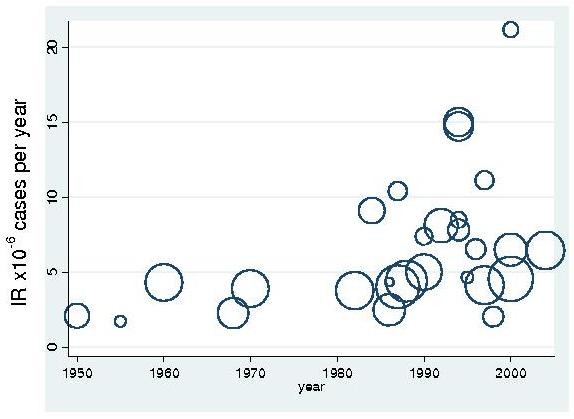
Incidence rate and prevalence rates with time. IR in cases per million person years and PR in cases per million persons are plotted against final year of study. The area each circle is proportional to the size of the study population.
There was no significant reduction in the heterogeneity of IR when studies were grouped by geographical area: Northern Europe I2 = 94% (C.I.:91, 95%), Southern Europe I2 = 97% (C.I.:96, 98%), North America and Canada I2 = 67% (C.I.:2, 89%). The limited numbers of studies in Asia, Africa or Central/South American sub-groups prevented similar analysis. There was no evidence of a difference (p = 0.47) between the area-specific pooled estimate IR: Northern Europe (eIR = 4.6 per million person-years, C.I.: 3.8, 5.7), Southern Europe (eIR = 4.9 per million person-years, C.I.: 4.9, 16.3), North America and Canada (eIR = 5.3 per million person-years, C.I.: 2.8, 10.0), Central and South America (eIR = 4.32 per million person-years, C.I.:3.8, 4.8) and Asia (eIR = 4.7 per million person-years, C.I.: 4.2, 5.3).
There was a trend to decreasing heterogeneity with increasing study quality: High (I2 = 76%, C.I.:49,89%), Moderate (I2 = 89%, C.I.:85,92%) and Low (I2 = 96%, C.I.:93, 97%). There was also evidence of a significant difference (p = 0.009) in the pooled estimate IR in the High quality studies (eIR = 9.4 per million person-years, C.I.:7.7, 11.4) and the pooled estimate IR in Intermediate and Low quality studies (eIR = 4.5 per million person-years, C.I.:3.8, 5.2).
Age and sex specific incidence rates
14 studies provided data required to allow analysis of incidence rate by age and sex (Figure 4). A bimodal distribution in IR in females was observed in 5 of 14 studies. IR in both sexes increased with age, peaking between 60 to 80 years in all but 2 studies, with apparent male predominance in the older age group. The Asian study stands out in that the proportion of childhood onset MG (onset < 15 years) appears to be higher in this population [26].
Figure 4.
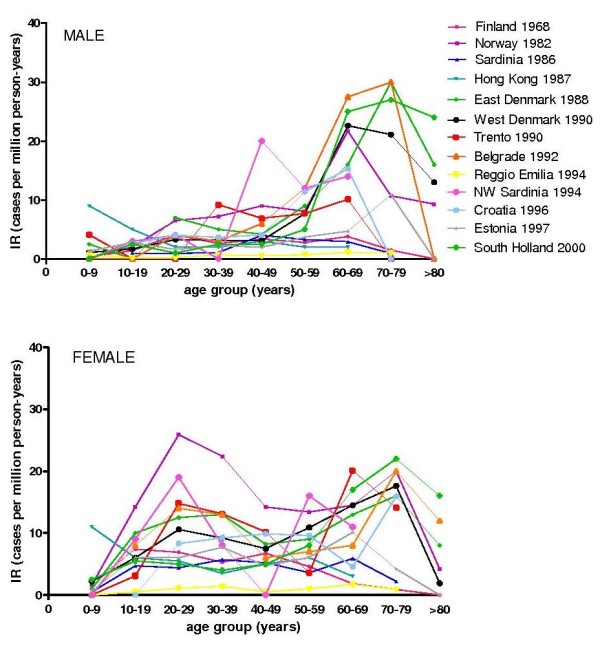
Age and sex specific incidence rates. Age and sex specific IR is shown in cases per million person years. An increasing frequency of disease with age is seen in all populations. However, only 5 out of 14 shown a bimodal distribution in IR with the peak in younger women not observed in the remaining 9 studies.
Prevalence rates
For the All MG group 44 studies examined prevalence. The observed PR ranged from 15 to 179 per million (Figure 5). The estimated pooled PR is 77.7 cases per million (C.I.: 63.98, 94.30) but, as for IR there was marked heterogeneity with I2 = 98% (C.I.: 97-98%) observed across studies limiting interpretation.
Figure 5.
Prevalence rates of myasthenia gravis. Prevalence rate (PR) is shown in cases per million persons with 95% confidence intervals (95% C.I.) according to the Poisson distribution and weighting according to the random effects model. All MG ePR = 77.67 cases per million (C.I.: 63.98, 94.30), range = 15 to 179 cases per million; AChR MG: range = 70.6 to 163.5; MuSK range = 1.9-2.9.
For the AChR MG group, 3 studies provided prevalence data. The prevalence of AChR MG ranged from 70.6 to 163.5 per million. The marked variation between rates is graphically depicted by the forest plot. The observed prevalence for MuSK MG in Southern Holland is1.9 per million (C.I.: 1.2, 2.6), representing 2% of prevalent MG cases in the region. MuSK-MG PR in Greece is higher at 2.9 per million (95% C.I.: 1.92, 3.92).
There was a linear trend to increasing PR with year of study (Meta-regression p = 0.0001, r2: 0.41). There is a doubling in PR after 1976; RR = 2.2 (C.I.: 1.36, 3.14). PR is on average 15-fold higher than IR for all studies (mean PR:IR ratio: 14.7, S.D.:6.0, range: 3.5 to 34.6), there is a trend to increasing PR:IR ratio with time (Linear regression p:0.0496, r2:0.12).
Mortality rates
Mortality rates as deaths due to MG per million person years were examined (Additional file 2). Seven All MG studies and 1 AChR MG study provided this information. The MR ranges from 0.06 to 0.89 per million person-years. The MR for AChR MG in Greece lies within this range: 0.43 per million person-years (C.I.:0.34, 0.55).
No trend was observed for MR with year of study.
Publication bias
Symmetrical funnel plots suggest absence of marked publication bias (Additional data file 1).
Discussion
Meta-analysis of observational studies is complicated by intrinsic differences between the populations being studied and variation in methodological quality of studies included; nevertheless the heterogeneity across studies in this review was marked. The degree of dissimilarity in IR, PR and MR of MG across populations renders the calculated pooled estimates of limited generalizability and validity. Nonetheless crude estimates are possible using the combination of pooled estimates and the range of observed frequencies. All MG eIR: 5.3 per million person-years (C.I.: 4.41, 6.12), range: 1.7 - 21.3; ePR: 77.67 cases per million (C.I.: 63.98, 94.30), range: 15 - 179; MR range: 0.06 - 0.89 per million person-years. AChR MG eIR: 7.3 per million person-years (C.I.: 5.5, 7.8), range: 4.3 - 18.0; PR range: 70.6 - 163.5 per million. MuSK MG IR range: 0.1 - 0.32 per million person-years; PR range: 1.9 - 2.9 cases per million.
In keeping with Phillips' observations [5] an increasing trend in IR and PR with time was observed. However this appears to be bimodal rather than linear in pattern, with estimated rates for incidence and prevalence approximately doubling around the mid-1980 s. This may be best explained by the influence of anti-AChR antibody receptor assay upon ascertainment but improving epidemiological methodology must also play a role. Our data shows a doubling of the pooled eIR in the 'High' quality studies when compared with that from the 'Low' and 'Intermediate' quality studies. As all 'High' quality studies were performed since 1987 it may be more accurate to use this data to estimate All MG incidence rate: eIR = 9.4 cases per million person years (C.I.:7.7, 11.4), range 7.4-14.7 cases per million person years. Despite a reduction in IR heterogeneity in this group it remained significant suggesting that these technical issues only contribute to the variation seen and do not explain it completely.
Several observations suggest that biological factors are important. Age and sex specific incidences show prominent differences between populations, with a peak in the incidence of MG in young women being observed in five of fourteen studies only. Childhood MG (onset < 15 years) was higher in the one Asian study [26] examined by age and sex. MuSK MG was described in only two population-based studies. Evidence from case series suggests differing frequency of MuSK MG across populations, with decreasing frequency with distance from the equator [63]. This suggests that at least some of the observed heterogeneity might be due to differing frequencies of MG subgroups between populations. Differences in population genetics may go some way to explain the variability in rates. For example, different HLA associations have been described in MG subsets: early onset MG, late onset MG, MuSK-MG. These HLA associations are different in North American/European populations versus Asian populations [1].
The greatest heterogeneity was observed in the AChR MG group. It might have been expected that these studies represent a more homogenous patient group than the broader All MG category. Five of nine studies in the AChR group were carried out with identical methodology. These 5 studies [55-58,60] were performed using records of anti-AChR testing from national or regional immunology laboratories, the assumption being that the first positive titre for an individual correlates with disease onset. If this were true for all AChR MG patients then any differences observed between the IR in the studies should be due to differences in population genetics and environmental factors influencing frequency of disease. For example the Greek study [57] and the UK study [58] were performed with identical methodology over similar time periods so the 3 fold difference in frequency is notable: Greek IR 4.8 per million person years (C.I.: 4.5, 5.2), UK IR 18.0 per million person years (C.I.: 17.4, 18.6). However this does not take into account the differing levels of access to a neurologist and access to serological tests between populations.
PRs were on average 15 fold higher than IR across studies. This difference could be explained by good survival and low mortality associated with MG, however, an older age of symptom onset probably limits overall survival therefore influencing PR. We also saw a trend to increasing prevalence compared to incidence (PR: IR ratio) with year of study which may suggest improving care and survival and increasing life expectancy.
It is notable that no data were available on seronegative MG. Its epidemiology, therefore, remains undescribed.
Conclusions
IR and PR of MG vary markedly between populations studied. Pooled incidence rates cannot readily be extrapolated to unstudied populations. The heterogeneity may be explained in part by methodological differences but the data also suggest that there are biological factors to account for differences. Detailed population-based data on serological and pathological sub-types of MG within complete populations are largely lacking.
Future studies should concentrate on accurate clinical case definition over adequate time periods in sufficiently sized populations. Factors influencing ascertainment such as equity of access to appropriate specialists in neurology and ophthalmology should be noted. National laboratory records of positive AChR or MuSK antibody titres used in isolation probably under-estimate rates so multiple sources of ascertainment should be used in order to optimise case identification. Serological sub-group classification is important and recently developed assays for the identification of MuSK MG and MG with antibodies to AChR clustered with rapsyn [64] will aid diagnosis and sub-group phenotype description.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AC designed and performed the literature review, collected data from individual studies, participated in statistical analysis, data interpretation and was primary author of the manuscript. CC participated in study design and performed statistical analysis. PMcC participated in study design and drafting of the final manuscript. JMcC conceived the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
MOOSE guidelines. Description of performance of the study in accordance to MOOSE (Meta-analysis of observational studies) guidelines including details of literature search.
All included studies. All included studies are listed with reference (ref), IR: Incidence rate (cases per million person years), PR: Prevalence rate (cases per millions), MR: Mortality rate (deaths due to MG per million person years), 95% C.I., S.E.: standard error and quality grading (1: High, 2: Intermediate, 3: Low).
All excluded studies. Table (iii)a List of studies excluded on examination of full text (or abstract only from proceedings papers) with reason for exclusion. Proceedings papers (P) Table (iii)b List of studies excluded on the basis of title and/or abstract from initial database searches (Medline, EMBASE and first 250 hits from Pubmed only).
Contributor Information
Aisling S Carr, Email: aisling.carr@belfasttrust.hscni.net.
Chris R Cardwell, Email: c.cardwell@qub.ac.uk.
Peter O McCarron, Email: peter.mccarron@sjog.ie.
John McConville, Email: john.mcconville@setrust.hscni.net.
Acknowledgements
The authors would like to acknowledge Professor Angela Vincent, Neurosciences Group, Oxford, UK for reviewing the list of included studies and for advice with this paper.
References
- Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–90. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom JM, Seybold ME, Lennon VA. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054–9. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Hoch WJ, McConville J, Helms S. Autoantibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Epidemiology of myasthenia gravis. Advances in Neurology. 1978;19:545–566. [PubMed] [Google Scholar]
- Phillips LH, Torner JC. Epidemiologic evidence for changing natural history of myasthenia gravis. Neurology. 1996;47:1233–1238. doi: 10.1212/wnl.47.5.1233. [DOI] [PubMed] [Google Scholar]
- Osserman KE, Genkins G. Studies in myasthenia gravis: review of twenty years experience in over 120 patients. Mt Sinai J Med. 1971;38(6):497–537. [PubMed] [Google Scholar]
- Age standardisation of rates: a new WHO standard (2000). Appendix D. [online] http://www.who.int/whosis/statistics/discussion_papers/pdf/paper31.pdf Accessed 2nd September 2008.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathisen Storm. Epidemiology of myasthenia gravis in Norway. Acta Neurol Scand. 1984. pp. 274–284. [DOI] [PubMed]
- Kurland LT. Descriptive epidemiology if selected neurologic and myopathic disorders with particular reference to a survey in Rochester Minnesota. J Chronic Disorders. 1958;8(4):378. doi: 10.1016/0021-9681(58)90002-X. [DOI] [PubMed] [Google Scholar]
- Garland H, Clark ANG. Myasthenia gravis, a personal study of 60 cases. BMJ. 1956;1:1259–1262. doi: 10.1136/bmj.1.4978.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter M, Rhett-Talbert O, Kurland LT. Myasthenia gravis in a southern community. Arch Neurol. 1960;3:65–69. doi: 10.1001/archneur.1960.00450040049006. [DOI] [PubMed] [Google Scholar]
- Pennington GW, Wilson A. In: Myasthenia Gravis. Proceedings of the second international symposium. Veits HR, editor. Springfield IL: Charles C Thomas; 1961. Incidence of myasthenia gravis in the Merseyside conurbation; pp. 337–45. [Google Scholar]
- Gudmundsson KR. The prevalence of some neurological diseases in Iceland. Acta Neurol Scand. 1968. pp. 55–69. [DOI] [PubMed]
- Oosterhuis. Epidemiologie dei myasthenie in Amsterdam. Neurologie Deutsche Jesells. 1977. pp. 103–108.
- Okinaka S, Reese HH, Katsuki S. The prevalence of multiple sclerosis and other neurological diseases in Japan. 1966. pp. 68–76. [DOI] [PubMed]
- Hokkanen E. Epidemiology of myasthenia gravis in Finland. J Neurol Sci. 1969. pp. 463–478. [DOI] [PubMed]
- Deffeminis Rospide HA, Petra de Mirabel M, Piazza de Silva N. Estudio epidemiologico de la miastenia en el Uruguay. Acta Neurol Latinoamer. 1975. pp. 53–65. [PubMed]
- Araki S, Uchino M, Yoshida O. Epidemiologic study of multiple sclerosis, myasthenia gravis and polymyositis in the city of Kumamoto, Japan. Clin Neurol. 1983;23:838–841. [PubMed] [Google Scholar]
- Phillips LH, Torner JC, Anderson MS, Cox GM. The epidemiology of myasthenia gravis in central and western Virginia. Neurology. 1992;42:1888–1894. doi: 10.1212/wnl.42.10.1888. [DOI] [PubMed] [Google Scholar]
- Kvirkveliia NB. Clinico-epidemiologic aspects of myasthenia in the Georgian SSR. Zh Nevropatol Psikhiatr Im S S Korsakova. 1986;86(3):327–30. [PubMed] [Google Scholar]
- Kondo K, Takasu T, Ahmed A. Neurological diseases in Karachi, Pakistan - elevated occurrence of subacute sclerosing panencephalitis. Neuroepidemiology. 1988;7:66–80. doi: 10.1159/000110138. [DOI] [PubMed] [Google Scholar]
- Giagheddu M, Puggioni G, Sanna G. Epidemiological study of myasthenia gravis in Sardinia Italy (1958-1986) Acta Neurol Scand. 1989;79:326–333. doi: 10.1111/j.1600-0404.1989.tb03793.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K, Thacker AK, Maloo JC, Gerryo SE, Mousa ME. Descriptive epidemiology of some rare neurological diseases in Benghazi, Libya. Neuroepidemiology. 1988;7(3):159–64. doi: 10.1159/000110150. [DOI] [PubMed] [Google Scholar]
- D'Alessamdro R, Granieri E, Benassi G. Comparative study on the prevalence of myasthenia gravis in the provinces of Bologna and Ferrera Italy. Acta Neurol Scand. 1991;83(2):83–88. doi: 10.1111/j.1600-0404.1991.tb04654.x. [DOI] [PubMed] [Google Scholar]
- Yu YL, Hawkins BR, Ip MS, Wong V, Woo E. Myasthenia gravis in Hong Kong Chinese: Epidemiology and adult disease. Acta Neurol Scand. 1992;86(2):113–119. doi: 10.1111/j.1600-0404.1992.tb05050.x. [DOI] [PubMed] [Google Scholar]
- Sorensen TT, Holm EB. Myasthenia gravis in the county of Viborg, Denmark. Eur Neurol. 1989. pp. 177–179. [DOI] [PubMed]
- Somnier FE, Keiding N, Paulson OB. Epidemiology of myasthenia gravis in Denmark: A longitudinal and comprehensive population survey. Archives of Neurology. 1991;48(7):733–739. doi: 10.1001/archneur.1991.00530190081019. [DOI] [PubMed] [Google Scholar]
- Christensen PB, Jensen TS, Tsiropoulos I. Mortality and survival in myasthenia gravis: a Danish population based study. JNNP. 1998;64(1):78–83. doi: 10.1136/jnnp.64.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Lovaste MG. Epidemiology of myasthenia gravis in the province of Trento (northern Italy) Neuroepidemiology. 1992;11(3):135–42. doi: 10.1159/000110923. [DOI] [PubMed] [Google Scholar]
- Christensen PB, Jensen TS, Tsiropoulos I. Incidence and prevalence of myasthenia gravis in western Denmark:1975-1989. Neurology. 1993;43(9):1779–83. doi: 10.1212/wnl.43.9.1779. [DOI] [PubMed] [Google Scholar]
- Cisernos AD, Luis RS, Leon R, Carrera PL. Some epidemiological aspects of myasthenia gravis in Cuba. Revista de Neurologia. 1996;24(128):435–9. [PubMed] [Google Scholar]
- Krivopusk ME. Clinico-epidemiological aspects of hereditary neuromuscular diseases in the Krasnodar territory. Zhurnal Nevropatologii I Psikhiatrii Imeni SS Korsakova. 1991;91(9):3–5. [PubMed] [Google Scholar]
- Lavrnic D, Jarebinski M, Rakocevic-Stojanovic V. Epidemiological and clinical characteristics of myasthenia gravis in Belgrade, Yugoslavia (1983-1992) Acta Neurologica Scandanavica. 1999;100(3):168–74. doi: 10.1111/j.1600-0404.1999.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Aiello I, Pastorino M, Sotgui S. Epidemiology of myasthenia gravis in northwestern Sardinia. Neuroepidemiology. 1997;16(4):199–206. doi: 10.1159/000109688. [DOI] [PubMed] [Google Scholar]
- Guidetti D, Sabadini R, Bondavalli M. Epidemiological study of myasthenia gravis in the province of Reggio Emilia, Italy. European Journal of Epidemiology. 1998;14(4):381–7. doi: 10.1023/A:1007449221638. [DOI] [PubMed] [Google Scholar]
- Study group on clinical and epidemiological problems in Neurology. Incidence of myasthenia gravis in the Emilia-Romagna region: a prospective multi-centre study. Neurology. 1998;51(1):255–8. doi: 10.1212/wnl.51.1.255. [DOI] [PubMed] [Google Scholar]
- Kyriallis K, Hristova A, Middleton I. What is the real epidemiology of myasthenia gravis? Neurology. 1995. p. A351.
- MacDonald BK, Sander WAS, Shorvom SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123:685–76. doi: 10.1093/brain/123.4.665. [DOI] [PubMed] [Google Scholar]
- Holtsema H, Mourik J, Rico RE. Myasthenia gravis on the Dutch Antilles: an epidemiological study. Clinical Neurology and Neurosurgery. 2000;102(4):195–198. doi: 10.1016/S0303-8467(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Villagra-Cocco A, Villagra-Cocco P. Prevalence of myasthenia gravis on the island of La Palma. Revista de Neurologia. 1997;25(148):2068–9. [PubMed] [Google Scholar]
- Zivadinov R, Jurjevic A, Willhein K, Cazzato G, Zorzon M. Incidence and prevalence of myasthenia gravis in the county of the coast and Gorski Kotar, Croatia. Neuroepidemiology. 1998;17(5):265–72. doi: 10.1159/000026179. [DOI] [PubMed] [Google Scholar]
- Robertson NP, Deans J, Compston DA. Myasthenia gravis: a population based epidemiological study in Cambridgeshire, England. J Neurol Neurosurg Psychiatry. 1998;65(4):492–496. doi: 10.1136/jnnp.65.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tallawy HN, Khedr EM, Qayed MH, Helliwell TR, Kamel NF. Epidemiological study of neuromuscular disorders in Assuit, Egypt. Neuroepidemiology. 2005;25(4):205–211. doi: 10.1159/000088674. [DOI] [PubMed] [Google Scholar]
- Oopik M, Kaasik AE, Jakobson J. A population based epidemiological study of myasthenia gravis in Estonia. JNNP. 2003;74(12):1638–43. doi: 10.1136/jnnp.74.12.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb B, Matell G, Pirskanen R, Lambe M. Epidemiology of myasthenia gravis: a population based epidemiological study in Stockholm, Sweden. Neuroepidemiology. 2002;21(5):221–5. doi: 10.1159/000065639. [DOI] [PubMed] [Google Scholar]
- Matuja WBP, Aris EA, Gabone J, Mgaya EM. Incidence and characteristics of myasthenia gravis in Dar Es Salaam, Tanzania. East African Medical Journal. 2001;78(9):473–476. doi: 10.4314/eamj.v78i9.8978. [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Uribe CS, Franco AF, Jimeniz ME, Arcos-Burgos OM, Palacio LG. Prevalence of myasthenia gravis in Antioquia, Colombia. Revista de Neurologia. 2002;34(11):1010–2. [PubMed] [Google Scholar]
- Wirtz PW, Nijnuis MG, Sotodeh M. The epidemiology of myasthenia gravis, Lambert Eaton myasthenic syndrome and their associated tumours in the northern part of Southern Holland. Journal of Neurology. 2003;250(6):698–701. doi: 10.1007/s00415-003-1063-7. [DOI] [PubMed] [Google Scholar]
- Matssuda M, Dohi-Iijima N, Nakamura A. Increase in incidence of elderly onset patients with myasthenia gravis in Nagano Prefecture, Japan. Internal Medicine. 2005;44(6):572–7. doi: 10.2169/internalmedicine.44.572. [DOI] [PubMed] [Google Scholar]
- Aragones JM, Bolibar I, Bonfill X. Myasthenia gravis: a higher than expected incidence in the elderly. Neurology. 2003;60(6):1024–6. doi: 10.1212/01.wnl.0000050461.05432.c5. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Rose NR, Kalaydjian A, Pederson MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. Journal of Autoimmunity. 2007;29(1):1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov SV, Neretin VI, Agafonov BV, Sidorova OP. Population based study of myasthenia in Moscow region. Zhurnal Nevrologii I Psikhiatrii Imeni SS Korsakova. 2006;106(5):52–5. [PubMed] [Google Scholar]
- Niks EH, Kuks JB, Verschuuren JJ. Epidemiology of myasthenia gravis with anti-muscle specific kinase antibodies in The Netherlands. JNNP. 2007;78(4):417–8. doi: 10.1136/jnnp.2006.102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somnier FE. Increasing incidence of late onset anti-AChR antibody-seropositive myasthenia gravis. Neurology. 2005;65:928–930. doi: 10.1212/01.wnl.0000176067.32186.a3. [DOI] [PubMed] [Google Scholar]
- Oger J, Wang L, Aziz T. The age of onset and the incidence of myasthenia gravis are increasing in British Columbia (Canada) Journal of the Neurological Sciences. 2002;199(suppl 1):S45–S45. [Google Scholar]
- Poulas K, Tsibri E, Kokla A. Epidemiology of seropositive myasthenia gravis in Greece. JNNP. 2001;71(3):352–6. doi: 10.1136/jnnp.71.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Clover L, Buckley C, Grimley-Evans J, Rothwell PM. Evidence of underdiagnosis of myasthenia gravis in older people. JNNP. 2003;74(8):1105–8. doi: 10.1136/jnnp.74.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia ME. A limited epidemiological study of seropositive myasthenia gravis in Tayside. Scottish Medical Journal. 2002;46(7):132–135. doi: 10.1177/003693300204700604. [DOI] [PubMed] [Google Scholar]
- Heldal AT, Owe JF, Gilhus NE. Acetylcholine receptor antibodies in Norwegian myasthenia gravis patients. European Journal of Neurology. 2008;15(suppl 3):154. [Google Scholar]
- Bateman KJ, Schinkel M, Little F, Leidenberg L, Vincent A, Heckmann JM. Incidence of seropositive myasthenia gravis in Cape Town and South Africa. South African Medical Journal. 2007;97(10):959–62. [PubMed] [Google Scholar]
- Tsiamalos P, Kordas G, Kokla A. Epidemiological and immunological profile of muscle-specific kinase myasthenia gravis in Greece. European Journal of Neurology. pp. 925–930. [DOI] [PubMed]
- Vincent A, Leite MI, Farrugia ME. Myasthenia Gravis seronegative for acetylcholine receptor antibodies. Ann N Y Acad Sci. 2008;1132:84–92. doi: 10.1196/annals.1405.020. [DOI] [PubMed] [Google Scholar]
- Leite MI, Jacob S, Veigas S. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain. 2008;131(7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MOOSE guidelines. Description of performance of the study in accordance to MOOSE (Meta-analysis of observational studies) guidelines including details of literature search.
All included studies. All included studies are listed with reference (ref), IR: Incidence rate (cases per million person years), PR: Prevalence rate (cases per millions), MR: Mortality rate (deaths due to MG per million person years), 95% C.I., S.E.: standard error and quality grading (1: High, 2: Intermediate, 3: Low).
All excluded studies. Table (iii)a List of studies excluded on examination of full text (or abstract only from proceedings papers) with reason for exclusion. Proceedings papers (P) Table (iii)b List of studies excluded on the basis of title and/or abstract from initial database searches (Medline, EMBASE and first 250 hits from Pubmed only).