Abstract
Mice deficient in superoxide dismutase 1 (Sod1−/− mice) develop many features seen in patients with age-related macular degeneration (AMD) including choroidal neovascularization (NV). We sought to determine if the absence of SOD1 contributes to the pro-angiogenic environment in the subretinal space or whether it is completely secondary to other changes in Bruch’s membrane and the retinal pigmented epithelium (RPE) that precede the development of choroidal NV. Expression of vascular endothelial growth factor (VEGF) in photoreceptors or ischemia resulted in significantly more NV in Sod1−/− compared to Sod1+/+ mice. The compromised antioxidant defense system in Sod1−/− mice contributes to the pro-angiogenic environment, because treatment of Sod1−/− mice with a mixture of antioxidants caused a significant reduction in ischemia-induced retinal NV. Wild type mice treated with the same antioxidants also showed reduced ischemia-induced retinal NV, reduced VEGF-induced subretinal NV, and reduced choroidal NV at Bruch’s membrane rupture sites. These data suggest that reactive oxygen species contribute to several types of ocular NV. This could explain why in the Age-Related Eye Disease Trial, antioxidant treatment reduced conversion from non-neovascular to neovascular AMD and severe vision loss, and suggest that potent antioxidants should be considered for other diseases complicated by ocular NV.
Introduction
Age-related macular degeneration (AMD) is a prevalent cause of vision loss in developed countries characterized by accumulation of extracellular deposits (drusen) beneath the retinal pigmented epithelium (RPE), diffuse thickening of Bruch’s membrane, gradual death of RPE cells and photoreceptors in the macula, and sporadic development of choroidal neovascularization (NV). The development of choroidal NV occurs in a minority of patients with AMD (10–20%), but is responsible for the majority of severe loss of vision. The pathogenesis of choroidal NV is not well understood. It is clear that increased production of vascular endothelial growth factor (VEGF) plays an important role, because VEGF antagonists suppress choroidal NV in animal models (Kwak et al., 2000; Kryzstolik et al., 2002; Saishin et al., 2003) and provide substantial benefit in patients with neovascular AMD (Brown et al., 2006; Rosenfeld et al., 2006). However, it is not known how VEGF is upregulated nor is it known if one or more of the other features of AMD contribute to the development of choroidal NV.
The Age Related Eye Disease Study (AREDS) has suggested that at least some of the other features of AMD may contribute to the development of choroidal NV, because large confluent drusen and/or pigmentary changes (hypopigmentation, a sign of RPE dropout, and hyperpigmentation, a sign of RPE proliferation) in the macula are risk factors for the development of choroidal NV (Age-Related Eye Disease Study Research Group, 2001). It also showed that antioxidants reduce progression to advanced stages of AMD, either choroidal NV or severe geographic atrophy (death of RPE and photoreceptors in the macula). Thus, it appears that macular drusen, pigmentary changes, and oxidative stress contribute to the conversion from non-neovascular to neovascular AMD, but it is not clear how they interact. It may be that oxidative stress simply accelerates progression of drusen, thickening of Bruch’s membrane, and death of RPE and photoreceptors which in turn promote choroidal NV by some other mechanism such as chemoattraction of pro-angiogenic macrophages. Alternatively, oxidative stress may contribute to a pro-angiogenic environment in the eye by a mechanism that is independent of anatomic changes which act to enable the development of choroidal NV in the presence of pro-angiogenic stimuli induced by oxidative stress.
The absence of an animal model of AMD has hampered the study of the pathogenesis of choroidal NV, but it has recently been observed that mice with targeted deletion of superoxide dismutase 1 (Sod1−/− mice) show many of the features of AMD, including drusen, thickening of Bruch’s membrane, pigmentary changes, and spontaneous development of choroidal NV (Imamura et al., 2006). SOD1 is a component of the antioxidant defense system of the retina and its absence results in increased basal levels of oxidative stress and reduced ability to cope with oxidants (Dong et al., 2006). In this study, we sought to determine if Sod1−/− mice develop a pro-angiogenic environment in the subretinal space and retina prior to the onset of visible anatomic changes characteristic of AMD.
Materials and Methods
Mice
Mice with targeted disruption of the Sod1 gene, 129S7-Sod1tmlLeb/J (Sod1−/− mice), β-actin promoter/Sod1 transgenic mice (C57BL/6-TgN(SOD1)3Cje/J; SOD1 overexpressors), and wild type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). These mice and rhopdopsin promoter/Vegf transgenic mice (Rho/VEGF mice) that express VEGF in photoreceptors under control of the rhodopsin promoter (Okamoto et al., 1997) were treated in accordance with the recommendations of the Association for Research in Vision and Ophthalmology and the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
VEGF-induced subretinal neovascularization
Male Sod1−/− mice were crossed with female Sod1+/+ mice that were homozygous at the Rho/Vegf transgene locus (RhoVegf+/−). Sod1+/−Rho/Vegf+/− offspring were crossed to obtain several litters. At postnatal day (P) 21, the mice were genotyped (Okamoto et al., 1997) and perfused with 1 ml of phosphate buffered saline (PBS) containing 50 mg/ml of fluorescein-labeled dextran (2 × 106 average molecular weight; Sigma-Aldrich, St. Louis, MO). Retinal flat mounts were prepared and the number of neovascular lesions on the outer surface of the retina and total area of neovascularization per retina were determined by image analysis as previously described (Tobe et al., 1998a) with the investigator masked with respect to genotype.
Oxygen-induced ischemic retinopathy
Oxygen-induced ischemic retinopathy was induced as previously described (Smith et al., 1994). Briefly, wild type C57BL/6 mice were crossed with Sod1−/− mice and Sod1+/− offspring were crossed to obtain several litters of mice that were placed in 75% oxygen at postnatal day (P) 7. They were returned to room air at P12 and at P17 retinal neovascularization was stained by in vivo immunostaining (Shen et al., 2007). Briefly, 1 μl containing 0.5 μg of rat anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM1) antibody (Pharmingen; San Jose, CA) was injected into the vitreous cavity under a dissecting microscope with a microinfusion pump (Harvard Pump Microinjection System; Harvard Apparatus Inc., South Natick, MA) and pulled glass micropipettes, as previously described (Mori et al., 2001). After 8 hours, mice were euthanized and eyes were removed and fixed in phosphate-buffered formalin at room temperature for at least 5 hours. Retinas were dissected intact and incubated with goat anti-rat IgG conjugated with Cy3 (1:800; Jackson ImmunoResearch Laboratory, West Grove, PA) or conjugated with Alexa 488 (1:400; Invitrogen, Carlsbad, CA) on a shaker at room temperature for 45 minutes. Retinas were washed, mounted on glass slides with mounting medium (Aquamount; Polysciences, Warrington, PA), and viewed with a fluorescence microscope (Nikon Instruments Inc., New York, NY) using imaging software (SPOT RT 3.4; Diagnostic Instruments, Sterling Heights, MI).
Treatment with antioxidants in models of ocular neovascularization
Starting at P7, Rho/VEGF transgenic mice were given daily intraperitoneal injections of vehicle or a mixture of antioxidants previously shown to suppress oxidative damage in the retina (Komeima et al., 2006) including α-tocopherol (200 mg/kg), ascorbic acid (250 mg/kg), and α-lipoic acid (100 mg/kg). At P21, the mice were perfused with fluorescein-labeled dextran and the number of neovascular lesions and total area of neovascularization per retina were measured as described above.
Four to five week old C57BL/6 female mice had laser-induced rupture of Bruch’s membrane at 3 locations in each eye as previously described (Tobe et al., 1998b). Daily intraperitoneal injections of vehicle or the mixture of 3 antioxidants was started the day prior to or immediately after laser photocoagulation. After 14 days, mice were perfused with fluorescein-labeled dextran and choroidal flat mounts were examined by fluorescence microscopy. Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used to measure the area of each CNV lesion with the investigator masked with respect to treatment group.
Results
Increased VEGF-induced subretinal NV in Sod1−/− mice
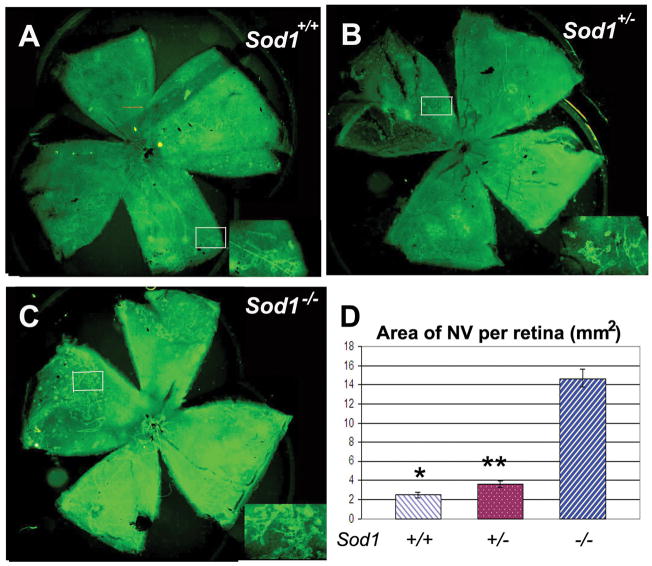
Mice deficient in SOD1 have been demonstrated to develop many of the phenotypic characteristics of age-related macular degeneration over time, including drusen, thickened Bruch’s membrane, and choroidal NV (Imamura et al., 2006). This raises an important question. Is the development of choroidal NV due solely to the other visible changes that precede it, or is the microenvironment in the subretinal space altered in a way that it is proangiogenic even prior to the development of visible changes in Bruch’s membrane? To address this question, Sod1−/− mice were crossed with Rho/VEGF transgenic mice in which the rhodopsin promoter drives expression of VEGF in photoreceptors (Okamoto et al., 1997). After the appropriate crosses, litters were obtained in which all mice were hemizygous at the transgene locus (RhoVegf+/−) and were either Sod1+/+, Sod1+/−, or Sod1−/−. At P21, mice were perfused with fluorescein-labeled dextran and fluorescence microscopy of retinal flat mounts showed a moderate number of neovascular tufts on the outer surface of the retinas of Sod1+/+RhoVegf+/− (Figure 1A) and Sod1+/−RhoVegf+/− mice (Figure 1B), while Sod1−/−RhoVegf+/− mice showed substantially more neovascular tufts (Figure 1C). At low magnification, the retinal vessels are seen in the background, but at high magnification, the narrow depth of field eliminates the background and the buds of neovascularization are more clearly seen on the outer surface of the retina (Figure 1D). Image analysis showed that compared to Sod1+/+RhoVegf+/− and Sod1+/−RhoVegf+/− mice, Sod1−/−RhoVegf+/− mice had significantly more neovascular lesions (Figure 1E) and significantly greater total area of NV per retina (Figure 1F). Thus Sod1−/− mice have a proangiogenic microenvironment in the subretinal space even before visible changes develop in Bruch’s membrane.
Figure 1. Mice deficient in superoxide dismutase 1 (Sod1−/−) show excessive amounts of VEGF-induced subretinal neovascularization compared to Sod1+/− or Sod1+/+ mice.
Rho/VEGF (RhoVegf+/−) transgenic mice that express VEGF in photoreceptors under control of the rhodopsin promoter were crossed with Sod1−/− and Sod1+/−RhoVegf+/− mice were crossed to obtain Sod1+/−RhoVegf+/+ mice which were crossed with Sod1+/−RhoVegf−/− mice to obtain several litters. At P21, the mice were genotyped and perfused with fluorescein-labeled dextran. The number of neovascular lesions and the total area of neovascularization on the outer surface of the retina were measured on retinal flat mounts by image analysis and afterward the genotype was unmasked. Sod1+/+RhoVegf+/− (A) and Sod1+/−RhoVegf+/− (B) appeared to have less neovascularization than Sod1−/−RhoVegf+/− mice (C). The neovascular tufts are indicated by arrows, but at this relatively low magnification the retinal vessels are seen in the background. At higher magnification (D), the narrow depth of field eliminates the background and the buds of neovascularization on the outer surface of the retina are seen more clearly (arrows) as is a feeder vessel (arrowhead) to the superior-most lesion. Image analysis showed that Sod1−/−RhoVegf+/− mice (n=10) had significantly greater mean (±SEM) number of neovascular lesions (E) and greater mean (±SEM) total area of neovascularization per retina (F) than Sod1+/+RhoVegf+/− (n=6) or Sod1+/−RhoVegf+/− (n=10).
*p=0.0015; **p=0.0007 by ANOVA with Dunnett’s correction for multiple comparisons. Bar = 100 μm
Increased ischemia-induced retinal NV in Sod1−/− mice
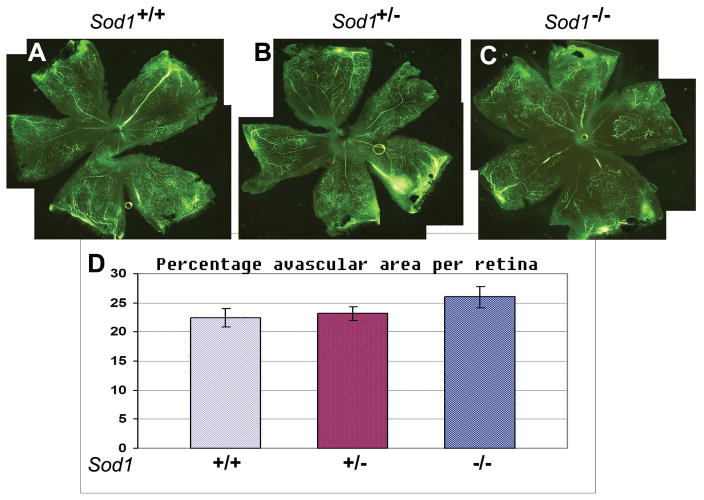
Next we wanted to determine if the proangiogenic environment in Sod1−/− mice was limited to the subretinal space or was generalized throughout the retina. To do this we utilized the murine model of oxygen-induced ischemic retinopathy in which ischemia-induced NV develops on the inner surface of the retina (Smith et al., 1994). Moderate amounts of NV were seen on the inner surface of the retina in Sod1+/+ (Figure 2A) and Sod1+/− mice (Figure 2B), while significantly greater amounts of NV were seen in Sod1−/− mice (Figure 2C and D). Similar experiments were done except the mice were perfused with fluorescein-labeled dextran at P12 after 5 days of hyperoxia. The amount of retinal nonperfusion appeared similar in Sod1+/+ (Figure 3A), Sod1+/− (Figure 3B), and Sod1−/− mice (Figure 3C). Measurement of the nonperfused area confirmed that there was no significant difference in the mean area of hyperoxia-induced nonperfusion among the 3 types of mice. This indicates that the increased retinal NV in Sod1−/− mice is not due to increased hyperoxia-induced nonperfusion and suggests that they have a proangiogenic environment throughout the retina and not just in the subretinal space.
Figure 2. Mice deficient in superoxide dismutase 1 (Sod1−/−) show excessive amounts of ischemia-induced retinal neovascularization compared to Sod1+/− or Sod1+/+ mice.
Litters containing Sod1−/−, Sod1+/−, and Sod1+/+ pups were placed in 75% oxygen at postnatal day (P) 7, returned to room air at P12, and euthanized at P17. Retinal neovascularization on the surface of the retina was visualized by staining for PECAM-1 as described in Sod1+/+ (A) and Sod1+/− mice (B), but in comparison, Sod1−/− mice appeared to have substantially more neovascularization (C). Insets show a high magnification view of the retinal neovascularization present within the box in the whole mounts. Measurement of the area of neovascularization per retina by image analysis with the investigator masked with respect to genotype showed a marked increase in the mean (± SEM) area of neovascularization in Sod1−/− mice (n=7) compared to Sod1+/+ (n=10) and Sod1+/− (n=8) mice (D).
*p=0.0005; **p=0.0005 by ANOVA with Dunnett’s correction for multiple comparisons.
Figure 3. Mice deficient in superoxide dismutase 1 (Sod1−/−) do not show increased hyperoxia-induced retinal nonperfusion compared to Sod1+/− or Sod1+/+ mice.
Litters containing Sod1−/− (n=8), Sod1+/− (n=10) and Sod1+/+ (n=10) pups were placed in 75% oxygen at postnatal day (P) 7, and perfused with fluorescein-labeled dextran at P12. Retinal flat mounts were visualized by fluorescence microscopy. The area of retinal nonperfusion appeared similar in Sod1+/+ (A), Sod1+/− (B), and Sod1−/− mice (C). Measurement of the areas of nonperfusion by image analysis with the observer masked with respect to genotype, showed no significant difference among the 3 types of mice (D).
Antioxidants reduce ischemia-induced retinal NV in Sod1−/− mice
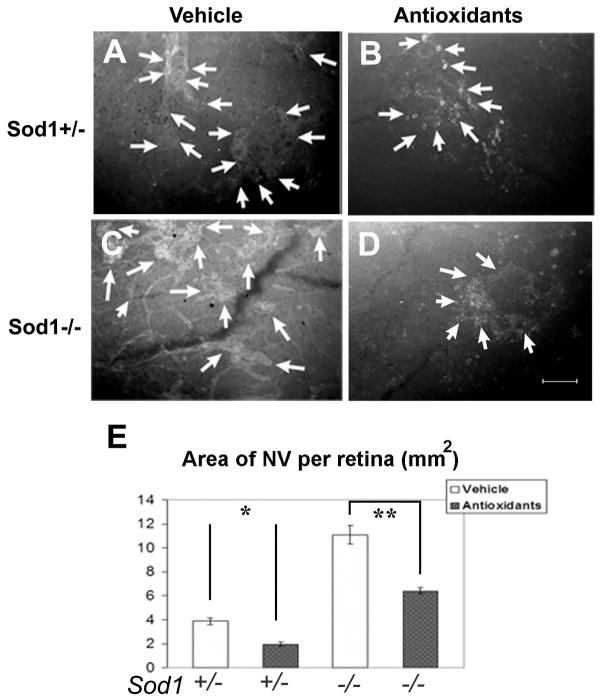
Sod1−/− mice have a compromised antioxidant defense system in the retina resulting in enhanced constitutive levels of oxidative stress and greater susceptibility to oxidative stress from administration of oxidants (Dong et al., 2006). To determine if it is the increased oxidative stress that contributes to the increase in NV in Sod1−/− mice, we used a mixture of antioxidants, α-tocopherol (200mg/kg), ascorbic acid (250mg/kg), and α-lipoic acid (100mg/kg), that reduce oxidative stress in the retina (Komeima et al., 2006). Compared to treatment with vehicle, treatment of Sod1−/− mice with the mixture of antioxidants resulted in a significant reduction in ischemia-induced retinal NV (Figure 4). This suggests that the increased oxidative stress in Sod1−/− mice contributes to the enhanced ocular NV seen in these mice.
Figure 4. Antioxidants reduce ischemia-induced retinal neovascularization in Sod1−/− mice.
Litters containing Sod1−/−, Sod1+/−, and Sod1+/+ pups were placed in 75% oxygen at P7 and and at P12 they were returned to room air and began receiving daily intraperitoneal injections of antioxidants or vehicle as described in Methods. At P17, retinal flat mounts were stained for PECAM-1 and the total area of neovascularization on the surface of the retina was measured. by image analysis. Sod1+/− treated with vehicle appeared to have a moderate amount of neovascularization (A) and Sod1−/− mice treated with antioxidants appeared to have smaller buds and less total area of neovascularization (B). Sod1−/− mice treated with vehicle had extensive neovascularization (C) and those treated with antioxidants appeared to have smaller buds and lesser area of neovascularization (D). Measurements by image analysis with the investigator masked with respect to genotype showed a marked decrease in the mean (± SEM) area of neovascularization per retina in Sod1−/− mice treated with antioxidants compared to those treated with vehicle(n=6 for each; **p<0.0001 by ANOVA with Dunnett’s correction for multiple comparisons). There was also a significant reduction in area of neovascularization in Sod1+/− mice treated with antioxidants compared to Sod1−/− treated with vehicle (n=6 for each; *p=0.0123 by ANOVA with Dunnett’s correction for multiple comparisons. Bar = 100 μm
Antioxidants suppress ischemia-induced retinal NV in wild type mice
Antioxidants suppressed ischemia-induced retinal NV in Sod+/− mice as well as Sod−/− mice (Figure 4), suggesting that even mice with mild levels of constitutive oxidative stress might develop less ischemia-induced retinal NV if treated with antioxidants. To test this, wild type C57BL/6 mice with oxygen-induced ischemic retinopathy were treated with antioxidants or vehicle at P12, the onset of ischemia. At P17, antioxidant-treated mice had significantly less retinal NV than those treated with vehicle (Figure 5).
Figure 5. Antioxidants suppress ischemia-induced retinal neovascularization in wild type mice.
At P7, litters of C57BL/6 mice were placed in 75% oxygen and at P12, they were returned to room air and started on daily intraperitoneal injections of vehicle or vehicle containing a mixture of 3 antioxidants, α-tocopherol, ascorbic acid, and α-lipoic acid. At P17, in vivo Immunofluorescent staining for PECAM1 was done as described in Methods, and the area of neovascularization on the surface of the retina was visualized on retinal flat mounts by fluorescence microscopy. Compared to mice treated with vehicle (A), those treated with antioxidants (B) had substantially less retinal neovascularization. Image analysis (C) showed that antioxidant-treated mice (n= 7) had a significant (*p<0.0001 by unpaired t-test) reduction in mean (±SEM) area of neovascularization per retina compared to vehicle-treated mice (n=5). Bar = 100 μm
Antioxidants reduce VEGF-induced subretinal NV
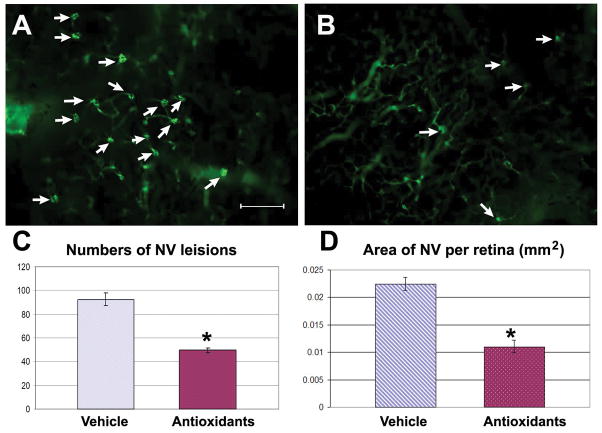
To determine if the lesser amounts of oxidative stress in Sod1-sufficient mice contributes to other types of ocular NV, Rho/VEGF transgenic mice were given daily intraperitoneal injections of the antioxidant mixture or vehicle between P7 and P21 when they were perfused with fluorescein-labeled dextran. Retinal flat mounts from vehicle-treated mice (Figure 6A) appeared to have more NV on the outer surface of the retina than those from antioxidant-treated mice (Figure 6B). Image analysis showed that mice treated with the antioxidant mixture had a significant reduction in neovascular lesions (Figure 6C) and the total area of NV per retina (Figure 6D). This indicates that reactive oxygen species contribute to VEGF-induced subretinal NV.
Figure 6. Antioxidants suppress subretinal neovascularization in Rho/VEGF transgenic mice.
Rho/Vegf transgenic mice were given daily intraperitoneal injections of vehicle or vehicle containing a mixture of 3 antioxidants, α-tocopherol, ascorbic acid, and α-lipoic acid between P7 and P21. At P21, the mice were perfused with fluorescein-labeled dextran and retinal flat mounts were examined by fluorescence microscopy. Retinas from vehicle-treated mice (A) showed more subretinal neovascularization than retinas from antioxidant-treated mice (B). Image analysis showed that antioxidant-treated mice (n=10) had a significant reduction in the mean (±SEM) area of neovascularization per retina (C) and number of neovascular lesions per retina (D) compared to vehicle-treated mice (n=6). Bar = 200 μm
*p < 0.0001 by unpaired t test.
Antioxidants reduce choroidal NV
Subretinal NV originating from the deep capillary bed of the retina like that seen in Rho/VEGF mice occurs in roughly 30% of patients with neovascular AMD, but more commonly, the NV originates from the choroid. The effect of antioxidants was tested in a murine model of choroidal NV in which Bruch’s membrane is ruptured by laser photocoagulation and after 14 days the amount of NV growing through the rupture site is measured (Tobe et al., 1998b). C57BL/6 mice were divided into 3 groups: one received daily intraperitoneal injections of vehicle starting the day prior to rupture of Bruch’s membrane (vehicle group), and the other two received daily intraperitoneal injections of the antioxidant starting the day prior to (pre-treatment group) or right after rupture of Bruch’s membrane (treatment group). At 14 days after rupture of Bruch’s membrane, the mice we perfused with fluorescein-labeled dextran and choroidal flat mounts were examined by fluorescence microscopy. Compared to mice in the vehicle group which had large choroidal neovascular lesions at Bruch’s membrane rupture sites (Figure 7A), mice in the pre-treatment group (Figure 7B) or treatment group (Figure 7C) had choroidal neovascular lesions that appeared smaller; however, only the pre-treatment group showed a statistically significant reduction in the area of choroidal NV compared to the vehicle group (Figure 7D).
Figure 7. Antioxidants suppress choroidal neovascularization.
Six week old C57BL/6 mice had rupture of Bruch’s membrane with laser photocoagulation at 3 locations in each eye. One day before laser treatment a group of mice were started on daily intraperitoneal injections of vehicle or vehicle containing a mixture of 3 antioxidants, α-tocopherol, ascorbic acid, and α-lipoic acid. Another group of mice was started on the injections of antioxidants after rupture of Bruch’s membrane. Fourteen days after rupture of Bruch’s membrane, the mice were perfused with fluorescein-labeled dextran and choroidal flat mounts were examined by fluorescence microscopy. Compared to eyes treated with vehicle (A), those started on antioxidants before laser treatment (B) or after laser treatment (C) appeared to have less choroidal neovascularization. Image analysis (D) confirmed that treatment with antioxidants starting prior to rupture of Bruch’s membrane (n= 54) caused a significant (*p< 0.0001 by general linear model) reduction in the mean (±SEM) area of choroidal neovascularization, but there was no significant difference between mice started on antioxidants after laser photocoagulation (n= 27) and mice treated with vehicle (n= 37). Bar = 100 μm.
Discussion
Epidemiologic studies have suggested that individuals who eat a diet rich in antioxidants have a lower incidence of AMD compared to those with an antioxidant-poor diet (Seddon et al., 1994). This led to the Age-Related Eye Disease Study which demonstrated that patients with AMD and confluent drusen and/or pigmentary changes in the macula have a significant reduction in progression to advanced AMD defined as choroidal NV or severe geographic atrophy or choroidal NV when treated with zinc or a combination of antioxidant vitamins and zinc (Age-Related Eye Disease Study Research Group, 2001).. These data suggest that oxidative stress may contribute to the progression of AMD. Oxidative stress has also been implicated by mass spectroscopy of drusen from postmortem eyes of patients with AMD, which shows many oxidized proteins (Crabb et al., 2002). The observation that Sod1−/− mice, which show increased constitutive and stimulated oxidative damage in the retina (Dong et al., 2006), develop many of the features of AMD has provided further evidence implicating oxidative stress in AMD (Imamura et al., 2006). However, it is not clear how oxidative stress contributes to AMD. Does oxidative stress lead to drusen, thickening of Bruch’s membrane, and death of RPE and photoreceptors, which then promote choroidal NV or does oxidative stress directly promote choroidal NV? In this study, we have demonstrated that increased expression of VEGF in photoreceptors results in greater amounts of subretinal NV in Sod1−/− mice than in Sod1+/+ mice, indicating that Sod1−/− mice have a proangiogenic environment in the subretinal space even before they develop visible changes that are associated with AMD. Interestingly, Sod1−/− mice also developed greater amounts of ischemia-induced retinal NV along the inner surface of the retina indicating that the proangiogenic environment occurs throughout the entire retina and not just the subretinal space. Since oxidative stress is increased in the retinas of Sod1−/− mice (Dong et al., 2006), we hypothesized that oxidative stress was contributing to the proangiogenic environment in Sod1−/− mice and that even the lower constitutive levels of oxidative stress in wild type mice might contribute to ocular NV. To test this hypothesis, we treated Sod1−/− mice with a mixture of antioxidants that we had previously shown markedly reduces oxidative stress in the retina (Komeima et al., 2006) and found that it significantly reduced ischemia-induced retinal NV indicating the oxidative stress is contributory. To determine if the lesser amounts of oxidative stress in wild type mice contributes to NV, we investigated the effect of the antioxidants in 3 models of ocular NV and found that the antioxidants significantly suppressed NV in each of the 3 models. These data indicate that oxidative stress enhances several types of ocular NV.
The suppression of subretinal NV in mice in which the rhodopsin promoter drives expression of VEGF in photoreceptors, suggests that antioxidants must act at least in part on downstream signaling events, because they cannot act by reducing VEGF levels in that model. This is consistent with the demonstration that VEGF stimulates production of reactive oxygen species (ROS) via Rac1-dependent, gp91phox-containing NAD(P)H oxidase in human umbilical vein endothelial (HUVEC) cells (Ushio-Fukai et al., 2002). Inhibitors of NAD(P)H oxidase reduce VEGF-induced autophosphorylation of VEGF receptor 2 (VEGFR2) suggesting a positive feedback loop whereby generation of ROS by VEGF results in enhanced VEGFR2 activity, possibly by inactivation of protein tyrosine phosphatases that contain cysteine residues in their active site (Finkel, 1999). Mice deficient in gp91phox show reduced NV associated with subcutaneously implanted VEGF-impregnated sponges, suggesting a role for gp91phox-containing NAD(P)H oxidase in VEGF-stimulated angiogenesis in dermis (Ushio-Fukai et al., 2002).
The effect of ROS is unlikely to be limited to modulation of VEGFR2 activity. Several transcription factors that enhance expression of Vegf are increased and/or activated by ROS, including nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), ATF3, and Ets-1 (Hsu et al., 2000; Okamoto et al., 2006; Wilson et al., 2005). Thus, there may be multiple mechanisms by which oxidative stress promotes ocular NV and future studies to determine their relative importance could potentially be useful.
The implication of oxidative stress in the pathogenesis of ocular NV suggests that the reduction in the rate of progression to choroidal NV in patients with drusen and pigmentary changes treated with the AREDS formulation is due in part to a direct reduction of oxidative stress and not solely to an indirect effect of reducing progression of thickening of Bruch’s membrane and other morphologic changes in the outer retina and subretinal space. A potentially important area for future investigations is to determine if more potent antioxidant mixtures have greater suppressive effects on choroidal NV. We found that antioxidants also decreased ischemia-induced retinal NV, suggesting that antioxidants may also have the potential to reduce conversion of nonproliferative diabetic retinopathy to proliferative retinopathy.
Our data suggest that oxidative stress may directly promote ocular NV, but they do not rule out concomitant indirect effects. Increased expression of VEGF in RPE cells is not sufficient to cause choroidal NV when Bruch’s membrane is normal, but markedly increases choroidal NV when Bruch’s membrane is compromised (Oshima et al., 2004). Thus, ROS may stimulate choroidal NV by fostering a proangiogenic environment in the retina and choroid while at the same time contributing to progressive compromise of Bruch’s membrane. The practical consequences of this dual effect may be that in patients with AMD who already have large confluent drusen which occur in association with thickening of Bruch’s membrane, even temporary withdrawal from antioxidants could promote a proangiogenic environment in the subretinal space and increase the risk of conversion to the neovascular form of AMD. This hypothesis could be tested by measuring antioxidant serum levels when patients on AREDS formulation first develop choroidal NV.
In addition to promoting the atrophic and neovascular forms of AMD, oxidative stress causes cone cell death in retinitis pigmentosa (Komeima et al., 2006) and may play a role in the pathogenesis of the early stages of diabetic retinopathy (Al-Shabrawey et al., 2008; Caldwell et al., 2005; Kowluru and Chan, 2007; Yamato et al., 2007) as well as retinal NV. Identifying more efficient ways to counter oxidative stress in the retina could potentially benefit an enormous number of patients with a variety of different diseases and should be a major priority.
Acknowledgments
Supported by EY05951 and EY12609 and core grant P30EY1765 from the NEI. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology.
References
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabrawey M, Bartoli M, El-Remessy A, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and STAT3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008 Mar 31; doi: 10.1167/iovs.08-1754. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Bartoli M, Behzadian RA, El-Remessy AEB, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West K, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natil Acad Sci USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Akiyama H, Hackett SF, Lai H, Campochiaro PA. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukoc Biol. 1999;65:337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28:1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natil Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Chan P-S. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007 doi: 10.1155/2007/43603:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryzstolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NM, Li W, Connolly E, O’Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- Okamoto A, Iwamoto Y, Maru Y. Oxidative stress-responsivetranscriptionfactor ATF3 potentially mediates diabetic angiopathy. Mol Cell Biol. 2006;26:108710–108797. doi: 10.1128/MCB.26.3.1087-1097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–91. [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Hackett SF, Melia M, Kaleko M, Connelly S, Esumi N, Zack DJ, Campochiaro PA. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge J, JWS, Campochiaro PA. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci. 2007;48:4335–4341. doi: 10.1167/iovs.07-0113. [DOI] [PubMed] [Google Scholar]
- Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998a;39:180–8. [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998b;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander W. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Wilson LA, Gemin A, Espiritu R, Singh G. Ets-1 is transcriptionally up- regulated by H2O2 via an antioxidant response element. FASEB J. 2005;19:2085–2087. doi: 10.1096/fj.05-4401fje. [DOI] [PubMed] [Google Scholar]
- Yamato M, Matsumoto S, Ura K, Yamada K, Naganuma T, Inoguchi T, Watanab T, Utsumi H. Are free radical reactions increased in the diabetic eye. Antioxid Redox Signal. 2007;9:367–373. doi: 10.1089/ars.2006.1502. [DOI] [PubMed] [Google Scholar]