Abstract
Solid tumors are not simply clones of cancer cells. Instead, they are abnormal organs composed of multiple cell types and extracellular matrix. Some aspects of tumor development resemble processes seen in developing organs, while others are more akin to tissue remodeling. Some microenvironments, particularly those associated with tissue injury, are favorable for progression of mutant cells while others restrict it. Cancer cells can also instruct surrounding tissues to undergo changes that promote malignancy. Understanding the complex ways in which cancer cells interact with their surroundings, both locally in the tumor organ and systemically in the body as a whole, has implications for effective cancer prevention and therapy.
INTRODUCTION
Cells and extracellular matrix (ECM) form tissues, and collections of tissues join together in structural and functional units to form organs. Different organs act together through blood and lymphatic vessels to form the organism. Solid tumors are not random mixtures of cells and ECM, but rather resemble organs, although structurally and functionally abnormal. They contain multiple cell types and extracellular matrix components and develop through complex interactions between these different components of the tissues using processes that often resemble those used by developing organs. Tumors interact with the rest of the organism, similarly to normal organs. However, whereas normal organs have functions that support the survival of the organism, the systemic effects of the tumor organ often are what ultimately kill the patient. Thinking of tumors as organs may allow us to better understand the processes that govern how solid tumors develop and progress.
STROMAL COMPONENTS OF THE TUMOR ORGAN
Organs are composed of the cells that perform the main organ function (e.g., secrete hormones or enzymes) and the stroma (from Latin or Greek, often translated as “mat” or “bed”), the supportive framework of an organ. The stroma can be divided into several classes: the ECM, which is composed of proteoglycans, hyaluronic acid and fibrous proteins (e.g., collagen, fibronectin and laminin), and stromal cells. The stromal cells include mesenchymal supporting cells (e.g., fibroblasts and adipocytes), cells of the vascular system, and cells of the immune system. Various peptide factors (e.g., growth factors, chemokines, cytokines, antibodies) and metabolites are also found in the stroma. The stroma is essential for normal organ development (e.g., Cunha, 2008; Puri and Hebrok, 2010; Wiseman and Werb, 2002). Different components of the tumor stroma similarly influence the progression of the tumor (Table 1). As tumors develop and progress, they undergo dramatic morphological changes (Figure 1A; Egeblad et al., 2008; Lin et al., 2003), which also involves the stroma (Figure 1B, C & D; Egeblad et al., 2008; Levental et al., 2009; Lin et al., 2006; Provenzano et al., 2006). The importance of stage-specific changes of the stroma is not yet completely clear. However, in most cases the stroma of the later stages is more supportive of tumor progression than the stroma of early stages. Examples of stromal components that have been proposed to have a more pronounced tumor-promoting function in advanced stages than in earlier stages are fibroblasts, type I collagen and the immune cell infiltrate, as described below.
Table 1.
The non-cancerous cells of the tumor organ
| Cell type | Effect on tumors | References |
|---|---|---|
| Normal epithelial cells | Inhibit | (Dong-Le Bourhis et al., 1997) |
| Myoepithelial cells | Inhibit (invasion, growth) | (Gudjonsson et al., 2002; Hu et al., 2008) |
| Fibroblasts | Promote (proliferation, angiogenesis, invasion) | (Bhowmick et al., 2004; Olumi et al., 1999; Orimo et al., 2005) |
| Mesenchymal stem cells | Promote (metastasis) | (Karnoub et al., 2007) |
| Adipocytes | Promote (tumor growth, survival, angiogenesis) | (Iyengar et al., 2005; Landskroner-Eiger et al., 2009) |
| Endothelial cells | Promote (angiogenesis, niche?) | (Ausprunk and Folkman, 1977; Calabrese et al., 2007) |
| Perivascular cells | Promote (vascularization) Inhibit (metastasis) |
(Song et al., 2005) (Xian et al., 2006) |
| Bone marrow-derived cells | Promote (proliferation, invasion angiogenesis) | (Coussens et al., 2000; Du et al., 2008; Lyden et al., 2001) |
| Dendritic cells | Inhibit (stimulate antitumor immunity) | (Knight et al., 1985; Mayordomo et al., 1995) |
| Myeloid derived suppressor cells and immature myeloid cells | Promote (angiogenesis, metastasis, reduce antitumor immunity) | (De Palma et al., 2005; Sinha et al., 2007; Yang et al., 2004; Yang et al., 2008b) |
| Macrophages, M1-like | Inhibit | (Sinha et al., 2005) |
| Macrophages, M2-like | Promote (invasion, angiogenesis) | (DeNardo et al., 2009; Lin et al., 2006; Lin et al., 2001) |
| Mast cells | Promote (angiogenesis) | (Coussens et al., 1999; Soucek et al., 2007; Yang et al., 2008a) |
| Neutrophils, N1 | Inhibits (stimulate antitumor immunity) | Fridlender et al., 2009 |
| Neutrophils, N2 | Promote (angiogenesis, reduce antitumor immunity) | (Nozawa et al., 2006; Schmielau and Finn, 2001; Shojaei et al., 2008) |
| T cells, CD4+, T helper 2 | Promote (metastasis) | (DeNardo et al., 2009) |
| T cells, CD8+, cytotoxic | Inhibit (tumoricidal) | (Romero et al., 1998) |
| T cells, CD4+CD25+ regulatory | Promote (reduce antitumor immunity) | (Casares et al., 2003; Curiel et al., 2004) |
| T cells, gamma/delta | Inhibit (stimulate antitumor immunity) | (Girardi et al., 2001) |
| T cells, Th17 | Promote (proliferation, angiogenesis) Inhibit (stimulate T cell antitumor immunity) |
(Numasaki et al., 2005) (Hirahara et al., 2001) |
| B cells | Promote (reduce antitumor immunity) | (Inoue et al., 2006) |
| B cells, Immunoglobulins | Promote (stimulate inflammation-associated progression) | (Andreu et al., 2010) |
| Platelets | Promote (metastasis) | (Camerer et al., 2004; Nieswandt et al., 1999) |
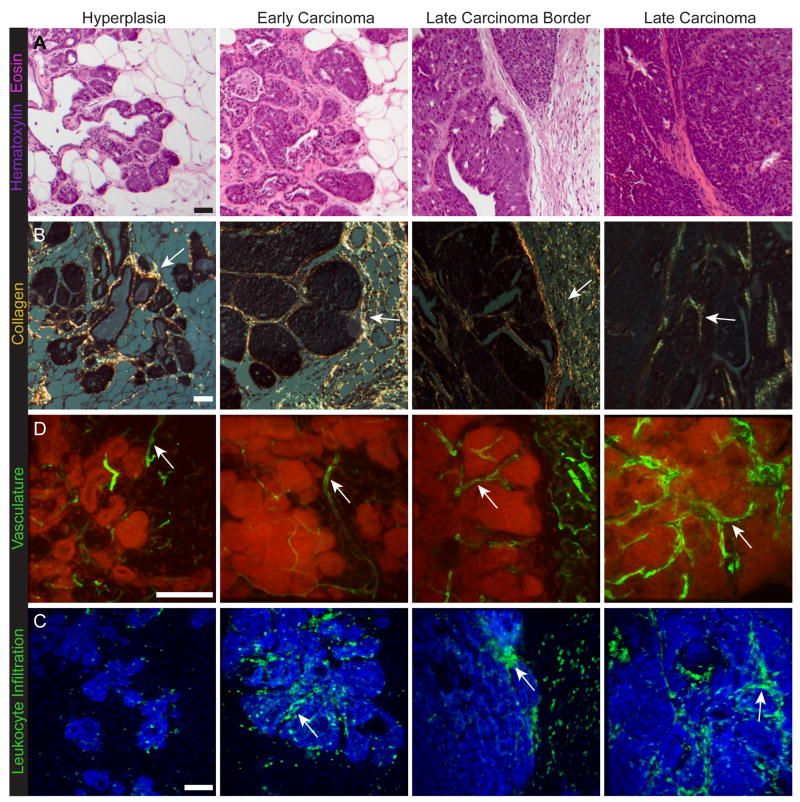
Figure 1. Changes in stromal organization during tumor progression.
(A) Changes in overall tissue composition with tumor stages in the MMTV-PyMT mouse mammary carcinoma model. Hematoxylin and eosin stained sections (H&E). With tumor progression, the architecture of the carcinoma cells bears less and less resemblance to the architecture of the tissue from which it was derived. The stromal tissue also changes, for example, from a tissue dominated by adipocytes to one dominated by extracellular matrix, fibroblasts and immune cells (adapted from Egeblad et al., 2008; Lin et al., 2003).
(B) Changes in extracellular matrix. Fibrillar collagens are stained with picrosirius red, shown as the birefringent stain under polarized light (arrows); counterstained with hematoxylin. With tumor progression, fibrillar collagen accumulates at the invasive edge of the tumor and surrounding nests of cancer cells (adapted from Levental et al., 2009; Provenzano et al., 2006).
(D) With increasing tumor stage, the tumor vasculature becomes increasingly abnormal, e.g., with dilated vessels. The vasculature is labeled by intravenous injection of FITC-tomato lectin, green (arrows). Nuclei stained with propidium iodide are shown in red. Scale bars: 50 μm (adapted from Lin et al., 2006).
(D) Leukocyte infiltration increases with tumor progression. Immune cells are labeled with antibodies against the common leukocyte marker CD45, green (arrows). Nuclei stained with propidium iodide are shown in blue (adapted from Egeblad et al., 2008).
Carcinoma-associated fibroblasts
Fibroblasts are mesenchymally derived cells present in the stroma of most tissues. During tissue development they or their progenitors are involved in epithelial-mesenchymal crosstalk, helping to shape organs. In adult animals, their major function is to deposit and turnover ECM, and they are activated by tissue injury. In tumors, activated fibroblasts that express smooth muscle actin are referred to as myofibroblasts and are a major population of carcinoma-associated fibroblasts (CAFs) (Kalluri and Zeisberg, 2006). CAFs share characteristics with embryonic fibroblasts or mesenchymal progenitors (Schor et al., 2003). CAFs stimulate cancer cell growth, inflammation, angiogenesis and invasion (Gaggioli et al., 2007; Kalluri and Zeisberg, 2006; Pietras et al., 2008). They can even promote tumor formation by immortalized, but otherwise non-tumorigenic, prostate epithelial cells (Olumi et al., 1999). In hyperplasia, fibroblasts may exert a tumor-inhibiting effect (Kalluri and Zeisberg, 2006; Kuperwasser et al., 2004). As the cancer cells begin to expand, they likely produce factors that activate and recruit CAFs (Figure 2A). Thus, normal fibroblasts do not promote experimental tumor take or initiation to the same extent as CAFs or fibroblasts that overexpress transforming growth factor-β (TGF-β), a factor involved in activation of fibroblasts, or hepatocyte growth factor (HGF), a factor secreted from activated fibroblasts (Kalluri and Zeisberg, 2006; Kuperwasser et al., 2004; Olumi et al., 1999). Although controversial, CAFs may become activated as a result of genetic alterations. Indeed, ablation of the tumor suppressor Pten in mammary stromal fibroblasts in mice results in increased fibrillar collagen, angiogenesis, macrophage infiltration and malignancy of mammary epithelial tumors (Trimboli et al., 2009). Communication between fibroblasts and cancer cells often involves other cell types: cancer cell-secreted platelet-derived growth factor (PDGF) can recruit macrophages, which then produce TGF-β that, in turn, induces development of reactive fibroblasts (Elenbaas and Weinberg, 2001). CAFs also promote tumor progression through communications with pericytes and endothelial cells that are mediated by secretion of growth factors and chemokines (e.g., CXCL12/SDF-1 and fibroblast growth factor-2 [FGF-2]) (Orimo et al., 2005; Pietras et al., 2008).
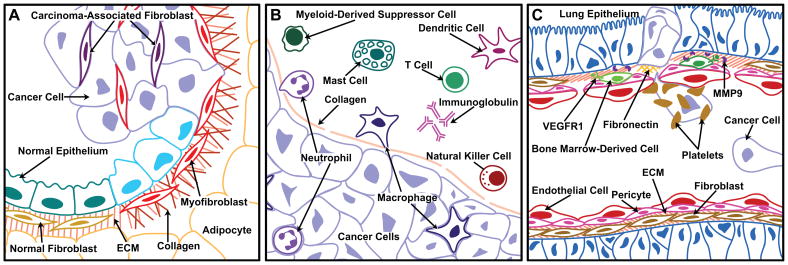
Figure 2. Interactions between cancer cells and stromal components influence tumor growth and progression.
(A) Interactions with mesenchymal cells. In hyperplastic tissues, normal epithelium and fibroblasts may exert a tumor-inhibiting effect. As cancer cells begin to expand, they also produce factors that activate myofibroblasts and recruit carcinoma-associated fibroblasts. These mesenchymal cell types, as well as adipocytes, are responsible for many of the tumor-associated changes in extracellular matrix (ECM).
(B) Recruitment of cells of the innate and adaptive immune compartment to a carcinoma. The immune cells are found both in the stroma at the invading edge of the carcinoma and infiltrating the tumor. Inflammatory cells, including neutrophils and macrophages, are frequently the first immune cells recruited to the tumor, and may be either tumor-promoting or tumor-inhibiting depending on their polarization. Another inflammatory cell type, the mast cell, is also recruited early and promotes tumor progression by releasing proteases that activate angiogenesis. Dendritic cells are primarily tumor-inhibiting as they support immunosurveillance and release signals that activate cytotoxic T cells. In contrast, myeloid-derived suppressor cells function to inhibit T cell activation. Natural killer cells and different types of T cells may have either pro- or anti-tumor functions, depending on their mode of activation. Immunoglobulins released by B cells promote tumor growth by initiation of the inflammatory response.
(C) Formation of metastases. At sites of vascular leakage, fibronectin is deposited and vascular endothelial growth factor receptor 1 (VEGFR1)-positive bone marrow-derived cells exit the circulation where they promote the establishment of the future metastases. They are involved in angiogenesis at the metastatic site through secretion of VEGF and degradation of the ECM by the release of MMP9. Circulating cancer cells reach the pre-metastatic site, sometimes covered by activated platelets, which protect them while they are in blood vessels and facilitate adhesion to the endothelial wall at the secondary site.
Many so-called fibroblasts may actually be pre-adipocytes, which also can affect cancer progression. Adipocytes secrete endocrine factors termed adipokines, such as adiponectin, which is proangiogenic (Landskroner-Eiger et al., 2009). Adipocytes can also stimulate tumor growth by secretion of the ECM protein type VI collagen, which binds to its receptor NG2 on cancer cells leading to activation of Akt and β-catenin and stabilization of cyclin D1 (Iyengar et al., 2005).
Extracellular matrix and tissue stiffness in the regulation of malignancy
Fibroblasts activated by the tumor microenvironment are largely responsible for tumor-associated changes in the ECM (Elenbaas and Weinberg, 2001; Kalluri and Zeisberg, 2006). These changes include upregulated ECM synthesis, post-translational modifications of ECM and extensive remodeling of ECM proteins by proteinases, e.g., matrix metalloproteinases (MMPs) (Figure 2A; Egeblad and Werb, 2002; Kalluri and Zeisberg, 2006; Kessenbrock et al., 2010). The altered ECM then influences tumor progression by architectural and signaling interactions.
The basal surface of epithelial cells is attached to a specialized ECM, the basement membrane, composed of laminins, type IV collagen, entactin/nidogen, and proteoglycans (reviewed in Xu et al., 2009). This basement membrane is breached when epithelial tumors proceed to invasive malignancy. In the mammary gland, myoepithelial cells produce the basement membrane in collaboration with stromal cells. They are regulators of normal mammary gland development due to their effect on luminal epithelial cell polarity, branching, and differentiation. The myoepithelial cells have tumor suppressor activity, which is progressively lost during the in situ to invasive carcinoma transition (Gudjonsson et al., 2002; Hu et al., 2008 and references therein). In vitro assays suggest that the tumor suppressor activity of myoepithelial cells is lost as they lose the ability to synthesize laminin-111 (Gudjonsson et al., 2002). Myoepithelial cells may also have negative effects on tumor progression via regulation of protease activation (Hu et al., 2008).
The interstitial matrix is the other major category of ECM and consists of macromolecules such as fibrillar collagens, fibronectin, and proteoglycans. It provides strength and structure to the tissue, but also binds growth factors and cytokines. The synthesis, and remodeling of the fibrillar type I collagen increases in tumors and is required for angiogenesis (reviewed in Erler and Weaver, 2009). The architecture of type I collagen also changes. Whereas collagen fibers are curly and oriented in parallel to normal or hyperplastic epithelium, there is a progressive change in the fibers so that they are straighter and mostly perpendicular to the tumor border in the late stages (Levental et al., 2009; Provenzano et al., 2006). This changed architecture may promote cell invasion by enabling cells to migrate along the collagen fibers or by enhancing integrin signaling (Condeelis and Pollard, 2006; Levental et al., 2009).
Several ECM proteins expressed embryonically during organ development are re-expressed during tumor progression. Tenascin-C is a major constituent of the ECM surrounding angiogenic blood vessels, and can increase endothelial cell migration, proliferation, sprouting and elongation (reviewed in Jones, 2001). Its expression at the invasion border predicts recurrence. An alternatively spliced version of fibronectin, which is normally expressed only during embryonic development (Schor et al., 2003), is expressed in tumors and facilitates angiogenesis (Avraamides et al., 2008).
The major ECM receptors, the integrins, mediate many of the effects of tumor-associated ECM on the cancer cells. Indeed, blocking β1 integrins blunts the malignant phenotype in culture and in vivo (Weaver et al., 1997), while ectopic expression of activated β1 integrin mutants compromises multi-cellular tissue morphogenesis and promotes tumorigenic behavior (reviewed in Erler and Weaver, 2009). In addition to direct signaling through receptors, the ECM also regulates signaling indirectly by sequestering growth factors and cytokines (e.g., FGF-2 and TGF-β), which are released by cleavage of the ECM molecules by proteinases (Egeblad and Werb, 2002). Cleavage of type I collagen or laminin-332 by MMPs also generates tumor-promoting fragments that stimulate cellular migration or survival (Giannelli et al., 1997; Montgomery et al., 1994). In contrast, fragments with anti-angiogenic, and thus tumor-inhibiting, activity are generated from type XVIII and type IV α3 collagens (Kalluri, 2003).
As a result of the changes in the ECM, tumors are often stiffer than normal tissues and stand apart from the surrounding tissue as hard nodules. The relative stiffness of a tissue can have profound effects on cellular function: Mammary epithelial cells cultured in compliant collagen matrices form polarized acini and differentiate when exposed to lactogenic hormones, but, as the collagen matrix is progressively stiffened, they transition to proliferating colonies with compromised polarity (Barcellos-Hoff et al., 1989). When rigidity approaches that of tumors, the non-malignant epithelial cells disorganize and invade (Paszek et al., 2005). ECM stiffening due to collagen crosslinking cooperates with oncogenes to promote invasive behavior. In vitro, sustained activation of the oncogene ErbB2 alone is insufficient to drive invasion of mammary epithelial cells, but promotes invasive growth in crosslinked, stiffened collagen. In vivo, reducing collagen crosslinking results in lower tumor incidence in a mouse model of mammary carcinoma (Levental et al., 2009).
Clinical data support a role for ECM molecules in cancer progression: Hierarchical clustering of the expression profile of 278 ECM-related genes in invasive breast carcinomas shows that favorable outcome is associated with overexpression of proteinase inhibitors, while poor prognosis is associated with high expression of integrins and metalloproteinases and low expression of several laminins (Bergamaschi et al., 2008).
Tumor vasculature and lymphatics
Recruiting a vasculature is a critical step in the development and differentiation of organs (Puri and Hebrok, 2010; Yamamoto et al., 2007). To assure growth, tumors also recruit blood vessels in a process termed the angiogenic switch, which can occur at different tumor progression stages (reviewed in Bergers and Benjamin, 2003). This process often involves angiogenic sprouting with the recruitment of perivascular cells (Bergers and Benjamin, 2003; Song et al., 2005). However, some tumors may rely more on vasculogenesis, the recruitment of bone marrow-derived endothelial precursor cells (Lyden et al., 2001).
The VEGF family members and FGF-2 are important in the regulation of the angiogenic response (Bergers and Benjamin, 2003). Although pro-angiogenic factors can be secreted by cancer cells, an important source is the tumor-infiltrating myeloid-derived cells, which are recruited to tumors by factors, e.g., CXCL12 or VEGF, secreted by hypoxic cancer cells (Du et al., 2008; Murdoch et al., 2008).
Tumor vessel density is not necessarily an indicator of tumor malignancy. For example, astrocytomas do not require neovascularization, but rather co-opt existing blood vessels by growing invasively along them (Bergers and Benjamin, 2003; Holash et al., 1999). Pancreatic adenocarcinomas also have a very low vessel density, but are aggressive (Olive et al., 2009). Yet some tumors, such as grade I pilocytic brain tumors, are slow-growing and do not metastasize even though they are highly angiogenic (Bergers and Benjamin, 2003).
In contrast to the normal vasculature, tumor blood vessels are irregular, dilated and can have dead ends. Furthermore, perivascular cells in tumors are loosely associated with endothelial cells (reviewed in Bergers and Benjamin, 2003). These changes result in abnormal blood flow and leaky blood vessels with extravasation of excess fluid and proteins from the capillaries. The excess fluid proteins are taken up by lymphatic vessels that transport these components back to the blood circulation. The lymphatic vessels also transport antigen-presenting cells to lymph nodes (reviewed in Tammela and Alitalo, 2010). Cancer cells too use lymphatic vessels to traffic to lymph nodes, and there is a positive correlation between the density of tumor lymphatic vessels and lymphatic metastases in some human cancers (Tammela and Alitalo, 2010). The VEGF family members VEGF-C and -D play major roles in tumor lymphangiogenesis and promote metastasis to lymph nodes (Skobe et al., 2001; Stacker et al., 2001). FGF-2, insulin-like growth factors (IGFs), and PDGF-B induce lymphangiogenesis in various contexts, but these effects may be secondary to the induction of VEGF-C and VEGF-D in inflammatory cells and fibroblasts (reviewed in Tammela and Alitalo, 2010). Targeting tumor lymphangiogenesis to inhibit lymphatic metastasis is complicated because destruction of lymphatic vessels also elevates the interstitial fluid pressure inside the tumors, thereby impairing the delivery of drugs to target the cancer cells.
The inflammatory response to cancer
Inflammatory cells are components of the microenvironment of normal tissues and organs, regulating various processes during development including epithelial growth and branching and clearance of apoptotic cells (reviewed in Pollard, 2009). They also play significant roles in the initiation and progression of cancer (Figure 2B). There is a progressive change in the composition of the immune cell infiltrate with tumor stage to one that is more conducive to tumor progression (e.g., Andreu et al., 2010; Coussens et al., 1999; DeNardo et al., 2009). Interactions between cancer cells and cells of the innate and adaptive immune system occur in the tumor organ and illustrate the complexity and dynamics of the tumor tissue (Figure 3, Movies S1, S2).
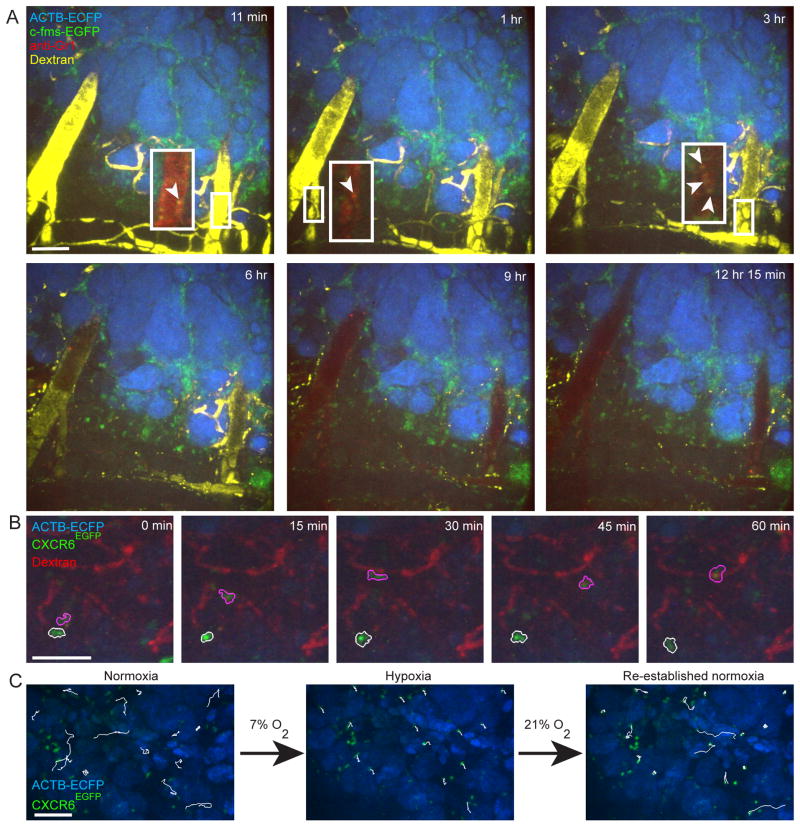
Figure 3. Dynamics of the interactions between cancer cells and stromal components in the tumor tissue.
(A) Dynamics of different subtypes of myeloid cells revealed by four-color time-lapse series of the same lesion of an MMTV-PyMT;ACTB-ECFP;c-fms-EGFP mouse co-injected with fluorescent 70 kD dextran and anti-Gr1 antibodies intravenously. Dextran (yellow) first labels the blood vessels (0–6 h post injection), but leaks out of the vessels over time and is taken up by M2-like macrophages (3–12 h). The anti-Gr1 antibody labels Gr1+ myeloid cells, including neutrophils, myeloid derived suppressor cells and monocytes (red, arrow heads). Inserts show higher magnifications of blood vessels without the dextran channel for identification of Gr1+ myeloid cells at early time points. All myeloid cells are green and cancer cells are blue (adapted from Egeblad et al., 2008). See also Movie S1 in supplementary material. Scale bars: 100 μm. (B) Examples of low-migratory (white outline) and high-migratory (pink outline) CXCR6+ T-cells (green) in proximity to the tumor vasculature (red) in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse injected with fluorescent dextran (red). Cancer cells are blue (adapted from Egeblad et al., 2008). See also Movie S2 in supplementary material for time-lapse recordings of the entire field. (C) Effects of acute hypoxia on T cell migration. CXCR6+ T cells (green) cease migration following acute systemic hypoxia in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse. Cancer cells are blue. The same 15 cells were tracked during 20 minutes of normoxia (21% inhaled oxygen), 20 minutes of systemic acute hypoxia (7% inhaled oxygen) and 20 minutes of re-established normoxia in the same field (tracking marks are white; adapted from Egeblad et al., 2008). See also Movie S3 in supplementary material.
Chronic inflammatory responses and infections are associated with many cancers, including liver and pancreatic cancer after alcohol abuse, mesothelioma caused by asbestos exposure, colon cancer associated with inflammatory bowel disease, gastric carcinoma after Helicobacter pylori infection, liver cancer caused by chronic viral hepatitis, cervical cancer after infection with human papilloma virus (HPV) and Burkitt’s lymphoma after infection with Epstein-Barr Virus (EBV) (reviewed in Lin and Karin, 2007). While chronic inflammation predisposes to cancer, long-term use of non-steroidal anti-inflammatory agents may protect against various tumors (Dannenberg and Subbaramaiah, 2003). Inflammation likely facilitates neoplasia rather than directly causing it. Nevertheless, reactive oxygen and nitrogen intermediates secreted by inflammatory cells can damage DNA and the frequency of random mutations is higher in inflamed than in normal tissues (reviewed in Colotta et al., 2009).
How does inflammation facilitate cancer progression? Both innate and acquired immune cells can be critical players. An immune cell infiltrate is present in most, if not all, tumors including those without an inflammatory basis (e.g., Figures 1C). This suggests that the genetic events that cause the cancers also result in an immune response). Indeed, instigation of inflammation has been reported after activation of RET, Ras and Myc, all common oncogenes in human cancer (Borrello et al., 2005; Soucek et al., 2007; Sparmann and Bar-Sagi, 2004). These data suggest that an important consequence of oncogene activation is to hijack the inflammation-based programs for tissue remodeling and regeneration.
Effects of myeloid-derived cells on cancer initiation and progression
Innate immune cells are largely responsible for inflammatory reactions. These cells include various myeloid-derived cells (e.g., macrophages, neutrophils, and mast cells, Figure 2B). While they are the first defense against foreign pathogens, they also facilitate tissue development and repair. In tumors, myeloid cells do not just regulate the immune response against the cancer cells, but they also regulate angiogenesis and metastatic spread. Tumor-associated myeloid cells are very heterogeneous, behaving differently and likely having different functions at the invading edge, along blood vessels and in hypoxic regions of tumors (Figure 3A, Movie S1; Egeblad et al., 2008; Lewis and Pollard, 2006; Wyckoff et al., 2007).
Macrophages originate from monocytes and have potent roles in the normal development of several organs, including bone and mammary gland (Pollard, 2009). They are also critically involved in regulating angiogenesis in wound healing (Pollard, 2009). The main activation states of macrophages are often referred to as M1 (classical) and M2 (alternative) (Mantovani et al., 2008b). Monocytes are polarized to M1 macrophages by cytokines secreted from T helper 1 (TH1) cells (including interferon [IFN]-γ, TNF-α, and granulocyte-monocyte-colony stimulating factor [GM-CSF]) or by microbial products. M1 macrophages produce reactive oxygen and nitrogen intermediates and inflammatory cytokines, and they have cytotoxic activity against intracellular parasites and cancer cells (Mantovani et al., 2008b). TH1/M1 dominated inflammatory diseases, such as psoriasis, do not appear to promote cancers, and this is likely because M1 macrophages have antitumor activity (Mantovani et al., 2008a).
Monocytes exposed to cytokines secreted from T helper 2 (TH2) cells (including IL-4 and IL-13) become polarized towards the M2 macrophage phenotype. M2 macrophages promote killing of parasites and tissue remodeling (Mantovani et al., 2008b). Tumor-associated macrophages (TAMs) mostly resemble M2 macrophages because M2 polarizing cytokines are common in tumors. However, it is clear that TAMs have properties that do not fit in a rigid classification of M1 and M2 macrophages (Pollard, 2009). Accumulation of TAMs is associated with poor prognosis as they promote tumor growth, invasion, metastasis and angiogenesis by releasing cytokines, growth factors, ECM-degrading enzymes and angiogenic factors, but also suppress cytotoxic T cell activity (Colotta et al., 2009; Condeelis and Pollard, 2006; Murdoch et al., 2008; Pollard, 2009). EGF or RANKL (receptor activator of NF-κB ligand) secreted by tumors constitute major mechanisms by which TAMs stimulate tumor dissemination (Condeelis and Pollard, 2006; DeNardo et al., 2009; Luo et al., 2007). Inhibition of polarization of monocytes to M2-like macrophages through inhibition of NF-κB signaling, results in an M1-like phenotype and reduced tumor growth (Hagemann et al., 2008).
Neutrophils are short-lived and have antimicrobial functions. Similarly to macrophages, tumor-associated neutrophils can be divided into N1 and N2 phenotypes that inhibit or promote cancer development, respectively. TGF-β polarizes neutrophils to an N2 pro-tumor phenotype, while inhibition of TGF-β results in a shift to the N1 phenotype (Fridlender et al., 2009). N2-effector neutrophils can be a major source of IL-13 (Neill et al., 2010), which may then polarize macrophages to the M2 phenotype. N1 neutrophils increase the activation status of cytotoxic CD8+ T cells and possibly dendritic cells (Fridlender et al., 2009 and references therein). In contrast, N2 neutrophils promote cancer by producing angiogenic factors and ECM-degrading enzymes (Nozawa et al., 2006; Shojaei et al., 2008) and by suppressing the anti-tumor immune response (Schmielau and Finn, 2001).
Myeloid-derived suppressor cells (MDSCs) are a poorly defined group of cells that have properties in common with both the macrophage and neutrophil lineages. With increased tumor burden, MDSCs accumulate in the bone marrow, spleen, peripheral blood and at the invasive tumor front (Gabrilovich and Nagaraj, 2009; Yang et al., 2008b). Among their reported functions, MDSCs inhibit natural killer cells, B cells, dendritic cells and cytotoxic T cells, induce regulatory T cells (TRegs), promote angiogenesis, and promote cancer cell invasion and metastasis, likely via MMP activity (Colotta et al., 2009; Gabrilovich and Nagaraj, 2009; Yang et al., 2004; Yang et al., 2008b). Other, overlapping myeloid cell populations with roles in cancer include CCR1+ myeloid cells, which promote tumor invasion (Kitamura et al., 2007), VEGFR1+ cells that are found at metastatic sites (Kaplan et al., 2005) and Tie2 receptor-expressing monocytes (TEMs) that have a major role in tumor angiogenesis (De Palma et al., 2005).
Mast cells infiltrate hyperplasias and invasive fronts of carcinomas, where they degranulate close to capillaries, releasing mast cell-specific proteinases, which can stimulate fibroblast proliferation and induce angiogenesis via MMP9 activation (Coussens et al., 1999). An intriguing example of collaboration between mutations in stromal and cancer cells involves mast cells: Individuals with germline mutations in the NF1 gene develop neurofibromas that are driven by biallelic loss of NF1 in Schwann cells due to loss-of-heterozygosity, but also require infiltration by non-neoplastic NF1 haploinsufficient mast cells for tumor initiation (Yang et al., 2008a).
The transcription factor NF-κB is a key mediator of inflammation that is important in both inflammatory and cancer cells (Karin, 2006). NF-κB is activated by the Toll-like receptor (TLR)-MyD88 pathway that senses microbes and tissue damage, by the inflammatory cytokines TNF-α and interleukin (IL)-1β, and by hypoxia. In asbestos-induced mesothelioma, macrophages phagocytose asbestos and then release TNF-α, which promotes the survival of asbestos-damaged, transformation-susceptible mesothelial cells through NF-κB–dependent antiapoptotic signaling (Yang et al., 2006). Activation of NF-κB promotes cancer in a mouse model of colon cancer initiated by a chemical carcinogen. NF-κB acts both within enterocytes by suppressing apoptosis and within myeloid cells by stimulating pro-inflammatory cytokines, which influence tumor growth (Greten et al., 2004). However, decreasing NF-κB activity can also promote cancer: In a model of carcinogen-induced liver cancer, decreased NF-κB activity in hepatocytes results in increased cell death, providing a stimulus that leads to production of pro-inflammatory cytokines by resident macrophages (Kupffer cells). These cytokines stimulate compensatory proliferation of the surviving hepatocytes, some of which will have carcinogen-induced mutations with the potential to generate transformed progeny (Maeda et al., 2005).
Effects of adaptive immune cells on cancer
Whereas the cells of the innate immune compartment are primarily tumor-promoting, the adaptive immune compartment (B and T cells) can be tumor-suppressing. These cells carry out tumor immune surveillance, eliminate early stage tumors and keep initiated cancer cells in check (Dunn et al., 2004; Mantovani et al., 2008a). Indeed, T cell depletion as late as 200 days after carcinogen treatment leads to tumor development in mice that until then have been tumor-free (Koebel et al., 2007). Furthermore, patients with chronic suppression of the adaptive immune compartment (e.g., patients with AIDS or organ transplants) have an increased risk of developing virus-associated cancers such as human herpes virus-8-associated Kaposi sarcoma, EBV-associated non-Hodgkin lymphoma, and HPV-associated squamous cell carcinoma (reviewed in de Visser et al., 2006). The mounting of a tumor antigen-specific cytotoxic T cell response is also important for an efficient response to chemotherapy and radiation therapy: a high-mobility-group box 1 protein that is released by dying cancer cells after therapy interacts with TLR4 on dendritic cells, resulting in efficient cytotoxic T cell activation (Apetoh et al., 2007).
While the role of the adaptive immune compartment in suppressing cancer is well established, there is also evidence that it plays a role in promoting cancer. Immune suppressed patients have diminished risk for non-virus-associated solid tumors of epithelial origin, including breast and prostate cancer (reviewed in de Visser et al., 2006). Furthermore, B cells initiate the chronic inflammatory response that is necessary for the development of epithelial hyperplasia in a mouse model of skin cancer, through deposition of autoantibodies (Andreu et al., 2010). TH2 cells are also tumor-promoting, as their cytokines polarize tumor-associated macrophages to the M2 type, which in turn promote invasive behavior of breast cancer cells (DeNardo et al., 2009).
CD4+CD25+ TRegs are a special class of immune suppressive T helper cells that can inhibit the activity of a variety of immune cells. They accumulate in tumors and directly suppress antitumor immunity of CD8+ cytotoxic T cells via secretion of IL-10 and TGF-β (Yu et al., 2005). Depletion of TRegs enhances anti-tumor T-cell responses and induces regression of experimental tumors (reviewed in de Visser et al., 2006).
THE ORGANIZATION OF TUMORS
During tumor progression, the organization of tumors evolves, and the tumor organ acquires architecture that resembles earlier or less differentiated states in normal tissues. While tumors are quite different from normal quiescent tissues, the multilayered epithelium with poor polarity seen in early mammary tumors resembles the actively proliferating and invading epithelium of the terminal end buds of the developing mammary gland (Ewald et al., 2008). The transcription factor GATA3 maintains epithelial differentiation, organization and survival in the developing and adult mammary gland. It has similar roles in early breast carcinoma (Kouros-Mehr et al., 2008 and references therein). However, as the carcinomas transition to later, less differentiated stages, there is a selection for GATA3-negative, progenitor-like cells. Remarkably, reintroducing GATA3 expressing in such late stage cancer cells, leads to acquisition of more differentiated and less metastatic tumors that re-acquire aspects of tissue organization resembling the adult mammary gland (Kouros-Mehr et al., 2008).
The different components of the tumor organ are not randomly distributed within tumors. ECM deposition and leukocyte infiltration are for example often very pronounced at the tumor- stroma border (Figure 1B, & C). Indeed, pathologists identify and categorize neoplasias not only by the morphology of the cancer cells but also by criteria that include the organization of the tissues: excess epithelial proliferation in the context of largely normal tissue organization usually represents benign lesions (Muthuswamy, 2009). In addition, CD8+ T cells within cancer cell nests predict a better survival for colorectal cancer patients than for those with T cells only at the tumor margins (Naito et al., 1998). T cells are dependent on normal oxygen levels for migration in the tumor tissue (Figure 3C, Movie S3, Egeblad et al., 2008), suggesting that hypoxia indirectly regulates antitumor immunity by restricting T cell access.
Tumor-promoting microenvironments
In normal tissues, the stem and progenitor cells that give rise to the organ reside in specific environments called stem cell niches. Stem cell niches are defined as sites where stem cells are sustained, self renew, and where differentiation is inhibited. Supporting cells (e.g., cap cells in the Drosophila ovary and endothelial cells in the brain), ECM and signaling molecules constitute classical stem cell niches (Sato et al., 2009; Scadden, 2006).
Tumors also have specialized microenvironments or niches that confer distinct functions to the cancer cells. It is therefore likely that cancer cell niches also involve interactions with the ECM and supporting cells. Indeed, the mechanisms defining stem cell and cancer cell niches are likely shared, since many genes (e.g., c-Myc, and members of the TGF-β, Hedgehog and Wnt signaling pathways) involved in regulation of classical stem cell niches also play a role in cancer (reviewed in Iwasaki and Suda, 2009). It is not clear how these microenvironments that support cancer cell renewal would arise. The niches may drive cancer cell progression, or the cancer cells may hijack stem cell niches or generate signals that instruct the development of new niches. Indeed, leukemic cancer cells residing in the bone marrow can induce the formation of new niches for normal CD34+ hematopoietic stem cell engraftment in the tumor microenvironment (Colmone et al., 2008).
The best examples suggesting that stem cell-like niches stimulate development of epithelial tumors come from gastrointestinal tumors. Wnt signaling is required for self-renewal of intestinal stem cells. Activation of the Wnt pathway by inactivating mutations in the APC gene, a signaling molecule that inhibits Wnt signaling, drives both familial and sporadic colorectal cancers (reviewed in Miller et al., 2005). Interestingly, germline mutations in SMAD4, a molecule downstream of TGF-β signaling, increase proliferation of stromal cells, resulting in formation of gastrointestinal polyps and ultimately epithelial cancer (Howe et al., 1998). In these tumors, the stromal cells contain genetic alterations, suggesting that the epithelial cancer is caused by amplification of the normal microenvironment (Kinzler and Vogelstein, 1998). Similarly, deletion of the TGF-β receptor in stromal cells results in gastrointestinal epithelial malignancies in mice (Bhowmick et al., 2004).
The tumor microenvironment resembles stem cell niches in terms of its functions. For example, antagonists of the bone morphogenic proteins (BMPs), which are members of the TGF-β superfamily, may be involved in defining such niches for cancer cells. The BMP inhibitor, noggin, maintains the intestinal stem cell population (Sato et al., 2009). Another BMP inhibitor Gremlin 1, an antagonist of BMPs that maintains the progenitor cell niche in normal skin, is expressed in stromal cells of human breast, lung, pancreas, colon and prostate carcinomas (Sneddon et al., 2006).
ECM components may provide an environment that supports the proliferation of cancer cells: hyaluronic acid, type I collagen and Matrigel (which contains basement membrane constituents such as laminin-111 and type IV collagen) all increase engraftment of cancer cells in mice (Del Buono et al., 1991; Iwasaki and Suda, 2009; Quintana et al., 2008). How the ECM components support tumor engraftment remains to be elucidated. One clue is that the percentage of cancer cells that express stem cell markers increases when the cells are grown on type I collagen (Kirkland, 2009). A supportive effect of ECM components, such as type I collagen, might explain why cancer stem-like cells are frequently enriched at the invasive front of tumors (Hermann et al., 2007), where the highest levels of type I collagen are found (Figure 1B). Interestingly, breast cancer cells with stem cell-like characteristics express increased levels of type I, IV and XVIII collagens, suggesting that cancer cells may contribute to the generation of their niche (Gupta et al., 2009).
In addition to or linked with their role in angiogenesis, endothelial cells may have a direct role in forming tumor-promoting niches: Medulloblastoma cells that express stem cell markers (CD133 and nestin) are preferentially located near capillaries and they adhere to endothelial cells in co-culture experiments (Calabrese et al., 2007). Furthermore, co-injecting the medulloblastoma cells with endothelial cells increases tumor growth and the number of cancer cells that express stem cell markers, while inhibition of angiogenesis has the opposite effect (Calabrese et al., 2007).
Oxygen tension has been proposed as a regulator of stem cell and cancer cell niches, however with two opposing views: one is that cancer stem-like cells are found close to capillaries (an oxygen-rich environment), consistent with a role for endothelial cells in defining the niche. The opposing view is that stem cells prefer a hypoxic environment (reviewed in Iwasaki and Suda, 2009). Indeed, hypoxia is linked with poor prognosis and increased risk of metastasis and cancer cells in areas of areas of hypoxia are often less differentiated (Erler et al., 2006).
Tumor-restricting microenvironments
Just as there are tumor-promoting microenvironments, or niches, certain microenvironments can restrict tumor progression. Thus, molecules involved in maintaining normal tissue architecture and keeping cancer cells quiescent can be regarded as tumor suppressors. A classical example of tumor repression by the microenvironment is the ability of teratocarcinoma cells without gross chromosomal abnormalities to contribute to normal tissues when injected into blastocysts (Mintz and Illmensee, 1975). In contrast, teratocarcinoma cell lines that have chromosomal abnormalities do generate tumors when injected into blastocysts (Papaioannou et al., 1975). Thus, both the microenvironment and mutations in the cancer cells contribute to the formation of tumors. Infection with Rous sarcoma virus, which readily results in tumor formation in newborn chickens, does not induce tumors in chick embryos unless combined with tissue injury or TGFβ1, another example of the niche restricting tumor initiation (Dolberg and Bissell, 1984; Dolberg et al., 1985; Sieweke et al., 1990). Finally, morphologically normal epithelium with the same gross chromosomal changes as adjacent breast carcinomas is found in about 25% of examined cases (Deng et al., 1996). While the authors do not rule out that the cancer cells have acquired additional point mutations, this observation suggests that the microenvironment in the areas with morphologically normal epithelium may keep the epithelium from developing into carcinomas. In support of this conjecture, antibodies to β1 integrins normalize the malignant phenotype of breast cancer cells in culture and in vivo (Weaver et al., 1997).
In normal epithelial tissues, cells have cell-cell junctions and form polarized sheets with asymmetric distribution of cellular components to basal, lateral and apical cell membranes. This polarization plays an important role in controlling epithelial proliferation, migration, adhesion, differentiation and cell death (O’Brien et al., 2002). Physiological conditions such as pregnancy-induced changes in the mammary gland and wound healing increase the rate of cell proliferation, but overall tissue organization and cellular polarization are usually maintained (Muthuswamy, 2009). Nevertheless, while these conditions typically do not result in tumor formation, they are associated with an increased risk of cancer (Esther et al., 1999; Polyak, 2006).
Oncogenes and tumor suppressors can regulate or collaborate with the loss of cell polarity. For example, the activation of c-Myc does not transform or induce proliferation of quiescent mammary acinar structures cultured in 3D matrices unless the normal epithelial organization is disrupted either by changing the ECM of the culture system or by silencing of the tumor suppressor and cell polarity protein LKB1 (Partanen et al., 2007). Indeed, Lkb1-deficient mice exhibit polarity defects in the pancreatic epithelium before tumors are observed, suggesting that polarity defects are important for tumor initiation (Hezel and Bardeesy, 2008). Loss or mislocalization of another cell polarity protein Scribble cooperates with c-Myc to transform epithelial cells and induce tumors in mouse models by blocking activation of apoptosis (Zhan et al., 2008). Human breast tumors often downregulate and mislocalize Scribble (Zhan et al., 2008). These results suggest that the tissue architecture can be a barrier for tumorigenesis and that organisms have mechanisms in place that preserve the normal tissue structure and must be overcome for the formation of tumors.
Metastasis-promoting microenvironments
Cancer cells from different types of tumors colonize specific organs (Paget 1889, reprinted as Paget, 1989). This is not simply because the cancer cells get trapped in the capillary network of the first organ that they encounter after dissemination; for example, prostate cancer almost exclusively metastasizes to bone and not the lungs (reviewed in Nguyen et al., 2009). Organ specificity of metastasis correlates with specific gene expression of the disseminating cancer cells (reviewed in Nguyen et al., 2009), but may also reflect that microenvironments of certain organs fit the requirements of specific cancer cells. Indeed, circulating cancer cells are able to re-infiltrate and grow in primary tumors, likely because a favorable microenvironment is already established (Kim et al., 2009a).
There is also evidence that primary tumors set up the distant microenvironment for colonization, primarily through recruitment of bone marrow-derived cells to and ECM remodeling at the distant site (Figure 2C). Intriguingly, factors secreted from primary tumors may assemble niches for distant metastasis, such that cancer cells with a restricted pattern of metastasis can colonize additional tissues if host mice are pre-treated with conditioned medium from another, more globally metastasizing, cell line (Kaplan et al., 2005). How these cancer niches are selected is not known, but a few significant molecules that are associated with these sites have been identified. Cancer cells in the primary tumor secrete VEGF, which induces MMP9 expression in endothelial cells and macrophages in the lungs, allowing the growth of metastases in the lungs (Hiratsuka et al., 2002). The primary tumor also controls the accumulation of the ECM protein fibronectin at sites in the metastastic organ, and this may promote homing of bone marrow-derived cells to the organ (Kaplan et al., 2005). Versican V1, another ECM protein at the metastatic site, binds to TLR2 on bone marrow-derived myeloid cells, which then produce cytokines, including TNF-α, that stimulate metastasis (Kim et al., 2009b). Lysyl oxidase secreted from primary tumors under hypoxic conditions (Erler et al., 2006) may crosslink collagen at the distant site, allowing for better adherence of myeloid cells, which in turn use MMPs to remodel the ECM of the metastatic site (Erler et al., 2009).
How specific tissues are targeted for metastatic colonization is still a major question in the field. A plausible hypothesis is that cancer cells may communicate with specific distant organs through secreted molecules or microvesicles. Microvesicles are shed by most cell types, including cancer cells, and can be found in sera from cancer patients (Cocucci et al., 2009; Skog et al., 2008). Since microvesicles can be taken up in a cell-specific manner (reviewed in Cocucci et al., 2009) and contain growth factors and receptors, functional mRNAs and miRNAs (Cocucci et al., 2009; Skog et al., 2008; Valadi et al., 2007) they could help set up the metastatic site.
PLASTICITY OF CANCER CELLS
Cancer cells can acquire stromal or embryonic phenotypes, allowing them to escape the primary tumor or to take on some of the supportive functions of stromal cells. This phenomenon creates therapeutic problems because cancer cells that masquerade as other cell types may be harder to target. To metastasize, cancer cells may exploit homing mechanisms normally utilized by leukocytes or stem cells by expressing chemokine receptors. The CXCR4 ligand, CXCL12 (SDF-1α), drives homing of CXCR4-expressing cancer cells to tissues such as lungs, liver, brain and bone marrow (Hermann et al., 2007; Zlotnik, 2004). Cancer cells also can utilize the CCR7 chemokine receptor, which is normally responsible for homing of dendritic cells and T cells to lymph nodes (Shields et al., 2007; Zlotnik, 2004). In addition, they can acquire other properties of leukocytes, such as the ability to phagocytose neighboring live cells (Lugini et al., 2006). This property may allow cancer cells to survive extreme hypoxic or acidic environments. One mechanism by which tumor cells may diversify their properties is by fusion with normal tissue cells (Lu and Kang, 2009).
“Vascular mimicry” describes the ability of cancer cells to form vessel-like structures (Hendrix et al., 2003). Although the degree to which cancer cells resemble endothelial cells is debatable, there is agreement that cancer cells can directly line the lumen of functional tumor blood vessels (Chang et al., 2000; McDonald et al., 2000).
Epithelial-mesenchymal transition and plasticity
Epithelial-derived cancer cells can acquire mesenchymal properties, such as loss of polarity, increased migratory and invasive capacity, and resistance to apoptosis, by undergoing epithelial-mesenchymal transition (EMT) (Kalluri and Weinberg, 2009; Polyak and Weinberg, 2009). It is intriguing that stem cells also express many of the same properties that are induced by EMT. Thus, induction of EMT increases the number of cells with stem cell-like traits (Mani et al., 2008). This suggests that EMT provides cancer cells with plasticity to switch between stages with more differentiated or less differentiated (and more stem cell-like) traits.
Canonical TGF-β signaling is one of the main inducers of EMT acting through SMAD molecules and leading to activation of the classical EMT-inducing transcription factors SNAI1, SNAI2 and Twist (Thuault et al., 2006). Activation of receptor tyrosine kinases, such as c-Met, can also mediate EMT. The downregulation or loss of E-cadherin, the primary molecule responsible for cell-cell adhesion of epithelial cells, is one overarching feature of EMT (Polyak and Weinberg, 2009; Yang and Weinberg, 2008). With the loss of E-cadherin, β-catenin is released from the cell membrane and translocates to the nucleus, where it activates the transcription factor lymphoid enhancer factor/T cell factor (LEF/TCF) and induces the expression of target genes that promote motility and inhibit adhesion (Yang and Weinberg, 2008).
Induction of EMT is linked with metastasis. Yet, metastatic colonies are mostly of epithelial phenotype, suggesting that the disseminated mesenchymal/stem cell-like cancer cells revert back to a differentiated, epithelial cell-like state once they colonize (reviewed in Polyak and Weinberg, 2009). One hypothesis would be that the invasive and mesenchymal-like behavior is transiently activated in the local microenvironment of the primary tumor, for example by secretion of the metastasis and EMT-inducer TGF-β by stromal cells. Indeed, live imaging of breast cancer cells expressing a TGF-β-dependent reporter gene demonstrates that TGF-β signaling is activated in breast cancer cells with a mesenchymal pattern of motility at the invasive margins of primary tumors, but not in cells once they have formed metastases in the lungs (Giampieri et al., 2009).
Induction of plasticity by the microenvironment
Since cancer cells can have a variety of different phenotypes, this raises the question as to how the diversity arises. Classically, it has been assumed that because cancer cells are genetically unstable, different microenvironments may select for cells with different genotypes. However, exposure to different stromal components (e.g., growth factors and chemokines, adhesion molecules, ECM components, oxygen levels or metabolites) may also change cancer cell gene expression. The microenvironments in areas of low oxygen/low pH and at the tumor-stroma edge appear particularly capable of selecting for or inducing cancer cells with more invasive properties (Hermann et al., 2007; Polyak and Weinberg, 2009; Staller et al., 2003). For example, in response to blockage of angiogenesis, cancer cells invade along the existing blood vessels deep into the brain parenchyma in a mouse model of glioblastoma (Du et al., 2008).
One potential mechanism of phenotypic plasticity in a genetically homogenous population is known from bacteria, which can react as collective groups of cells, a phenomenon known as quorum sensing. Concentrations of signaling molecules released from the bacterial colony increase proportionately with cell number. When they reach a threshold level, the bacteria respond with a population-wide alteration in gene expression and altered processes, which then involve bacterial escape from the primary site, motility to and survival at new sites (reviewed in Hickson et al., 2009). Interestingly, cancer cells grown at high confluency have increased metastatic ability compared to cells grown at lower confluency (reviewed in Hickson et al., 2009).
SYSTEMIC CHANGES RESULTING FROM CANCER
Just as normal developing organs such as liver and kidneys have systemic consequences for the organism, so does the tumor organ. The dramatic systemic effects of the tumor organ are not limited to metastatic spread, but also include effects on immunity, coagulation and metabolism (Figure 4). Indeed, it is these major systemic changes that cause the majority of cancer deaths, rather than effects of the direct overgrowth of the primary tumor or even the metastases. For example, cachexia, a syndrome of chronic wasting with severe fatigue and weight loss, due to loss of adipose tissue and muscle mass, is induced by factors secreted by tumors (Acharyya et al., 2004; Skipworth et al., 2007) and may account for nearly a third of cancer deaths (Acharyya et al., 2004 and references therein).
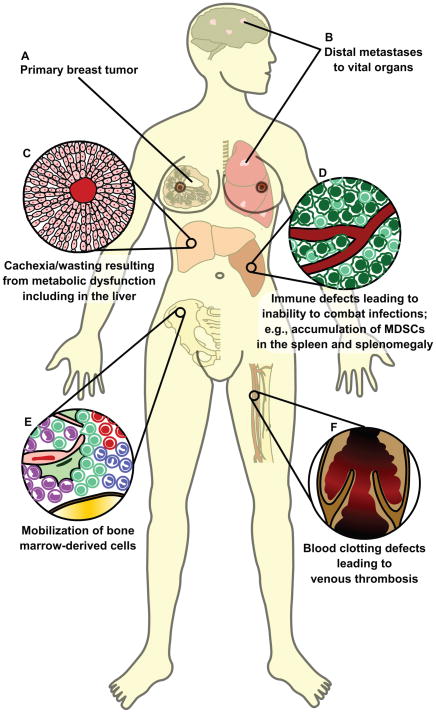
Figure 4. Systemic changes resulting from cancer.
The interactions between the tumor and the rest of the body are primary causes of cancer-related morbidity and mortality.
(A, B) Malignant cells from the primary tumor disseminate into circulation and colonize and expand into vital organs such as the lungs and brain.
(C) Tumor-secreted factors, such as TNF-α and IFN-γ, can disrupt the normal metabolic functions of the liver and lead to cachexia, a wasting away of body mass.
(D) The spleen has a major function in the mounting of immune responses. During cancer progression, myeloid-derived suppressor cells (MDSCs) accumulate, contributing to splenomegaly and an impaired immune response with increased susceptibility to infection.
(E) In the bone marrow, mobilization of bone marrow-derived cells is increased. These cells promote tumor progression by activating angiogenesis and increasing invasiveness of cancer cells.
(F) The primary tumors often activate pro-coagulatory factors and inhibit fibrinolytic factors, resulting in thrombosis formation. Clotting of the vasculature can be a sign of undiagnosed cancer, and is a major complication of late-stage cancer.
Immune suppression in cancer patients
Tumors severely suppress the immune system, resulting in significant patient mortality (Rolston and Bodey, 2006). Infections are common with hematological cancers, but patients with solid tumors are also at increased risk of developing infections (Rolston and Bodey, 2006). The changes to the immune system are rarely evident at the time of diagnosis, but as tumors progress, immune deficiencies develop and are further amplified by chemotherapy (Hadden, 2003). Tumor-induced increases in MDSCs may be a major factor in the global immune dysfunction of cancer patients (Gabrilovich and Nagaraj, 2009), as the percentage of MDSCs in the spleen increases from 2–4% in normal mice to 20–40% in tumor-bearing mice. Similarly, a ten-fold increase in the number of MDSCs is observed in the blood of human patients (Gabrilovich and Nagaraj, 2009). The adaptive immune system is also affected by tumors: T cells are functionally impaired and TRegs accumulate (reviewed in de Visser et al., 2006). Furthermore, tumor-bearing mice have a diminished ability to produce antibodies to foreign antigens and are unable to reject allogeneic tumors (Danna et al., 2004).
Altered coagulation in cancer, a link with angiogenesis and metastasis
Up to 50% of all cancer patients and 90% of those with metastatic disease have coagulation abnormalities (De Cicco, 2004). As a result, thromboembolism is estimated to be the second most common cause of cancer-related death (Caine et al., 2002). The pro-thrombotic state in cancer patients originates from the highly abnormal hemodynamic system in tumors with direct interactions between cancer cells and endothelial cells, platelets or monocytes. There is also an imbalance in pro-coagulatory and fibrinolytic (anti-coagulatory) factors induced by the tumor. Endothelial cells, macrophages and cancer cells in tumors express tissue factor and cancer procoagulant, which activates the coagulation cascade (Caine et al., 2002; De Cicco, 2004; Rickles and Falanga, 2001). Cancer-induced changes in the coagulation system are linked to tumor angiogenesis. For example, VEGF stimulates angiogenesis as well as secretion of tissue factor.
The increased procoagulant activity in tumors is also associated with increased risk of metastasis (Hejna et al., 1999). In mouse models, inhibitors of coagulation can inhibit metastasis, whereas platelet activation and release of fibrin enhance hematogenous metastasis (Camerer et al., 2004; Hejna et al., 1999; Nieswandt et al., 1999; Palumbo et al., 2000 and references therein). Platelet activation and fibrin formation promote the incorporation of cancer cells into mini-thrombi that protect the cancer cells from physical shear and attack from immune cells while they are in the blood stream. The activated platelets may also physically bridge the cancer cells to the vessel wall, thereby facilitating extravasation at a secondary site. Coagulation also affects the endothelial cells, which respond by displaying adhesion molecules that may help cancer cells adhere to the vessel wall (Camerer et al., 2004; Hejna et al., 1999). Finally, microvesicles, which could be involved in setting up the metastatic sites, are mediators of coagulation: upon exposure to collagen, platelets shed microvesicles, which provide a membrane surface for the assembly of pro-coagulant enzyme complexes (reviewed in Cocucci et al., 2009).
THE ORGANIZATION OF THE TUMOR TISSUE MAY INFLUENCE THE DRUG RESPONSE
Mutations in cancer cells can result in anti-cancer drug resistance. However, the microenvironment can also confer drug resistance for example by regulating drug distribution or by providing signals that lead to protection of the cancer cells against cell death. Understanding how the tumor tissue influences therapy responses may be critical for achieving better patient survival.
Tissue organization influences drug distribution
The abnormal organization of the tumor tissue affects the ability of anticancer drugs to reach the cancer cells (Minchinton and Tannock, 2006). In normal tissues, cells are within a few cell diameters of a blood capillary enabling efficient drug delivery. However, when cancer cells proliferate faster than the cells that form capillaries, the resulting increase in distance from blood vessels impairs drug delivery.
Drugs penetrate tissues with a net flow of fluid from blood vessels balanced by resorption into lymphatics. The tumor vasculature is often poorly organized with leaky vasculature and sparse or absent lymphatics. This leads to increased interstitial fluid pressure, thereby inhibiting drug distribution. The rate of diffusion through the tumor tissue is also affected by the properties of drugs (e.g., molecular weight, shape, charge and solubility), by uptake in cancer or stromal cells and by binding to the ECM (Figure 5A, Minchinton and Tannock, 2006). For example, type I collagen influences the distribution of antibodies and chemotherapeutic drugs (Loeffler et al., 2006). Although many drugs are of low molecular weight, they still may bind to plasma proteins such as albumin, rendering them high molecular weight for practical purposes.
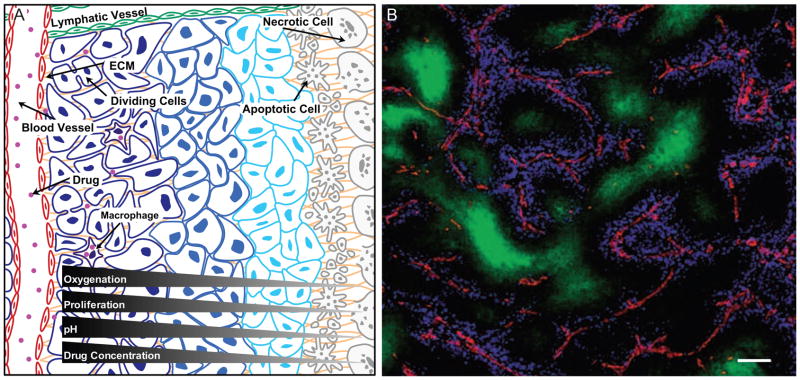
Figure 5. Tumor tissue organization influences drug response.
(A) The overall architecture of the tumor has a direct effect on the ability of a cancer drug to penetrate the tissue and reach the cancer cells. Abnormal leakage from blood vessels together with insufficient lymphatic drainage, especially from the middle of tumors, contribute to increased interstitial pressure in the tumor tissue that inhibits penetration of drugs into the deeper areas of the tumors. Cancer drug penetration is also limited by their binding to ECM proteins, such as collagen, or their uptake by stromal cells, such as macrophages. Cancer cells furthest away from the blood vessel not only are exposed to the lowest drug concentrations, but also receive the lowest amounts of nutrients and oxygen from the circulation and therefore have the lowest proliferative index. As many cancer cell drugs preferentially target actively proliferating cells, this effect contributes to the inability of drugs to target cells in hypoxic areas. Cancer cells further from the blood vessel are also exposed to a low pH microenvironment where many cytotoxic drugs become inactive. Thus, the organization of the tumor tissue results in limited drug availability and efficacy in the hypoxic areas of tumors, areas speculated to contain some of the most aggressive cancer cells. The changing microenvironment in different parts of the tumors therefore results in an apparent difference in drug sensitivity (indicated by the changing color of the cancer cells).
(B) Distribution of doxorubicin in vivo. Section from a mouse mammary tumor showing the distribution of doxorubicin (blue) in relation to tumor blood vessels (red) and regions of hypoxia (green). Note that doxorubicin is found only in close proximity to tumor blood vessels. Scale bar: 100 μm. Reprinted with permission from Primeau et al., 2005.
Doxorubicin is a classical chemotherapeutic drug with limited distribution in solid tumors due to binding to DNA and sequestration in cells close to the vasculature (Figure 5B; Primeau et al., 2005). The efficacy of doxorubicin, despite its poor penetration, may result from the practice that it is given in cycles, and therefore it may kill and remove consecutively deeper layers of cells. Alternatively, it may target the cancer cells with regenerative potential, which have been speculated to lie close to blood vessels (Calabrese et al., 2007).
Microenvironments that protect against therapy
Cancer cells in hypoxic areas of tumors are often refractory to therapy. These cells are furthest from the blood vessels and are exposed to the lowest drug concentrations. Furthermore, while cytotoxic drugs specifically target proliferating cells, cancer cells in hypoxic areas show lower proliferation as they are exposed to low concentrations of oxygen and nutrients (reviewed in Minchinton and Tannock, 2006). Several chemotherapeutic drugs are also less active in the acidic microenvironment associated with hypoxia (reviewed in Hockel and Vaupel, 2001; Minchinton and Tannock, 2006). Finally, hypoxia may produce resistance to chemotherapeutic drugs through gene expression, such as HIF-1α-mediated upregulation of anti-apoptotic proteins (e.g., Bcl-xL and heat shock proteins) or the multidrug resistance gene product P-glycoprotein (MDR1) (Baird et al., 2006; Chen et al., 2009; Comerford et al., 2002). Hypoxia is a special problem for radiation therapy because radiation-induced DNA damage occurs through the formation of oxygen free radicals (Hockel and Vaupel, 2001). The bone marrow microenvironment also protects cancer cells from cytotoxic drugs, likely through production of IL-6 and activation of Notch and integrin signaling (reviewed in Meads et al., 2008). Stimulation of integrins by ECM proteins can also reduce drug sensitivity in other microenvironments: Cell adhesion to fibronectin and collagen through the integrin VLA-4 mediates resistance to etoposide in small cell lung cancer (reviewed in Meads et al., 2008).
Targeting of the microenvironment for better drug responses
Chemoresistance may be overcome by targeting the stroma. Anticancer drugs reach tumors through the circulation and one might therefore expect that inhibiting angiogenesis in tumors would decrease the effectiveness of chemotherapy. Instead, angiogenesis inhibitors can enhance the effects of chemotherapy. Data from mouse models of cancer suggest that this is due to normalization rather than destruction of the tumor vasculature. Normalization improves blood flow and reduces interstitial fluid pressure thereby improving drug delivery (Tong et al., 2004). Increased vascular permeability of tumor vasculature can also be exploited by formulation of drugs, e.g., doxorubicin, into liposomes that preferentially leak into tissues with increased vascular permeability (Brown and Giaccia, 1998).
Increased chemotherapeutic drug penetration can be achieved by vaccinating mice against fibroblast-activating protein, a proteinase expressed by CAFs. As a result, the CAFs are killed, reducing the amount of type I collagen and improving drug delivery (Loeffler et al., 2006). Similarly, inhibition of Hedgehog signaling in a mouse model of pancreatic ductal adenocarcinoma leads to depletion of tumor-associated stroma, increased vascular density, and increased delivery and efficacy of gemcitabine (Olive et al., 2009).
Direct targets in the tumor microenvironment that have been or are being tested in clinical trials include the ECM, endothelial cells and pericytes, fibroblasts and innate immune cells (reviewed in Joyce, 2005). Furthermore, anticancer drugs that exploit the abnormal microenvironment are under development, including pro-drugs that are reduced under hypoxic conditions to active, cytotoxic forms (Brown and Giaccia, 1998; Minchinton and Tannock, 2006). Another approach is the targeting of the tumor-stroma communication through, for example, inhibition of the PDGF-FGF communication loops between epithelial cells, CAFs and pericytes (Pietras et al., 2008).
PERSPECTIVES AND CONCLUSIONS
Cancer cells and stromal components are organized into tissues, which in turn are organized into tumor organs that interact with the whole organismal system. Tumors may appear chaotic, but patterns are emerging for how ECM architecture, angiogenesis and inflammatory responses change with tumor progression. Many of the mechanisms responsible for the organization of tumors are unknown, but several processes employed during development (e.g., epithelial invasion; Wiseman and Werb, 2002) and during tissue remodeling (e.g., recruitment of bone marrow-derived cells; Pollard, 2009) are utilized. The communications between the many different components of the tumor organ are very complex and often involve more than two cell types. Thus, mathematical modeling may facilitate the prediction of malignant behavior based on knowledge of the tissue context (Anderson et al., 2006; Shields et al., 2007; Smallbone et al., 2007).
The importance of tissue organization in metastases is also emerging. ECM components (e.g., fibronectin and versican V1), inflammatory cells and bone marrow-derived vascular cells contribute critically to the initiation, establishment and growth of metastases. Interestingly, the recruitment and organization of one component is often critical for the organization of the components that follow. These findings raise the question of whether cancer cells have similar requirements for the organization of the distant environment as they do in the primary tumor.
Within the tumor organ there are microenvironments that confer promoting or inhibiting effects on cancer cells (e.g., areas of hypoxia). To address fully how such microenvironments influence the phenotype of cells, better markers are needed to allow for differentiation between subpopulations of cancer cells, including those with stem cell-like characteristics. Such markers may also facilitate studies to determine whether differences in, for example, tumorigenic potential or therapeutic responses between subpopulations of cancer cells reflect genetic, epigenetic or environmental differences. When coupled with appropriate markers, live imaging techniques may be used to track cancer cell fates over time in situ and allow for studies of how cancer cells interact with their microenvironment (e.g., Calabrese et al., 2007; Egeblad et al., 2008; Giampieri et al., 2009). Finally, in vivo tracking might be able to answer the question of whether carcinoma cells originate from epithelial stem cells or from differentiated cells that later acquire more stem cell-like characteristics.
The tumor-associated stromal cells (e.g., macrophages and fibroblasts) are different from their counterparts in the normal tissue. A major question is if these differences occur because of altered gene expression induced by the microenvironment or if some changes are caused by mutations (Polyak et al., 2009). The origin of most tumor-associated stromal cells is also unclear: do they originate from expansion of cells that originally were present in the tissue, are they recruited from the bone marrow, do they originate from mesenchymal stem cells present within the tissue, or have they transdifferentiated from the cancer cells?
Two out of five individuals will get cancer in their lifetime, and one out of five will succumb to the disease (Horner et al., 2009). We are learning that the context of the cancer cells plays a role in tumor initiation and progression, and ultimately in patient prognosis and treatment. An important aspect of tumor biology is therefore to understand the tumors, not just as clonal monocultures of cancer cells, but as deranged organs acting in the context of an organism. To determine how these interactions work is complicated and requires both advanced animal models, co-culturing models, and careful analysis of patient material. What we have learned so far is that tumors hijack programs that are part of normal tissue development, repair and regeneration, but use these programs in a deregulated manner. Why cancers form these abnormal organs remains a mystery. Hopefully, the further study of how cancer cells interact with and build the tumor organ will lead to the development of treatments that are both more effective and less toxic.
Supplementary Material
Gr1+ myeloid cells are a patrolling subpopulation of the tumor-infiltrating myeloid cells, related to Figure 3. Gr1+/c-fms+ myeloid cells (red/green) patrol in tumor vasculature (yellow) in the stroma surrounding the tumor (blue), while c-fms+/dextran+ macrophages (green/yellow) do not migrate in the same area. Gr1+ cells were labeled with Alexa-Fluor-647-conjugated anti-Gr1 antibodies (red), which were coinjected, i.v., with 70 kD rhodamine-conjugated dextran (yellow) in an MMTV-PyMT;ACTB-ECFP;c-fms-EGFP mouse. Dextran first labeled the blood vessels and was taken up by macrophages over time. Shown are the ECFP (blue), EGFP (green), rhodamine (yellow) and Alexa-Fluor-647 (red) channels. Scale bar is 100 μm. Play back is 1200x real-time, representing a 12 h 17 min long image sequence. Time indicates time after injection of antibody and dextran.
Migration of T-cells in the tumor microenvironment, related to Figure 3. Migration of CXCR6+ activated/memory T-cells (green) in proximity to blood vessels (red) in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse injected intravenously with rhodamine-conjugated dextran (red) to mark blood vessels. Cancer cells are labeled blue. Scale bar is 100 μm. Play back is 600x real-time, representing a 2 h 4 min long image sequence.
Migration of T-cells is stopped in response to hypoxia, related to Figure 3. CXCR6+ activated/memory T-cells (green) are seen migrating in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse. Cancer cells are labeled blue. The same field is shown during normoxia (21% inhaled oxygen), hypoxia (7% inhaled oxygen) and re-established normoxia (21% oxygen). Shown are the ECFP (blue) and EGFP (green) channels. Scale bar is 100 μm. Play back is 600x real-time, representing a 1 h 3 min long image sequence.
Acknowledgments
We thank Dr. Andrew J. Ewald and Hanne A. Askautrud for their contributions to Figure 1 and are grateful to Dr. Valery Krizhanovsky and our reviewers for helpful comments on the manuscript. We apologize for the omission of many significant references due to space constraints. This work was supported by a stipend from The San Cataldo Institution to M.E. and grants from NIH (R01 CA057621, R01 CA129523 and U01 ES012801 to Z.W.; U01 CA141451 to M.E.) and the Starr Cancer Consortium (M.E.). E.S.N. was supported by the Leslie C. Quick and William Randolph Hearst Fellowships at the Watson School of Biological Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–915. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, Denardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Turnbull DW, Johnson EA. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem. 2006;281:38675–38681. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, Auvinen P, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214:357–367. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Borrello MG, Alberti L, Fischer A, Degl’innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Chen X, Huang R, Zeng H, Gong J, Meng W, Lu Y, Zhao F, Wang L, Zhou Q. BCL-xL is a target gene regulated by hypoxia-inducible factor-1{alpha} J Biol Chem. 2009;284:10004–10012. doi: 10.1074/jbc.M805997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004;50:187–196. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Del Buono R, Pignatelli M, Hall PA. Control of differentiation in a rectal adenocarcinoma cell line: the role of diffusable and cell-associated factors. J Pathol. 1991;164:59–66. doi: 10.1002/path.1711640111. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230:676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- Dong-Le Bourhis X, Berthois Y, Millot G, Degeorges A, Sylvi M, Martin PM, Calvo F. Effect of stromal and epithelial cells derived from normal and tumorous breast tissue on the proliferation of human breast cancer cell lines in co-culture. Int J Cancer. 1997;71:42–48. doi: 10.1002/(sici)1097-0215(19970328)71:1<42::aid-ijc9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther RJ, Lamps L, Schwartz HS. Marjolin ulcers: secondary carcinomas in chronic wounds. J South Orthop Assoc. 1999;8:181–187. [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol. 2003;3:1061–1071. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- Hickson J, Diane Yamada S, Berger J, Alverdy J, O’Keefe J, Bassler B, Rinker-Schaeffer C. Societal interactions in ovarian cancer metastasis: a quorum-sensing hypothesis. Clin Exp Metastasis. 2009;26:67–76. doi: 10.1007/s10585-008-9177-z. [DOI] [PubMed] [Google Scholar]
- Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Horner M, Ries L, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse S, Feuer E, Huang L, Mariotto A, et al., editors. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL. Extracellular matrix and tenascin-C in pathogenesis of breast cancer. Lancet. 2001;357:1992–1994. doi: 10.1016/S0140-6736(00)05133-3. [DOI] [PubMed] [Google Scholar]
- Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Proteolytic regulators of the tumor microenvironment. Cell. 2010 doi: 10.1016/j.cell.2010.03.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, Massague J. Tumor Self-Seeding by Circulating Cancer Cells. Cell. 2009a;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009b;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101:320–326. doi: 10.1038/sj.bjc.6605143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- Knight SC, Hunt R, Dore C, Medawar PB. Influence of dendritic cells on tumor growth. Proc Natl Acad Sci U S A. 1985;82:4495–4497. doi: 10.1073/pnas.82.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW, Scherer PE. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini L, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008a;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008b;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol. 2000;156:383–388. doi: 10.1016/S0002-9440(10)64740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK. A new tumor suppressor that regulates tissue architecture. PLoS Med. 2009;6:e1000073. doi: 10.1371/journal.pmed.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nature. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- Papaioannou VE, McBurney MW, Gardner RL, Evans MJ. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975;258:70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Partanen JI, Nieminen AI, Makela TP, Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci U S A. 2007;104:14694–14699. doi: 10.1073/pnas.0704677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K. Pregnancy and breast cancer: the other side of the coin. Cancer Cell. 2006;9:151–153. doi: 10.1016/j.ccr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Cellular plasticity within the pancreas--lessons learned from development. Dev Cell. 2010;18:342–356. doi: 10.1016/j.devcel.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–224. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- Rolston KVI, Bodey GP. Infections in Patients with Cancer. In: Kufe DW, Frei E, Holland JF, Weichselbaum RR, Pollock RE, Bast RC Jr, Hong WKI, Hait WN, editors. Holland-Frei Cancer Medicine. 7. Columbia: BC Decker Inc; 2006. [Google Scholar]
- Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Lienard D, Cerottini JC, Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, Motegi K, Vojtesek B, Kankova K, Furrie E, et al. Migration-stimulating factor: a genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248:1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Crosstalk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- Skipworth RJ, Stewart GD, Dejong CH, Preston T, Fearon KC. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007;26:667–676. doi: 10.1016/j.clnu.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallbone K, Gatenby RA, Gillies RJ, Maini PK, Gavaghan DJ. Metabolic changes during carcinogenesis: potential impact on invasiveness. J Theor Biol. 2007;244:703–713. doi: 10.1016/j.jtbi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell. 2008a;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008b;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gr1+ myeloid cells are a patrolling subpopulation of the tumor-infiltrating myeloid cells, related to Figure 3. Gr1+/c-fms+ myeloid cells (red/green) patrol in tumor vasculature (yellow) in the stroma surrounding the tumor (blue), while c-fms+/dextran+ macrophages (green/yellow) do not migrate in the same area. Gr1+ cells were labeled with Alexa-Fluor-647-conjugated anti-Gr1 antibodies (red), which were coinjected, i.v., with 70 kD rhodamine-conjugated dextran (yellow) in an MMTV-PyMT;ACTB-ECFP;c-fms-EGFP mouse. Dextran first labeled the blood vessels and was taken up by macrophages over time. Shown are the ECFP (blue), EGFP (green), rhodamine (yellow) and Alexa-Fluor-647 (red) channels. Scale bar is 100 μm. Play back is 1200x real-time, representing a 12 h 17 min long image sequence. Time indicates time after injection of antibody and dextran.
Migration of T-cells in the tumor microenvironment, related to Figure 3. Migration of CXCR6+ activated/memory T-cells (green) in proximity to blood vessels (red) in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse injected intravenously with rhodamine-conjugated dextran (red) to mark blood vessels. Cancer cells are labeled blue. Scale bar is 100 μm. Play back is 600x real-time, representing a 2 h 4 min long image sequence.
Migration of T-cells is stopped in response to hypoxia, related to Figure 3. CXCR6+ activated/memory T-cells (green) are seen migrating in a live MMTV-PyMT;ACTB-ECFP;CXCR6EGFP mouse. Cancer cells are labeled blue. The same field is shown during normoxia (21% inhaled oxygen), hypoxia (7% inhaled oxygen) and re-established normoxia (21% oxygen). Shown are the ECFP (blue) and EGFP (green) channels. Scale bar is 100 μm. Play back is 600x real-time, representing a 1 h 3 min long image sequence.