Abstract
We report here the clinical, genetic and molecular characterization of four Chinese families with Leber’s hereditary optic neuropathy (LHON). There were variable severity and age-of-onset in visual impairment among these families. Strikingly, there were extremely low penetrances of visual impairment in these Chinese families. Sequence analysis of complete mitochondrial genomes in these pedigrees showed the homoplasmic T3394C (Y30H) mutation, which localized at a highly conserved tyrosine at position 30 of ND1, and distinct sets of mtDNA polymorphisms belonging to haplogroups D4b and M9a. The occurrence of T3394C mutation in these several genetically unrelated subjects affected by visual impairment strongly indicates that this mutation is involved in the pathogenesis of visual impairment. However, there was the absence of functionally significant mtDNA mutations in these four Chinese pedigrees carrying the T3394C mutation. Therefore, nuclear modifier gene(s) or environmental factor(s) may play a role in the phenotypic expression of the LHON-associated T3394C mutation.
Keywords: Mitochondrial DNA, ND1, Mutation, Leber’s hereditary optic neuropathy, Visual loss, Penetrance, Haplogroup, Maternally, Chinese
Introduction
Leber’s hereditary optic neuropathy (LHON) is a maternally inherited eye disease that generally affects young adults with the rapid, painless, bilateral loss of central vision [1–3]. Mutations in mitochondrial DNA (mtDNA) are the molecular bases for this disorder [4–7]. Since the landmark discovery of the first LHON-associated ND4 G11778A mutation [4], more than 30 mtDNA mutations have been associated LHON among various ethnic background [7]. Of these, the ND1 G3460A, ND4 G11778A and ND6 T14484C mutations, in the genes encoding the subunits of respiratory chain complex I, are the most commonly LHON-associated mtDNA mutations, accounting for more than 50% of LHON pedigrees in different ethnic origins worldwide [2,7–10]. Typical features in LHON pedigrees are incomplete penetrance and male bias among the affected subjects, reflecting the complex etiology of this disease [11,12]. The primary LHON-associated mtDNA mutations such as ND4 G11778A mutation by themselves are insufficient to produce a clinical phenotype. Therefore, other modifiers including the nuclear modifier genes, mitochondrial haplotypes and environmental factors modify the rick of visual loss [12,13].
It was anticipated that additional mutations causing LHON can be found in the mitochondrial genome in the Asian populations. To further elucidate molecular basis of LHON in the Chinese population, a systematic and extended mutational screening of mtDNA has been initiated in the large clinical population of Ophthalmology Clinic at the Wenzhou Medical College, China [11–19]. In the previous investigations, we showed that the LHON was associated with the ND4 G11778A mutation in 15 Chinese families with variable penetrance and severity and age-at-onset of visual impairment [11–16]. Furthermore, ND6 T14484C mutation and ND1 G3460A mutation were identified in three Han Chinese families and one Chinese families, respectively [18,19]. In addition, we showed that LHON is associated with the ND4 G11696A mutation in five Chinese families with extremely low penetrances of visual loss [17]. In the present study, we performed the clinical, genetic and molecular characterization of another four Chinese families with suggestively maternally transmitted LHON. Molecular analysis has led to identification of the T3394C mutation in ND1 gene in these Chinese families. To elucidate the role of mitochondrial haplotype in the phenotypic manifestation of the T3394C mutation, we performed a PCR-amplification of fragments spanning entire mitochondrial genome and subsequent DNA sequence analysis in the matrilineal relatives of those Chinese families.
Materials and methods
Patients
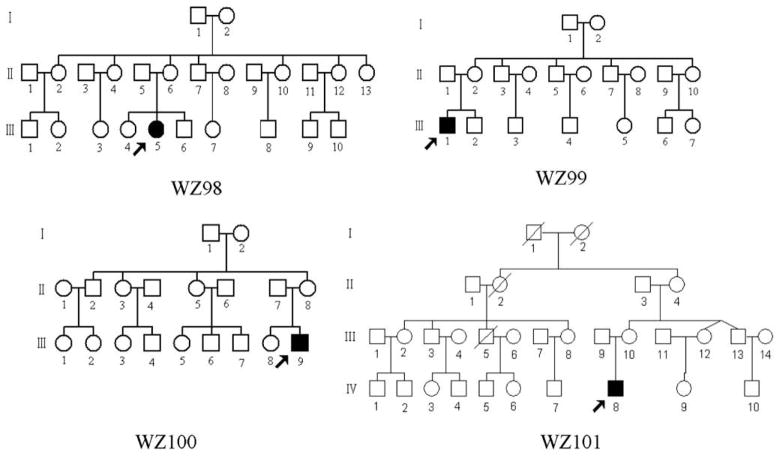
As a part of genetic screening program for visual impairment, four Chinese families (Fig. 1) were ascertained through the School of Ophthalmology and Optometry, Wenzhou Medical College, and Ophthalmology Clinic, Beijing Dongfang Hospital, respectively. Informed consent, blood samples, and clinical evaluations were obtained from all participating family members, under protocols approved by the Cincinnati Children’s Hospital Medical Center institute review board and the Wenzhou Medical College ethics committee. Members of those pedigrees were interviewed at length to identify both personal or family medical histories of visional impairments, and other clinical abnormalities. The 167 control DNA samples used for screening for the presence of mtDNA mutations were obtained from a panel of unaffected individuals from Chinese ancestry.
Fig. 1.
Four Chinese pedigrees with Leber’s hereditary optic neuropathy. Vision impaired individuals are indicated by filled symbols.
Ophthalmological examinations
The ophthalmologic examinations of probands and other members of these families were conducted, including visual acuity, visual field examination (Humphrey Visual Field Analyzer IIi, SITA Standard), visual evoked potentials (VEP) (Roland Consult RETI port gamma, flash VEP), and fundus photography (Canon CR6-45NM fundus camera). The degree of visual impairment was defined according to the visual acuity as follows: normal > 0.3, mild = 0.3–0.1; moderate < 0.1–0.05; severe < 0.05–0.02; and profound < 0.02.
Mutational analysis of the mitochondrial genome
Genomic DNA was isolated from whole blood of participants using the Puregene DNA Isolation Kits (Gentra Systems). The presence of the G3460A, G11778A and T14484C mutations was examined as detailed elsewhere [2]. Briefly, affected individuals’ DNA fragments spanning these mtDNA mutations were amplified by PCR using oligodeoxy-nucleotides corresponding to mtDNA at positions 3108–3717 for the G3460A mutation, 11654–11865 for the G11778A mutation, and 14260–14510 for the T14484C mutation [20], respectively. For the detection of the G3460A mutation, the amplified PCR segments were digested with a restriction enzyme BsaHI [2], while the presence of the T14484C mutation was examined by digesting PCR products with a restriction enzyme MvaI [2]. For the examination of the G11778A mutation, the amplified PCR segments were digested with the restriction enzyme Tsp45I [11–16].
The entire mitochondrial genome of four probands was PCR amplified in 24 overlapping fragments using sets of the light (L) strand and the heavy (H) strand oligonucleotide primers as described previously [21]. Each fragment was purified and subsequently analyzed by direct sequencing in an ABI 3100 automated DNA sequencer using the Big Dye Terminator Cycle sequencing reaction kit. These sequence results were compared with the updated consensus Cambridge sequence [20]. DNA and protein sequence alignments were carried out using seqweb program GAP (GCG). The allele frequency of T3394C mutation in ND1 gene was determined by PCR-amplification of fragments spanning the corresponding regions, using the genomic DNA derived from Chinese controls as templates and performing subsequent sequence analysis of PCR products, as described above.
Results
Clinical presentation
In family WZ98, the proband (III-5) complained of painless, progressive deterioration of bilateral visual impairment and came to the Ophthalmology Clinic at Wenzhou Medical College at the age of 14. Ophthalmological evaluation showed that her visual acuity was 0.1 in the both eyes. Fundus examination showed that both her temporal optic disks were pale and reflex on fovea centralis was normal. Visual field testing demonstrated large centrocecal scotomata in both her eyes. Therefore, she exhibited a typical clinical feature of LHON. No other abnormality was found on radiological and neurological examination. Furthermore, she had no other significant medical history. The family is originated from Zhejiang Province in Eastern China. However, none of other 16 matrilineal relatives in this family exhibited visual impairment.
In WZ99 pedigree, the proband (III-1) came to Ophthalmology Clinic at Wenzhou Medical College at the age of 21. He suffered from painless, progressive deterioration of bilateral visual impairment three months ago. His visual dysfunction occurred within a month, first in the left eye and then two weeks later in the right eye. He saw a dark cloud in the center of vision and had tinnitus. His visual acuity was 0.1 in the right eye and 0.06 in the left eye. Fundus examination showed that both his optic disks were abnormal: vascular tortuosity of the central retinal vessels, a circumpapillary telangiectatic microangiopathy, and no reflex on fovea centralis. Encephalon CT showed sphenoiditis. Visual field testing revealed large centrocecal scotomata in both his eyes. The flash VEP showed bilaterally decreased amplitudes with delayed latencies. Thus, he showed a typical clinical feature of LHON. He had no other significant medical history. The family is originated from Henan Province in Northwestern China. Further familiar history and clinical evaluation revealed that none of other 9 matrilineal relatives in this family exhibited a visual deficit.
In family WZ100, the proband III-9 came to the Ophthalmology clinic at the Wenzhou Medical College at the age of 14 years. He exhibited bilateral visual impairment at the age of 18-year-old. His visual acuity was 0.05 in the right eye and 0.03 in the left eye. Clinical evaluation revealed that he had a typical clinical feature of LHON. The family, originated from Zhejiang Province in Eastern China. Further familial history and clinical evaluation revealed that all other matrilineal relatives in this family exhibited normal vision.
In family WZ101, the proband (IV-8) was diagnosed as LHON by Ophthalmology clinic at the Dongfeng Hospital, Beijing at the age of 6. He began suffering bilateral visual impairment at the age of 1-year-old. Ophthalmological examination showed that his visual acuity was 0.4 in the right eye and 0.1 in the left eye. Visual field testing demonstrated large centrocecal scotomata in both eyes. None of other matrilineal relatives in this family living in Shandong Province in Eastern China had visual deficit.
Furthermore, there is no evidence that any member of those families had any other known cause to account for visual impairment. Comprehensive family medical histories of these individuals showed no other clinical abnormalities, including diabetes, muscular diseases, hearing impairment and neurological disorders.
Mitochondrial DNA analysis
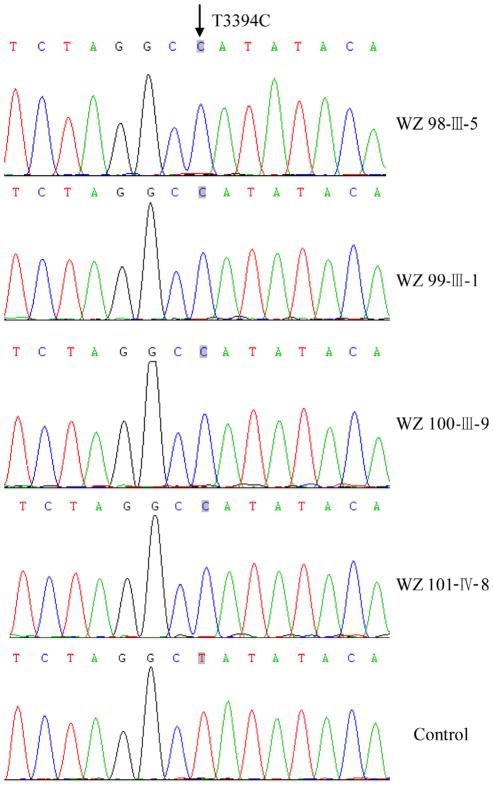
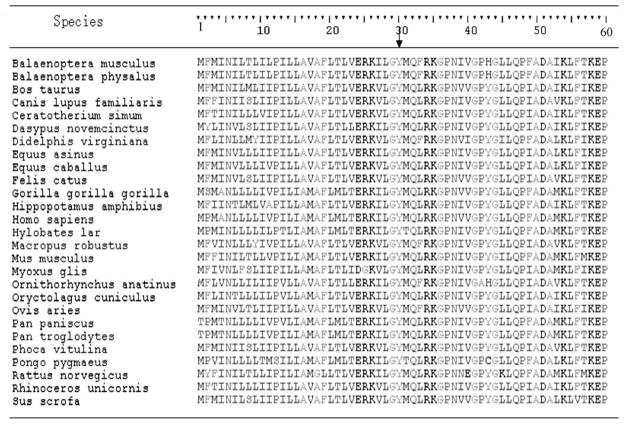
To elucidate the molecular basis of visual impairment, we have performed a mutational analysis of the mitochondrial genome in these families. First, we examined three commonly known LHON-associated mtDNA mutations (G3460A, G11778A and T14484C) by PCR-amplification and subsequent restriction enzyme digestion analysis of PCR fragments derived from each proband of those families. We failed to detect the presence of the G3460A, G11778A and T14484C mutations. We then performed a PCR-amplification of fragments spanning entire mitochondrial genome and subsequent DNA sequence analysis in these probands. As shown in Fig. 2, the T-to-C transition at position 3394 (T3394C) in ND1 gene, resulted in the substitution of a histidine for tyrosine (Y30H) at amino acid position 30, has been found in those subjects [22]. As shown in Fig. 3, the tyrosine at position 30 in ND1 is highly conserved among 27 organisms. Indeed, this mutation has been associated with LHON in a Finnish family [23], metabolic diseases [24] and deafness in a Chinese family [25]. Further sequence analysis, as shown in Fig. 2, confirmed the presence of the homplasmic T3394C mutation in matrilineal relatives of these families but not other members of these families. The allele frequency analysis of the T3394C mutation showed that one (20-year-old male) of 167 unrelated Chinese control subjects carried the T3394C mutation.
Fig. 2.
Identification of the T3394C mutation in the ND1 gene. Partial sequences chromatograms of ND1 gene from four affected individuals and one Chinese control. An arrow indicates the location of the base changes at position 3394.
Fig. 3.
Alignment of partial ND1 polypeptides from different species. Arrow indicates the tyrosine at position 30 (Y30) at the N-terminal of ND1 polypeptide, corresponding to the T3394C mutation.
In addition to the identical T3394C mutation, as shown in Table 2, these subjects exhibited distinct sets of mtDNA polymorphisms. Of other nucleotide changes in these mitochondrial genomes, there are 18 known variants in the D-loop, four known variants in 12S rRNA gene, two known variants in the 16S rRNA gene, 28 (2 novel and 26 known) silent variants in the protein encoding genes as well as 13 known missense mutations in the protein encoding genes [7]. These missense mutations are the G4491A (V8I) and C5178A (L237M) in the ND2 gene, the G6366A (V155I) in CO1 gene, the C8414T (L17F) in the A8 gene, the A8701G (T59A) and A8860G (T112A) in A6 gene, the A10398G (T114A) in the ND3 gene, the A12358G (T8A) and A12361G (T9A), in the ND5 gene, the A14417G (V86A) in the ND6, the C14766T (T7I), G15110A (A122T), and A15326G (T194A) in the Cytb gene. These variants in RNAs and polypeptides were further evaluated by phylogenetic analysis of these variants and sequences from other organisms including mouse [26], bovine [27], and Xenopus laevis [28]. However, all these variants showed no evolutionary conservation. Based on the nomenclature of mitochondrial haplogroups [29,30], we used the mtDNA sequence variations among 4 Chinese probands to establish the haplogroup affiliation of each mtDNA. Here, mtDNAs of pedigree WZ98, WZ99, WZ100 and WZ101 belong to the Eastern Asian halpogroups D4b2, M9a, M9a and M9a, respectively.
Table 2.
mtDNA variants in four Chinese pedigrees with Leber’s hereditary optic neuropathy.
| Gene | Position | Replacement | Conservationa (H/B/M/X) | CRSb | WZ98 | WZ99 | WZ100 | WZ101 | Previouslyc reported | |
|---|---|---|---|---|---|---|---|---|---|---|
| D loop | 73 | A to G | A | G | G | G | Yes | |||
| 153d | A to G | A | G | G | G | Yes | ||||
| 195 | T to C | T | C | Yes | ||||||
| 228 | G to A | G | A | Yes | ||||||
| 263 | A to G | A | G | G | G | G | Yes | |||
| 310 | T to CTC | T | CTC | TC | CTC | Yes | ||||
| 489 | T to C | T | C | C | C | C | Yes | |||
| 522 | C to Del | C | Del | Ins | Yes | |||||
| 523 | A to Del | A | Del | Ins | Yes | |||||
| 16051 | A to G | A | G | Yes | ||||||
| 16223 | C to T | C | T | T | T | Yes | ||||
| 16234 | C to T | C | T | T | T | Yes | ||||
| 16291 | C to T | C | T | Yes | ||||||
| 16311 | T to C | T | C | Yes | ||||||
| 16316 | A to G | A | G | G | G | Yes | ||||
| 16362 | T to C | T | C | C | C | Yes | ||||
| 16519 | T to C | T | C | Yes | ||||||
| 16526 | G to A | G | A | Yes | ||||||
| 12S rRNA | 750 | A to G | A/A/A/- | A | G | G | G | G | Yes | |
| 1041 | A to G | A/T/T/T | A | G | G | Yes | ||||
| 1382 | A to C | A/A/A/G | A | C | Yes | |||||
| 1438 | A to G | A/A/A/G | A | G | G | G | G | Yes | ||
| 16S rRNA | 2706 | A to G | A/G/A/A | A | G | G | G | G | Yes | |
| 3010 | G to A | G/G/A/A | G | A | Yes | |||||
| ND1 | 3394 | T to C | Tyr to His | Y/Y/Y/Y | T | C | C | C | C | Yes |
| ND2 | 4491 | G to A | Val to Ile | V/I/I/V | G | A | A | A | Yes | |
| 4769 | A to G | A | G | G | G | G | Yes | |||
| 4883 | C to T | C | T | Yes | ||||||
| 5178 | C to A | Leu to Met | L/T/T/T | C | A | Yes | ||||
| CO1 | 6366 | G to A | Val to Ile | V/V/V/I | G | A | Yes | |||
| 6815 | T to C | T | C | No | ||||||
| 7028 | C to T | C | T | T | T | T | Yes | |||
| 7142 | T to C | T | C | Yes | ||||||
| CO2 | 7861 | T to C | T | C | Yes | |||||
| 8020 | G to A | G | A | Yes | ||||||
| 8200 | T to C | T | C | Yes | ||||||
| 8167 | C to T | C | T | Yes | ||||||
| ATP8 | 8414 | C to T | Leu to Phe | L/F/M/W | C | T | Yes | |||
| ATP6 | 8701 | A to G | Thr to Ala | T/S/L/Q | A | G | G | G | G | Yes |
| 8860 | A to G | Thr to Ala | T/A/A/T | A | G | G | G | G | Yes | |
| 8964 | C to T | C | T | Yes | ||||||
| CO3 | 9242 | A to G | A | G | G | G | Yes | |||
| 9296 | C to T | C | T | Yes | ||||||
| 9540 | T to C | T | C | C | C | C | Yes | |||
| 9824 | T to C | T | C | Yes | ||||||
| ND3 | 10398 | A to G | Thr to Ala | T/T/T/A | A | G | G | G | G | Yes |
| 10400 | C to T | C | T | T | T | T | Yes | |||
| ND4 | 10873 | T to C | T | C | C | C | C | Yes | ||
| 11371 | A to G | A | G | No | ||||||
| 11719 | G to A | G | A | A | A | G | Yes | |||
| ND5 | 12358 | A to G | Thr to Ala | T/S/I/M | A | G | Yes | |||
| 12361 | A to G | Thr to Ala | T/L/L/L | A | G | Yes | ||||
| 12705 | C to T | C | T | T | T | T | Yes | |||
| 13260 | T to C | T | C | Yes | ||||||
| 12972 | A to G | A | G | Yes | ||||||
| ND6 | 14269 | A to G | A | G | No | |||||
| 14308 | T to C | T | C | C | C | Yes | ||||
| 14417 | A to G | Val to Ala | V/K/W/S | A | G | Yes | ||||
| 14668 | C to T | C | T | Yes | ||||||
| Cytb | 14766 | C to T | Thr to Ile | T/S/T/S | C | T | T | T | T | Yes |
| 14783 | T to C | T | C | C | C | C | Yes | |||
| 15043 | G to A | G | A | A | A | A | Yes | |||
| 15110 | G to A | Ala to Thr | A/T/A/L | G | A | Yes | ||||
| 15175 | C to T | C | T | No | ||||||
| 15301 | G to A | G | A | A | A | A | Yes | |||
| 15326 | A to G | Thr to Ala | T/M/I/I | A | G | G | G | G | Yes |
Conservation of amino acid for polypeptides or nucleotide for RNAs in human (H), bovine (B), mouse (M), and Xenopus laevis (X).
CRS, Cambridge reference sequence [20].
See online mitochondrial genome databases http://www.mitomap.org and http://www.genpat.uu.se/mtDB/.
Sequence variations used to estabilish the haplogroup affiliation of each mtDNA are in boldface.
Discussion
In the present study, we have performed the clinical, genetic, and molecular characterization of four Chinese families with Leber’s hereditary optic neuropathy. The bilateral visual impairment as a sole clinical phenotype was only present in the maternal lineage of those pedigrees, suggesting that the mtDNA mutation is the molecular basis for this disorder. Sequence analysis of the complete mitochondrial genomes in these pedigrees showed the distinct sets of mtDNA polymorphisms, in addition to the identical T3394C (Y30H) mutation in ND1 gene. Indeed, this mutation is present in homoplasy only in the maternal lineage of those pedigrees but not other members of these families. The tyrosine at amino acid position 30 is extremely conserved in ND1 polypeptide among different organisms [22]. This mutation has been associated with other clinical abnormalities including LHON [23], deafness [25] and metabolism disorders [24]. In fact, the occurrence of the T3394C mutation in these several genetically unrelated subjects affected by visual impairment strongly indicates that this mutation is involved in the pathogenesis of visual impairment.
In contrast to our previous data that there is a high penetrance of visual loss in some Chinese pedigrees carrying the G11778A mutation [13,15,16], the penetrances of visual impairment in these four Chinese families carrying the T3394C mutation were extremely low. In particular, only one matrilineal relative of each Chinese pedigree exhibited visual impairment. In addition, one of 167 Chinese subjects carried this mutation. Furthermore, as shown in Table 1, the age-at-onset for vision loss in those subjects varied from 1 to 21 years old, with an average of 10 years old. This data suggested that the age-at-onset for visual loss in four Chinese families carrying the T3394C mutation was younger than those in other Chinese families with LHON [11–19]. Unlike the fact that some Chinese subjects carrying the G11778A mutations exhibited profound visual loss [11,13,15,16], the severity of visual impairment in those affected subjects carrying the T3394C mutation varied from mild to moderate to severe.
Table 1.
Summary of clinical and molecular data for affected matrilineal relatives of four Chinese families with LHON.
| Subjects | Gender | Age of test (yrs) | Age of onset (yrs) | Visual acuity Right | Visual acuityLeft | Level of visual impairment | Number of matrilineal relatives | mtDNA haplogroup |
|---|---|---|---|---|---|---|---|---|
| WZ98-III-5 | F | 14 | 14 | 0.1 | 0.1 | Mild | 17 | D4b2 |
| WZ99-III-1 | M | 21 | 21 | 0.1 | 0.06 | Moderate | 10 | M9a |
| WZ100-III-9 | M | 14 | 14 | 0.05 | 0.03 | Severe | 12 | M9a |
| WZ101-IV-8 | M | 6 | 1 | 0.4 | 0.1 | Mild | 15 | M9a |
The extremely low penetrance of visual loss and the presence of one/167 controls indicted that the T3394C mutation, similar to ND4 G11778A and G11696A mutations [12,17], is itself insufficient to produce the clinical phenotype. Thus, the modifier factors including nuclear backgrounds, other environmental factors and mitochondrial haplotypes are necessary for the phenotypic manifestation of the T3394C mutation. In particular, the mitochondrial haplotypes have been shown to influence the penetrance and expressivity of visional loss associated with primary mtDNA mutations. mtDNA mutations at positions 4216 and 13708 labeled as second LHON mutations were implicated to increase the penetrance of the LHON-associated G11778A and T14484C mutations [31]. Furthermore, the mitochondrial haplogroup J can influence the phenotypic manifestation of the primary LHON G11778A and T14484C mutations in a very large cohort of families of European ancestry [32–33]. Most recently, the ND4 G11696A, tRNAThr A15951G and the tRNAMet A4435G mutations have been implicated to increase the penetrance of LHON in Chinese families [13,15,16]. Here, these four Chinese pedigrees shared the identical twenty one mtDNA variants. These mitochondrial genomes of W98, WZ99, WZ100 and WZ101 pedigrees belong to the Eastern Asian haplogroups D4b, M9a, M9a and M9a, respectively, while mtDNA of a Finnish family and one Chinese pedigree carrying the T3394C belonged to the haplogroups J and F2, respectively [23,25]. This implied that the T3394C mutation, similar to G11778A mutation [2,12], occurred sporadically and multiplied through evolution of the mtDNA [3,11–16]. However, there was the absence of functionally significant mutations in tRNA and rRNAs or secondary LHON mutations in Chinese pedigrees W98, WZ99, WZ100 and WZ101 pedigrees. Thus, these mtDNA variants may not have a potential modifying role in the development of visual impairment associated with T3394C mutation in those families. Therefore, nuclear modifier gene(s) or environmental factor(s) may play a role in the phenotypic expression of the LHON-associated T3394C mutation in these Chinese.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants RO1DC05230 and RO1DC07696 from the National Institute on Deafness and Other Communication Disorders to M.X.G., a Chinese Young Scholar Award (30628013) from National Science Foundation of China and a key research Grant Z204492 from Zhejiang Provincial Natural Science Foundation of China to M.X.G., a project Grant ZB0202 from Zhejiang Provincial Natural Science Foundation of China and a Key Research and Development Program project Grant 2004C14005 from Zhejiang Province, China to J.Q.
References
- 1.Newman NJ. Leber’s hereditary optic neuropathy. Ophthalmol Clin NA. 1993;4:431–447. [Google Scholar]
- 2.Brown MD, Torroni A, Reckord CL, Wallace DC. Phylogenetic analysis of Leber’s hereditary optic neuropathy mitochondrial DNA’s indicates multiple independent occurrences of the common mutations. Hum Mut. 1995;6:311–325. doi: 10.1002/humu.1380060405. [DOI] [PubMed] [Google Scholar]
- 3.Man PY, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet. 2002;39:162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 5.Howell N. LHON and other optic nerve atrophies: the mitochondrial connection. Dev Ophthalmol. 2003;37:94–108. doi: 10.1159/000072041. [DOI] [PubMed] [Google Scholar]
- 6.Servidei S. Mitochondrial encephalomyopathies: gene mutation. Neuromuscul Disord. 2004;14:107–116. doi: 10.1016/s0960-8966(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 7.Brandon MC, Lott MT, Nguyen KC, Spolim S, Navathe SB, Baldi P, Wallace DC. MITOMAP: a human mitochondrial genome database-2004 update. Nucleic Acids Res. 2005;33:D611–D613. doi: 10.1093/nar/gki079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackey DA, Oostra RJ, Rosenberg T, Nikoskelainen E, Bronte-Stewart J, Poulton J, Harding AE, Govan G, Bolhuis PA, Norby S, Bleeker-Wagemakers EM, Savontaus ML, Cahn C, Howell N. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59:481–485. [PMC free article] [PubMed] [Google Scholar]
- 9.Mashima Y, Yamada K, Wakakura M, Kigasawa K, Kudoh J, Shimizu N, Oguchi Y. Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber’s hereditary optic neuropathy. Curr Eye Res. 1998;17:403–408. doi: 10.1080/02713689808951221. [DOI] [PubMed] [Google Scholar]
- 10.Man PY, Griffiths PG, Brown DT, Howell N, Turnbull DM, Chinnery PF. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72:333–339. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu J, Li R, Tong Y, Hu Y, Zhou X, Qian Y, Lu F, Guan MX. Only male matrilineal relatives with Leber’s hereditary optic neuropathy in a large Chinese family carrying the mitochondrial DNA G11778A mutation. Biochem Biophys Res Commun. 2005;328:1139–1145. doi: 10.1016/j.bbrc.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 12.Qu J, Zhou X, Zhang J, Zhao F, Sun YH, Tong Y, Wei QP, Cai W, Yang L, West CE, Guan MX. Extremely low penetrance of Leber’s hereditary optic neuropathy in eight Han Chinese families carrying the ND4 G11778A mutation. Ophthalmology. 2009;116:558–564. doi: 10.1016/j.ophtha.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu J, Li R, Zhou X, Tong Y, Lu F, Qian Y, Hu Y, Mo JQ, West CE, Guan MX. The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation in a Chinese family. Invest Ophth Vis Sci. 2006;47:475–483. doi: 10.1167/iovs.05-0665. [DOI] [PubMed] [Google Scholar]
- 14.Qian Y, Zhou X, Hu Y, Tong Y, Li R, Lu F, Yang H, Mo JQ, Qu J, Guan MX. Clinical evaluation and mitochondrial DNA sequence analysis in three Chinese families with Leber’s hereditary optic neuropathy. Biochem Biophys Res Commun. 2005;332:614–621. doi: 10.1016/j.bbrc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Qu J, Zhou X, Tong Y, Lu F, Qian Y, Hu Y, Mo JQ, West CE, Guan MX. The mitochondrial tRNAThr A15951G mutation may influence the phenotypic expression of the LHON-associated ND4 G11778A mutation in a Chinese family. Gene. 2006;376:79–86. doi: 10.1016/j.gene.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Qu J, Li R, Zhou X, Tong Y, Yang L, Chen J, Zhao F, Qian Y, Lu F, West CE, Guan MX. Cosegregation of the ND4 G11696A mutation with the LHON-associated ND4 G11778A mutation in a four generation Chinese family. Mitochondrion. 2007;7:140–146. doi: 10.1016/j.mito.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Wei QP, Hu Y, Tong Y, Zhao F, Lu C, Qian Y, Sun YH, Lu F, Qu J, Guan MX. Leber’s hereditary optic neuropathy is associated with the mitochondrial ND4 G11696A mutation in five Chinese families. Biochem Biophys Res Commun. 2006;340:69–75. doi: 10.1016/j.bbrc.2005.11.150. [DOI] [PubMed] [Google Scholar]
- 18.Sun YH, Wei QP, Zhou X, Qian Y, Zhou J, Lu F, Qu J, Guan MX. Leber’s hereditary optic neuropathy is associated with the mitochondrial ND6 T14484C mutation in three Chinese families. Biochem Biophys Res Commun. 2006;347:221–225. doi: 10.1016/j.bbrc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 19.Tong Y, Mao Y, Zhou X, Yang L, Zhang J, Cai W, Zhao F, Wang X, Lu F, Qu J, Guan MX. The mitochondrial tRNAGlu A14693G mutation may influence the phenotypic manifestation of ND1 G3460A mutation in a Chinese family with Leber’s hereditary optic neuropathy. Biochem Biophys Res Commun. 2007;357:524–530. doi: 10.1016/j.bbrc.2007.03.189. [DOI] [PubMed] [Google Scholar]
- 20.Andrews RM, Kubacka I, Chinerry PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 21.Rieder MJ, Taylor SL, Tobe VO, Nickerson DA. Automating the identification of DNA variations using quality-based fluorescence re-sequencing: analysis of the human mitochondrial genome. Nucleic Acids Res. 1981;26:967–973. doi: 10.1093/nar/26.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearnley IM, Walker JE. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992;1140:105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- 23.Puomila A, Hamalainen P, Kivioja S, Savontaus ML, Koivumaki S, Huoponen K, Nikoskelainen E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur J Hum Genet. 2007;15:1079–1089. doi: 10.1038/sj.ejhg.5201828. [DOI] [PubMed] [Google Scholar]
- 24.Saxena R, de Bakker PI, Singer K, Mootha V, Burtt N, Hirschhorn JN, Gaudet D, Isomaa B, Daly MJ, Groop L, Ardlie KG, Altshuler D. Comprehensive association testing of common mitochondrial DNA variation in metabolic disease. Am J Hum Genet. 2006;79:54–61. doi: 10.1086/504926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Yuan H, Lu J, Liu X, Wang G, Zhu Y, Cheng J, Wang X, Han B, Yang L, Yang S, Yang A, Sun Q, Kang D, Zhang X, Dai P, Zhai S, Han D, Young WY, Guan MX. Mutations at position 7445 in the precursor of mitochondrial tRNASer(UCN) gene in three maternal Chinese pedigrees with sensorineural hearing loss. Mitochondrion. 2008;8:285–292. doi: 10.1016/j.mito.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981;26:167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- 27.Gadaleta G, Pepe G, De Candia G, Quagliariello C, Sbisa E, Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989;28:497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- 28.Roe A, Ma DP, Wilson RK, Wong JF. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985;260:9759–9774. [PubMed] [Google Scholar]
- 29.Tanaka M, Cabrera VM, Gonzalez AM, Larruga JM, Takeyasu T, Fuku N, Guo LJ, Hirose R, Fujita Y, Kurata M, Shinoda K, Umetsu K, Yamada Y, Oshida Y, Sato Y, Hattori N, Mizuno Y, Arai Y, Hirose N, Ohta S, Ogawa O, Tanaka Y, Kawamori R, Shamoto-Nagai M, Maruyama W, Shimokata H, Suzuki R, Shimodaira H. Mitochondrial genome variation in eastern Asia and the peopling of Japan. Genome Res. 2004:1832–1850. doi: 10.1101/gr.2286304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong QP, Bandelt HJ, Sun C, Yao YG, Salas A, Achilli A, Wang CY, Zhong L, Zhu CL, Wu SF, Torroni A, Zhang YP. Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet. 2006;15:2076–2086. doi: 10.1093/hmg/ddl130. [DOI] [PubMed] [Google Scholar]
- 31.Torroni A, Petrozzi M, D’Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet. 1997;60:1107–1121. [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MD, Starikovskaya E, Derbeneva O, Hosseini S, Allen JC, Mikhailovskaya IE, Sukernik RI, Wallace DC. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup. J Hum Genet. 2002;110:130–138. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- 33.Howell N, Oostra RJ, Bolhuis PA, Spruijt L, Clarke LA, Mackey DA, Preston G, Herrnstadt C. Sequence analysis of the mitochondrial genomes from Dutch pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 2003;72:1460–1469. doi: 10.1086/375537. [DOI] [PMC free article] [PubMed] [Google Scholar]