Abstract
The neurotoxicity of methylmercury (MeHg) is well documented in both humans and animals. MeHg causes acute and chronic damage to multiple organs, most profoundly the central nervous system (CNS). Microglial cells are derived from macrophage cell lineage, making up ∼12% of cells in the CNS, yet their role in MeHg-induced neurotoxicity is not well defined. The purpose of the present study was to characterize microglial vulnerability to MeHg and their potential adaptive response to acute MeHg exposure. We examined the effects of MeHg on microglial viability, reactive oxygen species (ROS) generation, glutathione (GSH) level, redox homeostasis, and Nrf2 protein expression. Our data showed that MeHg (1–5μM) treatment caused a rapid (within 1 min) concentration- and time-dependent increase in ROS generation, accompanied by a statistically significant decrease in the ratio of GSH and its oxidized form glutathione disulfide (GSSG) (GSH:GSSG ratio). MeHg increased the cytosolic Nrf2 protein level within 1 min of exposure, followed by its nuclear translocation after 10 min of treatment. Consistent with the nuclear translocation of Nrf2, quantitative real-time PCR revealed a concentration-dependent increase in the messenger RNA level of Ho-1, Nqo1, and xCT 30 min post MeHg exposure, whereas Nrf2 knockdown greatly reduced the upregulation of these genes. Furthermore, we observed increased microglial death upon Nrf2 knockdown by the small hairpin RNA approach. Taken together, our study has demonstrated that microglial cells are exquisitely sensitive to MeHg and respond rapidly to MeHg by upregulating the Nrf2-mediated antioxidant response.
Keywords: methylmercury, microglial cells, reactive oxygen species, glutathione, Nrf2
Mercury is a global pollutant universally found within the environment. Once mercury has been introduced into the aquatic environment, it is methylated by sulfate-reducing bacteria into methylmercury (MeHg) (Jensen and Jernelov, 1969), which is rapidly taken up by living organisms and biomagnified through the food chain, reaching concentrations 10,000–100,000 times greater in fish than in the water itself (U.S. EPA, 1997). MeHg accumulates in humans, especially in fish-eating populations (Dorea, 2008; Kuntz et al., 2009). It readily crosses the blood-brain barrier via the L-type large neutral amino acid transporter (Yin et al., 2008). Congenital Minamata disease is the most well-documented neurological disorder caused by severe MeHg poisoning due to industrial pollution (Irukayama et al., 1963).
In the past several decades, the majority of studies on MeHg-induced central nervous system (CNS) damage have focused on its effects on neurons and astrocytes (Shanker et al., 2001; Tamm et al., 2006; Yin et al., 2007). It has been reported that MeHg disrupts cellular redox homeostasis through excessive reactive oxygen species (ROS) generation. The interaction of MeHg with the mitochondrial electron transport chain represents a major mechanism of MeHg-induced ROS generation (Yee and Choi, 1996). It causes a concentration-dependent reduction of the inner mitochondrial membrane potential (Yin et al., 2007) by altering Ca2+ homeostasis (Levesque and Atchison, 1991). MeHg also decreases adenosine triphosphate synthesis in neurons by inhibiting both glycolysis and the Krebs cycle (Kauppinen et al., 1989) thereby contributing to neuronal dysfunction and death. Furthermore, MeHg inhibits astrocytic glutamate uptake, while stimulating glutamate efflux (Aschner et al., 1993), consequently resulting in an excessive concentration of synaptic glutamate, which ultimately leads to neuronal excitotoxicity and cell death (Mutkus et al., 2006).
Microglial cells are another major glial cell type in the brain. They possess phagocytic activity (Smith, 2001; Witting et al., 2000), and they are the first cell type to become activated in the presence of inflammation, infection, trauma, and degenerative diseases (Gehrmann et al., 1995). The activation of microglial cells is inherent to Alzheimer's disease (Shie et al., 2009), Parkinson's disease (Kim and Joh, 2006), HIV dementia (Dheen et al., 2007), and MeHg exposure (Garg and Chang, 2006; Nishioku et al., 2000; Sakamoto et al., 2008). Upon activation, microglial cells produce free radicals (Long et al., 2007) and release proinflammatory cytokines including interleukin (IL) 6, tumor necrosis factor-α (TNF-α), and prostaglandin E2, all of which influence neighboring cells (Kim and Joh, 2006). Comparative studies carried out in animals treated with neurotoxins have shown that microglial cells react at lower concentrations (Monnet-Tschudi et al., 1995a,b) and that such reactions occur prior to astrocytic dysfunction and neuronal death (Kuhlmann and Guilarte, 2000; Maier et al., 1995). Monnet-Tschudi (1998) reported that the earliest sign of MeHg-induced neurotoxicity was inherent to microglial cells by using three-dimensional brain cell cultures. Consistently, Charleston et al. (1995) reported that microglial cells accumulated the largest concentration of mercury following MeHg exposure in nonhuman primates. Although some studies have assessed the effects of high concentrations of MeHg after long times of exposure on immortalized microglial cell lines (Eskes et al., 2002; Garg and Chang, 2006), these results may not represent the acute response of primary microglial cells to lower concentrations of MeHg as detected in the early stage of Minamata disease patients. Given existing limitations, it is necessary to characterize microglial vulnerability to MeHg and to test the role of microglial cells in exhibiting an acute response to low concentrations of MeHg after short times of exposure. In this study, we assessed microglial functional changes after short-term exposure to MeHg concentrations that failed to elicit detrimental effects in other cell types. For example, MeHg treatment at concentrations less than 5μM did not cause decrease in formazan production [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay] or oxidative stress in rat striatal synaptosomes (Dreiem and Seegal, 2007). Similar results were also observed in primary rat astrocytes, where MeHg at concentrations less than 5μM failed to elicit ROS production (Shanker and Aschner, 2003).
Analogous to other cell types, the generation of ROS in microglial cells by MeHg plays a critical role in its toxicity (Garg and Chang, 2006). The Nrf2-mediated antioxidant machinery is likely used in microglial cells to detoxify free radicals. Nrf2 maintains redox balance in microglial cells after exposure to the murine leukemia virus (Qiang et al., 2004) and a variety of environmental toxicants, such as lipopolysaccharides (LPS) (Koh et al., 2009) and kainic acid (Kraft et al., 2006). Under physiological conditions, inactive Nrf2 is bound to the Kelch-like ECH-associating protein 1 (Keap1) in the cytoplasm (Kensler and Wakabayashi, 2010). Keap1 functions both as an Nrf2 cytosolic repressor (Kahn et al., 2008) and as an adaptor for cullin 3–based E3 ubiquitin ligase, leading to rapid proteasomal degradation of Nrf2 (McMahon et al., 2003, 2004). Upon oxidative stress, the interaction between Nrf2 and Keap1 is disrupted, decreasing the rate of proteasomal degradation of Nrf2. This causes de novo Nrf2 to build up within the cells, leading to increased translocation of Nrf2 into the nuclei (Li and Kong, 2009). In the nucleus, Nrf2 forms heterodimers with small Maf proteins, such as FosB, c-Jun, JunD, activating transcription factor 2, and activating transcription factor 4 (Itoh et al., 2003; McMahon et al., 2003; Nguyen et al., 2003). These heterodimers interact with the antioxidant response element to initiate the transcription of target genes including the light chain of the cysteine/glutamate transporter (xCT), γ-glutamyl cysteine synthase, hemeoxygenase-1 (Ho-1), and nicotinamide adenine dinucleotide phosphate:quinone oxidoreductase-1 (Nqo1), in order to detoxify xenobiotics and endogenous reactive electrophiles (Itoh et al., 1999; Prestera and Talalay, 1995; Prestera et al., 1993). Furthermore, Nrf2 knockout mice show increased sensitivity to a variety of pharmacological and environmental toxicants, such as carcinogens and acetaminophen (Enomoto et al., 2001; Ramos-Gomez et al., 2001). Therefore, it is important to explore the role of Nrf2 in MeHg toxicity in microglial cells.
The present study was carried out to test the hypothesis that microglial cells are exquisitely sensitive to MeHg and that they undergo rapid activation upon being treated with low concentrations of MeHg (0.1–5μM), representative of common environmental exposure levels (Akagi et al., 1998). We found that acute MeHg treatment immediately resulted in ROS generation, GSH depletion, and, consequently, the upregulation of both Nrf2 protein and the expression of Ho-1, Nqo1, and xCT in primary microglial cells. Nrf2 knockdown attenuated the upregulation of such genes, resulting in increased microglial death upon MeHg exposure.
MATERIALS AND METHODS
Primary microglial culture.
Primary microglial cells were isolated and cultured according to a published protocol (Ni and Aschner, 2010). Briefly, the cerebral hemispheres of postnatal day 1 neonatal Sprague-Dawley rats were removed and the meninges were dissected off. The cortical tissue was digested with dispase (BD Biosciences, Two Oak Park Drive Bedford, MA). The mixed glial cell culture was maintained in minimum essential medium (MEM) (Invitrogen, Carlsbad, CA), supplemented with 5% heat-inactivated fetal bovine serum (Hyclone, South Logan, UT) and 5% horse serum (Invitrogen). The media were changed once a week. After 2 weeks in culture, microglial cells were separated by gentle shaking for 20 min at room temperature and then plated in six-well plates and cultured at 37°C in a 95% air/5% CO2 incubator for additional 48 h in MEM containing 10% fetal bovine serum (Hyclone) and 1% penicillin and streptomycin (Invitrogen). The purity of the cells was determined by immunostaining for the microglia-specific marker, OX42 (sc-53086, Santa Cruz Biotechnology, Santa Cruz, CA); cell nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI) (VECTASHIELD Mounting Medium with DAPI, H-1200; VECTOR, Burlingame, CA).
MTT assay and lactate dehydrogenase assay.
The cytotoxic effect of MeHg in microglial cells was evaluated by MTT assay (In vitro Toxicology Assay Kit, MTT based, M-5655; Sigma, St Louis, MO). MTT stocking solution (10×) was prepared by reconstituting 15 mg stock MTT reagent in 3 ml of OPTI-MEM culture media (Invitrogen) in the absence of phenol red immediately before the experiment. Primary cultured microglial cells were maintained in 96-well plates at a density of 20,000 cells per well for 2 days prior to experiment. Cells were treated for 6 h. Treatment with 100μM H2O2 was used as a positive control of cell death. After treatment, 10× MTT stocking solution was directly added to each well at a final concentration of 0.5 mg/ml. The formazan crystal precipitates were dissolved by adding an equal volume of MTT solubilization solution (Sigma, M-8910) and gently shaking for 20 min. The absorbance was measured by spectrophotometer (Molecular Devices, VMax Kinetic Microplate Reader, Sunnyvale, CA) at a wavelength of 570 nm. The background absorbance was measured at 690 nm and subtracted from the 570 nm measurement.
Cellular membrane integrity was measured by the lactate dehydrogenase (LDH) assay. After treatment, the culture media were collected for LDH analysis. The LDH assay substrate (L 2402; Sigma) was always freshly prepared. The assay mixture was added in an amount equal to twice of the volume of the culture medium and incubated at room temperature for 30 min in the dark. The reaction was terminated by adding 1/10 volume of 1N HCl. The absorbance was measured by a spectrophotometer (Molecular Devices, VMax Kinetic Microplate Reader) at a wavelength of 490 nm, and the background absorbance was measured at 690 nm.
Detection of intracellular ROS formation.
Microglial cells were preincubated with 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA) (C6827; Invitrogen) at a final concentration of 25μM for 30 min at 37°C. The MeHg solution was directly added in the MEM culture media containing H2DCF-DA. After treatment, the microglial cells were washed with 4°C PBS twice and precipitated by centrifugation at 400 × g. The cell pellets were then dissolved in 1% Triton X-100 (Promega, Madison, WI). Fluorescence was measured at a wavelength of 480/530 nm (excitation/emission) by SpectraMax M5 (Molecular Devices). The DCF fluorescent signal in microglial cells was obtained with a Nikon microscope (Nikon Eclipse 80i, Shinagawa-ku, Tokyo, Japan) at 480/530 nm.
Measurement of GSH.
The intracellular GSH concentration was measured by high-performance liquid chromatography (HPLC) as previously described (Wang et al., 2009). After MeHg treatment, microglial cells were derivatized with iodoacetic acid and dansyl chloride. HPLC analysis was carried out with a propylamine column (YMC Pack, NH2; Waters Corporation, Milford, MA) and an automated HPLC system (Alliance 2695; Waters Corporation). The GSH and GSSG concentrations were normalized to the protein concentration of the samples as measured by colorimetric detection and quantitation using Bicinchoninic Acid Protein Assay Reagent (Pierce 23225, Rockford, IL).
Immunohistochemistry.
Microglial cells were cultured on coverslips coated with poly-L-lysine. After MeHg treatment, cells were fixed with ice-cold 4% paraformaldehyde (PFA) for 15 min, followed by two washes with 30mM glycine to quench the PFA. Cells were permeated for 3 min by 0.25% Triton X-100 in PBS and blocked in 5% bovine serum albumin/PBS for 45 min. The samples were incubated for 1 h with 1:200 rabbit anti-Nrf2 (31136; Abcam, Cambridge, MA) and washed in PBS three times before incubation with 1:400 secondary antibody, donkey anti-rabbit IgG conjugated with fluorescein isothiocyanate (AP182F; Millipore, Billerica, MA). Coverslips were mounted with VECTASHIELD Mounting Medium with propidium iodide (PI) (H-1300; VECTOR). The fluorescence signal was detected using a Zeiss confocal microscope (LSM 510; Zeiss, Dublin, CA) at the following settings: detector gain 900, amplifier offset −0.085, and pinhole 98. The PI signal was detected with the following settings: detector gain 844, amplifier offset −0.068, and pinhole 110.
Measurement of Nrf2 by Western blot analysis.
Western blot analysis was conducted with the primary antibodies, rabbit anti Nrf2 (1:400) (sc-722; Santa Cruz Biotechnology) and mouse anti β-actin (1:2000) (A1978; Sigma). Secondary antibodies were donkey anti-rabbit IgG conjugated with horseradish peroxidase (HRP) (1:1000) (W4011; Promega) and donkey anti-mouse IgG conjugated with HRP (1:4000) (W4021; Promega). The cytosolic and nuclear fractions were prepared with NE-PER Nuclear and Cytosolic Extraction Reagents (78833; Thermo Scientific, Rockford, IL). Briefly, after treatment, microglial cells were lysed in ice-cold hypotonic buffer containing 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 1.5mM MgCl2, 10mM KCl, 1mM dithiothreitol (DTT), and cocktail of protease inhibitors (Thermo Scientific). After 30 min incubation on ice, the samples were centrifuged at 4°C at 12,000 × g and the supernatant was collected as the cytosolic fraction. The pellet was further extracted for 30 min in a high salt lysis buffer containing 50mM HEPES, 500mM NaCl, 1mM DTT, and protease inhibitors (Thermo Scientific). After 10 min centrifugation at 4°C at 12,000 × g, the supernatant was collected as the nuclear fraction. The density of the Nrf2-specific bands was normalized to β-actin.
Measurement of Ho-1, Nqo1, and xCT genes expression by quantitative real-time PCR.
Messenger RNA (mRNA) of Ho-1, Nqo1, and xCT were measured by real-time PCR using the Universal Probe Library (UPL; Roche, Indianapolis, IN). Table 1 lists the primer sequences and UPL probes. Average threshold cycle (ΔCt) values were used to determine the relative difference between control and treated samples. All data were normalized to β-actin levels.
TABLE 1.
Primer Sequences and UPL Probe Numbers Used to Measure Expression Levels of Ho-1, Nqo1, and xCT in Real-Time PCR
| Primer sequences used for real-time PCR analysis | |||
| Gene | Forward (5′–3′) | Backward (5′–3′) | UPL probe |
| xCT | 5′-TCC ATG AAC GGT GGT GTG T-3′ | 5′-CCC TTC TCG AGA TGC AAC AT-3′ | #80 |
| Ho-1 | 5′-GTC AGG TGT CCA GGG AAG G-3′ | 5′-CTC TTC CAG GGC CGT ATA GA-3′ | #9 |
| Nqo1 | 5′-AGC GCT TGA CAC TAC GAT CC-3′ | 5′-CAA TCA GGG CTC TTC TCA CC-3′ | #50 |
| β-actin | 5′-CCC GCG AGT ACA ACC TTC T-3′ | 5′-CGT CAT CCA TGG CGA ACT-3′ | #17 |
Nrf2 knockdown by small hairpin RNA.
Primary microglial cells at ∼50–60% confluence were infected with 15 μl lentiviral particles with 1 × 106 infectious units of virus for 24 h. The lentiviral particles contained an expression construct encoding specific small hairpin RNA (shRNA) against Nrf2 (sc-37049-V; Santa Cruz Biotechnology). The infection rate was tested by copGFP Control Lentiviral particles (sc-108084; Santa Cruz Biotechnology). Primary microglial cells were also infected with control lentiviral particles containing scrambled shRNA sequence (sc-108080; Santa Cruz Biotechnology) as a negative control.
Statistical analysis.
All results were expressed as means ± SEs. Unless otherwise specified, differences among treatment groups were analyzed by one-way ANOVA, followed by Bonferroni's post hoc test. Statistical significance was set at p < 0.05. All experiments were repeated with at least three independently isolated microglial cultures, and data analysis was carried out with GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Overall Cytotoxic Effects of MeHg on Microglia
As the first step, the purity of primary microglial cultures was assessed by immunostaining with the microglial-specific marker, OX42 (Reenila et al., 1997) (Fig. 1A). As shown in the merged image (Fig. 1C), when co-stained with DAPI (Fig. 1B), a nuclear dye, nearly all cells appeared to be of the microglial cells lineage. In addition, cells exhibited characteristic microglial morphology in the differential interference contrast image (Fig. 1D).
FIG. 1.
The purity of primary microglial culture was tested on day 3 after separation from mixed glial culture by immunostaining for OX42. (A) Microglial cells were fixed and immunostained for OX42. (B) Microglial nuclei were counterstained with DAPI. (C) Merged image of OX-42 and DAPI demonstrated the purity of microglial culture close to 100%. (D) Differential interference contrast image of microglial cells. Photographs show representative fields observed in 12 coverslips from three independent experiments.
Next, we tested the overall cytotoxic effects of MeHg on microglial cells with the MTT assay. The absorbance of MTT (wavelength of 570 nm) is positively correlated with cell viability. As shown in Figure 2A, after MeHg treatment for 6 h, the absorbance was indistinguishable between control and 0.1μM MeHg-treated cells. However, higher concentrations of MeHg (1 and 5μM) reduced the absorbance to 0.86 ± 0.09 (p < 0.05) and 0.59 ± 0.08 (p < 0.001), respectively, reflecting reduced cell viability. As the positive control, H2O2 at 100μM reduced MTT absorbance to 0.27 ± 0.07 (p < 0.001). Cell viability was also measured by the LDH assay. The absorbance of LDH (wavelength of 490 nm) is reversely correlated with cell viability. As shown in Figure 2B, the absorbance of LDH increased after 6-h treatment with 1 (p < 0.01) and 5μM MeHg (p < 0.001), respectively, reflecting decreased cell viability.
FIG. 2.
Effect of MeHg cytotoxicity on microglial cells was measured by MTT assay (A) and LDH assay (B). (A) Result of MTT assay of microglial cells after MeHg treatment for 6 h showed the concentration-dependent decrease in absorbance. Microglial cells were treated with MeHg with the concentrations indicated in the graph; 100μM H2O2 was used as a positive control. (B) Result of LDH assay using microglial culture media after the same treatment demonstrated a concentration-dependent increase in absorbance. Values are expressed as the mean ± SEM derived from five independent experiments.
MeHg Caused Acute Oxidative Stress in Microglia
Next, we tested whether MeHg induces ROS generation in microglial cells with the H2DCF-DA assay. The lipophilic H2DCF-DA diffuses freely through the cell membrane, and it is intracellularily converted to hydrophilic DCF. Once in the cells, DCF reacts with ROS and fluoresces, providing a tool for intracellular ROS level analyses. As shown in Figure 3, a significant increase in fluorescence intensity was readily detectable after 1 min treatment with 5μM MeHg (222.6 ± 39.01, p < 0.01) and 100μM H2O2 (229.03 ± 40.08, p < 0.001). Treatments with 1 and 5μM MeHg for 10 min further increased DCF fluorescence in a concentration-dependent manner (Fig. 3). Furthermore, increased DCF fluorescence in living microglial cells was also visualized under a Nikon microscope at 480/530 nm corroborating the findings in Figure 3 (see Fig. 4).
FIG. 3.
ROS production was measured by DCF fluorescence in microglial cells. Microglial cells were treated with MeHg at the concentrations indicated in the graph; 100μM H2O2 was used as a positive control. DCF fluorescence levels were measured after 1 and 10 min treatment, respectively. The results demonstrated that higher MeHg concentration and longer exposure time led to higher ROS level. Values are expressed as the mean ± SEM derived from six independent experiments.
FIG. 4.
DCF fluorescence levels in living microglial cells were visualized by microscopy. (A) Microglial cells were treated with MeHg at the concentrations indicated in the figures; 100μM H2O2 was used as a positive control for 1 min. (B) Microglial cells were treated under the same concentrations for 10 min. The intensity of the fluorescence signal was indicative of the intracellular ROS production. Photographs show representative fields observed from three independent experiments.
MeHg Caused Microglial GSH Reduction
GSH can detoxify ROS, resulting in the formation of GSSG, the oxidized form of GSH. Therefore, we sought to determine if microglial ROS generation could cause a decline in the GSH level. As shown in Figure 5, after 0.1μM MeHg treatment for 1 min, the GSH:GSSG ratio remained statistically unchanged as compared with the control. However, higher concentrations of MeHg (1 and 5μM) produced a concentration-dependent reduction in the GSH:GSSG ratio (69.01 ± 16.9% and 51.32 ± 20.9% of the control level, respectively). Further reduction in the GSH:GSSG ratio was observed after prolonged MeHg treatment for 10 min and 1 h in a concentration- and time-dependent manner.
FIG. 5.
GSH and GSSG levels were measured by HPLC after MeHg treatment. Microglial cells were treated with MeHg at the concentrations indicated in the graph for 1 min, 10 min, and 1 h; 100μM H2O2 was used as a positive control. The ratios of GSH:GSSG were calculated, and the percentages of control were used to plot the graph. Each treatment group was compared with its corresponding control level. The results demonstrated that MeHg caused acute and robust reduction in microglial GSH level in a concentration- and time-dependent manner. Values are expressed as the mean ± SEM derived from three independent experiments.
MeHg-Induced Oxidative Stress Upregulated Nrf2 and Promoted Its Nuclear Translocation
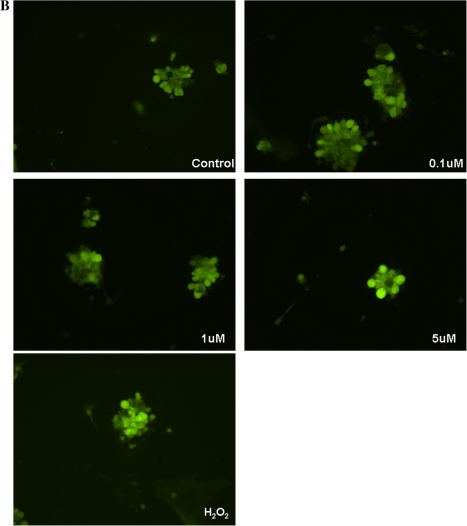
As mentioned before, Nrf2 plays a pivotal role in maintaining redox balance. Therefore, we sought to determine if Nrf2 was altered in microglial cells post MeHg exposure in order to counteract ROS generation. Indeed, the upregulation of the Nrf2 protein level and its nuclear translocation were detected in microglial cells by confocal microscopy after MeHg exposure. As shown in Figure 6, the basal level of Nrf2 (green fluorescence) was low. After 10 min treatment with 0.1μM MeHg, Nrf2 staining was sparsely detected in the cytosol. In contrast, treatment with 1 or 5μM MeHg increased Nrf2 staining in both the cytosol and the nuclei. Compared with 1μM MeHg treatment, the staining intensity was greater in 5μM MeHg-treated cells. The colocalization of Nrf2 and PI staining in the nuclei indicated the nuclear translocation of Nrf2.
FIG. 6.
Nrf2 protein level and intracellular localization were assessed by immunostaining. (A) Control group showed minimal Nrf2 (green) in the cytosol. Cell nuclei were stained by PI (red); (B) 0.1μM MeHg treatment increased Nrf2 in the cytosol but not in the nuclei; (C) 1μM MeHg treatment for 10 min increased Nrf2 in both the cytosol and the nuclei; (D) 5μM MeHg treatment for 10 min resulted in a higher level of Nrf2 in both cytosol and nuclei, as compared with 1μM MeHg-treated cells. The yellow color indicates the colocalization of Nrf2 and DNA in microglial nuclei. Photographs show representative fields observed from four independent experiments.
Next, we delineated the dynamic changes in Nrf2 level in both the cytosol and the nuclei. As shown in Figure 7, cytosolic Nrf2 protein increased in a concentration-dependent manner after 1 min MeHg treatment. However, under the same conditions, the nuclear Nrf2 protein level remained unchanged. After 10 min, Nrf2 protein level was increased in both the cytosolic and the nuclear fractions after 1 and 5μM MeHg treatments as well as 100μM H2O2 treatment. After 1 h of MeHg treatment, increased Nrf2 accumulated in the nuclei but not in the cytosol. The quantitative results in Figure 7 show that 1 and 5μM MeHg treatment for 1 h increased nuclear Nrf2 by 2.60 ± 0.56-fold (p < 0.05) and 3.99 ± 1.32-fold (p < 0.01) over the control value, respectively. The representative Western blot bands are shown below the quantified data in Figure 7.
FIG. 7.
Cytosolic and nuclear fractions of Nrf2 were analyzed by Western blot. Microglial cells were treated with MeHg at the concentrations indicated in the graph for 1 min, 10 min, and 1 h; 100μM H2O2 was used as a positive control. Nrf2 protein levels in cytosolic and nuclear fractions were measured by Western blot. Density of each band was measured by Image J, and the values were normalized with β-actin. After 1 min MeHg treatment, Nrf2 increased in the cytosolic fraction but not in the nuclear fraction. After 10 min MeHg treatment, however, Nrf2 increased in both subcellular fractions. After 1 h MeHg treatment, Nrf2 remained increased in the nuclear fraction but returned to control level in the cytosol. Values are expressed as the mean ± SEM derived from three independent experiments.
MeHg-Induced Nrf2 Nuclear Accumulation Upregulated the Transcription of Ho-1, Nqo1, and xCT Genes
After treatment with MeHg for 30 min, the mRNA levels of Ho-1, Nqo1, and xCT were quantified by real-time PCR. The treatment time was based on the observation that Nrf2 was translocated to the nuclei commencing at 10 min post MeHg treatment and remained detectable at 1 h posttreatment. The transcription level of Ho-1 (Fig. 8A) and Nqo1 (Fig. 8B) was upregulated in response to MeHg treatment with 1 and 5μM. However, xCT gene expression was increased by 2.3 ± 1.46-fold over the control value (p < 0.01) after treatment with the highest concentration of MeHg (5μM), as shown in Figure 8C.
FIG. 8.
The expression of Ho-1, Nqo1, and xCT was measured by real-time PCR after MeHg treatment. Microglial cells were treated with MeHg at the concentrations indicated in the graph for 30 min; 100μM H2O2 was used as a positive control. The mRNA levels of Ho-1 (A), Nqo1 (B), and xCT (C) were measured by real-time PCR. The differences in the average threshold cycle (ΔCt) values were determined and normalized to the expression of β-actin. Values are expressed as the mean ± SEM derived from five independent experiments.
Next, we carried out an Nrf2 knockdown experiment to confirm that the transcriptional upregulation of these tested genes is dependent on Nrf2 activity. Compared with the control group, 5μM MeHg treatment caused a significant increase in the Ho-1 mRNA levels in both uninfected and scramble shRNA-infected microglial cells (p < 0.05). In contrast, the Nrf2 knockdown group did not demonstrate an increased Ho-1 mRNA level after the same treatment as compared with the control level (p > 0.05) (Fig. 9A). Figure 9B shows that no significant increase in Nqo1 mRNA level was present in the Nrf2 knockdown group after 5μM MeHg, in contrast to uninfected or scramble shRNA-treated groups. Similar changes in xCT mRNA level were also observed after Nrf2 knockdown (Fig. 9C). Therefore, the upregulation of Ho-1, Nqo1, and xCT transcription upon MeHg treatment is dependent upon nuclear Nrf2 activity.
FIG. 9.
The effects of Nrf2 knockdown on MeHg-induced Ho-1, Nqo1, and xCT expression were analyzed by real-time PCR. Primary microglial cells were first infected with lentivirus as indicated for 24 h and then treated with 5μM MeHg for 30 min. Then the mRNA levels of Ho-1 (A), Nqo1 (B), and xCT (C) were measured by real-time PCR. The difference in the average threshold cycle (ΔCt) values was determined and normalized to the expression of β-actin. The uninfected and untreated microglial cells were used to determine the basal gene expression levels. The uninfected cells treated with 5μM MeHg for 30 min served as a positive control. The random virus with no known gene target was also used to infect microglial cells in order to reveal any possible effects of lentiviral backbone on gene expression. Microglial cells treated with Nrf2-specific shRNA showed no significant increase in downstream gene expression compared with basal levels after 5μM MeHg treatment for 30 min. Values are expressed as the mean ± SEM derived from three independent experiments.
Nrf2 Protected Microglial Cells against MeHg Toxicity
To further evaluate the cytoprotective function of Nrf2 against MeHg-induced cell death, we assessed cell viability upon MeHg treatment under Nrf2 knockdown condition. As shown in Figure 10A, compared with uninfected microglial cells in the absence of MeHg exposure, both uninfected cells and random virus–infected cells treated with 5μM MeHg for 6 h demonstrated a significant decrease in MTT absorbance; knockdown of Nrf2 further decreased MTT absorbance after the same treatment (p < 0.001). Notably, compared with uninfected cells post MeHg treatment, knockdown of Nrf2 caused a significant decrease in MTT absorbance (p < 0.001, compare second column with fourth column in Fig. 10A). Consistent with the MTT assay results, the Nrf2 knockdown group demonstrated the highest absorbance after 5μM MeHg treatment for 6 h in the LDH assay (Fig. 10B). Furthermore, compared with uninfected microglial cells treated with MeHg, Nrf2 knockdown of microglia displayed increased LDH absorbance, indicating decreased viability in Nrf2-deficienct cells (p < 0.001, compare second column with fourth column of Fig. 10B).
FIG. 10.
The effects of Nrf2 knockdown on microglial cell viability were assessed by MTT and LDH assay. Primary microglial cells were first infected with lentivirus for 24 h and then treated with 5μM MeHg for 6 h. The uninfected microglial cells without MeHg exposure were used to measure the maximal cell viability. The uninfected cells treated with 5μM MeHg for 6 h served as a positive control for cell death. The random virus with no known gene target was also used to infect microglial cells in order to reveal any possible cytotoxicity effects of the lentiviral backbone. (A) Result of MTT assay showed lowest absorbance in Nrf2 knockdown cells, indicative of lowest cell viability. (B) Result of LDH assay using culture media from the same treatment groups demonstrated the highest absorbance in Nrf2 knockdown cells, which indicated the highest level of LDH released from damaged microglial cells to culture media. Values are expressed as the mean ± SEM derived from four independent experiments.
DISCUSSION
To our knowledge, this is the first study to investigate the response of primary microglial cells to acute MeHg exposure. We have demonstrated that MeHg leads to rapid ROS generation and GSH depletion commencing at 1 min post MeHg exposure, well before changes in astrocytes or neurons are observed (data not shown). In response, microglial Nrf2 is upregulated and undergoes nuclear translocation to activate the expression of downstream genes such as Ho-1, Nqo1, and xCT. Our data suggest that microglial cells are the first line of cellular defense against MeHg toxicity in the CNS.
Previous studies have reported an increase in ROS in microglial cells after prolonged MeHg treatment at high concentrations (Chang, 2007; Eskes et al., 2002; Garg and Chang, 2006). However, these studies do not capture the acute microglial response to lower concentrations of MeHg as detected in early stage of Minamata disease patients. Here, we have revealed that 5μM MeHg exposures cause a significant increase in ROS generation in primary microglial cells commencing at 1 min post MeHg exposure (Figs. 3 and 4). Similar acute ROS generation in microglial cells was also observed after TiO2 exposure (Long et al., 2007). Although increased generation of ROS also occurs in neurons (Mundy and Freudenrich, 2000; Sanfeliu et al., 2001) and astrocytes (Wang et al., 2009; Yin et al., 2007), it is only detected after much longer exposure to MeHg.
GSH is used to detoxify ROS and itself is converted to the oxidized form, GSSG. In response to MeHg, microglial ROS is increased (Figs. 3 and 4) and, correspondingly, the GSH:GSSG ratio is decreased (Fig. 5). Previous studies have established the cytoprotective effects of GSH in MeHg-induced toxicity (Mullaney et al., 1993, 1994;). GSH binds to Hg compounds via its sulfhydryl groups, and the conjugated products are actively pumped out of the cells by multidrug resistance proteins, leading to the decrease in intracellular Hg and its toxicity (Konig et al., 1999). Our data have also revealed that a lower intracellular GSH level is correlated with greater microglial death. For example, treatment with 5μM MeHg causes maximal reduction in GSH levels (Fig. 5) as well as maximal cell death (Fig. 2). In agreement with our observations, Miura and Clarkson reported a significant inverse correlation between the GSH level and MeHg toxicity using a MeHg-resistant rat pheochromocytoma PC12 cell line. The levels of GSH in these resistant cells were fourfold higher than in the nonresistant cells, which led to greater efflux and less intracellular retention of MeHg (Miura and Clarkson, 1993; Miura et al., 1994). Furthermore, microglial GSH levels are only 25% of those in astrocytes (data not shown), which likely reflects their diminished capability to buffer MeHg-induced ROS generation. In addition, lower GSH levels in microglial cells may lead to reduced MeHg efflux and increased intracellular Hg levels. This may explain previous findings of the earliest and highest accumulation of Hg deposits in microglial cells in the rat brain (Garman et al., 1975) and nonhuman primates (Charleston et al., 1995).
Intracellular ROS generation is the pivotal event in regulating Nrf2 levels (Wang et al., 2009). As the major regulator of the antioxidant system in cells, Nrf2 protein levels in microglia increased rapidly within 1 min of MeHg treatment (Fig. 7). The increase in Nrf2 (Fig. 7) protein levels was paralleled by the MeHg-induced increase of ROS (Figs. 3 and 4), as well as the decrease in GSH (Fig. 5). We also observed Nrf2 nuclear translocation commencing at 10 min post MeHg exposure. Our data corroborate Li's model supporting a role for oxidative stress in disrupting the interaction between Nrf2 and Keap1, which leads to decreased rate of proteasomal degradation of Nrf2 in the cytosolic fraction. This model explains why the increase of cytosolic Nrf2 was detected as early as 1 min post MeHg exposure (Li and Kong, 2009). Based on the fact that Nrf2 activation was not observed in primary astrocytes until 90 min after MeHg treatment at the same concentration (Wang et al., 2009), our results support the notion that microglia are the first glial cell type to respond to MeHg (Gehrmann et al., 1995).
We observed increased microglial death upon Nrf2 knockdown with the shRNA approach (Fig. 10). Consistent with our results, Toyama et al. (2007) reported that primary mouse hepatocytes isolated from Nrf2-deficient mice were highly susceptible to MeHg-induced cytotoxicity, and Nrf2 overexpression attenuated MeHg-induced cytotoxicity in SH-SY5Y neuroblastoma cells. Nuclear translocation of Nrf2 is critical for attenuation of oxidative stress. Once in the nuclei, Nrf2 orchestrates the expression of Ho-1, Nqo1, and xCT, all of which detoxify xenobiotics and endogenous reactive electrophiles. Previous studies have also confirmed the critical function of Nrf2 in regulating the expression of these genes in other cell types. For example, in human lymphoma cells, gallium nitrate upregulated the Ho-1 mRNA level as a result of Nrf2 activation (Wang et al., 2009; Yang and Chitambar, 2008). Similarly, Nrf2 in renal epithelial cells upregulated the Nqo1 reporter construct after exposure to hypoxia/reoxygenation (Leonard et al., 2006).
Upon activation, microglial cells secrete numerous bioactive factors such as ILs, prostaglandin, TNF-α, and nitric oxide (NO). The nature of the released factors appears to be dependent on the nature of the causative agents. For example, LPS and trimethyltin were reported to increase the microglial release of IL-6, TNF-α, and NO (Eskes et al., 2002). In contrast, MeHg leads only to IL-6 release, leaving TNF-α and NO unchanged (Chang, 2007). As the first cell type to respond to MeHg, the microglial response to this metal may influence other cell types via the secretion of IL-6. Current literature suggests that IL-6 has differential effects on different cell types. For example, microglial IL-6 induces astrogliosis, associated with MeHg-induced microglial clusters in three-dimensional brain cell cultures. In contrast, neurons were decreased in the vicinity of such clusters (Eskes et al., 2002). Interestingly, as a proinflammatory factor, IL-6 may have a neuroprotective function. IL-6 coadministered with MeHg prevented MeHg-induced degeneration of the neuronal cytoskeleton (Eskes et al., 2002). Similar protective effects of IL-6 against the toxic effects of glutamate have also been previously described (Yamada and Hatanaka, 1994) and 1-methyl-4-phenylpyridinium (Akaneya et al., 1995). Furthermore, Nrf2 has been shown to regulate the production of microglial biofactors (Thimmulappa et al., 2006). Thus, in future studies, it is essential to address the functions of microglial IL-6 and the regulatory effect of Nrf2 in response to MeHg treatment.
On the other hand, microglial activity can be modulated by other cell types in the brain. Neurons can increase microglial reactivity (Eskes et al., 2003). Coculturing with neurons leads microglial cells to differentiate to a macrophagic state, which suggests that certain diffusible factors released from neurons could result in the activation of microglial cells (Thomas, 1992). In contrast to neurons, astrocytes appear to dampen microglial reaction. The current study was performed in purified microglial cells after acute MeHg treatment, given the cross talk between the various cell types. It is imperative to extend the findings regarding MeHg's function on microglial cells to more complex cellular networks, such as cocultures, and, ultimately, to in vivo preparations.
In summary, our work demonstrates that microglial cells are the first CNS cell type to respond to MeHg. Future studies on either the attenuation of intracellular ROS generation or the upregulation of GSH and Nrf2 in microglial cells could afford potential therapeutic value and more efficacious treatment techniques and protocols to address and ameliorate MeHg poisoning at early stages of exposure.
FUNDING
National Institute of Environmental Health Sciences (10563) to M.A.
References
- Akagi H, Grandjean P, Takizawa Y, Weihe P. Methylmercury dose estimation from umbilical cord concentrations in patients with Minamata disease. Environ. Res. 1998;77(2):98–103. doi: 10.1006/enrs.1997.3822. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Takahashi M, Hatanaka H. Interleukin-1 beta enhances survival and interleukin-6 protects against MPP+ neurotoxicity in cultures of fetal rat dopaminergic neurons. Exp. Neurol. 1995;136(1):44–52. doi: 10.1006/exnr.1995.1082. [DOI] [PubMed] [Google Scholar]
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602(2):181–186. doi: 10.1016/0006-8993(93)90680-l. [DOI] [PubMed] [Google Scholar]
- Chang JY. Methylmercury causes glial IL-6 release. Neurosci. Lett. 2007;416(3):217–220. doi: 10.1016/j.neulet.2007.01.076. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Mottet NK, Vahter ME, Burbacher TM. Autometallographic determination of inorganic mercury distribution in the cortex of the calcarine sulcus of the monkey Macaca fascicularis following long-term subclinical exposure to methylmercury and mercuric chloride. Toxicol. Appl. Pharmacol. 1995;132(2):325–333. doi: 10.1006/taap.1995.1114. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dorea JG. Persistent, bioaccumulative and toxic substances in fish: human health considerations. Sci. Total Environ. 2008;400(1–3):93–114. doi: 10.1016/j.scitotenv.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Seegal RF. Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology. 2007;28(4):720–726. doi: 10.1016/j.neuro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59(1):169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Eskes C, Honegger P, Juillerat-Jeanneret L, Monnet-Tschudi F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia. 2002;37(1):43–52. doi: 10.1002/glia.10019. [DOI] [PubMed] [Google Scholar]
- Eskes C, Juillerat-Jeanneret L, Leuba G, Honegger P, Monnet-Tschudi F. Involvement of microglia-neuron interactions in the tumor necrosis factor-alpha release, microglial activation, and neurodegeneration induced by trimethyltin. J. Neurosci. Res. 2003;71(4):583–590. doi: 10.1002/jnr.10508. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. Methylmercury causes oxidative stress and cytotoxicity in microglia: attenuation by 15-deoxy-delta 12, 14-prostaglandin J2. J. Neuroimmunol. 2006;171(1–2):17–28. doi: 10.1016/j.jneuroim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Garman RH, Weiss B, Evans HL. Alkylmercurial encephalopathy in the monkey (Saimiri sciureus and Macaca arctoides): a histopathologic and autoradiographic study. Acta Neuropathol. 1975;32(1):61–74. doi: 10.1007/BF00686067. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 1995;20(3):269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Irukayama K, Kai F, Kondo T, Ushigusa S, Hashiguchi M. [Consideration of the toxicity of methylmercuric compounds and the causative agent of Minamata disease.] Nisshin Igaku Jpn. J. Med. Prog. 1963;50:491–505. [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8(4):379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Jensen S, Jernelov A. Biological methylation of mercury in aquatic organisms. Nature. 1969;223(5207):753–754. doi: 10.1038/223753a0. [DOI] [PubMed] [Google Scholar]
- Kahn NW, Rea SL, Moyle S, Kell A, Johnson TE. Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem. J. 2008;409(1):205–213. doi: 10.1042/BJ20070521. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Komulainen H, Taipale H. Cellular mechanisms underlying the increase in cytosolic free calcium concentration induced by methylmercury in cerebrocortical synaptosomes from guinea pig. J. Pharmacol. Exp. Ther. 1989;248(3):1248–1254. [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp. Mol. Med. 2006;38(4):333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Koh K, Cha Y, Kim S, Kim J. tBHQ inhibits LPS-induced microglial activation via Nrf2-mediated suppression of p38 phosphorylation. Biochem. Biophys. Res. Commun. 2009;380(3):449–453. doi: 10.1016/j.bbrc.2009.01.082. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1999;1461(2):377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J. Neurochem. 2006;98(6):1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J. Neurochem. 2000;74(4):1694–1704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- Kuntz SW, Hill WG, Linkenbach JW, Lande G, Larsson L. Methylmercury risk and awareness among American Indian women of childbearing age living on an inland northwest reservation. Environ. Res. 2009;109(6):753–759. doi: 10.1016/j.envres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20(14):2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Disruption of brain mitochondrial calcium sequestration by methylmercury. J. Pharmacol. Exp. Ther. 1991;256(1):236–242. [PubMed] [Google Scholar]
- Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ. Health Perspect. 2007;115(11):1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier WE, Brown HW, Tilson HA, Luster MI, Harry GJ. Trimethyltin increases interleukin (IL)-1 alpha, IL-6 and tumor necrosis factor alpha mRNA levels in rat hippocampus. J. Neuroimmunol. 1995;59(1–2):65–75. doi: 10.1016/0165-5728(95)00026-x. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278(24):21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279(30):31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- Miura K, Clarkson TW. Reduced methylmercury accumulation in a methylmercury-resistant rat pheochromocytoma PC12 cell line. Toxicol. Appl. Pharmacol. 1993;118(1):39–45. doi: 10.1006/taap.1993.1006. [DOI] [PubMed] [Google Scholar]
- Miura K, Clarkson TW, Ikeda K, Naganuma A, Imura N. Establishment and characterization of methylmercury-resistant PC12 cell line. Environ. Health Perspect. 1994;102(Suppl. 3):313–315. doi: 10.1289/ehp.94102s3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet-Tschudi F. Induction of apoptosis by mercury compounds depends on maturation and is not associated with microglial activation. J. Neurosci. Res. 1998;53(3):361–367. doi: 10.1002/(SICI)1097-4547(19980801)53:3<361::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Pithon E, van Melle G, Honegger P. Microglial responsiveness as a sensitive marker for trimethyltin (TMT) neurotoxicity. Brain Res. 1995a;690(1):8–14. doi: 10.1016/0006-8993(95)00509-o. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Riederer BM, Honegger P. Effects of trimethyltin (TMT) on glial and neuronal cells in aggregate cultures: dependence on the developmental stage. Neurotoxicology. 1995b;16(1):97–104. [PubMed] [Google Scholar]
- Mullaney KJ, Fehm MN, Vitarella D, Wagoner DE, Jr, Aschner M. The role of -SH groups in methylmercuric chloride-induced D-aspartate and rubidium release from rat primary astrocyte cultures. Brain Res. 1994;641(1):1–9. doi: 10.1016/0006-8993(94)91808-2. [DOI] [PubMed] [Google Scholar]
- Mullaney KJ, Vitarella D, Albrecht J, Kimelberg HK, Aschner M. Stimulation of D-aspartate efflux by mercuric chloride from rat primary astrocyte cultures. Brain Res. Dev. Brain Res. 1993;75(2):261–268. doi: 10.1016/0165-3806(93)90030-e. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM. Sensitivity of immature neurons in culture to metal-induced changes in reactive oxygen species and intracellular free calcium. Neurotoxicology. 2000;21(6):1135–1144. [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Shanker G, Sonnewald U, Aschner M. Mercuric chloride inhibits the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biol Trace Elem. Res. 2006;109(3):267–280. doi: 10.1385/BTER:109:3:267. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 2003;278(7):4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- Ni M, Aschner M. Neonatal rat primary microglia: isolation, culturing and selected applications. Curr. Protoc. Toxicol. 2010;12.17(Suppl. 43) doi: 10.1002/0471140856.tx1217s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioku T, Takai N, Miyamoto K, Murao K, Hara C, Yamamoto K, Nakanishi H. Involvement of caspase 3-like protease in methylmercury-induced apoptosis of primary cultured rat cerebral microglia. Brain Res. 2000;871(1):160–164. doi: 10.1016/s0006-8993(00)02436-7. [DOI] [PubMed] [Google Scholar]
- Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1993;90(7):2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1995;92(19):8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, et al. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J. Virol. 2004;78(21):11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenila I, Tuomainen P, Soinila S, Mannisto PT. Increase of catechol-O-methyltransferase activity in rat brain microglia after intrastriatal infusion of fluorocitrate, a glial toxin. Neurosci. Lett. 1997;230(3):155–158. doi: 10.1016/s0304-3940(97)00502-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Miyamoto K, Wu Z, Nakanishi H. Possible involvement of cathepsin B released by microglia in methylmercury-induced cerebellar pathological changes in the adult rat. Neurosci. Lett. 2008;442(3):292–296. doi: 10.1016/j.neulet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Ki SU. Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. Neurotoxicology. 2001;22(3):317–327. doi: 10.1016/s0161-813x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001;914(1–2):159–165. doi: 10.1016/s0006-8993(01)02791-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Brain Res. Mol. Brain Res. 2003;110(1):85–91. doi: 10.1016/s0169-328x(02)00642-3. [DOI] [PubMed] [Google Scholar]
- Shie FS, Nivison M, Hsu PC, Montine TJ. Modulation of microglial innate immunity in Alzheimer's disease by activation of peroxisome proliferator-activated receptor gamma. Curr. Med. Chem. 2009;16(6):643–651. doi: 10.2174/092986709787458399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc. Res. Tech. 2001;54(2):81–94. doi: 10.1002/jemt.1123. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J. Neurochem. 2006;97(1):69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006;351(4):883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res. Brain Res. Rev. 1992;17(1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Toyama T, Sumi D, Shinkai Y, Yasutake A, Taguchi K, Tong KI, Yamamoto M, Kumagai Y. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem. Biophys. Res. Commun. 2007;363(3):645–650. doi: 10.1016/j.bbrc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Mercury Study Report to Congress, Office of Air Quality Planning & Standards and Office of Research and Development. Health Effects of Mercury and Mercury Compounds V. Washington, DC: EPA-452/R-97–007. U.S. EPA; 1997. [Google Scholar]
- Wang L, Jiang H, Yin Z, Aschner M, Cai J. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol. Sci. 2009;107(1):135–143. doi: 10.1093/toxsci/kfn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J. Neurochem. 2000;75(3):1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Hatanaka H. Interleukin-6 protects cultured rat hippocampal neurons against glutamate-induced cell death. Brain Res. 1994;643(1–2):173–180. doi: 10.1016/0006-8993(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Yang M, Chitambar CR. Role of oxidative stress in the induction of metallothionein-2A and heme oxygenase-1 gene expression by the antineoplastic agent gallium nitrate in human lymphoma cells. Free Radic. Biol. Med. 2008;45(6):763–772. doi: 10.1016/j.freeradbiomed.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S, Choi BH. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996;17(1):17–26. [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J. Neurochem. 2008;107(4):1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131(1):1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]