Abstract
Valproic acid (VPA) is used worldwide to treat epilepsy, migraine headaches, and bipolar disorder. However, VPA is teratogenic and in utero exposure can lead to congenital malformations. Using inbred C57BL/6J (B6) and DBA/2J (D2) mice, we asked whether genetic variation could play a role in susceptibility to VPA teratogenesis. Whereas B6 fetuses were more susceptible than D2 fetuses to digit and vertebral malformations, D2 fetuses were more susceptible to rib malformations. In a reciprocal cross between B6 and D2, genetically identical F1 mice carried in a B6 mother had a greater percentage of vertebral malformations following prenatal VPA exposure than F1 mice carried in a D2 mother. This reciprocal F1 difference is known as a maternal effect and shows that maternal genotype/uterine environment is an important mediator of VPA teratogenecity. VPA is a histone deacetylase inhibitor, and it is possible that the differential teratogenesis in B6 and D2 is because of strain differences in histone acetylation. We observed strain differences in acetylation of histones H3 and H4 in both embryo and placenta following in utero VPA exposure, but additional studies are needed to determine the significance of these changes in mediating teratogenesis. Our results provide additional support that genetic factors, both maternal and fetal, play a role in VPA teratogenesis. Lines of mice derived from B6 and D2 will be a useful model for elucidating the genetic architecture underlying susceptibility to VPA teratogenesis.
Keywords: valproic acid, teratology, inbred strain, maternal effect, histone deacetylase, inhibitor
Valproic acid (VPA) is one of the most frequently used antiepileptic drugs (AEDs) worldwide and has developed into a first-line treatment for migraine headaches and mania associated with bipolar disorder. Although VPA has a good side-effect profile with little sedation and few behavioral effects, it is teratogenic. in utero VPA exposure can produce growth retardation and congenital malformations, including facial dysmorphology, spina bifida and other neural tube defects (NTDs), urogenital abnormalities, heart anomalies, and skeletal malformations (Duncan, 2007; Meador et al., 2008b). Recently revised estimates indicate that in the United States, there are one-half million women of childbearing age with epilepsy, most requiring AEDs; they give birth to 25,000 offspring each year (Harden et al., 2009; Meador et al., 2008a). The number of children exposed to AEDs in utero is likely double that because of AED use in treating mood disorders and migraines (Pennell, 2004). VPA warrants particular attention because it is more teratogenic than other AEDs (Harden et al., 2009; Meador et al., 2008a,b), and an increasing number of women of reproductive age are taking VPA for various therapeutic reasons (Koren et al., 2006).
The vast majority of women who take VPA during pregnancy give birth to normal children. It is likely that many factors play a role in susceptibility to the teratogenic effects of VPA. Studies, although few in number, have shown that genetic variation can play a role in the development of morphological and cognitive/behavioral abnormalities in children exposed to VPA in utero (Hockey et al., 1996; Kini et al., 2007; Kozma, 2001; Malm et al., 2002). Animal models have shown conclusively that genotype can affect susceptibility to VPA teratogenesis. Inbred strains of mice differ on several teratogenic outcomes, including skeletal malformations and NTDs (Faiella et al., 2000; Finnell et al., 1988). Significant changes in expression of many genes have also been reported following prenatal VPA exposure (Kultima et al., 2004; Massa et al., 2005). Although genetic variation in VPA teratogenesis has been observed, the range of variation has not been well characterized, in humans or mice, and susceptibility genes remain unknown.
We examined skeletal teratogenesis in inbred C57BL/6J (B6) and DBA/2J (D2) mice following in utero exposure to one of several doses of VPA. We chose B6 and D2 because they have been among the most widely used inbred strains of mice in biomedical research. Their genomes have been well characterized, which makes lines of mice derived from them valuable for identifying genetic variation mediating many traits. In order to investigate the effects of maternal genotype and uterine environment on VPA teratogenesis, we reciprocally mated B6 and D2 and examined F1 litters. VPA is a histone deacetylase inhibitor (HDACi), and increased histone acetylation/deacetylation inhibition has been linked to VPA teratogenesis (Eikel et al., 2006; Menegola et al., 2005). Therefore, we used Western blotting to quantify changes in acetylation of histone proteins H3 and H4 following prenatal VPA in both embryo and placenta. Finally, we looked at messenger RNA (mRNA) levels of several HDAC genes following prenatal VPA.
MATERIALS AND METHODS
Dose-response study.
Male and female B6 and D2 mice were obtained from the Jackson Laboratory and housed in the animal facility at the Institute for Behavioral Genetics, Boulder, CO. Males were individually housed, whereas females were housed —three to five per cage. Mice were maintained on a 12-h light/dark cycle, with lights on at 7:00 A.M. The temperature was kept at a constant 22 ± 2°C. All procedures were approved by the University of Colorado Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health guidelines.
From 7:00 to 9:00 A.M., two females were placed with a male and then examined for a seminal plug as evidence of mating. The morning of plug detection was designated gestational day (GD) 0. Mated females were weighed and single-housed. At noon on GD 9, females were weighed to ascertain a 2-g minimum weight gain as evidence of pregnancy. Dams were then given an ip injection of either saline or 200 mg/kg (0.4 ml/kg), 400 mg/kg (0.8 ml/kg), or 800 mg/kg (1.6 ml/kg) VPA (sodium salt; Sigma). On GD 18, females were sacrificed and caesarean-sectioned (c-sectioned) between 1:30 and 2:30 P.M.; uterine horns were exposed and a count was made of live, dead, and resorbed fetuses. Live fetuses were weighed, sexed, and examined for gross morphological malformations. Fetuses were eviscerated and placed in 95% alcohol for 2 weeks. They were subsequently macerated in a 1% KOH solution for 72 h and then placed in a 1% KOH solution containing alizarin red for 6–8 h. Stained fetuses were placed first in a 25% and then a 75% glycerin solution for subsequent skeletal examination.
Maternal variables included maternal weight gain and prenatal mortality. Data were analyzed using ANOVA with strain (B6 or D2) and treatment (saline, 200, 400, and 800 mg/kg VPA) as between-group factors. Offspring variables included fetal weight at c-section and digit, rib, and vertebral malformations. Digit malformations included fused or missing digits. Rib malformations included fused, missing, bifurcated, or wavy/bulbous ribs. Vertebral malformations included fused, missing, or asymmetrical arches and centra. Between-group variables for offspring data included strain, treatment, and sex. Litter means (percent litter malformed) were the unit of analysis, and litters with only one live fetus were not used in the analyses of offspring variables. With the exception of weight at c-section, we saw no effect of sex on any measures of teratogenesis, so we collapsed across sex for means in Table 2. Post hoc analyses consisted of Bonferroni-corrected t-tests (treatment) within strain.
TABLE 2.
Mean (±SEM) Litter Percent for Digit, Rib, and Vertebral Malformations. Sample Sizes (number of litters) are Indicated in Parentheses under Strain and Treatment
| B6 |
D2 |
B6D2 |
D2B6 |
|||||||||
| Treatmenta | Saline | 200 | 400 | 800 | Saline | 200 | 400 | 800 | Saline | VA | Saline | VA |
| Litters | (11) | (11) | (10) | (10) | (10) | (9) | (11) | (10) | (12) | (11) | (12) | (10) |
| Digitb | 3 (1) | 0 | 0 | 27 (7) | 0 | 6 (6) | 2 (2) | 0 | 0 | 23 (11) | 0 | 8 (3) |
| Ribc | 5 (3) | 4 (3) | 9 (6) | 55 (11) | 7 (5) | 3 (3) | 47 (10) | 63 (15) | 5 (2) | 18 (8) | 12 (7) | 58 (11) |
| Vertebrald | 1 (1) | 1 (1) | 39 (11) | 94(6) | 0 | 11 (6) | 28 (10) | 53 (13) | 2 (2) | 91 (9) | 0 | 49 (12) |
C57BL/6J (B6) and DBA/2J (D2) litters were exposed to saline or 200, 400, or 800 mg/kg VPA on GD 9; B6D2 and D2B6 litters were exposed to saline or 600 mg/kg VPA.
Digit malformations (percent litter malformed) include fused or missing digits. At the 800 mg/kg dose, B6 fetuses had a significant increase in digit malformations (p < 0.01). B6D2 fetuses also had a significant increase in digit malformations (p < 0.01).
Rib malformations included fused, missing, bifurcated, or wavy/bulbous ribs. At the 800 mg/kg dose, B6 fetuses had a significant increase in rib malformations (p < 0.01), whereas D2 fetuses had a significant increase at the 400 and 800 mg/kg doses (ps < 0.01). D2B6 fetuses had a larger increase in rib malformation compared with B6D2 fetuses (p < 0.03).
Vertebral malformations included fused, missing, or asymmetrical arches and centra. B6 fetuses had a significant increase in vertebral malformations at the 400 and 800 mg/kg doses (ps < 0.01), as did D2 fetuses (ps < 0.02 and 0.01). B6D2 fetuses had a larger increase compared with D2B6 fetuses (p < 0.01).
Reciprocal cross.
When it became apparent that there was a strain difference in response to VPA, we reciprocally bred B6 and D2 mice. On GD 9, pregnant dams were ip injected with either saline or 600 mg/kg (1.2 ml/kg) VPA. We chose the 600 mg/kg dose because of the high rate of prenatal mortality at the 800 mg/kg dose (B6 = 41%, D2 = 31%). Dams were sacrificed on GD 18 and fetuses prepared and examined as described above.
Western blots for acetylated histones.
In order to investigate the effects of in utero VPA exposure on histone acetylation in B6 and D2, mice were mated as described above. At noon on GD 9, pregnant dams were ip injected with 600 mg/kg VPA. We included two additional groups in this study, one group of pregnant dams that was intragastrically intubated with 5.8 g/kg ethanol (2.9 ml/kg) and one that received an isocaloric amount of maltose-dextrin (3.5 ml/kg). We included these additional groups because we have previously shown that, similar to our findings with VPA, B6 are susceptible to digit and vertebral malformations following prenatal alcohol exposure, whereas D2 are resistant (Boehm et al., 1997; Downing and Gilliam, 1999). Strain differences in histone acetylation following prenatal VPA exposure and prenatal ethanol exposure, although far from conclusive, would suggest a possible common teratogenic mechanism.
Four hours after intubation or injection, dams were sacrificed and embryos and placentae were excised and frozen. Three litters per strain and treatment were produced, and within a litter, all embryos were pooled for protein extraction and all placentae were pooled for protein extraction. Tissues were minced with a scissor and then homogenized in a sucrose lysis buffer using a Teflon homogenizer. The homogenate was washed, and nuclear and cytoplasmic fractions were separated using low-speed centrifugation. Nuclear pellets were resuspended in an H2SO4 buffer and gently stirred on a shaker overnight at 4°C. Pelleted, non–acid-soluble proteins were removed by centrifugation, and the supernatants containing the acid-soluble proteins were precipitated with trichloroacetic acid. Precipitated proteins were then centrifuged, and the subsequent protein pellet was washed by centrifugation in ice-cold acetone; this step was repeated twice. Pellets were then resuspended in 10mM Tris-HCl, stirred gently overnight at 4°C, and stored at −80°C for subsequent analyses. Protein concentrations were determined using a bicinchoninic acid assay with bovine serum albumin as the standard.
Nuclear protein extracts (5 μg) were subjected to SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (LC2000; Invitrogen). Membranes were blocked in tris buffered saline containing 5% powdered milk and 0.1% Tween 20. Membranes were then incubated with antibodies against histone H3 (ab1791, 1:2500 dilution; Abcam), histone H4 (05-858, 1:2500 dilution; Upstate Biotechnology), acetylated histone H3K9 (ab4441, 1:2500 dilution; Abcam), and acetylated H4 (H4ac; 06-866, 1:2500 dilution; Upstate Biotechnology). The H4ac antibody recognizes four different acetylated lysines in the first 19 amino acids. After incubation with a primary antibody, membranes were incubated with a secondary antibody (anti-rabbit IgG peroxidase, #A0545; Sigma-Aldrich) and visualized using an electrochemiluminescence Western Blotting Detection System (RPN2132; Amersham). Immunoreactive bands were visualized by exposure to autoradiographic film. Films were scanned and band densities were quantified using ImageJ software (http://rsb.info.nih.gov/ij).
Quantitative PCR.
We examined expression of several HDAC genes following prenatal VPA or alcohol exposure. B6 and D2 mice were mated as described above. At noon on GD 9, pregnant dams were ip injected with 600 mg/kg VPA or intragastrically intubated with either 5.8 g/kg ethanol or maltose-dextrin. Four hours later, dams were sacrificed and embryos and placentae were excised. Three litters per strain and treatment were produced, and tissues were pooled for extraction. RNA was extracted using a Qiagen RNeasy Midi Kit. Total RNA was then reverse transcribed to generate single-stranded complementary DNA (cDNA) using a Promega ImProm II Reverse Transcription Kit. Primers for Hdac1, Hdac2, Hdac3, Hdac4, and Hdac8 were designed using Primer Express software (ABI; Applied Biosystems). Quantitative PCR was performed on cDNAs using SYBR green chemistry and an ABI Prism 7000 system. Relative quantification of mRNA levels was determined by normalizing against a control gene, Gapdh, using the 2−ΔΔCt method (Livak and Schmittgen, 2001). All determinations were replicated three times.
We chose the 4-h time point to examine changes in histone acetylation and gene expression for several reasons. Although many studies have shown increased acetylation or changes in gene expression by exposing cell lines or embryos to VPA in culture, to the best of our knowledge, only one study has looked at acetylation in vivo in mouse embryos following an acute dose of VPA prenatally (Menegola et al., 2005); they showed increased acetylation 1 h after VPA exposure. The Menegola laboratory has also observed increased acetylation in embryos exposed to other HDAC inhibitors in utero at several different time points, including 4 h later (Di Renzo et al., 2007a,b, 2008). Two studies have examined changes in gene expression in mouse embryos following an acute dose of VPA in utero (Massa et al., 2005; Stodgell et al., 2006) and have shown changes in expression at the 4-h time point. In our own work with C57BL/6J (B6) mice, we have shown changes in DNA methylation and gene expression in embryos 4 h after intrauterine exposure to ethanol (Downing, Johnson, Larson, Leakey, Siegfried, Rafferty, and Cooney, in preparation).
RESULTS
Dose Response
During pregnancy, B6 dams gained more weight than D2 dams (p < 0.01; Table 1). Compared with saline controls, only the 800 mg/kg dose of VPA significantly decreased weight gain (p < 0.01; Table 1) and increased embryolethality (p < 0.01; Table 1) in B6 and D2 litters. B6 fetuses exposed to either 400 or 800 mg/kg VPA weighed less than saline controls (ps < 0.01; Table 1), whereas for D2, only the 800 mg/kg dose decreased fetal weight (p < 0.01; Table 1).
TABLE 1.
Mean (±SEM) Percent Maternal Weight Gain (PMWG), Prenatal Mortality, and Offspring Weight at c-Section (CSWT). Sample Size is Shown in Parentheses under Treatment for Each Strain
| B6 |
D2 |
B6D2a |
D2B6a |
|||||||||
| Treatmentb | Saline | 200 | 400 | 800 | Saline | 200 | 400 | 800 | Saline | VPA | Saline | VPA |
| Litters | (11) | (11) | (10) | (10) | (10) | (9) | (11) | (10) | (12) | (11) | (12) | (10) |
| PMWGc | 58 (3) | 55 (3) | 55 (2) | 41 (4) | 47 (4) | 40 (5) | 37 (3) | 31 (2) | 68 (4) | 53 (4) | 50 (4) | 45 (4) |
| ELd | 4 (2) | 5 (3) | 9 (4) | 41 (8) | 12 (5) | 21 (6) | 11 (6) | 31 (8) | 2 (2) | 12 (5) | 11 (5) | 20 (6) |
| ♀ CSWTe | 1.16 (0.02) | 1.13 (0.02) | 1.03 (0.01) | 0.90 (0.01) | 0.91 (0.02) | 0.91 (0.03) | 0.86 (0.04) | 0.83 (0.03) | 1.30 (0.08) | 1.08 (0.02) | 1.20 (0.02) | 1.14 (0.03) |
| ♂ CSWTe | 1.19 (0.02) | 1.17 (0.01) | 1.11 (0.01) | 0.98 (0.03) | 0.97 (0.03) | 0.91 (0.02) | 0.97 (0.03) | 0.87 (0.04) | 1.33 (0.01) | 1.10 (0.02) | 1.25 (0.03) | 1.15 (0.03) |
B6 = C57BL/6J; D2 = DBA/2J; B6D2 = B6♀ × D2♂; D2B6 = D2♀ × B6♂.
B6 and D2 litters were exposed to saline or 200, 400, or 800 mg/kg VPA on GD 9; B6D2 and D2B6 litters were exposed to saline or 600 mg/kg VPA.
Percent maternal weight gain = (weight day 18 − weight day 9)/weight day 9. B6 dams gained more weight than D2 dams (p < 0.01). At the 800 mg/kg dose, both B6 and D2 dams gained less weight than saline controls (ps < 0.01). B6D2 dams gained more weight than D2B6 dams (p < 0.01), and saline-treated dams gained more weight than VPA-treated dams (p < 0.02).
Embryolethality (EL) = [(resorptions + dead)/implantation sites] × 100. At the 800 mg/kg dose, both B6 and D2 had a significant increase in EL (p < 0.01). VPA-treated B6D2 and D2B6 dams had greater EL compared with saline controls (ps < 0.02).
Fetal weight at c-section. Male fetuses weighed more than female fetuses (p < 0.01). At the 400 and 800 mg/kg doses, B6 fetuses weighed less than saline controls (ps < 0.01). At the 800 mg/kg dose, D2 fetuses weighed less than saline controls (p < 0.01). Male B6D2 and D2B6 fetuses weighed more than female fetuses, and saline-treated fetuses weighed more than VPA-treated fetuses (ps < 0.01).
D2 fetuses had a very low incidence of fused or missing digits after VPA treatment, whereas an increase was observed in B6 fetuses at 800 mg/kg (p < 0.01; Table 2). In utero VPA increased rib malformations only at the highest dose in B6 fetuses (p < 0.01), but both 400 and 800 mg/kg produced a significant increase in D2 (ps < 0.01; Table 2). Although VPA affected vertebral development in both B6 and D2, the effect was greater in B6. B6 fetuses had 39 and 94% vertebral malformation rates at the 400 and 800 mg/kg doses (ps < 0.01), whereas D2 had 28 and 53% vertebral malformation rates at these doses (ps < 0.02 and 0.01; Table 2). Indeed, following intrauterine exposure to 800 mg/kg VPA, most B6 fetuses had at least one vertebral defect.
In addition to the malformations described above, we observed several other anomalies (data not shown). We found several supernumerary ribs; there were no differences between strains or among treatments. There are six components to the fetal sternum: the manubrium or first segment, segments two through five, and the xiphoid or sixth segment. Sternebral anomalies are not uncommon, even in unexposed mice, and are generally considered as variations rather than malformations; we did not include sternebral anomalies in vertebral or rib malformations described above. However, sternebral anomalies are frequently reported following in utero VPA exposure. We observed fused, bipartite, incompletely ossified, and asymmetrical sternebrae in both strains. These sternebral anomalies occurred at a higher frequency in B6 fetuses and were observed at all doses, including saline. In addition, at the 800 mg/kg dose, two D2 and three B6 fetuses had exencephaly.
Reciprocal Cross
We reciprocally mated B6 and D2, which produced B6D2 and D2B6 fetal genotypes (maternal genotype is first). On GD 9, dams were ip injected with saline or 600 mg/kg VPA. Dams were sacrificed on GD 18, and fetuses were processed and examined as described above. B6D2 dams gained more weight than D2B6 dams (p < 0.01) and saline-treated dams put on more weight than VPA-treated dams (p < 0.02), but there was no significant genotype × treatment interaction (Table 1). Both genotypes had greater embryolethality when treated with VPA compared with saline controls (p < 0.02; Table 1). Saline-treated fetuses weighed more than VPA-treated fetuses (p < 0.01).
Prenatal VPA exposure significantly increased digit malformations in B6D2 fetuses but not in D2B6 fetuses (ps < 0.01 and 0.07, respectively; Table 2). Although in utero VPA increased vertebral malformations in both B6D2 and D2B6 fetuses, the increase was greater in B6D2 fetuses (p < 0.01; Table 2). These results demonstrate that B6 maternal genotype and/or uterine environment increase susceptibility to digit and vertebral malformations, whereas the D2 genotype/uterine environment is less susceptible. There appears to be a threshold dose of VPA, somewhere between 400 and 600 mg/kg, above which fetuses carried in a B6 mother are particularly susceptible to vertebral malformations. B6 fetuses exposed to 400 mg/kg VPA had a 39% malformation rate, whereas the malformation rate at 800 mg/kg was 94%; B6D2 fetuses exposed to 600 mg/kg VPA had a 91% malformation rate. Interestingly, for rib malformations, the difference between saline- and VPA-exposed fetuses was greater in the D2B6 genotype compared with the B6D2 genotype (p < 0.03; Table 2). In this case, the D2 genotype and/or uterine environment increased susceptibility to rib malformations.
Western Blots and Quantitative PCR
VPA is an HDACi, and increased histone acetylation/deacetylation inhibition has been linked to VPA teratogenesis (Eikel et al., 2006; Menegola et al., 2005). It is possible that the differences in VPA teratogenesis observed in B6 and D2 are due, at least in part, to strain differences in VPA-induced acetylation/deacetylation inhibition. Therefore, we examined acetylation of histone proteins H3 and H4 in both placenta and embryo following intrauterine VPA or ethanol exposure. We chose H3 and H4 because most previous studies that have examined acetylation/deacetylation with VPA have examined these two histone proteins and not H1, H2A, or H2B. There were no differences in band intensity in histones H3 or H4, either among treatments or between strains, so band densities for acetylated H3 (H3ac) or H4ac were normalized to H3 or H4.
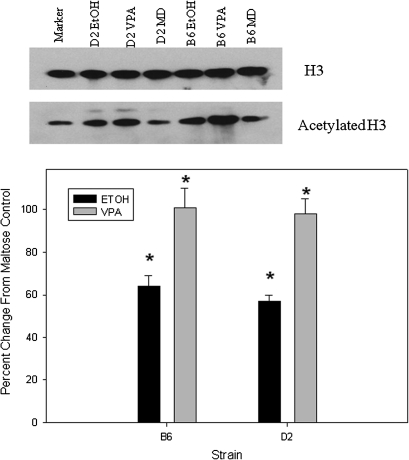
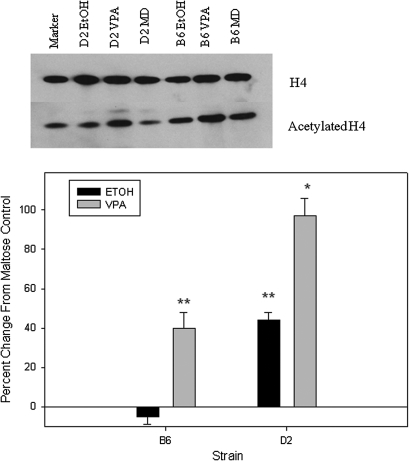
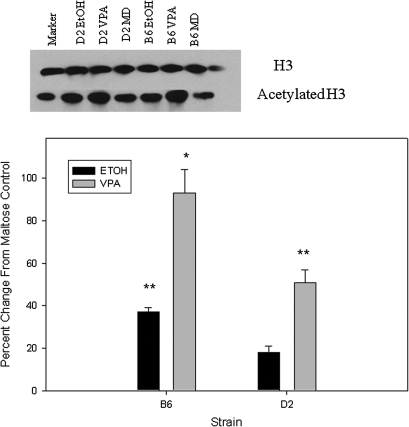
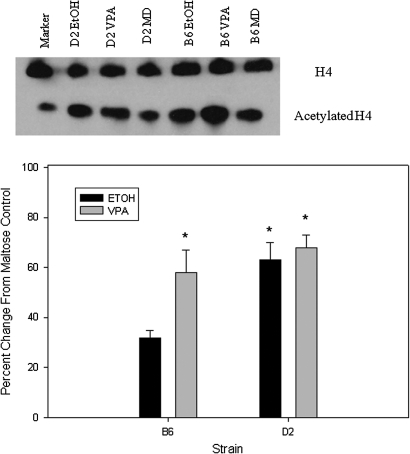
Compared with maltose-treated controls, embryos from both strains exhibited a similar increase in H3ac following prenatal VPA (98–101%) and prenatal ethanol (57–64%; Fig. 1). D2 embryos showed a greater increase in H4ac compared with B6 following VPA (97 vs. 40%; p < 0.01; Fig. 2). Following prenatal alcohol exposure, D2 embryos had a 44% increase in H4ac (p < 0.01; Fig. 2), whereas B6 embryos actually showed a slight decrease. Increases in acetylation were smaller in placenta, compared with embryo, following in utero VPA or ethanol exposure (Figs. 1–4). B6 placentae had a 93% increase in H3ac following prenatal VPA compared with 51% for D2 (p < 0.02); the increases in H3ac following prenatal alcohol were 37 and 18%, respectively (Fig. 3). Following in utero VPA, B6 and D2 had similar increases in H4ac in placentae, but D2 had a larger increase than B6 following prenatal alcohol (63 vs. 32%, p < 0.05; Fig. 4).
FIG. 1.
Western blot for H3ac in embryo following in utero exposure to VPA or ethanol. There were no differences in H3 among treatments or between strains, so band densities for each sample were normalized to H3. Results are presented as the percent change from maltose-treated controls. Asterisk indicates a significant increase compared with maltose control, p < 0.01.
FIG. 2.
Western blot for acetylated histone H4 (H4ac) in embryo following in utero exposure to VPA or ethanol. There were no differences in H4 among treatments or between strains, so band densities for each sample were normalized to H4. Results are presented as the percent change from maltose-treated controls. Asterisks indicate a significant increase compared with maltose controls, *p < 0.01; **p < 0.05.
FIG. 3.
Western blot for H3ac in placenta. Band densities for H3ac were normalized to H3 for each sample, and results are presented as the percent change from maltose-treated controls. Asterisks indicate a significant increase, *p < 0.01; **p < 0.05.
FIG. 4.
Western blot for H4ac in placenta. Band densities for H4ac were normalized to H4 for each sample, and results are presented as the percent change from maltose-treated controls. Asterisk indicates a significant increase, p < 0.01.
VPA preferentially inhibits class I (1, 2, 3, and 8) HDACs (Gottlicher et al., 2001). It is possible that if VPA inhibits HDAC and effectively increases acetylation, and HDAC inhibition/increased acetylation is correlated with teratogenesis, there would be a compensatory increase in HDAC mRNA and protein. In culture, VPA has been shown to increase expression of several HDAC mRNAs (Kim et al., 2008). Therefore, we examined expression of Hdac1, Hdac2, Hdac3, and Hdac8 in embryo and placenta following in utero exposure to maltose, ethanol, or VPA. We also examined expression of Hdac4 because it plays a role in skeletal development (Vega et al., 2004). Cycle threshold values were normalized to Gapdh, and relative fold changes were determined in comparison to maltose-treated controls. Fold changes were small, ranging from a 0.31-fold increase to a 0.22-fold decrease (Table 3). There were no significant fold changes in any of the Hdac genes in either strain (placenta or embryo) following prenatal VPA or alcohol.
TABLE 3.
Results from Quantitative PCR. Data Were Analyzed Using the 2−ΔΔCt Method of Livak and Schmittgen, Normalized to Gapdh and Presented as Mean Fold Changes (SEM) from Maltose-Dextrin Control. V = VPA, 600 mg/kg; E = ethanol, 5.8 g/kg. Positive Values Indicate an Increase, Whereas Negative Values Indicate a Decrease
| Hdac1 V | Hdac1 E | Hdac2 V | Hdac2 E | Hdac3 V | Hdac3 E | Hdac4 V | Hdac4 E | Hdac8 V | Hdac8 E | |
| Embryo | ||||||||||
| C57BL/6J | 0.12 (0.01) | −0.11 (0.01) | 0.06 (0.004) | −0.09 (0.008) | 0.13 (0.02) | 0.01 (0) | 0.31 (0.04) | 0.22 (0.02) | 0.05 (0) | 0.02 (0) |
| DBA/2J | 0.33 (0.03) | 0.26 (0.03) | 0.02 (0) | 0.02 (0) | 0.11 (0.01) | 0.08 (0.01) | −0.20 (0.04) | −0.13 (0.03) | 0.08 (0.01) | 0.26 (0.02) |
| Placenta | ||||||||||
| C57BL/6J | −0.09 (0.008) | −0.08 (0.006) | 0.13 (0.009) | 0.18 (0.01) | −0.02 (0) | −0.04 (0) | 0.10 (0.009) | 0.01 (0) | −0.24 (0.04) | −0.03 (0) |
| DBA/2J | 0.28 (0.06) | 0.31 (0.08) | 0.13 (0.01) | 0.14 (0.03) | 0.30 (0.03) | 0.26 (0.04) | 0.04 (0.006) | 0.09 (0.008) | 0.11 (0.01) | 0.19 (0.02) |
DISCUSSION
VPA is one of the most frequently used drugs to treat epilepsy, bipolar disorder, and migraine headaches, but VPA is teratogenic. Whereas few studies have looked for genetic variation in susceptibility to VPA teratogenesis in humans, several studies have demonstrated genetic variation in mice. We found that following intrauterine VPA exposure, fetuses from C57BL/6J (B6) and DBA/2J (D2) inbred strains of mice showed altered skeletal development but differed in degree of susceptibility to specific malformations. Whereas B6 fetuses showed an increase in fused and missing digits at the highest dose of VPA, D2 fetuses were resistant to digit malformations. Rib development was altered in both strains, but the effect was greater in D2 fetuses. In contrast, although vertebral development was also altered in both strains, the effect was greater in B6 fetuses. Results from our study agree with Faiella et al. (2000), who reported that B6 fetuses were more susceptible to digit and vertebral malformations than D2 following in utero VPA, whereas D2 fetuses were more susceptible to rib malformations. These studies have identified genetic variation in response to prenatal VPA but do not reveal to what extent maternal and embryonic genes play a role in teratogenesis.
One simple strategy for determining maternal and fetal genetic contribution is to reciprocally cross two inbred strains. If the reciprocally bred, genetically identical F1 offspring differ on the trait, this is known as a maternal effect and suggests that maternal genotype can play an important role in the trait. We found that F1 offspring carried in a B6 mother were more susceptible to vertebral malformations than F1 offspring carried in a D2 mother, whereas F1 offspring carried in a D2 mother were more susceptible to rib malformations. Beck (1999, 2001) reported a maternal effect following in utero VPA, with fetuses carried in a B6 mother being comparatively resistant to exencephaly compared with fetuses carried in susceptible SWV mothers. In a reciprocal cross between D2 and P/J inbred mice, fetuses carried in a D2 mother were relatively resistant to vertebral malformations (Faiella et al., 2000). These findings indicate that maternal effects can be an important determinant of VPA teratogenesis and the same maternal genotype/uterine environment can increase susceptibility to some teratogenic effects but not others. Embryo transfers can be used to evaluate the effects of uterine environment.
One potential mechanism that can account for a maternal effect is genomic imprinting, an epigenetic phenomenon in which only one of two parental alleles at a locus is expressed. At the molecular level, genomic imprinting is mediated in large part by differential DNA methylation and covalent modification of histone proteins. One of the best-characterized histone modifications is acetylation, where acetyl groups are attached to histones by histone acetyltransferases (HATs) and removed by HDACs. HDACs have been shown to play a role in somitogenesis, skeletogenesis, and limb/digit development (Morrison and D'Mello, 2008; Westendorf, 2007). VPA is an HDACi and increases acetylation, an effect that is correlated with its teratogenic properties (Menegola et al., 2005). It is possible that the differential teratogenesis in B6 and D2 fetuses is due, at least in part, to differential HDACi activity of VPA in these two strains. As a first step in testing this hypothesis, we examined acetylation of lysine residues on histones H3 and H4 following in utero exposure to VPA. Embryos from both strains showed a nearly twofold increase in H3ac following intrauterine exposure to VPA. Whereas D2 embryos also showed a nearly twofold increase in H4ac, the increase in B6 was much smaller. In placenta, where overall acetylation levels were higher, the increases in acetylation following VPA exposure were smaller and the strain differences were reversed. Both strains showed similar increases in H4ac, whereas B6 had a nearly twofold increase in H3ac compared with D2. These strain differences in acetylation following in utero VPA exposure need to be examined in greater depth before the relationship between differential acetylation/deacetylation and teratogenesis can be determined.
Analogs of VPA that have HDACi activity are teratogenic, whereas analogs of VPA that do not have HDACi activity are not teratogenic (Eikel et al., 2006; Gurvich et al., 2005). Furthermore, other teratogens such as sodium salicylate, sodium butyrate, apicidin, the synthetic benzamide derivative MS-275, and boric acid all have HDACi activity and produce similar vertebral malformations (Di Renzo et al., 2007a,b, 2008). This provides convincing evidence that histone acetylation/deacetylation can play an important role in vertebral teratogenesis. Given that alcohol produces similar vertebral malformations when administered in utero, we examined histone acetylation in B6 and D2 following prenatal alcohol exposure. There were increases in H3ac and H4ac in both embryo and placenta following prenatal alcohol exposure, but the increases were clearly not as large as those observed with VPA. This suggests that, prenatally, alcohol does not have as great of HDACi activity as VPA, although other mechanisms for increased acetylation may be responsible, as discussed below. The only strain difference in acetylation that was consistent between VPA and ethanol was a greater increase in H4ac in D2 embryos compared with B6 embryos.
Our study is a first step toward characterizing genetic variation in teratogenesis and histone acetylation/deacetylation following prenatal VPA exposure. One shortcoming of our study is that we did not examine VPA metabolism in pregnant dams or embryos. It is possible that strain differences in VPA metabolism could contribute, at least in part, to strain differences in VPA teratogenesis. To the best of our knowledge, no studies have looked at VPA metabolism in pregnant B6 or D2 dams or embryos. Additional studies are also needed to clarify the role that increased acetylation and deacetylase inhibition play in VPA teratogenesis. We observed changes in global acetylation, but it is likely that there are specific regions of the genome, perhaps imprinted loci, where histone acetylation/deacetylation plays a crucial role in VPA teratogenesis. Chromatin immunoprecipitation assays can be used to interrogate acetylation in specific genomic regions. Acetylation levels will also need to be measured at different time points following VPA exposure and in more specific tissues (i.e., developing vertebra). Although studies often infer HDACi activity by observing increased acetylation, assays that measure the rate of deacetylation are available and can be used to assess the extent to which increased acetylation is because of HDACi activity. Levels of HATs and HAT activity will also need to be examined.
Acetylation and HDAC inhibition may play a role in differential susceptibility to VPA teratogenesis, but additional mechanisms are almost certainly involved. Observing genetic variation will identify strains for further investigation but does not provide information on causal genes, polymorphisms, or genetic pathways. Quantitative trait locus (QTL) mapping can be used to identify regions on chromosomes that harbor genes or DNA polymorphisms mediating variation in VPA teratogenesis. B6 and D2 have been two of the most frequently used inbred strains in biomedical research, and their genomes have been extensively sequenced. This makes lines of mice derived from B6 and D2, such as the BXD recombinant inbred strains, a valuable resource for QTL mapping. There is a high degree of synteny and homology between mouse and human genomes, so putative causal polymorphisms and epigenetic modifications identified in mice can be examined in humans. Given the paucity of research investigating genetic variation in humans, mice will be a valuable tool for elucidating genetic mechanisms mediating susceptibility to VPA teratogenesis.
FUNDING
National Institute on Alcohol Abuse and Alcoholism (R01 AA016676 and R24 AA01466).
References
- Beck SL. Contributions of dam and conceptus to differences in sensitivity to valproic acid among C57 black and SWV mice. Reprod. Toxicol. 1999;13:353–360. doi: 10.1016/s0890-6238(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Beck SL. Does genomic imprinting contribute to valproic acid teratogenecity? Reprod. Toxicol. 2001;15:43–48. doi: 10.1016/s0890-6238(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J and A/J inbred mouse strains. Alcohol. 1997;14:389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Di Renzo F, Broccia ML, Giavini E, Menegola E. Relationship between embryonic histonic hyperacetylation and axial skeletal defects in mouse exposed to the three HDAC inhibitors apicidin, MS-275, and sodium butyrate. Toxicol. Sci. 2007a;98:582–588. doi: 10.1093/toxsci/kfm115. [DOI] [PubMed] [Google Scholar]
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol. Appl. Pharmacol. 2007b;220:178–185. doi: 10.1016/j.taap.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. The inhibition of embryonic histone deacetylases as the possible mechanism accounting for axial skeletal malformations induced by sodium salicylate. Toxicol. Sci. 2008;104:387–404. doi: 10.1093/toxsci/kfn094. [DOI] [PubMed] [Google Scholar]
- Downing C, Gilliam DM. Cytoplasmic factors do not contribute to a maternal effect on ethanol teratogenesis. Behav. Genet. 1999;29:31–39. doi: 10.1023/a:1021485821842. [DOI] [PubMed] [Google Scholar]
- Duncan S. Teratogenesis of sodium valproate. Curr. Opin. Neurol. 2007;20:175–180. doi: 10.1097/WCO.0b013e32805866fb. [DOI] [PubMed] [Google Scholar]
- Eikel D, Lampen A, Nau H. Teratogenic effects mediated by inhibition of histone deacetylases: evidence from quantitative structure activity relationships of 20 valproic acid derivatives. Chem. Res. Toxicol. 2006;19:272–278. doi: 10.1021/tx0502241. [DOI] [PubMed] [Google Scholar]
- Faiella A, Wernig M, Consalez GC, Hostick U, Hofmann C, Hustert E, Boncinelli E, Balling R, Nadeau JN. A mouse model for valproate teratogenecity: parental effects, homeotic transformations, and altered HOX expression. Hum. Mol. Genet. 2000;9:227–236. doi: 10.1093/hmg/9.2.227. [DOI] [PubMed] [Google Scholar]
- Finnell RH, Bennett GD, Karras SB, Mohl VK. Common hierarchies of susceptibility to the induction of neural tube defects in mouse embryos by valproic acid and its 4-propyl-4-penetnoic acid metabolite. Teratology. 1988;38:313–320. doi: 10.1002/tera.1420380403. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JBA. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 2005;19:1166–1188. doi: 10.1096/fj.04-3425fje. [DOI] [PubMed] [Google Scholar]
- Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, Wiebe S, Thurman D, Koppel BS, Kaplan PW, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes. Epilepsia. 2009;50:1237–1246. doi: 10.1111/j.1528-1167.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Hockey A, Bower C, Goldblatt J, Knowles S. Fetal valproate embryopathy in twins: genetic modification of the response to a teratogen. Birth Defects Orig. Artic Ser. 1996;30:401–405. [PubMed] [Google Scholar]
- Kim B, Rincon Castro LM, Jawed S, Niles LP. Clinically relevant concentrations of valproic acid modulate melatonin MT1. receptor, HDAC and MeCP2 mRNA expression in C6 glioma cells. Eur. J. Pharmacol. 2008;589:45–48. doi: 10.1016/j.ejphar.2008.04.058. [DOI] [PubMed] [Google Scholar]
- Kini U, Lee R, Jones A, Smith S, Ramsden S, Fryer A, Clayton-Smith J. Influence of the MTHFR genotype on the rate of malformations following exposure to antiepileptic drugs in utero. Eur. J. Med. Genet. 2007;50:411–420. doi: 10.1016/j.ejmg.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Koren G, Nava-Ocampo AA, Moretti ME, Sussman R, Nulman I. Motherisk update: major malformations with valproic acid. Can. Fam. Physician. 2006;52:441–447. [PMC free article] [PubMed] [Google Scholar]
- Kozma C. Valproic acid embryopathy: report of two siblings with further expansion of the phenotypic abnormalities and a review of the literature. Am. J. Med. Genet. 2001;98:168–175. [PubMed] [Google Scholar]
- Kultima K, Nystrom A-M, Scholz B, Gustafson A-L, Dencker L, Stigson M. Valproic acid teratogenecity: a toxicogenomics approach. Environ. Health Perspect. 2004;112:1225–1235. doi: 10.1289/txg.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malm H, Kajantie E, Kivirikko S, Kaariainen H, Peippo M, Somer M. Valproate embryopathy in three sets of siblings: further proof of hereditary susceptibility. Neurology. 2002;59:630–633. doi: 10.1212/wnl.59.4.630. [DOI] [PubMed] [Google Scholar]
- Massa V, Cabrera RM, Menegola E, Giavini E, Finnell RH. Valproic acid-induced skeletal malformations: associated gene expression cascades. Pharmacogenet. Genomics. 2005;15:787–800. doi: 10.1097/01.fpc.0000170914.11898.3a. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Pennell PB, Harden CL, Gordon JC, Tomson T, Kaplan PW, Holmes GL, French JA, Hauser WA, Wells PG, et al. Pregnancy registries in epilepsy. Neurology. 2008a;71:1109–1117. doi: 10.1212/01.wnl.0000316199.92256.af. [DOI] [PubMed] [Google Scholar]
- Meador K, Reynolds MW, Ceran S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008b;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola E, Di Renzo F, Broccia ML, Prudenziati M, Minucci S, Massa V, Giavini E. Inhibition of histone deacetylase activity on specific embryonic tissues as a new mechanism for teratogenecity. Birth Defects Res. 2005;74:392–398. doi: 10.1002/bdrb.20053. [DOI] [PubMed] [Google Scholar]
- Morrison BE, D'Mello SR. Polydactyly in mice lacking HDAC9/HDRP. Exp. Biol. Med. 2008;233:980–988. doi: 10.3181/0802-RM-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell PB. Pregnancy in women who have epilepsy. Neurol. Clin. 2004;22:799–820. doi: 10.1016/j.ncl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O'Bara M, Tisdale BK, Nau H, Rodier PM. Induction of the homeotic gene Hoxa1 through valproic acid's teratogenic mechanism of action. Neurotoxicol. Teratol. 2006;28:617–624. doi: 10.1016/j.ntt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Westendorf J. Histone deacetylases in control of skeletogenesis. J. Cell. Biochem. 2007;102:332–340. doi: 10.1002/jcb.21486. [DOI] [PubMed] [Google Scholar]