Abstract
Acute systemic toxicity studies are carried out in many sectors in which synthetic chemicals are manufactured or used and are among the most criticized of all toxicology tests on both scientific and ethical grounds. A review of the drivers for acute toxicity testing within the pharmaceutical industry led to a paradigm shift whereby in vivo acute toxicity data are no longer routinely required in advance of human clinical trials. Based on this experience, the following review was undertaken to identify (1) regulatory and scientific drivers for acute toxicity testing in other industrial sectors, (2) activities aimed at replacing, reducing, or refining the use of animals, and (3) recommendations for future work in this area.
Keywords: 3Rs, acute toxicity, harmonization, hazard labeling, limit dose, redundancy, regulatory classification, systemic toxicity
This review has been carried out under the auspices of the European Partnership for Alternative Approaches to Animal (EPAA) Testing, an unprecedented collaboration between the European Commission (EC), European industry trade associations, and companies from seven industrial sectors. The partners are committed to pooling knowledge and resources to accelerate the development, validation, and acceptance of alternative approaches to further the reduction, refinement, and replacement (3Rs) of animal use in regulatory testing.
The term “acute toxicity” is used to describe the adverse effects of a substance that may result from a single exposure or multiple exposures within a 24-h period. Acute effects may be local (e.g., skin or eye irritation) and/or systemic in nature. This review focuses on the latter, with emphasis on regulatory required high-dose studies carried out via oral, dermal, and inhalation routes of exposure for the purpose of identifying or estimating doses that cause lethality. Other types of acute studies such as nonlethal single-dose studies (e.g., for derivation of an acute reference dose), acute ecotoxicological studies in fish and avian species, testing for marine biotoxins, and safety/potency testing of vaccines are not explored in this paper.
Acute systemic toxicity studies are rooted in the post-World War I era concept of the “LD50,” which was defined by Trevan (1927) as the single dose of a substance that can be expected to cause death in 50% of the animals in an experimental group. Initially developed to provide a relative index of toxicity for plant and biological extracts, LD50-type studies achieved general acceptance as a basis of comparing and classifying the toxicities of chemicals (FDA, 1988) and have become a routine testing requirement in a number of regulatory sectors (Botham, 2004). According to EC (2007) animal use statistics, acute toxicity studies remain the most prevalent class of toxicological test in use today.
Acute lethality studies have been among the most heavily criticized of all regulatory toxicity tests, both on scientific and on ethical grounds (Ekwall et al., 1998; Langley, 2005; Lorke, 1983; Zbinden and Flury-Roversi, 1981). In response to criticisms, there has been a gradual evolution in study designs for acute systemic toxicity consistent with the 3Rs principle (Russell and Burch, 1959), coupled with increasingly sophisticated efforts to move away from animal testing altogether (Table 1). Notably, reduction and in part refinement methods have been introduced as Organization for Economic Cooperation and Development (OECD) Test Guidelines for oral and inhalation routes, although no such approach for dermal exposure is currently available. And despite efforts over many years, acute toxicity testing remains a core regulatory requirement in many sectors.
TABLE 1.
Acute Toxicity Testing: 1927 Through the Present
| Date | Milestone |
| 1927 | British pharmacologist John Trevan publishes first paper describing the LD50 test |
| 1930s | LD50 test becomes gradually accepted for the standardization of toxic plant and biological extracts and other chemicals (FDA, 1988) |
| 1959 | Publication of The Principles of Humane Experimental Technique outlining the “3Rs” principle of replacement, reduction, and refinement of animal use |
| 1973 | Swiss toxicologist Zbinden (1973) publishes a review that concludes there is little justification for conducting the classical LD50 |
| 1981 | The OECD adopts Test Guidelines 401–403, the “classical” oral, dermal, and inhalation LD/LC50 tests |
| 1984 | The British Toxicology Society (BTS, 1984) concludes that precisely determined LD50 values are rarely justified and proposes the alternative Fixed Dose Procedure |
| 1987 | OECD 401 is revised to reduce number of animals used, e.g., only one sex required, and in some regions, the limit dose is reduced from 5000 to 2000 mg/kg |
| 1988 | US Food and Drug Administration publishes a policy on the LD50 stating “The scientific community agrees that the “classical” LD50 test is not necessary for determining acute toxicity. The agency supports efforts to discontinue conduct of the “classical” LD50 test and to reduce the numbers of animals used in acute toxicity testing without sacrificing information necessary in the interest of human safety” (FDA, 1988) |
| 1992 | Adoption of OECD 420: Fixed Dose Procedure, a reduction and refinement alternative to OECD 401, the classical oral LD50 test |
| 1996 | Adoption of OECD 423: Acute Toxic Class Method, a second reduction alternative to OECD 401 |
| 1998 | Adoption of OECD 425: Up-and-Down Procedure, a third alternative to OECD 401 |
| Results of the Multicentre Evaluation of In Vitro Cytotoxicity are released by Ekwall et al. (1998) illustrating a strong concordance between a battery of in vitro cytotoxicity assays and human lethal blood concentrations for 50 chemicals | |
| 1999 | OECD member countries agree in principle to delete TG 401 |
| British Home Office discontinues issuing licenses for LD50 if a suitable alternative is available (HO, 1999) | |
| 2000 | ICCVAM/NICEATM (2001b) convene an International Workshop on In Vitro Methods for Assessing Acute Systemic Toxicity to explore the potential to use nonanimal methods to predict LD50 values |
| 2001 | ICCVAM/NICEATM (2001a) publish a Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity |
| 2002 | OECD (2002) officially deletes TG 401 from its internationally harmonized guidelines |
| Commencement of the joint ICCVAM/ECVAM international validation study of in vitro cytotoxicity test methods for estimating acute oral systemic toxicity | |
| 2003 | ECVAM holds a Workshop on Strategies to Replace In Vivo Acute Systemic Toxicity Testing (Gennari et al., 2004) |
| European pharmaceutical company/NC3Rs working group formed | |
| 2005 | Pharmaceutical companies and NC3Rs organize a regulatory workshop to discuss the requirement for acute toxicity tests in the development of new human medicines (Chapman and Robinson, 2007) |
| Launch of the 15 million Euro, pan-European ACuteTox integrated project (“ACuteTox.org”) | |
| Pesticide regulators propose a new nonlethal study design for the derivation of an “acute reference dose” (Solecki et al., 2005) | |
| 2006 | Publication of the peer review report of the joint ICCVAM/ECVAM international validation study of in vitro cytotoxicity test methods for estimating acute oral systemic toxicity (ICCVAM/NICEATM, 2006) |
| NC3Rs organizes a regulatory workshop to discuss drivers for acute toxicity testing within the pharmaceutical sector (Chapman and Robinson, 2007) | |
| 2007 | US National Research Council report “Toxicity Testing in the 21st Century” calls for a transition toward a mechanistic, and predominantly animal-free, paradigm in toxicology, which offers a possible path forward for replacing in vivo systemic toxicity testing (NRC, 2007) |
| US Environmental Protection Agency “ToxCast” program launched to build computational models to forecast human toxicity (EPA, 2008b, 2009) | |
| 2008 | Publication of a review of the scientific drives for acute toxicity testing within the pharmaceutical industry (Robinson et al., 2008) |
| EPAA establishes a cross-sector task force on acute toxicity | |
| ECVAM funds a follow-up validation study of the 3T3 Neutral Red Uptake cytotoxicity assay (ICCVAM/NICEATM, 2006) to evaluate the predictive capacity of the assay to identify substances with acute oral LD50 > 2000 mg/kg (Kinsner-Ovaskainen et al., 2009) | |
| NC3Rs establishes an expert working group to develop the scientific evidence needed to support regulatory acceptance of the Fixed Concentration Procedure for acute inhalation toxicity testing | |
| ICCVAM (2008) issues test method recommendations to U.S. agencies regarding use of two in vitro methods for estimating starting doses for acute oral toxicity studies | |
| U.S. federal agencies announce “Tox21” collaboration on high throughput screening, toxicity pathway profiling, and biological interpretation of findings (HHS & EPA, 2008) | |
| 2009 | Adoption of ICH M3(R2) test guideline, including a reduction of the standard limit dose to 1000 mg/kg (ICH, 2009) |
| Adoption of OECD 436: Acute Toxic Class Method, a reduction alternative to OECD 403, the classical inhalation LC50 test, together with a revision to 403 (OECD, 2009c) | |
| OECD publishes a Draft Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests (OECD, 2009a) |
In 2003, a working group comprised 18 international pharmaceutical companies and contract testing laboratories, together with the U.K. National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs), was established to evaluate the utility of acute systemic toxicity studies in the development of new medicines. The expert group determined that “the information obtained from acute toxicity studies is of little or no value in the pharmaceutical development process,” a conclusion subsequently considered and endorsed by pharmaceutical regulators and scientists via the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) process (ICH, 2009; Robinson et al., 2008). In light of these findings, and in view of the requirement of acute toxicity testing across multiple industry sectors, the EPAA established a task force to examine scientific and regulatory drivers for such testing and to promote the use of 3Rs approaches that the task force considers are currently available. This publication is one of several products of that effort.
CURRENT PRACTICE
Survey of EPAA Member Companies
A questionnaire designed to gather information regarding current practices in the conduct of acute toxicity studies and companies’ experiences in this area was sent to all EPAA members except those in the pharmaceutical sector, which has already participated in such an exercise (Robinson et al., 2008). The EPAA survey questions covered the scientific and regulatory objectives of the studies, routes of administration, preferred test guideline, parameters examined, dose limit, and regulatory experience (a link to the EPAA questionnaire is included under “Supplementary data”). Seventeen companies responded, and the number of companies responding per sector is outlined as follows: agrochemicals (four companies), animal health (two companies), consumer products/cosmetics (nine companies), industrial chemicals (four companies), and together with two contract research organizations that conduct studies to support the various sectors. The total number of responding companies appears greater than 17 because some companies represent more than one sector. The aggregated responses are not detailed in this publication because the number of companies responding within each sector was relatively small. The limited nature of the survey means that generalized qualitative responses rather than quantitative data are used to support the points made in relevant sections of this publication. Reference to the earlier pharmaceutical company survey is also made when relevant.
Number of Animals and End Points Measured in Guideline Studies
Table 2 compares current and proposed protocols for acute toxicity studies and identifies study designs used to determine LD50 point estimates versus range estimates, as well as how many animals are typically used under each protocol. A full statistical breakdown of animal use across industry sectors and in various classes of acute toxicity studies is available elsewhere (EC, 2007).
TABLE 2.
Comparison of OECD Guidelines for Acute Systemic Toxicity (adapted from Botham, 2004)
| Route | Orala | Dermalb | Inhalationc | ||||||
| OECD test guideline (year of adoption) | 401 LD50 (1981; deleted 2001) | 420 fixed dose (1992) | 423 acute toxic class (1996) | 425 up and down (1998) | 402 LD50 (1981) | Draft 434 fixed dose (on hold) | 403 LC50 (1981) | 403 LC50 (revised 2009) | 436 acute toxic class (2009) |
| Sighting study required? | Yes | 1 animal per [ ] | No | No | Yes | Yes | ≤ 3 ♂ and ≤ 3 ♀ (or ≤ 3 of susceptible sex) per [ ]. At least 3 ♂ and 3 ♀ per [ ] to test sex differences if unknown | ≤ 3 ♂ and ≤ 3 ♀ per [ ] | No |
| Dose levels | At least 3, spaced appropriately to produce test groups with a range of toxic effects and mortality rates. The data should be sufficient to produce a dose-response curve and, where possible, permit an acceptable determination of the LD50. May also be used as a limit test. At least 5 rodents of the same sex per dose level | Fixed doses of 5, 50, 300, and 2000 (5000) mg/kg; 5 animals/dose level | Fixed doses of 5, 50, 300, and 2000 (5000) mg/kg; 3 animals/dose level | Starting dose at best estimate of LD50 (or 175 mg/kg) and using dose progression factor of 3.2, single animals dosed until one of three stopping criteria met | At least 3, spaced appropriately to produce test groups with a range of toxic effects (including death). Data should be sufficient to produce a dose-response curve and, where possible, permit an acceptable determination of the LD50. May also be used as a limit test. At least 5 rodents of the same sex per dose level | Fixed doses of 5, 50, 300, and 2000 (5000) mg/kg; 5 animals/dose level | At least 3, spaced appropriately to produce test groups with a range of toxic effects and mortality rates. Data should be sufficient to produce a dose-response curve and, where possible, permit an acceptable determination of the LC50. May also be used as a limit dose test. At least 10 rodents (at least 5 of each sex) per dose level | At least 3, spaced to produce a range of toxic effects (including death). The data should be sufficient to produce a dose-response curve and permit an acceptable determination of the LC50.—or––as a C × T protocol for deriving AEGL, ERPG, or AETL values for emergency response planning, or land use planning. May also be used as a limit dose test. A limit version of the C × T protocol may also be performed. At least 5 rodents of the same sex per dose level should be used in the traditional protocol. One animal/sex/interval under the C × T protocol | Fixed doses during and exposure period of 4 h; 3 animals/sex/dose level or 6 animals of the more sensitive sex/dose level |
| Average number of animals | > 20 | 5 (limit test) to 7 | 6 (limit test) to 7 | 5 (limit test) to 9 | 10 (limit test) to 30 | 5 (limit test) to 7 | 40 | If 4 concentrations tested: | 6–9 |
| —In case of 1 animal/sex/(C × T) point: both sexes = 40; susceptible sex = 40 | |||||||||
| —In case of 2 animals/sex/ (C × T) point: both sexes = 80; susceptible sex = 80 | |||||||||
| Aim | Identify the LD50 and the range of associated toxic effects | Identify lowest fixed dose causing evident toxicity | Identify lowest fixed dose causing mortality | Calculated LD50 | Identify the LD50 and the range of associated toxic effects | Identify lowest fixed dose causing evident toxicity | Identify the LC50 and the range of associated toxic effects | Identify the LC50 and the range of associated toxic effects | Identify the LC50 and the range of associated toxic effects |
| Output | Point estimate of LD50 with confidence intervals; signs of acute toxicity; target organ(s) | Range estimate of LD50; signs of acute toxicity; target organ(s) | Range estimate of LD50; signs of acute toxicity; target organ(s) | Point estimate of LD50 with confidence intervals; signs of acute toxicity; target organ(s) | Point estimate of LD50 with confidence intervals. Signs of acute toxicity. Target organ(s) | Range estimate of LD50; signs of acute toxicity; target organ(s) | Point estimate of LC50 with confidence intervals; signs of acute toxicity; target organ(s) | Range estimate of LC50; signs of acute toxicity; target organ(s) | Range estimate of LC50; signs of acute toxicity; target organ(s) |
Note. ♂, male; ♀, female; [ ], concentration; C × T, concentration × time protocol; AEGL/AETL, acute exposure guideline/threshold level; ERPG, emergency response planning guideline.
Single bolus. Young adult rats (“one sex”). Oral gavage with constant volume or concentration, clinical observations, bodyweight, and mortality over 14 days. Necropsy at termination. It is recommended that only one sex needs to be tested initially followed by a second group of the other sex tested to investigate sex differences unless data exist to show the first sex is the more sensitive.
Young adult rats (“one sex”). Dermal application to 10% of skin surface area (clipped free of hair) for 24 h under a gauze and tape dressing. Clinical observations, bodyweight, and mortality over 14 days. Necropsy at termination. It is recommended that only one sex needs to be tested initially followed by a second group of the other sex tested to investigate sex differences unless data exist to show the first sex is the more sensitive.
Young adult rats (“one sex”). Inhalation exposure for at least 4 h in rat and mice in the current 403 or up to 6 h for rats in the proposed revised 403 (up to 4 h for mice only). Clinical observations, bodyweight, and mortality over 14 days. Necropsy at termination. It is recommended that only one sex needs to be tested initially followed by a second group of the other sex tested to investigate sex differences unless data exist to show the first sex is the more sensitive. Alternative protocol under proposed revision to 403: C × T protocol: young adult rats, exposed to a test article at several concentration levels and for multiple time durations (1 animal/sex/interval). All testing is performed in a nose-only chamber.
Contemporary test guidelines offer greater flexibility for generating data fit for purpose, potentially using fewer animals than the older guidelines, such as the now-deleted OECD Test Guideline 401 (OECD, 2009c). It is also possible to use clinical signs such as “evident toxicity” rather than death as an end point for classification, e.g., in the U.K.-pioneered Fixed Dose Procedure (OECD 420).
Choice of test guideline is driven in large part by national and sector-specific regulatory requirements but can also be influenced by what the LC50 or LD50 might reasonably be expected to be. For example, if there is reason to expect that the acute toxicity will be greater than the limit dose for classification, OECD 420 would be a suitable choice in using the fewest animals to achieve this end. If this is not absolutely certain, the German-developed Acute Toxic Class Method (OECD 423) may ultimately use the fewest animals. If on the other hand a point estimate of the oral LD50 is required, the U.S.-developed Up-And-Down Procedure (OECD 425) would be required. According to EPAA’s survey of members, many European companies and contract research organizations default to OECD 423 unless a specific regulatory authority requires a more humane method or a point estimate of the LD50.
For acute dermal toxicity, the only guideline currently available is the classic dermal LD50 study (OECD 402). An OECD dermal fixed dose guideline was proposed in 2004 but has since been withdrawn. Acute dermal studies are normally performed after oral or inhalation testing, and as discussed later in this publication, dermal toxicity is rarely greater than what is observed in oral or inhalation studies. Thus, a limit test is normally sufficient.
For acute inhalation toxicity, a revised version of the classic mammalian LC50 study (OECD 403) has recently been adopted, together with a new Acute Toxic Class guideline (OECD 436) as an animal reduction measure. An inhalation Fixed Concentration Procedure has also been proposed, and work to develop the scientific evidence needed to support the adoption of this method is currently ongoing (see Table 1).
SCIENTIFIC AND REGULATORY DRIVERS FOR ACUTE TOXICITY STUDIES
Regulatory Drivers in Different Sectors and Regions
By sector.
Most countries examined have enacted legislation and regulations governing the testing and marketing of agricultural and industrial chemicals, biocides, cosmetics, food additives, medicinal products, and other substances for the protection of human health and the environment. A multisector and multiregional overview of regulatory data requirements for acute systemic toxicity is presented in Table 3. This illustrates the complexity of the regulatory arena across sectors and countries and the challenges this creates for those seeking to reduce the numbers of animals used in acute toxicity studies while generating globally acceptable registration data packages.
TABLE 3.
Regulatory Drivers for Acute Toxicity Testing Across Agrochemicals, Biocides, Chemicals, Cosmetics, and Medicinal Products Sectors
| Sector | Europe | United States | Japan | China |
| Agrochemicals | Regulation (EC) No 1107/2009 (OJ, 2009); data requirements specified in Annexes II and III. The recently revised EU regulation makes sharing of vertebrate data between applicants and notifiers obligatory (in a manner similar to REACH) so that duplicate testing is avoided. At the time of this writing, data requirements are still being revised via an independent comitology process. Annex II requires acute oral and dermal data for each active substance, and an inhalation study must be performed except where exposure via this route can be ruled out. Annex III requirements for formulated products prescribe separate acute oral and dermal studies; however, classification by calculation should be a viable alternative for most formulations. | Federal Insecticide, Fungicide, and Rodenticide Act (USC, 2008b); data requirements for active substances/formulations specified in 40 CFR § 158 (EPA, 2007b); guidance on determining data needs for other ingredients provided by EPA (2002). Part 158 prescribes acute systemic toxicity studies via oral, dermal, and inhalation routes for the active substance. Additionally, each finished product/formulation is normally also required to undergo separate acute toxicity testing via the oral, dermal, and inhalation routes for labeling purposes, although data waivers may be granted in cases where a scientifically sound argument can be made (EPA, 2001). Acute systemic toxicity data are not normally required in the United States for nonactive ingredients in a pesticide formulation (EPA, 2002). | Agricultural Chemicals Regulation Law (MAFF, 1948); Appendix to Data Requirements for Supporting Registration of Pesticides (FAMIC, undated). Acute toxicity data requirements are generally consistent with those of other countries listed here, with the proviso that dermal studies may be waived if a substance is corrosive, and inhalation studies may be waived “when it is determined that there is no danger that users will be exposed to the relevant agricultural chemical through inhalation.” | Regulation on Pesticide Administration (SC, 2001); Requirements of the Pesticide Registration Document (MOA, 2001). Acute toxicity data requirements are generally consistent with those of the EU and the United States. |
| Biocides | Directive 98/8/EC (OJ, 1998); data requirements specified in Annexes II and III. At the time of writing, Directive 98/8/EC is in the process of being replaced by the Biocidal Products Regulation (EC, 2009). With respect to acute systemic toxicity of the active ingredient, oral data are normally required except where inhalation data area available, and inhalation and dermal data are required except where exposure via these routes can be ruled out. Annex III requirements (for formulated products) prescribe acute testing via at least two routes. Article 13 of the Biocides Directive, however, does specify mechanisms by which vertebrate data sharing must occur for existing actives. | Federal Insecticide, Fungicide, and Rodenticide Act (USC, 2008b); data requirements for active substances/formulations specified in 40 CFR Part 158W (EPA, 2008a); guidance on determining data needs for other ingredients provided by EPA (2002). Acute toxicity requirements are the same as for agrochemicals. | Regulated as industrial chemicals | Regulated as industrial chemicals |
| Cosmetics | Directive 76/768/EEC, as amended (OJ, 2003, 2006); guidance regarding data needs provided by the Scientific Committee on Consumer Safety (SCCP, 2006). At the time of this writing, the Cosmetics Directive is being recast as an EU regulation. This process does not alter existing or future marketing bans on products containing animal tested ingredients. Animal testing for acute systemic toxicity is banned in the EU as of March 2009, as is the marketing of cosmetic products containing ingredients that have been subject to acute testing on animals after that date. | Federal Food, Drug, and Cosmetic Act (USC, 2008a). Cosmetics are not subject to specific testing requirements or premarket approval in the United States. However, the Federal Food, Drug and Cosmetic Act broadly prohibits the marketing of adulterated or misbranded cosmetics, including any product (other than a hair dye) that “bears or contains any poisonous or deleterious substance which may render it injurious to users under the conditions of use prescribed in the labeling thereof, or under conditions of use as are customary and usual.” Companies are encouraged to register their establishments and file Cosmetic Product Ingredient Statements with FDA’s Voluntary Cosmetic Registration Program | Pharmaceutical Affairs Law; Standards for Cosmetics (MHLW, 2000). Cosmetics are not subject to specific testing requirements. However, they “shall not contain anything that may cause infection or that otherwise makes the use of the cosmetics a potential health hazard.” | Regulations Concerning The Hygiene Supervision Over Cosmetics (MPH, 1989). A distinction is made between “ordinary cosmetics” such as shampoos, deodorants, and lipstick and “special use cosmetics” such as sunscreens, depilatory creams, and weight loss products, as well as between domestic and imported cosmetics (RPA, 2004). Strict premarket requirements are imposed in China for all imported cosmetics, with special cosmetics (both domestic and imported) being subject to a safety assessment including acute toxicity testing. China is not a party to the OECD Council Decision Regarding the Mutual Acceptance of Data (OECD, 1981) and by extension does not typically accept foreign data. As a consequence, cosmetic products produced by foreign companies may therefore be subject to duplicate testing within China (RPA, 2004). |
| Chemicals | Regulation (EC) No 1907/2006 (REACH) (OJ, 2007); data requirements are specified in Annexes VII–XI. REACH data requirements are tonnage triggered, with no requirement for acute toxicity data for substances produced or imported in volumes of less than 1 metric ton per annum (tpa). Acute toxicity data via a single exposure route are required for substances at volumes above 1 tpa, and data for a second route are required for substances at levels of 10 tpa and above. REACH specifies that in vivo testing in vertebrates should only be considered as a “last resort” and provides specific criteria for waiving or adapting certain in vivo data requirements, e.g., the requirement for an oral study may be waived if an acute inhalation study is available or the material is corrosive. A case for a data waiver could also be made if testing by a particular route is not relevant based on human exposure scenarios, e.g., testing a gaseous substance via the oral route. | The Toxic Substances Control Act (TSCA; USC, 1976). No specific testing or data requirements are imposed for new chemicals, although companies are required to file a pre-manufacture notice. For existing chemicals, EPA has the authority to require companies to submit “all existing data concerning the environmental and health effects of [a chemical] or mixture”; however, this authority is seldom used. Instead, EPA has launched a series of voluntary programs: | Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. Two components: a premanufacturing evaluation of new chemical substances and monitoring/regulations based on the properties of chemical substances (METI, 2005). The hazard-based premanufacturing evaluation is primarily concerned with a chemical’s biopersistence and potential sub/chronic risks to human health and the environment. Acute toxicity studies are not specifically listed as a premanufacturing data requirement. | Measures for the Environmental Administration of New Chemical Substances (MEP, 2003); guideline for the hazard evaluation of new chemical substances (MEP, 2004a). Notification and registration provisions specify a premarket hazard evaluation by the MEP Chemical Registration Center, which normally requires the submission of acute toxicity data (MEP, 2004a). The hazard assessment principles and test guidelines mirror those set out by the OECD. Data requirements may be waived on a case-by-case basis provided a compelling scientific rationale can be provided (MEP, 2004b). Where test data originate from laboratories outside China, the laboratories involved must be Good Laboratory Practice accredited by the competent authority of the country in which the laboratory is located (MEP, 2003). Because China is not a party to the OECD Mutual Acceptance of Data, foreign data may or may not be accepted and chemicals may be subject to duplicate testing within China (RPA, 2004). |
| —High Production Volume (HPV) Chemical Challenge Program | ||||
| —Voluntary Children’s Chemical Evaluation Program | ||||
| —HPV “orphan chemicals” test rule (March 2006) | ||||
| —Extended HPV Program | ||||
| —Chemical Assessment and Management Program (ChAMP—on hold pending reauthorization/revision of TSCA); Each of these programs calls for the submission of at least Screening Information Data Set-level data (EPA, 2007a), which in all cases include an acute systemic toxicity study by at least one exposure route. |
For “agrochemicals and biocides,” acute data for three routes of administration (oral, dermal, and inhalation) are generally required for all active substances and in many cases for formulated products and certain other chemical ingredients as well (EPA, 2007b, 2008a; FAMIC, undated; GC, 2006; MOA, 2001; OJ, 1992, 1998). Requirements for “industrial chemicals” are generally less rigid, with most countries examined requiring testing by a single route or possibly two routes for higher tonnage substances (GC, 2005; MEP, 2004a; OJ, 2007). Some countries currently impose no specific data requirement for acute toxicity testing of industrial chemicals (EPA, 2007a; METI, 2005) or no testing below a specified production volume, e.g., one metric ton in the European Union (EU) (OJ, 2007). Within the EU, the only officially recognized methods for the determination of acute oral toxicity of industrial chemicals are OECD TG 420 and OECD 423 (OJ, 2004, 2008), which is a consideration when determining a test to be used across geographical regions and regulatory frameworks. The EPAA survey confirmed implementation of these regulatory requirements in practice.
For “cosmetics,” acute toxicity testing of both finished products and raw ingredients is now prohibited in the EU (OJ, 2003) and not specifically required in the United States or Canada, although information on systemic effects may be obtained using other methods to ensure the legally required safety of the product. In Japan, for cosmetics consisting of ingredients already on an approved list, there is no requirement for additional testing. In contrast, China and certain South American countries require premarket registration of cosmetic finished products, which may entail some level of acute toxicity testing above and beyond the safety assessment of raw ingredients (RPA, 2004). Additionally, some of these countries do not consistently accept foreign data, which may result in cosmetic products produced by foreign companies being subject to duplicate testing.
For “food additives, flavorings, and food-contact materials,” a specific requirement to generate acute systemic toxicity data could not be found in applicable legislation, regulations, or guidance in any of the countries surveyed (EC, 2001a, 2001b; FDA, 2002, 2006; MHLW, 2009).
For the development of new “human medicines,” the requirement for acute toxicity tests is now largely historic because the revised text of ICH Test Guideline “M3 R2” was adopted last year (ICH, 2009). All that remains is for the regional guidelines in Europe, the United States, and Japan to be updated to reflect the text of the revised ICH M3. Many pharmaceutical companies have not conducted acute toxicity studies for new medicines for some time because data generated from other more refined study types (e.g., in vivo genetic toxicology studies, safety pharmacology studies, and dose-range finding studies), which are already conducted as part of the development of new medicines, are considered to provide a better assessment of potential human safety risks in advance of clinical trials. The same is true regarding the protection of workers in manufacturing and production plants, such that most companies are now using data from other studies to inform Material Safety Data Sheets and other worker protection measures.
With respect to “veterinary medical products,” acute toxicity studies are not specifically required for the demonstration of safety either to target animals or to human consumers (EMEA, 2009; OJ, 1990; VICH, 2008). However, acute studies may be carried out on a voluntary basis to obtain information on other aspects of safety for veterinary medical product (e.g., worker protection), though as above, other available data could be used for these purposes.
Across all sectors and countries examined, it is generally accepted that acute toxicity studies may be waived if a substance is known to be corrosive or if there is a low risk of human exposure (ECHA, 2008b). Route-specific waivers may be granted on the basis of physicochemical properties, such as volatility, particle size, molecular weight and volume, and log Kow (ECHA, 2008c). A notable exception is for agrochemical and biocide active substances in the EU, where acute toxicity studies must usually be carried out for hazard classification of the active substances regardless of the expected exposure. For formulations, waivers may be granted in cases where a scientifically sound case can be made, e.g., when the outcome of the study is highly predictable based on the properties and concentration of individual ingredients (EPA, 2001; OJ, 1999). Weight-of-evidence and read-across approaches might also be used to estimate acute toxicity (discussed further in the “Alternative Approaches” section below).
Classification and labeling.
Classification and labeling of substances and products is relevant to various sectors. Regulatory authorities across the globe have also developed frameworks for the classification and labeling of chemical hazards for the protection of workers, consumers, and the environment. In many cases, the regulatory requirement for acute toxicity data is for classification and labeling purposes only, a fact confirmed by the EPAA survey, with the majority of companies identifying classification and labeling as a primary reason for conducting acute toxicity testing.
When testing is conducted solely to meet classification and labeling requirements, precise LD50/LC50 values are not necessary because testing to the upper boundary of a hazard category (i.e., limit dose) is sufficient to establish a regulatory classification. Therefore, there is no scientific necessity to establish a dose-response curve for mortality.
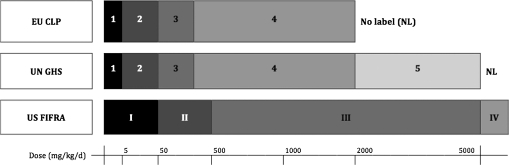
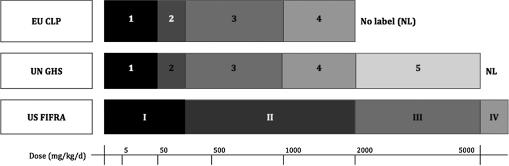
The Globally Harmonized System of Classification and Labeling (GHS) was developed under the auspices of the United Nations (UN, 2007) to promote increased consistency among diverse national and sectoral frameworks. To date, the GHS has been or is being implemented in the EU, New Zealand, Korea, China, India, Japan, and the United States (OECD, 2007a), although in certain cases, the flexibility provided by the GHS modular design has led to continued differences in implementation. For example, European authorities and the U.S. Occupational Safety and Health Administration accept a limit dose of 2000 mg/kg (i.e., GHS category 4), beyond which a substance or product is not required to bear an acute hazard label (OJ, 2008; OSHA, 2009), whereas other authorities require testing to a limit dose of 5000 mg/kg (i.e., GHS category 5) to support a no-label designation. Figures 1 and 2 illustrate the different hazard class cutoffs between the GHS, EU, and U.S. pesticide (EPA, 2004) classification schemes.
FIG. 1.
Hazard classification schemes for acute oral toxicity as defined under the UN GHS, EU CLP, and U.S. pesticide regulations.
FIG. 2.
Hazard classification schemes for acute dermal toxicity as defined under the UN GHS, EU CLP, and U.S. pesticide regulations.
The majority of European companies surveyed reported using 2000 mg/kg as the default limit dose, and the GHS itself expressly discourages testing beyond 2000 mg/kg for animal welfare reasons (UN, 2007). Regulatory guidance is also available to support extrapolation of data gained with a limit dose of 2000 to GHS category 5 without retesting (OJ, 2004). However, ongoing geopolitical differences continue to inspire duplicative animal testing (e.g., to retain a no-label designation in a country or sector where GHS category 5 is considered mandatory).
Scientific drivers.
There have been several scientific reasons proposed for conducting acute toxicity studies. Potential drivers have been gathered from the 2003 pharmaceutical industry initiative, as well as the more recent EPAA survey of member companies in other sectors, and appear to be common across industrial sectors. These are listed below, together with a discussion of their merit.
First indication of systemic toxicity during substance development
Assessing hazard for workers in manufacturing/production plants
Selection criterion (e.g., to avoid development of highly toxic compounds)
Establishing dose levels for subsequent repeated dose toxicity studies
Each of the above statements has some merit when acute lethality studies are conducted for regulatory purposes anyway (e.g., to support hazard classification and labeling). However, it is not necessary to conduct an acute toxicity study to address these scientific objectives per se. In fact, in terms of dose setting for a repeated dose study, the use of lethality as a specific end point is counterintuitive. Other study types with more refined end points (e.g., a dose escalation study to identify maximum tolerated dose) can equally address these objectives. This also holds true in cases where repeated dose toxicity data are available.
Supporting single accidental exposure/overdose
This statement assumes that the data obtained from acute toxicity studies provide information on the likely effects of acute overdose or accidental exposure in humans. However, the EPAA and pharmaceutical industry surveys demonstrated that these studies do not normally include clinical pathology, microscopic pathology, or toxicokinetic evaluation, which would provide useful information to aid in risk assessment. In addition, clinical observations seen in rodents at doses above 1000 mg/kg are often nonspecific and do not add information that would support measures to be taken in overdose or accidental exposure situations in humans.
A pilot survey of European and U.S. poison centers conducted by the NC3Rs and AstraZeneca indicated that 6 of 10 do not use the acute toxicity data in animals to manage cases of overdose in humans (Robinson and Chapman, 2009). Four centers stated that they do use animal acute toxicity data. However, the data that these poison centers thought were useful, such as target organ or mode of toxicity, are not normally provided by conventional acute toxicity studies. To explore this issue further, the NC3Rs held a workshop in January 2010, bringing together representatives from international poison centers, the pharmaceutical and chemical industries, and regulatory bodies to discuss whether and how acute toxicity data are used to assess and treat cases of pharmaceutical overdose and chemical poisoning. The discussions from this workshop are currently being written up for publication elsewhere.
Specific organs affected and mechanism of toxic action
The EPAA and pharmaceutical industry surveys have shown that microscopic pathology is not routinely performed during acute toxicity studies, which essentially negates their value in identifying target organs or mechanisms of toxic action.
In conclusion, it is evident that the scientific drivers listed above may have some merit when acute toxicity tests are conducted for regulatory purposes, such as classification and labeling. However, in the absence of a specific regulatory requirement, the scientific objectives can equally be met by other study designs that do not include lethality as the end point and that include parameters that could assist risk assessment (e.g., histopathology, clinical pathology, and measures of systemic exposure).
ALTERNATIVE APPROACHES
This section outlines accepted and emerging strategies with the potential to affect an immediate and substantial reduction in the number of animals used in regulatory acute toxicity testing (Table 3). A more extensive listing of ongoing and historic activities aimed at refinement, reduction, and replacement of animal use in acute toxicity studies is provided in Table 1.
Discontinuing Redundant Multiroute Testing
Retrospective data analyses have been undertaken by Creton et al. (2010) and Seidle, Prieto, and Bulgheroni (submitted for publication elsewhere) to ascertain the value of regulatory requirements prescribing multiroute testing for acute systemic toxicity. These analyses have examined the concordance among regulatory classifications for acute oral, dermal, and/or inhalation toxicity for ∼500 agrochemical and biocidal active substances and nearly 2000 industrial chemicals. The findings from these two independent reviews have revealed that acute dermal studies of pure substances do not add value above and beyond oral data for hazard classification of pesticides, biocides, or chemicals. Follow-up work is currently under way by Seidle to ascertain whether this conclusion holds true for multicomponent formulations. Concordance between oral and inhalation data sets was also reasonably high for certain substance classes, suggesting that it may be possible to develop waiver criteria for inhalation testing, subject to further review and analysis including consideration of factors, such as physicochemical properties, bioavailability, etc. An international workshop to discuss the findings on redundancy of the dermal route with industry and regulators is planned for September 2010.
Nontesting Approaches
A range of nontesting approaches, including chemical grouping and read across, weight of evidence, exposure-based waiving, and various calculation methods, could be put to immediate use to satisfy regulatory requirements for acute toxicity data without new testing. These approaches are commonly accepted under most regulatory frameworks including EU and U.S. pesticide and chemical regulations and international regulations implementing the GHS.
“Chemical grouping and read across” is based on the recognition that substances with similar molecular structures often share similar toxicological profiles, and where end point data are available for one member of a chemical family, these data may be used to bridge a gap for another member of the same chemical family. This approach requires expert judgment, which may be augmented by “in silico” tools, such as the OECD (quantitative) structure-activity-relationship ((Q)SAR) toolbox (OECD, 2009b) or the Ambit 2.0 database (“http://ambit.sourceforge.net”).
“Weight of evidence” recognizes that data exist which on their own would not be sufficiently robust or reliable for regulatory purposes but that when relevant data from different sources (e.g., animal studies that were not performed to current standards, in vitro data, (Q)SARs predictions, and threshold considerations) are combined using expert judgment, sound regulatory conclusions can be drawn. Further information on how read-across and weight-of-evidence approaches may be implemented can be found in ECHA (2008a) guidance documents and elsewhere (OECD, 2007b; Worth et al., 2007).
Calculation approaches for mixtures and formulations.
For formulated products containing mixtures of chemicals, a number of organizations, and regulations, including the GHS, provide guidance on the use of calculation methods to determine the toxicity and appropriate classification, thus avoiding the need for acute toxicity testing (UN, 2007; WHO, 2005). Classification can be determined on the basis on the toxicological properties of the individual ingredients and their relative proportions within the mixture or formulation.
Exposure-based adaptation.
Where exposure can be demonstrated to be negligible, or the risk of exposure is low, it could be argued that hazard characterization, i.e., an acute toxicity study, is unnecessary.
Estimation of Acute Oral Toxicity from 28-Day General Toxicity Studies
In 2008, European Centre for the Validation of Alternative Methods performed an investigation to explore whether it is possible to identify nontoxic compounds (LD50 > 2000 mg/kg) using information from 28-day repeated dose toxicity studies. Taking into account the high prevalence of nontoxic substances (87% of 4219) in the EU’s New Chemicals Database (Bulgheroni et al., 2009), a No Observed Adverse Effect Level threshold was set that allowed the correct identification of 63% of nontoxic compounds, while less than 1% of harmful compounds were misclassified as nontoxic. The proposed approach could permit the waiving of acute oral testing of more than 50% of chemical substances. Although the research focused on using the proposed approach for cosmetic ingredients, it could potentially also be applied for chemicals in other sectors where 28-day studies are performed.
Use of In vitro Data to Set Starting Doses
Based on an analysis showing strong concordance between in vitro cytotoxicity data and human lethal blood concentrations, i.e., R2 = 0.77–0.83 (Ekwall et al., 1998), it was recommended in 2000 that basal cytotoxicity tests be put to immediate use in establishing starting doses for acute oral toxicity studies in animals as a means of reducing animal use, e.g., by up to 40% in relation to OECD 425 (ICCVAM/NICEATM, 2001b). The following year, U.S. validation authorities published a guidance document on the use of in vitro data to estimate oral starting doses (ICCVAM/NICEATM, 2001a), although to date, this approach does not appear to have been widely taken up in practice. More recently, the OECD (2009a) has undertaken to update this guidance for an international audience to promote wider awareness and use of this animal reduction strategy.
CONCLUSIONS AND RECOMMENDATIONS
In the following, conclusions and recommendations are listed in hierarchical order according to the authors’ perspective:
-
1.
Before considering any acute toxicity test, it is recommended that all relevant information (from historical animal tests or other sources) on a substance or product, as well as on similar substances or products, be thoroughly evaluated to determine whether one or more nontesting approaches could be used to satisfy regulatory needs.
-
2.
Acute lethality testing is now largely historic in three regulatory sectors (i.e., pharmaceuticals, food additives/flavorings/contact materials, and in the EU, cosmetics). EPAA’s survey of 18 member companies revealed that the primary driver for conducting acute toxicity studies is to meet regulatory requirements for classification and labeling. While some companies cited other scientific drivers (e.g., first estimate of systemic toxicity, dose selection for other animal studies, or target organ identification), this information can be obtained from studies that do not use lethality as an end point. There would therefore appear to be little or no scientific basis for the continued use of death as an end point. It is recommended that regulators and policy makers worldwide critically examine whether conventional approaches to acute toxicity testing could not be replaced by nonlethal approaches for making classification and labeling determinations.
-
3.
Recent studies have demonstrated that there is little or no value in performing an acute dermal study where oral data are already available and that a significant opportunity exists to reduce animal for this purpose. It is recommended at a minimum that requirements for acute dermal testing of chemicals and pesticide and biocide active substances be deleted from relevant legislation, regulations, and implementing guidance. Similar steps should also be considered for mixtures and formulations, except perhaps where a penetration enhancer is present.
-
4.
While the majority of European companies reported using a limit dose of 2000 mg/kg in oral and dermal studies, a small number reported testing up to 5000 mg/kg to meet regulatory requirements elsewhere in the world. Meanwhile, in the pharmaceutical sector, the standard limit dose has been reduced to 1000 mg/kg. The ultimate goal of acute toxicity testing, i.e., to provide information on potential hazards and reduce the risk of accidental poisoning, was the same for all sectors, and it is therefore questionable whether a need exists for different limit doses. OECD guidelines and the GHS state that testing above 2000 mg/kg is discouraged for reasons of animal welfare and should only be considered when there is a strong likelihood that results would have a direct relevance for protecting health or the environment. With this in mind, and given that EPAA’s survey suggests acute toxicity testing is rarely used for scientific purposes including risk assessment, it is recommended that the limit dose of 5000 mg/kg be reduced to at most 2000 mg/kg or preferably 1000 mg/kg. This should be considered in cases where no data on acute toxicity are yet available and not lead to retesting.
-
5.
Within the pharmaceutical sector, the recent revision of the ICH M3 guideline to remove the requirement for acute toxicity studies to support the first clinical trial in humans represents a significant advance in reducing animal use for this purpose. To ensure full implementation of this change, it is recommended that regional regulatory requirements and guidance (i.e., EMEA, 1987; FDA, 1996) be revised and brought into line with current ICH guidelines as a matter of priority.
-
6.
Our review highlighted the existence of substantially discordant regulatory policies and testing requirements in certain emerging markets (e.g., mandatory finished product testing, the requirement to carry out some tests within the country to which a product is to be exported, and failure to routinely accept foreign-generated data). It is recommended that regulatory authorities worldwide strive to enhance international harmonization of data requirements in affected sectors and to ensure mutual recognition of test results among authorities in both existing and emerging markets.
-
7.
An historical data review has demonstrated that 28-day repeated dose toxicity studies can be used to identify compounds that are not acutely toxic (LD50 > 2000 mg/kg), which suggests that in vivo testing could be avoided for these substances, thereby substantially reducing animal use in acute toxicity testing. It is recommended that opportunities to implement this approach in practice be explored, e.g., under REACH and other chemical assessment programs where the 28-day study is legally required.
-
8.
Where acute toxicity testing cannot be avoided, it is recommended that account be taken of the study objective (i.e., point estimation or simple classification), as well as the reasonably expected LD50 or LC50, when choosing the protocol in order that the fewest animals will be used in achieving the objective.
As this paper highlights, the regulatory landscape across industry sectors and geographical regions is complex, and multiple efforts are ongoing to promote the 3Rs in acute toxicity testing across sectors and parts of the globe. However, there remains a need for greater cross-sector and international cooperation to ensure that developments that can reduce, refine, and ultimately replace the use of animals in acute toxicity testing, while assuring safety, are fully implemented.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by the authors’ affiliated institutions, together with a grant for the lead author from the Doerenkamp-Zbinden Foundation, Switzerland.
Acknowledgments
The authors are members of the EPAA Acute Toxicity Task Force and would like to thank the other members for their input and advice: David Dreher (Covance), Nigel Moore (Dow), Sally Old (Sanofi Aventis), Andrea Paetz (Bayer), Andreas Schnurstein (Evonik), Thomas Skripsky (Novartis Animal Health), and Susanne Thun-Battersby (Solvay). The authors would also like to acknowledge support from members of EPAA working group 4 (dealing with implementation of 3Rs in legislation), as well as additional support from representatives of industry sectors and the EPAA steering committee. Further information about the EPAA and its current initiatives can be found at “http://www.epaa.eu.com.”
References
- Botham PA. Acute systemic toxicity––prospects for tiered testing strategies. Toxicol. In Vitro. 2004;18:227–230. doi: 10.1016/s0887-2333(03)00143-7. [DOI] [PubMed] [Google Scholar]
- British Toxicology Society (BTS) A new approach to the classification of substances and preparations on the basis of acute toxicity. Hum. Toxicol. 1984;3:85–92. doi: 10.1177/096032718400300202. [DOI] [PubMed] [Google Scholar]
- Bulgheroni A, Kinsner-Ovaskainen A, Hoffmann S, Hartung T, Prieto P. Estimation of acute oral toxicity using the No Observed Adverse Effect Level (NOAEL) from the 28-day repeated dose toxicity studies in rats. Regulat. Toxicol. Pharmacol. 2009;53:16–19. doi: 10.1016/j.yrtph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Chapman K, Robinson S. Challenging the Regulatory Requirement for Acute Toxicity Studies in the Development of New Medicines: A Workshop Report. London: National Centre for the Replacement, Refinement and Reduction of Animals in Research; 2007. [Google Scholar]
- Chinese Ministry of Agriculture (MOA) Requirements of the Pesticide Registration Document. 2001. Available at: www.chinapesticide.gov.cn/en/2.pdf. Accessed March 15, 2010. [Google Scholar]
- Creton S, Dewhurst IC, Earl LK, Gehen SC, Guest R, Hotchkiss JA, Indans I, Woolhiser MR, Billington R. Acute toxicity testing of chemicals: opportunities to avoid redundant testing and use alternative approaches. Crit. Rev. Toxicol. 2010;40:50–83. doi: 10.3109/10408440903401511. [DOI] [PubMed] [Google Scholar]
- European Commission (EC) Guidance on Submissions for Food Additive Evaluations by the Scientific Committee on Food. 2001a. European Commission, Brussels, Belgium. Available at: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf. Accessed March 15, 2010. [Google Scholar]
- European Commission (EC) Guidelines of the Scientific Committee on Food for the Presentation of an Application for Safety Assessment of a Substance to be Used in Food Contact Materials prior to Its Authorization. 2001b. European Commission, Brussels, Belgium. Available at: http://ec.europa.eu/food/fs/sc/scf/out82_en.pdf. Accessed March 15, 2010. [Google Scholar]
- European Commission (EC) Annex to the Fifth Report on the Statistics on the Number of Animals Used for Experimental and Other Scientific Purposes in the Member States of the European Union. 2007. European Commission, Brussels, Belgium. Available at: http://ec.europa.eu/environment/chemicals/lab_animals/pdf/staff_work_doc_sec1455.pdf. Accessed March 15, 2010. [Google Scholar]
- European Commission (EC) Proposal for a Regulation of the European Parliament and of the Council Concerning the Placing on the Market and Use of Biocidal Products. 2009. European Commission, Brussels, Belgium. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2009:0267:FIN:EN:PDF. Accessed March 15, 2010. [Google Scholar]
- Ekwall B, Barile FA, Castano A, Clemendson C, Clothier RH, Dierickx P, Ekwall B, Ferro M, Friskesjö G, Garza-Ocanas L, et al. MEIC evaluation of acute systemic toxicity. Part VI. The prediction of human toxicity by rodent LD50 values and results from 61 in vitro methods. Alt. Lab. Anim. 1998;26(Suppl. 2):617–658. [PubMed] [Google Scholar]
- European Chemicals Agency (ECHA) Guidance on Information Requirements and Chemical Safety Assessment. 2008a. Available at: http://guidance.echa.europa.eu/docs/guidance_document/information_requirements_en.htm?time=1258583530. Accessed March 15, 2010. [Google Scholar]
- European Chemicals Agency (ECHA) Technical Guidance Document for Preparing the Chemical Safety Assessment. Chapter R.5: Adaptation of Information Requirements. 2008b. Available at: http://guidance.echa.europa.eu/docs/guidance_document/information_requirements_r5_en.pdf?vers=20_08_08. Accessed March 15, 2010. [Google Scholar]
- European Chemicals Agency (ECHA) Technical Guidance Document for Preparing the Chemical Safety Assessment. Chapter R.7: Endpoint Specific Guidance. 2008c. Available at: http://guidance.echa.europa.eu/docs/guidance_document/information_requirements_r7a_en.pdf?vers=20_08_08. Accessed March 15, 2010. [Google Scholar]
- European Commission Scientific Committee on Consumer Products (SCCP) The SCCP’s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation. 2006. 6th Revision. European Commission, Brussels, Belgium. Available at: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_03j.pdf. Accessed March 15, 2010. [Google Scholar]
- European Medicines Agency (EMEA) Single Dose Toxicity. 1987. European Medicines Agency, London, UK. Available at: http://www.emea.europa.eu/pdfs/human/swp/3bs1aen.pdf. Accessed March 15, 2010. [Google Scholar]
- European Medicines Agency (EMEA) Scientific Guidelines for Veterinary Medicinal Products, Safety and Residues Guidelines and Efficacy Guidelines. 2009. European Medicines Agency, London, UK. Available at: http://www.emea.europa.eu/htms/vet/vetguidelines/background.htm. Accessed March 15, 2010. [Google Scholar]
- Gennari A, ven den Berghe C, Casati S, Castell J, Clemedson C, Coecke S, Colombo A, Curren R, Dal Negro G, Goldberg A, et al. Strategies to replace in vivo acute systemic toxicity testing. Alt. Lab. Anim. 2004;32:437–459. doi: 10.1177/026119290403200417. [DOI] [PubMed] [Google Scholar]
- Government of Canada (GC) New Substances Notification Regulations (Chemicals and Polymers) 2005. Canada Gazette, ON, Ottawa, Canada. Available at: http://www.gazette.gc.ca/archives/p2/2005/2005-09-21/html/sor-dors247-eng.html. Accessed March 15, 2010. [Google Scholar]
- Government of Canada (GC) Pest Control Products Act. 2006. Department of Justice, Ottawa, ON, Canada. Available at: http://laws.justice.gc.ca/en/P-9.01/. Accessed March 15, 2010. [Google Scholar]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Validation Study of In Vitro Cytotoxicity Test Methods: Recommendations and Agency Responses. 2008. National Toxicology Program, Research Triangle Park, NC. Available at: http://iccvam.niehs.nih.gov/methods/acutetox/inv_nru_recommend.htm. Accessed March 15, 2010. [Google Scholar]
- Interagency Coordinating Committee on the Validation of Alternative Methods/National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (ICCVAM/NICEATM) Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity. 2001a. National Toxicology Program, Research Triangle Park, NC. Available at: http://iccvam.niehs.nih.gov/docs/acutetox_docs/guidance0801/iv_guide.pdf. Accessed March 15, 2010. [Google Scholar]
- Interagency Coordinating Committee on the Validation of Alternative Methods/National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (ICCVAM/NICEATM) Report of the International Workshop on In Vitro Methods for Assessing Acute Systemic Toxicity. 2001b. National Toxicology Program, Research Triangle Park, NC. Available at: http://iccvam.niehs.nih.gov/methods/acutetox/inv_cyto_wksp.htm. Accessed March 15, 2010. [Google Scholar]
- Interagency Coordinating Committee on the Validation of Alternative Methods/National Toxicology Program Interage ncy Center for the Evaluation of Alternative Toxicological Methods (ICCVAM/NICEATM) Validation Study of In Vitro Cytotoxicity Test Methods. 2006. National Toxicology Program, Research Triangle Park, NC. Available at: http://iccvam.niehs.nih.gov/methods/acutetox/inv_nru_tmer.htm. Accessed March 15, 2010. [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Revised ICH Topic M3(R2): Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. 2009. International Federation of Pharmaceutical Manufacturers and Associations, Geneva, Switzerland. Available at: http://www.ich.org/LOB/media/MEDIA5544.pdf. Accessed March 15, 2010. [Google Scholar]
- International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) VICH GL 43 (Target Animal Safety). 2008. International Federation for Animal Health, Brussels, Belgium. Available at: http://www.vichsec.org/pdf/0708/GL43-st7.doc. Accessed March 15, 2010. [DOI] [PubMed] [Google Scholar]
- Japan Food and Agricultural Materials Inspection Center (FAMIC) Appendix to Data Requirements for Supporting Registration of Pesticides. undated. Government of Japan, Saitama, Japan. Available at: http://www.acis.famic.go.jp/eng/shinsei/8147appendix.pdf. Accessed March 15, 2010. [Google Scholar]
- Japan Ministry of Agriculture, Forestry and Fisheries (MAFF) Agricultural Chemicals Regulation Law. 1948. Government of Japan, Tokyo, Japan. Available at: http://www.env.go.jp/en/chemi/pops/Appendix/05-Laws/agri-chem-laws.pdf. Accessed March 15, 2010. [Google Scholar]
- Japan Ministry of Economy, Trade and Industry (METI) Act on The Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. 2005. Government of Japan, Tokyo, Japan. Available at: http://www.meti.go.jp/english/policy/mono_info_service/kagaku/chemical_substances/chemical_substances03.html. Accessed March 15, 2010. [Google Scholar]
- Japanese Ministry of Health, Labor and Welfare (MHLW) Standards for Cosmetics. 2000. Government of Japan, Toxyo, Japan. Available at: http://www.mhlw.go.jp/english/topics/cosmetics/index.html. Accessed March 15, 2010. [Google Scholar]
- Japanese Ministry of Health, Labor and Welfare (MHLW) The Guidelines for Designation of Food Additives, and for Revision of Standards for Use of Food Additives. 2009. Government of Japan, Tokyo, Japan. Available at: http://www.mhlw.go.jp/english/topics/foodsafety/foodadditives/index.html. Accessed March 15, 2010. [Google Scholar]
- Kinsner-Ovaskainen A, Bulgheroni A, Hartung T, Prieto P. ECVAM’s ongoing activities in the area of acute oral toxicity. Toxicol. In Vitro. 2009;23:1535–1540. doi: 10.1016/j.tiv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Langley G. Acute Toxicity Testing Without Animals: More Scientific and Less of a Gamble. 2005 BUAV, London. [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People's Republic of China (MHP) Regulations Concerning The Hygiene Supervision Over Cosmetics. 1989. Ministry of Health, Beijing, China. Available at: http://tradeinservices.mofcom.gov.cn/en/b/1989-11-13/26891.shtml. Accessed March 15, 2010. [Google Scholar]
- Ministry of Environmental Protection, Peoples’ Republic of China (MEP) Provisions for the Environmental Administration of New Chemical Substances. 2003. Ministry of Health, Beijing, China. Available at: http://www.crc-mep.org.cn/newchem/enewchem.htm. Accessed March 15, 2010. [Google Scholar]
- Ministry of Environmental Protection, Peoples’ Republic of China (MEP) Guideline for the Hazard Evaluation of New Chemical Substances: HJ/T 154–2004. 2004a. Beijing, China. Available at: http://www.mep.gov.cn/image20010518/4342.pdf. Accessed March 15, 2010. [Google Scholar]
- Ministry of Environmental Protection, Peoples’ Republic of China (MEP) Guideline for the Testing of Chemicals: HJ/T 153–2004. 2004b. Available at: http://english.mep.gov.cn/standards_reports/standards/Solid_Waste/other_standards1/200710/t20071024_111961.htm. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Council Regulation (EEC) No. 2377/90 Laying Down a Community Procedure for the Establishment of Maximum Residue Limits of Veterinary Medicinal Products in Foodstuffs of Animal Origin. 1990. EU Publications Office, Brussels, Belgium. Available at: http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-5/reg_1990_2377/reg_1990_2377_en.pdf. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Council Directive Concerning the Placing of Plant Protection Products on the Market. 1992. EU Publications Office, Brussels, Belgium. Available at: http://europa.eu/eur-lex/en/consleg/pdf/1991/en_1991L0414_do_001.pdf. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. 1998. EU Publications Office, Brussels, Belgium. Available at: http://ec.europa.eu/environment/biocides/pdf/dir_98_8_biocides.pdf. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Directive 1999/45/EC Concerning the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Classification, Packaging and Labelling of Dangerous Preparations. 1999. EU Publications Office, Brussels, Belgium. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31999L0045:EN:HTML. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Directive 2003/15/EC of the European Parliament and of the Council Amending Council Directive 76/768/EEC on the Approximation of the Laws of the Member States Relating to Cosmetic Products. 2003. EU Publications Office, Brussels, Belgium. Available at: http://ec.europa.eu/enterprise/cosmetics/doc/200315/200315_en.pdf. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Corrigendum to Commission Directive 2004/73/EC adapting to technical progress for the 29th time Council Directive 6/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Official Journal of the European Union. 2004 EU Publications Office, Brussels, Belgium. L 216, 3–310. [Google Scholar]
- Official Journal of the European Communities (OJ) Council Directive on the Approximation of Laws of the Member States Relating to Cosmetic Products. 2006. EU Publications Office, Brussels, Belgium. Available at: http://eurlex.europa.eu/LexUriServ/site/en/consleg/1976/L/01976L0768-20060809-en.pdf. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Regulation (EC) No. 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). 2007. EU Publications Office, Brussels, Belgium. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:EN:PDF. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Regulation (EC) No. 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures. 2008. EU Publications Office, Brussels, Belgium. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:353:0001:1355:EN:PDF. Accessed March 15, 2010. [Google Scholar]
- Official Journal of the European Communities (OJ) Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. 2009. EU Publications Office, Brussels, Belgium. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:EN:PDF. Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals. 1981. OECD, Paris, France. Available at: http://www.oecd.org/document/41/0,3343,en_2649_34365_1890473_1_1_1_1,00.html. Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) OECD Test Guideline 401 was Deleted in 2002: A Major Step in Animal Welfare: OECD Reached Agreement on the Abolishment of the LD50 Acute Toxicity Test. 2002. OECD, Paris, France. Available at: http://www.oecd.org/document/52/0,3343,en_2649_34377_2752116_1_1_1_1,00.html. Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Report on Preparation of GHS Implementation by the OECD Countries. OECD Series on Testing and Assessment No. 70. Paris, France: OECD; 2007a. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Series on Testing and Assessment Number 80: Guidance on Grouping of Chemicals. 2007b. OECD, Paris, France. Available at: http://www.olis.oecd.org/olis/2007doc.nsf/LinkTo/NT0000426A/$FILE/JT03232745.PDF. Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Draft Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests. 2009a. OECD, Paris, France. Available at: http://www.oecd.org/dataoecd/17/0/43325517.pdf. Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Guidance Document for Using the OECD (Q)SAR Application Toolbox to Develop Chemical Categories According to the OECD Guidance on Grouping of Chemicals, OECD Environment Health and Safety Publications, Series on Testing and Assessment No. 102. 2009b. OECD, Paris, France. Available at: http://www.olis.oecd.org/olis/2009doc.nsf/linkto/env-jm-mono(2009). Accessed March 15, 2010. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) OECD Guidelines for the Testing of Chemicals. Section 4: Health Effects. 2009c. OECD, Paris, France. Available at: http://masetto.sourceoecd.org/vl=1083876/cl=12/nw=1/rpsv/cw/vhosts/oecdjournals/1607310x/v1n4/contp1-1.htm. Accessed March 15, 2010. [Google Scholar]
- Risk & Policy Analysts Limited. Comparative Study on Cosmetics Legislation in the EU and Other Principal Markets with Special Attention to so-called Borderline Products. Final Report. RPA Ltd., Norfolk, UK. 2004. Available at: http://ec.europa.eu/enterprise/newsroom/cf/itemshortdetail.cfm?item_id=3519&lang=nl. Accessed May 25, 2010. [Google Scholar]
- Robinson S, Chapman K. Are acute toxicity studies required to support overdose for new medicines? Regul. Toxicol. Pharmacol. 2009;55:110. doi: 10.1016/j.yrtph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Robinson S, Delongeas J-L, Donald E, Dreher D, Festag M, Kervyn S, Lampo A, Nahas K, Nogues V, Ockert D, et al. A European pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. Regul. Toxicol. Pharmacol. 2008;50:345–352. doi: 10.1016/j.yrtph.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The Principles of Humane Experimental Technique. 1959 Methuen & Co. Ltd. London. [Reissued: 1992, Universities Federation for Animal Welfare, Herts, England.] [Google Scholar]
- Solecki R, Davies L, Dellarco V, Dewhurst I, van Raaij M, Tritscher A. Guidance on setting of acute reference dose (ARfD) for pesticides. Food Chem. Toxicol. 2005;43:1569–1593. doi: 10.1016/j.fct.2005.04.005. [DOI] [PubMed] [Google Scholar]
- State Council of the People’s Republic of China. Regulations on Pesticide Administration. 2001. State Council of P.R. China, Beijing, China. Available at: http://www.gov.cn/english/laws/2005-08/24/content_25760.htm. Accessed March 15, 2010. [Google Scholar]
- Trevan JW. The error of determination of toxicity. Proc. R. Soc. Lond. 1927;1.1B:483. [Google Scholar]
- U.K. Home Office (HO) LD50 Test—Changes To Licensing Procedures. 1999. Available at: http://www.apc.gov.uk/press_releases/991021.htm. Accessed March 15, 2010. [Google Scholar]
- United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS). 2007. Available at: http://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html. Accessed March 15, 2010. [Google Scholar]
- United States Code (USC) The Toxic Substances Control Act (TSCA) 1976. 15 U.S.C. §§ 2601 et seq. United States Government, Washington, DC. Available at: http://www.epa.gov/compliance/civil/tsca/tscaenfstatreq.html. Accessed March 15, 2010. [Google Scholar]
- United States Code (USC) Federal Food, Drug and Cosmetic Act (FFDCA) of 1938. 2008a. 21 U.S.C. § 301 et seq. United States Government, Washington, DC. Available at: http://www.law.cornell.edu/uscode/21/usc_sup_01_21_10_9.html. Accessed March 15, 2010. [Google Scholar]
- United States Code (USC) Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) of 1947. 2008b. 7 U.S.C. § 136 et seq. United States Government, Washington, DC. Available at: http://agriculture.senate.gov/Legislation/Compilations/Fifra/FIFRA.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Environmental Protection Agency (HHS & EPA) Memorandum of Understanding on High Throughput Screening, Toxicity Pathway Profiling, and Biological Interpretation of Findings. 2008. HHS & EPA, Research Triangle Park, NC. Available at: http://ntp.niehs.nih.gov/files/ntpncgcepamou.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Pesticide Registration Notice 2001-2: Acute Toxicity Data Requirements for Granular Pesticide Products, Including Those with Granular Fertilizers in the Produce. 2001. U.S. EPA, Washington, DC. Available at: http://www.epa.gov/PR_Notices/pr2001-2.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Guidance Document on Methodology for Determining the Data Needed and the Types of Assessments necessary to make FFDCA Section 408 Safety Determinations for Lower Toxicity Pesticide Chemicals. 2002. U.S. EPA, Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Chemical Hazard Classification and Labeling: Comparison of OPP Requirements and the GHS. 2004. U.S. EPA, Washington, DC. Available at: http://www.epa.gov/oppfead1/international/global/ghscriteria-summary.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) OECD SIDS Manual Sections 3.4 and 3.5. 2007a. U.S. EPA, Washington, DC. Available at: http://www.epa.gov/HPV/pubs/general/sidsappb.htm. Accessed March 15, 2010. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Pesticides; data requirements for conventional chemicals, technical amendments, and data requirements for biochemical and microbial pesticides; final rules. 27 Fed. Regist. 2007b 60934. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Data requirements for antimicrobial pesticides; proposed rule. 73 Fed. Regist. 2008a 59382. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) ToxCast™ Program: Predicting Hazard, Characterizing Toxicity Pathways, and Prioritizing the Toxicity Testing of Environmental Chemicals. 2008b. U.S. EPA, Washington, DC. Available at: http://www.epa.gov/ncct/toxcast/index.html. Accessed March 15, 2010. [Google Scholar]
- U.S. Environmental Protection Agency (EPA) The U.S. Environmental Protection Agency’s Strategic Plan for Evaluating the Toxicity of Chemicals. 2009. U.S. EPA, Washington, DC. Available at: http://www.epa.gov/osa/spc/toxicitytesting/docs/toxtest_strategy_032309.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Food and Drug Administration (FDA) FDA Policy Statement on the LD50. 1988. Docket No. 86P-0224, 53 FR 39650. U.S. FDA, Rockville, MD. [Google Scholar]
- U.S. Food and Drug Administration (FDA) Guidance for Industry: Single Dose Acute Toxicity Testing for Pharmaceuticals. 1996. U.S. FDA, Rockville, MD. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079270.pdf. Accessed March 15, 2010. [Google Scholar]
- U.S. Food and Drug Administration (FDA) Preparation of Food Contact Notifications for Food Contact Substances: Toxicology Recommendations. 2002. September 1999; Revised April 2002 Guidance for Industry. U.S. FDA, Rockville, MD. Available at: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/ucm081825.htm. Accessed March 15, 2010. [Google Scholar]
- U.S. Food and Drug Administration (FDA) Summary Table of Recommended Toxicological Testing for Additives Used in Food June 2006 Guidance for Industry. 2006. U.S. FDA, Rockville, MD. Available at: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/ucm054658.htm. Accessed March 15, 2010. [Google Scholar]
- U.S. National Research Council (NRC) Toxicity Testing in the Twenty-First Century: A Vision and a Strategy. Washington, DC: National Academies Press; 2007. [Google Scholar]
- U.S. Occupational Safety and Health Administration (OSHA) Hazard communication; proposed rule. 74 Fed. Regist. 2009 50280. [Google Scholar]
- World Health Organization (WHO) The WHO recommended classification of pesticides by hazard and guidelines to classification. International Programme on Chemical Safety. 2005. WHO, Geneva, Switzerland. Available at: http://www.who.int/ipcs/publications/pesticides_hazard_rev_3.pdf. Accessed March 15, 2010. [Google Scholar]
- Worth AP, Bassan A, De Bruijn J, Gallegos Saliner A, Netzeva T, Patlewicz G, Pavan M, Tsakovska I, Eisenreich S. The role of the European Chemicals Bureau in promoting the regulatory use of (Q)SAR methods. SAR QSAR Environ. Res. 2007;18:111–125. doi: 10.1080/10629360601054255. [DOI] [PubMed] [Google Scholar]
- Zbinden G. Progress in Toxicology. Berlin, Germany: Springer-Verlag; 1973. [Google Scholar]
- Zbinden G, Flury-Roversi M. Significance of the LD50 test for the toxicological evaluation of chemical substances. Arch. Toxicol. 1981;47:77–99. doi: 10.1007/BF00332351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.