Abstract
Air pollution is a critical factor in the development and exacerbation of pulmonary diseases. Ozone, automobile exhaust, cigarette smoke, and metallic dust are among the potentially harmful pollution components that are linked to disease progression. Transition metals, such as cobalt, have been identified at significant levels in air pollution. Cobalt exerts numerous biological effects, including mimicking hypoxia. Similar to hypoxia, cobalt exposure results in the stabilization of hypoxia-inducible factors (HIFs), a family of proteins that regulate the cellular response to oxygen deficit. HIFs also play an important role in innate immunity and inflammatory processes. To characterize the role of HIF1α, the most ubiquitously expressed HIF, in the early events during cobalt-induced lung inflammation, an inducible lung-specific HIF1α deletion model was employed. Control mice showed classical signs of metal-induced injury following cobalt exposure, including neutrophilic infiltration and induction of Th1 cytokines. In contrast, HIF1α-deficient mice exhibited pronounced eosinophil counts in bronchoalveolar lavage fluid and lung tissue complemented with Th2 cytokine induction. The timing of these results suggests that the loss of epithelial-derived HIF1α alters the lung's innate immune response and biases the tissue toward a Th2-mediated inflammation.
Keywords: hypoxia, HIF1α, cobalt, lung inflammation
Postnatal lungs require pollution-free ambient air for proper function and development. Air pollution is also a critical factor in the occurrence and exacerbation of pathological conditions of the lung, such as airway hypersensitivity, asthma, chronic obstructive pulmonary disease (COPD), and lung cancer (Yang and Omaye, 2009). A plethora of airborne particles are injected into the environment through anthropogenic activities.
One line of defense against airborne particles is innate immunity. Various lung diseases, such as asthma and COPD, occur because of detrimental effects of these particles on epithelial integrity, resident macrophage activation, and recruitment of inflammatory cells. Hard metal lung disease (HMLD) and cobalt asthma are occupational respiratory diseases affecting workers involved in the manufacture and maintenance of hard metals (material consisting of tungsten carbide cemented in a matrix of cobalt), diamond polishing, and coal mining. These workers are exposed to cobalt dust and manifest airway constriction, alveolitis, fibrosis, and associated giant cell interstitial pneumonitis (Lison et al., 1996). The mechanism for the cobalt-induced pathology remains largely unknown; however, several possibilities have been proposed. One of these possibilities is that ability of cobalt to promote a hypoxic-like response in cells. Given the link between hypoxia and inflammation, cobalt-induced hypoxia mimicry offers a logical link between metal exposure and the observed pathologies of HMLD and cobalt asthma (Jain and Sznajder, 2005; Vengellur et al., 2005).
Hypoxia, a decrease in available oxygen reaching the tissues of the body, can influence normal cellular homeostasis, cellular repair, and inflammation. One component of the cellular response to hypoxia is regulated by a family of transcription factors called the hypoxia-inducible factors (HIFs) (Bunn and Poyton, 1996). There are five mammalian HIFs, of which HIF1α is the most ubiquitously expressed and studied (Jain and Bradfield, 1998). HIF1α is primarily regulated at the level of protein stability by a family of prolyl hydroxylases. These prolyl hydroxylase domain proteins (PHDs), upon exposure to decreases in oxygen availability, become inhibited and the HIF1α becomes stabilized (Epstein et al., 2001). Once stable, HIF1α translocates to the nucleus and heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (also known as HIF1β) forming the functional transcription factor HIF1. HIF1 regulates the expression of over 100 genes, including ones involved in energy metabolism, matrix/barrier function, angiogenesis, and inflammation (Forsythe et al., 1996; Jung et al., 2003; Mojsilovic-Petrovic et al., 2007; Semenza et al., 1994). Similar to hypoxia, cobalt has been shown to inhibit PHDs, and this inhibition causes very similar transcriptional outputs to that of hypoxia (Salnikow et al., 2004; Vengellur et al., 2005). A recent study in human peripheral blood mononuclear cells has shown similar transcriptional overlap upon tungsten carbide-cobalt particle treatment, linking HMLD to hypoxia signaling (Lombaert et al., 2008).
Recently, using a lung-specific HIF1α-deficient mouse model, a compromised HIF signaling system was shown to bias the lung's inflammatory response toward a Th2-mediated process following a 14-day exposure paradigm. These HIF1α-deficient mice displayed an asthma-like phenotype, including pronounced eosinophil infiltration, mucus cell metaplasia of airway epithelium, and increased levels of the chitinase-like proteins YM1 and YM2 following cobalt challenge. These results suggest that airway epithelial-derived HIF1α signaling plays a critical role in modulating the inflammatory response of the lung. These studies were undertaken to test the hypothesis that HIF1α plays a developmental role in establishing the innate immunity of the lung, and this is the reason for the switch in inflammatory response observed in the subchronic exposure study. To test this hypothesis, the early events of cobalt-induced inflammation in control and HIF1α-deficient mice were assessed. In the present study, control and HIF1α-deficient mice were exposed to cobalt daily for 1, 2, or 5 days. Bronchoalveolar lavage fluid (BALF) cellularity from HIF1α-deficient mice displayed a progressive eosinophilic infiltration whereas control mice displayed a transient increase in neutrophils. Histological analysis revealed accelerated tissue injury following acute cobalt challenge in HIF1α-deficient mice. Finally, BALF cytokine analysis showed Interleukin-4 (IL-4), IL-5, and IL-10 elevation specific to cobalt-treated HIF1α-deficient mice. In contrast, control mice showed specific induction of IL-6 and tumor necrosis factor α (TNF-α) following cobalt treatment. These results suggest that epithelial-derived HIF1α is essential for regulating early inflammatory events following cobalt challenge, and loss of this regulation biases the lung toward a Th2-mediated process and an asthma-like pathology following metal exposure. More importantly, the results suggest that HIF1α is essential for establishing the normal innate immunity of the lungs. This raises the possibility that HIF1α is involved in asthma susceptibility.
MATERIALS AND METHODS
Description of mice.
The mice used in these studies were created by mating HIF1αflox/flox (a generous gift of Dr Randall Johnson, University of California-San Diego) and SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg transgenic mice (a generous gifts of Dr Jeffrey A. Whitsett, Cincinnati Children's Hospital Medical Center) (Lobe et al., 1999; Perl et al., 2002; Ryan et al., 1998, 2000). The generated triple transgenic mice, SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox, are capable of respiratory epithelium-specific conditional recombination in the floxed HIF1α gene upon exposure to doxycycline (20). All the mice genotypes used in this study have been maintained in a mixed C57/BL6 and FVB/N background. Genotyping of the mice was performed by PCR for the three loci as previously described (Saini et al., 2008).
Doxycycline treatment and animal husbandry.
Postnatal recombination was carried out by exposing lactating dams to doxycycline through feed (625 mg doxycycline/kg; Harlan Teklad, Madison, WI) and drinking water (0.8 mg/ml; Sigma Chemicals Co.) until weaning. Triple transgenic mice were then maintained on the same food and water until they were approximately 7 weeks of age. In order to eliminate the effects of doxycycline (DOX), the treatment was terminated 7–10 days prior to first exposure to metals. This DOX exposure paradigm has been demonstrated to produce almost complete loss of HIF1α expression in the adult lung (Saini et al., 2009). These mice will be referred to as HIF1α deficient or HIF1αΔ/Δ. Genotypic designation of these mice is SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αΔ/Δ. Animals of the same genotype that were maintained on normal food and water ad libitum were used as controls. Mice used in this study were kept at the animal housing facility under the strict hygienic and pathogen-free conditions approved by the University Laboratory Animal Resource (ULAR) regulatory unit. All the animal handling and necropsy protocols were approved by the ULAR regulatory unit of Michigan State University.
Cobalt exposure, tissue harvesting, and processing.
Control and HIF1αΔ/Δ male mice were randomly assigned to one of 6 groups. Mice were treated with saline or 10-mM cobaltous chloride in 25-μl volume by oropharyngeal aspiration daily for 1, 2, or 5 days. The 10-mM cobalt chloride concentrations correspond to daily exposure of 60 μg of CoCl2 (corresponding to an average exposure of 2 mg/kg). This dose of cobalt was chosen because it had previously been demonstrated to induce robust inflammation (Saini et al., 2009). Moreover, this dose falls within the levels used in the National Toxicology Programs’ assessment of cobalt sulfate and shown to induce injury (Bucher, 1991). Animals were sacrificed 24 h following the final exposure (Fig. 1). In the case of the 1-day treatment group, a single dose of cobalt was administered and mice were sacrificed 24 h later. For the 2-day time point, two doses were delivered at 24-h interval and animals were sacrificed 24 h after second dose. Finally, for the 5-day time point, five doses were delivered at 24 h intervals and animals were sacrificed 24 h after fifth dose. Following exposure, mice were anesthetized with sodium pentobarbital (50 mg/ml), and a midline laparotomy was performed. The trachea was exposed and cannulated. The lung and heart were removed en bloc, and the lungs were lavaged with two successive 1-ml volumes of sterile saline. These fractions were combined, and total cell counts were performed using a hemocytometer. Differential cell counts were performed in cytospin samples using Diff-Quik reagent (Baxter, FL). The remaining BALF was frozen for cytokine profiling. The right lung lobe was removed and stored in RNAlater RNA stabilizing reagent (Qiagen, Valencia, CA) for protein and RNA isolation. The left lobe was fixed in 10% neutral buffered formalin for histopathological analysis.
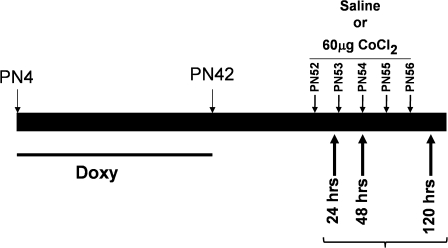
FIG. 1.
Experimental design. HIF1αΔ/Δ mice were generated through postnatal doxycycline treatment paradigm (doxycycline given from PN4 to PN42). Control (n = 36) and HIF1αΔ/Δ (n = 36) male mice randomly assigned to three different treatment groups (24, 48, and 120 h). For each time point, mice were challenged with saline (n = 6) or cobalt chloride (60 μg, n = 6) via oropharyngeal aspiration. Animals were euthanized 24 h after their first dose (24-h treatment group), second dose (48-h treatment group), or fifth dose (120-h treatment group).
Protein assay.
The total amount of protein in the BALF was quantified using the Bradford assay (Bradford, 1976). Briefly, BALF samples were diluted in distilled water and mixed with dye reagent via manufacturer's instructions (Bio-Rad, Hercules, CA). Absorbance was read at 595nm using spectrophotometer (GeneQuant 100, GE Healthcare Piscataway, NJ). Protein concentrations were determined by comparison to a standard curve created from serially diluted bovine serum albumin standards of known concentrations.
Histopathology and immunohistochemistry.
At least four mice from each genotype and treatment group were analyzed for histopathological changes. Formalin-fixed left lung lobe tissues were paraffin embedded, and 5-μm-thick sections were mounted on glass slides and stained with hematoxylin and eosin or major basic protein (MBP) (1:500 dilution, Mayo Clinic, AZ), 40-kDa antigen of neutrophils (MCA771GA 1:100 dilution, Serotec, Raleigh, NC) as previously described (Saini et al., 2008).
Determination of cytokine levels by Bead array.
Levels of cytokines (IL-2, keratinocyte chemoattractant [KC], IL-4, IL-5, IL-13, TNF-α, IL-6, IL-10, interferon-γ [ INF-γ], and Rantes) from acellular BALF samples were measured using BD CBA Mouse Soluble Protein Flex Sets and FACSCalibur flow cytometer according to the manufacturer's instruction (CBA; BD Biosciences, San Diego, CA). Briefly, BALF was mixed with capture beads and incubated for 1 h at room temperature. Subsequently, PE detection reagent was added and incubated for 1 h at room temperature. Following extensive washing, samples were analyzed on a BD FACSArray Bioanalyzer (BD Biosciences) according to the manufacturer's instruction.
Quantitative analysis.
All cell counts and cytokine and gene expression data were analyzed by ANOVA followed by a Bonferroni post-test. All the data from the study were presented as SEM. Statistical difference of p value less than 0.05 was considered as significant.
RESULTS
BALF Protein Exudation and Cellularity
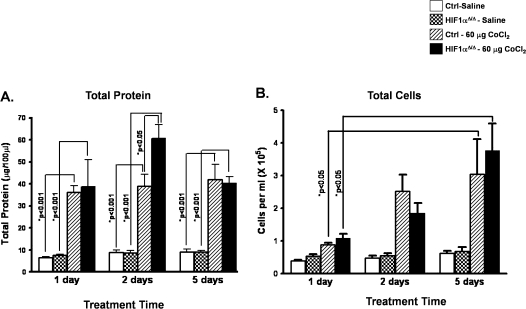
To characterize the role of HIF1α in cobalt-induced lung injury, control and HIF1αΔ/Δ mice were exposed to saline or cobalt for varying times. Total BALF protein concentration was measured as an index of lung epithelial permeability and, hence, lung injury. Cobalt-treated control and HIF1αΔ/Δ mice showed significantly higher protein concentration when compared with saline-treated mice, indicating that lung injury initiates as soon as 24 h after the initial exposure (Fig. 2A). The level of protein exudation in cobalt-treated control mice remained within narrow range of 35–45 μg/100 μl at all the three time points. In contrast, cobalt-treated HIF1αΔ/Δ mice showed significantly higher protein exudation (60.71 ± 6.2) at the 48-h time point as compared with respective control (38.9 ± 5.3) counterparts (Fig. 2A), suggesting these mice are more prone to metal-induced lung injury. The total cell count from BALF was also measured to follow the progression of injury. Total cells in BALF showed a significant increase in both control and HIF1αΔ/Δ mice following 5 days of cobalt exposure. There was no difference between control and HIF1αΔ/Δ mice at any time point (Fig. 2B).
FIG. 2.
BALF protein and total cell counts from control and HIF1αΔ/Δ mice. Total protein concentrations (A) and cell counts (B) were assessed from BALF of saline-treated (white bars) and cobalt (hatched bars)-treated control mice and saline-treated (checkered bars) and cobalt (black bars)-treated HIF1αΔ/Δ mice as described in Materials and Methods section. n > 5 mice per group. Data are expressed as mean ± SE. Significance is noted where applicable.
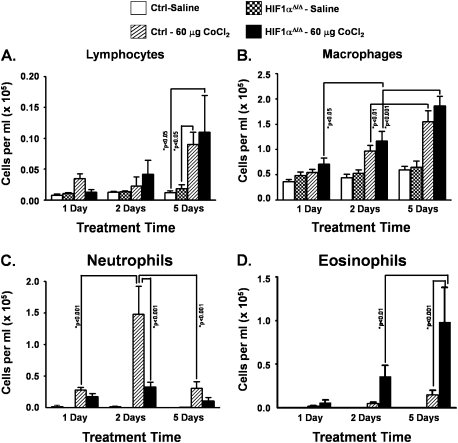
To characterize the types of inflammatory cell infiltration that made up the total cells in BALF, differential cell counts were performed. Similar to total cell counts, total macrophages and lymphocytes showed time-dependent increase in numbers; however, no significant difference was noticed between cobalt-treated control and HIF1αΔ/Δ mice at any of the three time points (Figs. 3A and 3B). In contrast, there was a distinct difference between the two genotypes in the numbers of neutrophils and eosinophils found in the BALF. The control mice displayed a significant increase in neutrophils following cobalt exposure at the 48-h time point that was not seen in the HIF1α-deficient mice. Moreover, this increase was resolved by the 5-day treatment time (Fig. 3C). The HIF1αΔ/Δ mice displayed a large and sustained increase in eosinophil infiltration into the lung following cobalt exposure. This increase was observed as early as 2 days post-treatment and reached significance by 5 days of treatment (Fig. 3D). These genotype-specific changes in inflammatory cell infiltrates suggest that epithelium-derived HIF1α is an important regulator in the inflammatory responses to metal insults.
FIG. 3.
Effect of cobalt treatment on inflammatory cells recovered in BALF. Differential cell counts were performed for lymphocytes (A), macrophages (B), neutrophils (C), and eosinophils (D) from BALF of saline-treated (white bars) and cobalt (hatched bars)-treated control mice and saline-treated (checkered bars) and cobalt (black bars)-treated HIF1αΔ/Δ mice. n > 5 mice per group. Data are expressed as mean ± SE. Significance is noted where applicable.
Histopathology of Cobalt-Induced Injury
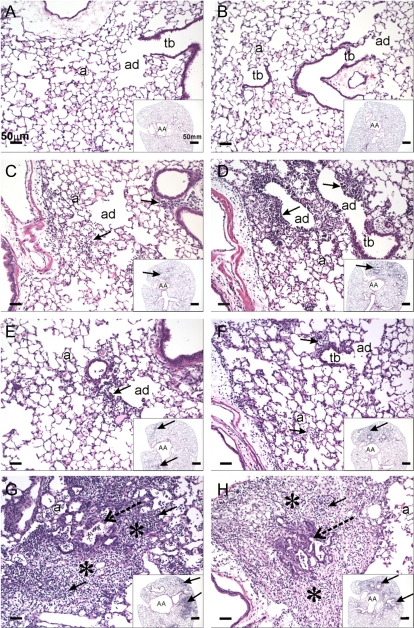
No exposure-related histopathology was found in the lungs of any of the mice instilled with the saline vehicle alone (Figs. 4A and 4B). In contrast, cobalt-instilled mice had both inflammatory and epithelial lesions in the lung, the character and severity of which were both treatment (number of days of instillations) and genotype dependent.
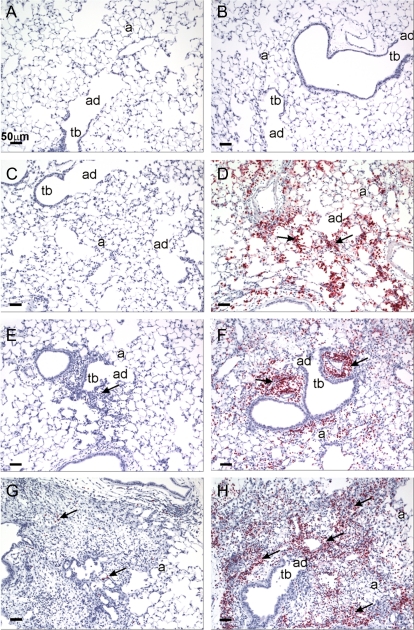
FIG. 4.
Histopathology staining of saline- and cobalt-treated control and HIF1αΔ/Δ mice. Light photomicrographs of hematoxylin and eosin–stained lung sections taken from control (A, C, E, G) and HIF1αΔ/Δ mice (B, D, F, H) that were dosed by oropharyngeal aspiration with cobalt in saline (C, D, E, F, G, H) or saline alone (saline-vehicle controls; A and B). Mice were exposed once a day for 1 (C, D), 2 (E, F), or 5 (A, B, G, H) days. Small insert in the right lower corner of each figure is a photomicrograph at low magnification of the transverse left lung lobe (proximal aspect) taken at generation five of the intrapulmonary axial airway (AA). Solid arrows identify areas of cobalt-induced mixed inflammatory cell infiltration. In (G and H), focal areas of epithelial hyperplasia lining terminal bronchioles and/or alveolar ducts (stippled arrows) are circumscribed by areas of cellular inflammation and alveolar interstitial fibrosis (asterisks). Tb, terminal bronchiole; ad, alveolar duct; and a, alveolus.
After 1 day of cobalt instillation, control mice had a mild, acute bronchopneumonia (bronchiolitis and alveolitis) that was most prominent in the hilar region of the left lung lobe (proximal G5 section). Few cobalt-associated airway or alveolar lesions were found in the distal lung section (G11). The inflammatory cell infiltrate was characterized by a mononuclear cell infiltrate (mainly small and large lymphocytes, monocytes, and occasional plasma cells) admixed with neutrophils and a few widely scattered eosinophils, in interstitial tissues surrounding large- and small-diameter conducting airways (i.e., axial, preterminal, and terminal bronchioles) and extending into the centriacinar regions of the lung (alveolar ducts and adjacent alveoli) (Fig. 4C). Associated with this acute mixed inflammatory cell response, there were airway epithelial degeneration and necrosis that were most prominent in the affected terminal bronchioles and proximal alveolar ducts (Fig. 4C). These cobalt-induced morphologic changes were also present in the lungs of similarly treated HIF1αΔ/Δ mice. These HIF1α-deficient mice, however, had a greater number of eosinophils in the mixed inflammatory cell infiltrate and slightly more epithelial degeneration, necrosis, and exfoliation in terminal bronchioles in the affected regions of the lung (Fig. 4D).
After 2 days of cobalt treatment, the character and extent of the peribronchiolar inflammation and alveolitis in control mice were similar to that observed after 1 day of cobalt treatment with the exception that there were more infiltrating neutrophils (Fig. 4E). In comparison, cobalt-treated HIF1αΔ/Δ mice had less neutrophils and more eosinophils in the areas of bronchiolitis and alveolitis after 2 days of cobalt instillations (Fig. 4F). In addition, the degenerative/necrotic epithelium lining the affected terminal bronchioles that was observed after 1 day of cobalt instillation was replaced by conspicuous regenerative, basophilic, noncilitated cuboidal epithelium after 2 days of treatment. This specific epithelial change was most prominent in the HIF1αΔ/Δ mice (Fig. 4F). In both genotypes, there was a mild increase in the size and number of alveolar macrophages in the affected regions of the alveolar parenchyma.
After 5 days of cobalt instillations, hilar lung lesions in both the control and HIF1αΔ/Δ mice involved more of the proximal lung section (G5) and was characterized by a marked interstitial fibrosis, focal areas of type 2 cell hyperplasia, regenerative epithelial hyperplasia in terminal bronchioles, and a marked mixed inflammatory cell infiltrate in affected regions of the alveoli and bronchioles (chronic alveolitis and bronchiolitis, respectively) (Figs. 4G and 4H). As after 1 or 2 days of toxicant instillations, few cobalt-associated airway or alveolar lesions were found in the distal lung section (G11). The most dramatic difference in the pulmonary histopathology between control and HIF1αΔ/Δ mice after 5 days of cobalt treatment was the number of eosinophils, with knockout mice having conspicuously more eosinophils in the inflammatory cell infiltrate compared with that in controls (Fig. 4H). The amount of the other inflammatory cell types (e.g., lymphocytes, monocytes, neutrophils) in these areas of chronic alveolitis and bronchiolitis were similar in both genotypes. A mild to moderate accumulation of hypertrophic alveolar macrophages was present in the cobalt-induced lung lesions of control and HIF1αΔ/Δ mice.
To verify the cell infiltration, lung tissues from control and HIF1αΔ/Δ mice were analyzed via immunohistochemistry using an antibody specific to MBP, an eosinophilic-specific marker. No eosinophils were observed in saline-treated mice of either genotype (Figs. 5A and 5B). Control mice, following single or repeated doses of cobalt, showed little if any increase in MBP positive cells in the interstitium of proximal lung (Figs. 5C, 5E, and 5G). In contrast, HIF1α-deficient mice showed a pronounced and prolonged eosinophilia following cobalt exposure (Figs. 5D, 5F, and 5G). The level of neutrophilic infiltration was also assessed using immunohistochemistry. Again, there was little polymorphonuclear neutrophil (PMN)-positive staining observed in either control or HIF1αΔ/Δ saline-treated mice (data not shown). In control mice exposed to cobalt, there was substantial PMN-positive staining (Fig. 6A). HIF1αΔ/Δ mice also showed PMN-positive staining following cobalt; however, this staining was drastically decreased compared with control mice (Fig. 6B). These results are in agreement with the differential cell counts and suggest that a compromised HIF1α causes a shift in the inflammatory response of the lungs following cobalt exposure.
FIG. 5.
MBP immunohistochemistry in lungs from saline- and cobalt-treated control and HIF1αΔ/Δ mice. Light photomicrographs of lung sections immunohistochemically stained for eosinophil-specific MBP (inflammatory cells with cytoplasmic red staining). Sections were taken from the left lung lobe of control mice (A, C, E, G) and HIF1αΔ/Δ mice (B, D, F, H) that were dosed by oropharyngeal aspiration with cobalt in saline (C, D, E, F, G, H) or saline alone (saline-vehicle controls; A and B). Mice were instilled once a day for 1 (C, D), 2 (E, F), or 5 (A, B, G, H) days. All tissue sections were counter stained with hematoxylin. Tb, terminal bronchiole; ad, alveolar duct; and a, alveolus. Large peribronchiolar and alveolar infiltrates of immunohistochemically positive eosinophils are present in cobalt-instilled lungs after 1, 2, and 5 days of cobalt instillation (arrows). Only a few eosinophils (individual cells or small aggregates) are present in the mixed inflammatory cell infiltrates of control mice similarly instilled with cobalt (arrows).
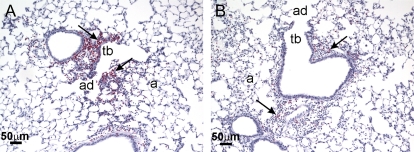
FIG. 6.
Neutrophil (PMN) immunohistochemistry in lungs from control and HIF1αΔ/Δ mice. Light photomicrographs of lung sections immunohistochemically stained for neutrophils (inflammatory cells with red chromagen). (A) Tissue section from the left lung lobe of a control mouse that received 2 days of cobalt aspiration. A mixed inflammatory cell infiltration containing numerous neutrophils (arrows) is present in the centriacinar region that includes the terminal bronchiole (tb), alveolar duct (ad), and surrounding alveoli (a). (B) Tissue section from the left lung lobe of a HIF1αΔ/Δ mouse that also received 2 days of cobalt aspiration. In contrast to the lung section in (A), the centriacinar inflammatory cell influx in (B) contains markedly less neutrophils.
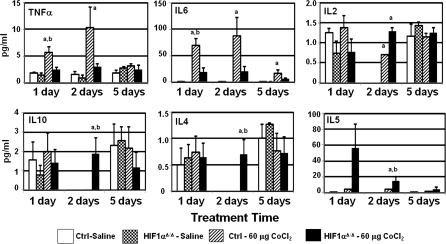
Cytokine Profiling
The pathology of the lung and the BALF cellularity patterns suggested a change in the inflammatory response upon loss of HIF1α from type II and Clara cells. To determine the mechanism underlying these patterns, 10 cytokines (namely, IL-2, KC, IL-4, IL-5, IL-13, TNF-α, IL-6, IL-10, INF-γ, Rantes) were profiled in the cell-free BALF. Out of these profiled cytokines, six showed significant difference when compared across genotype or within treatment groups (Fig. 7). IL-4, IL-5, and IL-10 were elevated only in HIF1αΔ/Δ mice following two doses of cobalt. IL-5, a key mediator in eosinophil activation, was also elevated in BALF collected from HIF1αΔ/Δ mice following a single dose of cobalt as compared with control mice; however, this increase did not reach significance (Fig. 7). In contrast, cobalt-treated control mice had significantly higher levels of TNF-α following one and two doses of metals and elevate IL-6 at all three time points (Fig. 7). Finally, IL2 was elevated in both genotypes following two doses of cobalt (Fig. 7). These results suggest that loss of HIF1α from ATII and Clara cells alters the cytokine response following cobalt exposure.
FIG. 7.
Cytokine levels in BALF from cobalt-treated control and HIF1αΔ/Δ mice. The levels of cytokines in BALF were assessed using BD CBA Mouse Soluble Protein Flex Sets and FACSCalibur flow cytometer following exposure of saline-treated (white bars) or cobalt (hatched bars)-treated control mice and saline-treated (checkered bars) or cobalt (black bars)-treated HIF1αΔ/Δ mice. n > 5 mice per group. Outliers were removed by Grubb's test, and ANOVA was performed with Boneferroni post-test. a = significance at p < 0.05 when cobalt treated group is compared with controls within genotype. b = significance at p < 0.05 when cobalt treated groups are compared across genotypes.
DISCUSSION
HMLD and cobalt asthma are occupational respiratory diseases affecting workers involved in manufacture and maintenance of hard metals (material consisting of tungsten carbide cemented in a matrix of cobalt), diamond polishing, and coal mining. The incidence of HMLD in diamond polishers who were exposed to cobalt-containing dust suggests cobalt as a sole etiological agent in HMLD (Lison et al., 1996). Despite the countless incidences of cobalt-induced toxicities, a complete mechanistic understanding is still lacking. Several in vitro studies have shown that cobalt acts as a stabilizer of HIFs (Karovic et al., 2007; Vengellur and LaPres, 2004). In addition, a defined role for HIFs in inflammation and tissue injury has been established. HIF1α target genes such as VEGF and matrix metalloproteinase (MMPs) have been shown to be unregulated in inflammatory conditions (Elson et al., 2000). Similarly, hypoxia-induced activation of genes involved in inflammatory processes suggests links between hypoxia and inflammation (Walmsley et al., 2005). Based on the links between cobalt and hypoxia as well as hypoxia and inflammation, cobalt-induced HIF1α stabilization might be an important player in inflammatory manifestations of metal-induced lung disease.
In recent years, clinical and experimental studies have generated evidence highlighting various pathological changes in the lungs of exposed humans as well as experimental animals. The pathology observed in cobalt-induced inhalation toxicity includes degeneration of the olfactory epithelium, hyperplasia, and squamous metaplasia in the epithelium of the respiratory turbinates and larynx, hemorrhage, and macrophage infiltration in the alveolar spaces, lung edema (Bucher, 1991), and fibrosing alveolitis (Lison, 1996). In clinical cases of cobalt-fume exposure, positive correlation between TNF-α and cobalt pneumoconiosis has been reported that suggests TNF-α’s potential role in the pathogenesis of interstitial lung disease (Rolfe et al., 1992). However, a similar elevation of TNF-α was seen neither in rat alveolar macrophage culture nor in in vivo intranasal exposure studies in rats (Huaux et al., 1995). Interestingly, levels of TNF-α are specifically higher in control mice after 1 and 2 days of cobalt chloride treatments that were not observed in their HIF1αΔ/Δ counterparts (Fig. 7). These results suggest that HIF1α is capable of regulating the cytokines correlated with manifestations of lung disease.
Early in the course of injury, differences in cellular infiltrates were already evident between the control and HIF1αΔ/Δ mice (Figs. 4, 5, and 6). Control mice displayed a significant increase in neutrophils that peaked 48 h after the initial exposure to cobalt whereas HIF1αΔ/Δ mice displayed eosinophilia that peaked at 5 days (Figs. 3, 5, and 6). Neutrophilic influx in to the lung and alveolar space is the characteristic feature of acute lung injury where they act as a key player in the pathogenesis of the injury. Although important for the immune response, neutrophil-predominant inflammatory responses are involved in diffused alveolar tissue damage through the release of proteases (MMPs, protease 3, neutrophil elastase, cathepsin G) and reactive oxygen metabolites (hydrogen peroxide, hypohalous acids, hydroxyl radicals) (Nathan, 2006). In contrast to control mice, HIF1αΔ/Δ mice displayed more pronounced eosinophil infiltration following cobalt challenge (Figs. 5D, 5F, and 5H). However, the BALF eosinophilia in cobalt-treated HIF1α-deficient mice reached significance only after five exposures (Fig. 3D). Eosinophils are differentiated from myeloid precursor cells under the influence of interleukin IL-3, granulocyte-macrophage colony–stimulating factor, and IL-5. These cells are selectively recruited into the airways through Th2 cytokines. The fact that this eosinophilic infiltration was specific to HIF1α-deficient mice suggests an epithelial origin of the inflammatory switch. To date, the role of HIFs in eosinophilic inflammation is unclear. Moreover, the role of epithelial-derived mediators of inflammatory responses has not been examined directly. It is interesting, however, to speculate that loss of epithelial HIF1α during development plays a role in the hygiene hypothesis (Strachan, 1989). The hygiene hypothesis posits that lack of developmental exposure to various allergens makes a child more susceptible to allergic diseases, such as asthma. If this hypothesis is taken one step further, one outcome of these allergen exposures is inflammation and subsequent localized hypoxia. In the absence of these incidences of inflammation-induced hypoxia, the HIF1α-mediated signaling does not occur early in development (i.e., similar to developmental deletion of HIF1α in the lungs of the mice in these experiments). This lack of HIF1α-mediated signaling and its ability to modulate Th1-type cytokines (Fig. 7) might explain why the immunity of affected individuals remains biased toward a Th2 inflammatory response.
The results also suggest that HIF1α is a susceptibility gene for allergic diseases, such as asthma. Genome-wide and gene screens have identified several loci that play a role in allergic disease, including toll-like receptor 2, CHI3L1, and TGFβ1 (Ahmad-Nejad et al., 2004; Li et al., 2007; Rathcke et al., 2009). To date, none have specifically identified HIF1α; however, several have linked chromosome 14q24 (HIF1α’s chromosomal localization is 14q21-q24) to susceptibility to asthma (Anonymous, 1997; Hakonarson et al., 2002; Mansur et al., 1999). In addition, single nucleotide polymorphisms within the HIF1α gene have been linked to other diseases that have strong innate immunity components, including diabetes and systemic sclerosis (Nagy et al., 2009; Wipff et al., 2009). Overall, these results suggest that genetic modulation of HIF1α activity is linked to disease states whose etiology can be linked to dysregulated innate immunity and/or inflammation.
Taken together, the findings from the current study and recent reports suggest a critical role of epithelial-derived HIF1α signaling in establishing the lung's innate immunity, resulting in the observed inflammatory switch. The data support a model in which epithelial-derived HIF1α activity regulates the lung's reaction to metal challenge by controlling the expression of cytokines necessary to recruit the proper inflammatory response. Loss of this regulatory mechanism leads to the tissue being biased toward a Th2-mediated inflammation. The mechanism that leads to this alteration in the inflammatory response remains unknown and is the focus of new investigations.
FUNDING
National Institutes of Health (R01-ES12186 and P42 ES04911-17).
Acknowledgments
The authors appreciate the gift of the conditional HIF1α mice from Dr Randall Johnson (University of California-San Diego) and the SP-C-rtTA/(tetO)7-CMV-Cre transgenic from Dr Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center).
References
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, Heeg K, Neumaier M, Renz H. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J. Allergy. Clin. Immunol. 2004;113:565–567. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucher JR. NTP technical report on the toxicity studies of cobalt sulfate heptahydrate in F344/N rats and B6C3F1 mice (Inhalation Studies) (CAS No. 10026-24-1) Toxic. Rep. Ser. 1991;5:1–38. [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Phys. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonarson H, Bjornsdottir US, Halapi E, Palsson S, Adalsteinsdottir E, Gislason D, Finnbogason G, Gislason T, Kristjansson K, Arnason T. A major susceptibility gene for asthma maps to chromosome 14q24. Am. J. Hum. Genet. 2002;71:483–491. doi: 10.1086/342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F, Lasfargues G, Lauwerys R, Lison D. Lung toxicity of hard metal particles and production of interleukin-1, tumor necrosis factor-alpha, fibronectin, and cystatin-c by lung phagocytes. Toxicol. Appl. Pharmacol. 1995;132:53–62. doi: 10.1006/taap.1995.1086. [DOI] [PubMed] [Google Scholar]
- Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc. Am. Thorac. Soc. 2005;2:202–205. doi: 10.1513/pats.200501-006AC. [DOI] [PubMed] [Google Scholar]
- Jain S, Bradfield CA. Developmental profiles of basic helix-loop-helix PAS proteins in mice. Mech. Dev. 1998;73:117–123. [Google Scholar]
- Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem. J. 2003;370:1011–1017. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karovic O, Tonazzini I, Rebola N, Edström E, Lövdahl C, Fredholm BB, Daré E. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1alpha. Biochem. Pharmacol. 2007;73:694–708. doi: 10.1016/j.bcp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Li H, Romieu I, Wu H, Sienra-Monge JJ, Ramírez-Aguilar M, del Río-Navarro BE, del Lara-Sánchez IC, Kistner EO, Gjessing HK, London SJ. Genetic polymorphisms in transforming growth factor beta-1 (TGFB1) and childhood asthma and atopy. Hum. Genet. 2007;121:529–538. doi: 10.1007/s00439-007-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lison D. Human toxicity of cobalt-containing dust and experimental studies on the mechanism of interstitial lung disease (hard metal disease) Crit. Rev. Toxicol. 1996;26:585–616. doi: 10.3109/10408449609037478. [DOI] [PubMed] [Google Scholar]
- Lison D, Lauwerys R, Demedts M, Nemery B. Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur. Respir. J. 1996;9:1024–1028. doi: 10.1183/09031936.96.09051024. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lombaert N, Lison D, Van Hummelen P, Kirsch-Volders M. In vitro expression of hard metal dust (WC-Co)—responsive genes in human peripheral blood mononucleated cells. Toxicol. Appl. Pharmacol. 2008;227:299–312. doi: 10.1016/j.taap.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Mansur AH, Bishop DT, Markham AF, Morton NE, Holgate ST, Morrison JF. Suggestive evidence for genetic linkage between IgE phenotypes and chromosome 14q markers. Am. J. Respir. Crit. Care Med. 1999;159:1796–1802. doi: 10.1164/ajrccm.159.6.9804036. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J. Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Kovacs-Nagy R, Kereszturi E, Somogyi A, Szekely A, Nemeth N, Hosszufalusi N, Panczel P, Ronai Z, Sasvari-Szekely M. Association of hypoxia inducible factor-1 alpha gene polymorphism with both type 1 and type 2 diabetes in a Caucasian (Hungarian) sample. BMC Med. Genet. 2009;10:79. doi: 10.1186/1471-2350-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl Acad. Sci. U.S.A. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathcke CN, Holmkvist J, Husmoen LL, Hansen T, Pedersen O, Vestergaard H, Linneberg A. Association of polymorphisms of the CHI3L1 gene with asthma and atopy: a populations-based study of 6514 Danish adults. PLoS ONE. 2009;4:e6106. doi: 10.1371/journal.pone.0006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe MW, Paine R, Davenport RB, Strieter RM. Hard metal pneumoconiosis and the association of tumor necrosis factor-alpha. Am. Rev. Respir. Dis. 1992;146:1600–1602. doi: 10.1164/ajrccm/146.6.1600. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Saini Y, Harkema JR, LaPres JJ. HIF1alpha is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J. Biol. Chem. 2008;283:33650–33657. doi: 10.1074/jbc.M805927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini Y, Kim KY, Lewandowski R, Bramble L, Harkema JR, LaPres JJ. The role of hypoxia inducible factor 1 (HIF1) in modulating cobalt-induced lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;298:L139–L147. doi: 10.1152/ajplung.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. Br. Med. J. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Collaborative Study on the Genetics of Asthma (CSGA) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat. Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1 alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol. Sci. 2004;82:638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- Vengellur A, Phillips JM, Hogenesch JB, LaPres JJ. Gene expression profiling of hypoxia signaling in human hepatocellular carcinoma cells. Physiol. Genomics. 2005;22:308–318. doi: 10.1152/physiolgenomics.00045.2004. [DOI] [PubMed] [Google Scholar]
- Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff J, Dieude P, Avouac J, Tiev K, Hachulla E, Granel B, Diot E, Sibilia J, Mouthon L, Meyer O, et al. Association of hypoxia-inducible factor 1A (HIF1A) gene polymorphisms with systemic sclerosis in a French European Caucasian population. Scand. J. Rheumatol. 2009;38:291–294. doi: 10.1080/03009740802629432. [DOI] [PubMed] [Google Scholar]
- Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat. Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]