Abstract
Background. Haemodialysis (HD) catheter-related blood stream infections are a major cause of morbidity and mortality in patients with acute and chronic renal failure.
Methods. We conducted a randomized, prospective, double-blinded trial investigating the clinical value of bismuth-coated non-tunneled HD catheters in patients in need of temporary short-term vascular access. A standard catheter (SC) was compared to a surface-modified, bismuth-film-coated catheter (FCC). After removal of the catheter for any reason, both arterial and venous lumina were rinsed and the fluid cultured for detection of bacterial colony-forming units (CFU). The catheter tip was placed in a tube containing sterile saline, sonicated and shortly centrifuged to remove debris (3 min at 1000 g). The supernatant was cultured and assayed for DNA content.
Results. Seventy-seven patients in three HD units were randomized. Thirteen patients suffered from acute renal failure, 60 patients from chronic renal failure, and four patients without renal insufficiency were treated with plasma exchange. The time to catheter removal was not significantly different between groups, with a mean of 18.5 ± 2 days for SC and 15.1 ± 2 days for FCC. In most cases, the reasons for catheter removal were related to no further need for extracorporeal therapy or establishment of a permanent vascular access. Six catheters for SC and four catheters for FCC were removed because of presumed infection. Bacterial colonization was significantly lower for coated catheters compared to standard catheters, both for cultured catheter tips as well as for CFU in rinse fluids (P < 0.05).
Conclusions. Surface modification with bismuth film reduces bacterial colonization of temporary non-tunneled HD catheters in a clinical trial. Larger trials with these modified catheters are justified to further investigate the effect on catheter-related infections, complications and costs.
Keywords: bismuth, catheters, haemodialysis, infection, sepsis
Introduction
The use of central vein catheters (CVC) for haemodialysis (HD) access is related to significant morbidity and mortality. In the USA, 70% of patients start HD with a CVC as the vascular access, and 30% of the prevalent patients are being dialyzed using a permanent catheter [1]. The use of CVC is associated with an increased mortality. Foley and colleagues reported that annual mortality was 26% for patients, and adjusted mortality hazard ratio was 2.18 with a CVC compared to patients with an arteriovenous (AV) fistula [2]. Results of the HEMO study indicate that patients with CVC at the start and end of the observation period have an increased relative mortality risk of 3.4 compared to patients with AV fistula at both time points. Patients who switched from CVC to an AV fistula had a mortality risk of only 1.4 [3]. Thus, the increased mortality risk seems to be directly associated with the use of CVC. One possible and likely cause of the increased mortality is infection and sepsis related to the CVC. Indeed, exit site infection is significantly related to death by sepsis [4]. Thus, preventing CVC infection is of utmost importance.
Bismuth is a substance whose antimicrobial effects have been known for many years. Bismuth inhibits the growth of both Gram-negative and Gram-positive bacteria [5–7]. The mechanism of antimicrobial action is not completely known but involves inhibition of the Escherichia coli rho transcription termination factor [8] and iron transport [9]. More importantly, bismuth inhibits biofilm production by Staphylococci and Pseudomonas species [5,6]. We therefore evaluated the impact of bismuth-coated CVC on clinical outcome in patients in need of vascular access for extracorporeal therapy in a randomized controlled trial.
Materials and methods
The study was conducted in the nephrology departments of three different clinics (Berlin, Munich and Bottrop). All patients in need of vascular access for acute extracorporeal therapy of presumably more than 1 week duration were eligible for the study. Exclusion criteria were age <18 or >75 years, known hepatitis B or C, known HIV infection, a positive blood culture within 10 days before randomization, pregnancy, hospitalization of more than 14 days, respirator therapy, treatment with antibiotics or participation in another clinical study within 30 days before randomization.
Patients were informed by the study coordinators and gave written informed consent. The local ethics committee of all three clinics approved the study protocol. The study has been registered under ISRCTN 81821807.
End points were catheter survival, i.e. days to removal for any cause as well as bacterial contamination, DNA content of the catheter segments and laboratory results such as whole blood count, fibrinogen, antithrombin III and C-reactive protein.
After obtaining written informed consent, patients were randomized to either standard catheters (SC, GamCath®, Gambro) or to bismuth-film-coated catheters (FCC, GamCath Dolphin® Protect, Gambro). In order to obtain the Conformité Européene (CE)-sign, the catheters had been extensively tested with respect to bismuth release and toxicity before market authorization. The catheters meet the requirements according to current ISO 10993 (no risk to patient of toxicity from the exposure to Dolphin Protect® catheters). Randomization was performed by a list provided before the start of the study. The patients and clinicians who inserted the catheters were blinded to the study group assignment. Catheters were used according to clinical needs and placed considering universal hygienic precautions according to the European Best Practice Guidelines [10]. Between treatments, 30% citrate solution was used as a locking solution. Before each treatment, blood was obtained for whole blood count, fibrinogen, antithrombin III and C-reactive protein. These parameters were measured in the central laboratory of each clinic using routine methods.
Catheters were removed due to the discretion of the treating physician either when there was no longer need for extracorporeal therapy, when an alternative vascular access became available or when infection was suspected on clinical grounds.
After aseptic removal of the catheters, both lumina were flushed with 10 ml of sterile saline, and the effluent fluid was collected and stored at −20°C. Then, the catheters were cut in three pieces under aseptic conditions and stored at −20°C.
Microbiology
Rinse fluids (10 ml) were filtered through membrane filters (Millipore, 0.45 µm, F4PN04370), and filters were subsequently cultured on sheep blood agar for 48 h at 36°C. The catheter tips (app. 2 cm) were placed in 10 ml of sterile saline and subjected to ultrasound sonication for 2 min at 35 kHz (Dentil, SonoRex RK100 H). The fluid supernatant was then filtered as described above, and filters were cultured on sheep blood agar for 48 h at 36°C. Colony-forming units (CFU) were counted and characterized with standard microbiological methods.
Detection of oligonucleotides
For detection of DNA sticking to the catheter surface, the tip of the catheters as described above as well as the next 2-cm piece (called shaft piece) were cut and placed in Tris–EDTA buffer (10 mM Tris, 1 mM EDTA pH 7.5). The catheter pieces were subjected to sonication for 30 min and the buffer then assayed for oligonucleotides (ODN). ODN were detected using the Quant-it OliGreen kit (Molecular Probes, Eugene, OR, USA) that labels ODN with a fluorescent dye and subsequently detects the bound amount of dye using a FLUOtest Optima fluorescent plate reader. The test was performed as described by the manufacturer; the sensitivity of the assay was 1.2 ng/ml.
Statistics
Catheter survival was analyzed by Kaplan–Meier analysis (log rank Mantel–Cox test). Microbiological results for the catheter tip segments were compared using the chi-square test. Microbiological results in rinse fluids from both (arterial and venous) lumina were combined and also compared using the chi-square test. The DNA content was compared using the Mann–Whitney test for unpaired, nonparametric samples. Data are given as means ± SEM or median and range as indicated.
Results
A total of 103 patients were randomized (53 for SC and 50 for FCC). Results could be analyzed for 77 patients; 26 patients had to be excluded because of screening failure (n = 1), loss to follow-up (n = 1) and failure of collecting catheter and rinse fluid samples (n = 24). Samples were not collected mainly when catheters were removed during the night shift or when samples thawed before/during transport.
Table 1 gives the demographic details of the patient population. Two in each group received catheters for plasma exchange, the remaining patients for renal replacement therapy. Of these, the minority had acute renal failure, while the majority had decompensated chronic renal failure or were chronic HD patients with temporary failure of their usual vascular access.
Table 1.
Patient characteristics
| Standard catheter (n = 39) | Bismuth-film-coated catheter (n = 38) | |
|---|---|---|
| Age | 57 ± 2 | 60.7 ± 2 |
| Gender | 63% male | 61% male |
| Diabetes | 37% | 32% |
| Plasma exchange | 2 | 2 |
| Renal replacement therapy | 37 | 36 |
| Acute renal failure | 5 | 8 |
| Chronic renal failure | 32 | 28 |
| Days in place (median) | 18.5 ± 2.2 (15) | 15.1 ± 1.7 (12) |
| Number of sessions (median) | 7.7 ± 1 (7) | 6.8 ± 1 (6) |
| Reason for catheter removal | ||
| Suspected infection | 6 | 4 |
| Malfunction | 3 | 2 |
| No further need | 12 | 13 |
| Functioning fistula/graft | 14 | 16 |
| Death | 1 | 0 |
| Others | 3 | 3 |
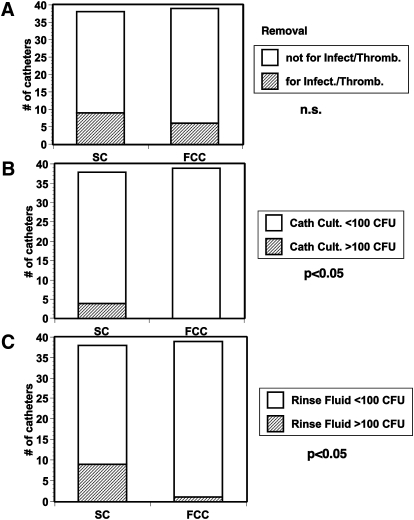
Catheter survival was not significantly different between groups, mean 18.2 ± 2 days for SC and 15.1 ± 2 days for FCC (n.s., Table 1). The number of extracorporeal treatments was also not significantly different between groups (Table 1). In most cases, the reason for catheter removal was not related to infection (six patients with SC and four patients with FCC) but rather to no further need for extracorporeal therapy (11 and 14, respectively), malfunction (three vs. two) or establishment of an AV fistula or a prosthetic graft for vascular access (14 and 16 patients, respectively, Table 1 and Figure 1). The Kaplan–Meier analysis for catheter survival was not significant different between groups.
Fig. 1.
(A) Number of catheters removed for suspected infection/thrombosis. (B) Number of direct catheters with bacterial cultures >100 CFU. (C) Number of catheters with rinse fluids containing >100 CFU/ml. P < 0.05 denotes significance between groups; n.s., not significant.
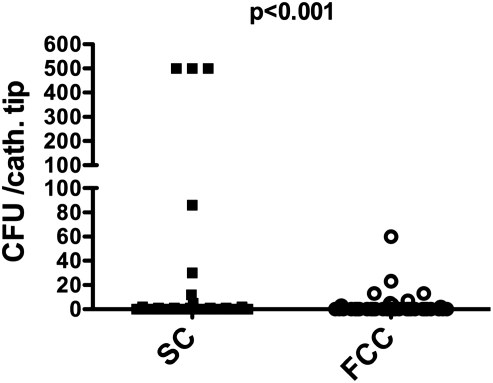
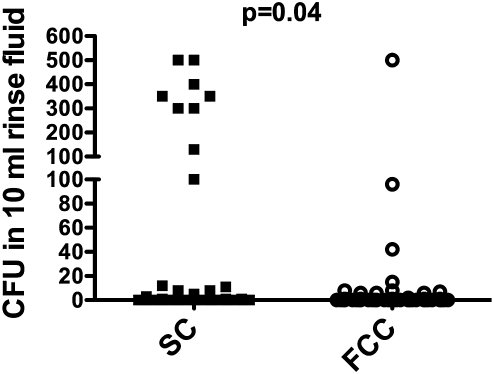
Bacterial colonization of the catheter tip was higher in SC compared to FCC [mean ± SEM; median (range) 63 ± 29 CFU; 0 (0–600) for SC vs. 3.5 ± 1.6 CFU; 0 (0–60) for FCC, P < 0.001, Figures 1 and 2]. The number of CFU in 10 ml rinse fluid was also significantly higher in SC compared to FCC [39 ± 13; 0 (0–500) for SC vs. 9.2 ± 6; 0 (0–500) for FCC, P = 0.04, Figures 1 and 3]. Characterization of the bacterial strains revealed mostly coagulase-negative Staphylococci (total number for all positive samples n = 76), the incidence of other strains was much lower (two Streptococci; one Acinetobacter; two Micrococci; one E. coli, two Corynebacter).
Fig. 2.
CFU of the cultured segments of FCC and SC catheters.
Fig. 3.
Number of CFU in 10 ml rinse fluids for FCC and SC catheters.
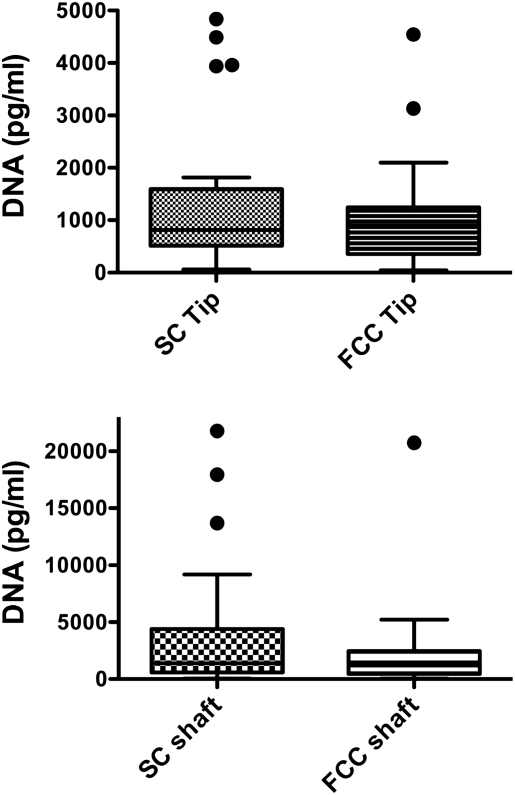
The DNA content of the catheter tip and adjacent shaft segments was not significantly different between groups (Figure 4). The median DNA content for catheter tips was 0.81 ng/ml for SC and 0.89 ng/ml for FCC; for catheter shaft the median was 1.39 ng/ml for SC and 1.2 ng/ml for FCC (Figure 4, n.s).
Fig. 4.
Boxplots of the DNA content of tip or shaft segments of FCC and SC catheters
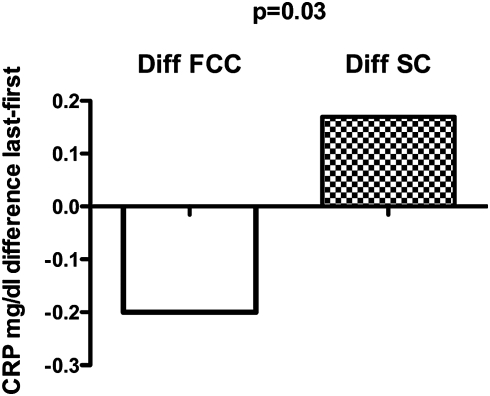
There were no overall differences between groups regarding leukocyte count, fibrinogen and antithrombin III. However, when the course of C-reactive protein (CRP) was analyzed during the study, there was a significant trend for CRP to decrease in patients with FCC catheters and a trend to increase in patients with SC. The difference in CRP between the last and the first extracorporeal treatment was significantly lower for FCC compared to SC (median −0.2 mg/dl for FCC and +0.17 mg/dl for SC, P = 0.03, Figure 5).
Fig. 5.
Median difference in CRP between the first and the last treatment with the assigned catheter. The range of the differences was for FCC, −20 to +2.7; and for SC, −4 to +11 mg/dl.
Discussion
In the present study, catheter survival was not different between coated and standard catheters. On the other hand, the secondary end point bacterial colonization was significantly lower for coated catheters compared to standard catheters indicating that bismuth coating of catheters was effective. The reason why less bacterial colonization did not translate into the clinical end point of catheter survival was the non-anticipated relatively short duration of catheter requirement in the present study. The mean duration of catheters in place was 18 and 15 days, respectively. Most patients received a catheter for acute malfunction of their usual vascular access or for acute renal failure; removal of catheters was mostly related to no further need instead of infections. Thus, the advantages of bismuth-film-coated catheters regarding colonization likely did not have enough time to turn into improved catheter survival. Indeed, when comparing tunneled and non-tunneled catheters, a difference in catheter survival was evident only at 2 weeks and later after catheter insertion [11]. In another study comparing different lock solutions, the difference in catheter survival was only evident after 50 days and later [12]. The present study demonstrates advantages of bismuth-coated catheters regarding bacterial colonization; further trials are warranted in patients with longer requirement of catheters to establish the benefits of coated catheters on catheter survival, morbidity and costs. In addition, bismuth has potential toxic effects such as cytotoxicity or neurotoxicity [13] that may only be observed after treating a larger population. However, the total amounts of bismuth on the catheter surface is very low; we did not observe local reactions, and bismuth inhibits bacterial growth substantially at concentrations well below the toxic threshold in tissue culture [7].
Central venous catheters are widely used as vascular access. Data from the DOPPS study indicate that in Germany, 25% of incident HD patients start dialysis with catheters, while in other countries the use of catheters is even more common (France 40%, USA 70%). During recent years, the use of catheters in prevalent HD patients has even increased further [1]. Catheter-related blood stream infections are a significant cause for mortality in patients in intensive care units with up to 35% mortality [14]. Besides increasing mortality, catheter-related infections are associated with additional costs. It was calculated that one episode of catheter-related blood stream infections increases hospital expenditure between $11 000 and $54 000 [15,16]. Thus, preventing catheter-related infections is of enormous clinical importance.
There are several options to prevent catheter infections. The use of antibiotics or bactericidal substances in lock solutions is more effective than heparin alone to prevent infections [12,17]. The implementation of simple but strict standards for catheter placement and care significantly decreases catheter-related infections [15]. The present study indicates that the use of bismuth-coated catheters decreases bacterial colonization of HD catheters and may prevent potentially lethal catheter-related blood stream infections.
In the present study, many subjects (26 of 103) had to be excluded from analysis. Reasons for exclusion were mainly due to failure of proper catheter preservation/collection after removal. This large number was not anticipated and may have been caused by the complex procedure of catheter collection. However, exclusion occurred equally in all three participating centers and was equally distributed in both groups. We therefore do not consider exclusion of subjects as a major bias influencing our results.
A proposed strategy to reduce catheter-related blood stream infections catheter-related blood stream infections is the use of catheters impregnated or coated with antimicrobial substances such as chlorhexidine, bismuth, platinum and silver sulfadiazine or the antibiotics cefazolin or minocycline and rifampicin (for review, see [18]). A number of studies during the last two decades are available on the effectiveness of antimicrobial catheters in preventing catheter-related blood stream infections and show that externally coated chlorhexidine–silver catheters significantly reduce the risk of catheter-related blood stream infections in the intensive care unit [19]. In a study on silver-coated, tunneled HD catheters, Trerotola et al. reported slightly but not significantly lower infection and colonization rates compared to uncoated catheters [20]. Minocycline–rifampicin-coated catheters appear to be even more effective in preventing catheter-related blood stream infections. Despite these studies, coated catheters are not commonly used. There are reservations about the quality of the trial evidence, e.g. most studies were not blinded and were driven by manufacturers [21]. In addition, all studies used either catheter colonization or catheter-related blood stream infections as end points; none of these studies investigated hard clinical end points such as mortality, length of hospital stay etc. Other factors related to cost, toxicity and increased microbial resistance prevented the recommendation for these catheters. Also, the use of these devices may divert resources needed to implement other evidence-based preventive measures that are more likely to be effective than coated catheters [15].
Our study demonstrated significant differences in catheter colonization between a noncoated and a bismuth-coated catheter in a clinical setting. We did not choose mortality as an end point because a much larger number of subjects would have been needed to demonstrate a significant difference in mortality. However, the present study was randomized, prospective and fully blinded to both patients and participant physicians. Thus, the design was superior to most previous studies on coated catheters. It is also one of the first studies in patients in need of extracorporeal therapy exclusively and the first one using bismuth-coated catheters. We could demonstrate that bismuth coating of central vein catheters for HD reduces bacterial colonization without showing signs of adverse events. Bismuth-coated catheters may not eliminate the problem of catheter-related blood stream infections but at least improve its rate. Given the great importance of CVC infections for both prevalent and incident HD patients, further and larger trials may prove that Bismuth-coated catheters may contribute to lower morbidity and possibly mortality of HD patients.
Conflict of interest statement. U.H., R.D. and W.B. are full-time employees of Gambro. R.S. received research grants and lecture honorarium from Gambro.
References
- 1.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2008;23:3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first”. Hemodial Int. 2009;13:533–542. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 3.Allon M, Daugirdas J, Depner TA, et al. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47:469–477. doi: 10.1053/j.ajkd.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Mokrzycki MH, Zhang M, Cohen H, et al. Tunnelled haemodialysis catheter bacteraemia: risk factors for bacteraemia recurrence, infectious complications and mortality. Nephrol Dial Transplant. 2006;21:1024–1031. doi: 10.1093/ndt/gfi104. [DOI] [PubMed] [Google Scholar]
- 5.Domenico P, Baldassarri L, Schoch PE, et al. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrob Agents Chemother. 2001;45:1417–1421. doi: 10.1128/AAC.45.5.1417-1421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CT, Stewart PS. Reduction of polysaccharide production in Pseudomonas aeruginosa biofilms by bismuth dimercaprol (BisBAL) treatment. J Antimicrob Chemother. 1999;44:601–605. doi: 10.1093/jac/44.5.601. [DOI] [PubMed] [Google Scholar]
- 7.Wu CL, Domenico P, Hassett DJ, et al. Subinhibitory bismuth-thiols reduce virulence of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2002;26:731–738. doi: 10.1165/ajrcmb.26.6.2001-00020oc. [DOI] [PubMed] [Google Scholar]
- 8.Brogan AP, Verghese J, Widger WR, et al. Bismuth-dithiol inhibition of the Escherichia coli rho transcription termination factor. J Inorg Biochem. 2005;99:841–851. doi: 10.1016/j.jinorgbio.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Domenico P, Reich J, Madonia W, et al. Resistance to bismuth among gram-negative bacteria is dependent upon iron and its uptake. J Antimicrob Chemother. 1996;38:1031–1040. doi: 10.1093/jac/38.6.1031. [DOI] [PubMed] [Google Scholar]
- 10.Tordoir J, Canaud B, Haage P, et al. EBPG on vascular access. Nephrol Dial Transplant. 2007;22:ii88–ii117. doi: 10.1093/ndt/gfm021. [DOI] [PubMed] [Google Scholar]
- 11.Weijmer MC, Vervloet MG, ter Wee PM. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant. 2004;19:670–677. doi: 10.1093/ndt/gfg581. [DOI] [PubMed] [Google Scholar]
- 12.Weijmer MC, van den Dorpel MA, Van de Ven PJ, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol. 2005;16:2769–2777. doi: 10.1681/ASN.2004100870. [DOI] [PubMed] [Google Scholar]
- 13.Noach LA, Eekhof JL, Bour LJ, et al. Bismuth salts and neurotoxicity. A randomised, single-blind and controlled study. Hum Exp Toxicol. 1995;14:349–355. doi: 10.1177/096032719501400405. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel RP, Edmond MB. Team-based prevention of catheter-related infections. N Engl J Med. 2006;355:2781–2783. doi: 10.1056/NEJMe068230. [DOI] [PubMed] [Google Scholar]
- 15.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 16.Warren DK, Quadir WW, Hollenbeak CS, et al. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006;34:2084–2089. doi: 10.1097/01.CCM.0000227648.15804.2D. [DOI] [PubMed] [Google Scholar]
- 17.Allon M. Prophylactic effect of antibiotic lock solution on bacteremia related to use of uncuffed hemodialysis catheters. Nat Clin Pract Nephrol. 2006;2:418–419. doi: 10.1038/ncpneph0224. [DOI] [PubMed] [Google Scholar]
- 18.Ramritu P, Halton K, Collignon P, et al. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control. 2008;36:104–117. doi: 10.1016/j.ajic.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Maki DG, Stolz SM, Wheeler S, et al. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Trerotola SO, Johnson MS, Shah H, et al. Tunneled hemodialysis catheters: use of a silver-coated catheter for prevention of infection—a randomized study. Radiology. 1998;207:491–496. doi: 10.1148/radiology.207.2.9577500. [DOI] [PubMed] [Google Scholar]
- 21.Anaissie E. Antimicrobial coating of central venous catheters: show me the data. Crit Care Med. 2007;35:1197–1199. doi: 10.1097/01.CCM.0000259546.48827.A2. [DOI] [PubMed] [Google Scholar]