Abstract
Background. Patients with chronic kidney disease (CKD) often present with iron depletion and iron deficiency anaemia (IDA) because of frequent blood (and iron) loss. Therapy consists of repletion of iron stores and intravenous (i.v.) iron has become the standard care in this setting. However, older i.v. iron preparations have their limitations. This study primarily investigated the safety, and also the efficacy, of ferric carboxymaltose (FCM), a next-generation i.v. iron formulation, given as a bolus–push injection in patients with CKD undergoing maintenance haemodialysis (HD).
Methods. Patients (aged 18–65 years) with IDA undergoing HD received 100–200 mg of iron as FCM via an i.v. bolus–push injection into the HD venous line, two to three times weekly for ≤6 weeks. Safety assessments included incidence of adverse events (AEs). Treatment responders were patients attaining ≥1.0 g/dl increase in haemoglobin (Hb) from baseline at any time during the study. Enrolled patients (safety population) receiving ≥1 dose of study medication were included in the efficacy analyses [intent-to-treat (ITT) population].
Results. Of 163 patients enrolled, 150 (92%) completed the study. The mean ± SD total cumulative dose of iron as FCM administered was 2133.3 ± 57.7 mg. In total, 193 AEs were reported in 89 out of 163 (54.6%) patients. Almost three-quarters of patients (73.6%) received erythropoiesis-stimulating agents (ESAs), but the dose remained stable during the study. Serious AEs occurred in 12 out of 163 (7.4%) patients and two patients died; none of these was considered by the investigator to be related to the study medication. Only five out of 163 (3.1%) patients discontinued study medication due to an AE. Overall, 100 out of 162 (61.7%; ITT population) patients were treatment responders, and mean Hb levels increased from 9.1 ± 1.30 g/dl at baseline to 10.3 ± 1.63 g/dl at follow-up.
Conclusions. FCM is well-tolerated and effective in the correction of Hb levels and iron stores in patients with IDA undergoing HD. As changes in anaemia treatment other than i.v. FCM (e.g. increased ESA doses) were not permitted during the study, the clinically relevant increase in Hb in the majority of patients can be solely attributed to efficient iron utilization. The incidence of AEs was as expected for this population.
Keywords: clinical trial, efficacy, ferric carboxymaltose, haemodialysis, iron deficiency anaemia, safety
Introduction
Annual blood losses of around 2.5 l place patients with chronic kidney disease (CKD) undergoing haemodialysis (HD) at particularly high risk of iron store depletion with subsequent iron deficiency anaemia (IDA) [1,2]. Iron deficiency can be defined as absolute or functional [1,3]. Absolute iron deficiency develops as the body's iron stores become depleted to such a low level that not enough iron is available for the production of haemoglobin (Hb) [4,5]. This is usually indicated by a decline in serum ferritin levels to ∼<15 µg/l in patients with normal kidney function, but is much higher in patients undergoing HD as a result of chronic inflammation, and is associated with elevated levels of C-reactive protein (CRP) [4,5]. This functional iron deficiency describes the state when iron cannot be mobilized from stores (despite an adequate dietary supply) to meet the demand for erythropoiesis [4]. Serum ferritin levels can appear normal (200–500 µg/l) or increased in chronic inflammatory disorders [4], while levels of transferrin saturation (TSAT), which is serum iron divided by total iron-binding capacity, will be low (typically <20%), indicating limited transport of iron to the erythron for erythropoiesis [4].
The primary therapeutic aim in anaemic patients undergoing HD is to restore Hb levels and iron stores to internationally recommended target ranges. To attain the Hb target range of 11.0–12.0 g/dl (not exceeding 13.0 g/dl), patients are given supplemental intravenous (i.v.) iron as an adjunct to erythropoiesis-stimulating agents (ESAs) [1,6]. For patients undergoing HD, i.v. iron substitution is the recommended route of administration [1] and has become the standard treatment for optimizing a patient's iron status [3,7]. Nevertheless, older i.v. iron formulations have their limitations, including the potential for immunogenic reactions induced by dextran molecules (iron dextran) [8], dose limitations, a slow rate of administration (to prevent acute, labile iron-induced toxicity and vasoactive reactions) [4,8] and the compulsory requirement for a test dose (iron dextrans in USA [9, 10] and Europe [11]). All-event reporting rates were 29.2, 10.5 and 4.2 reports per million 100 mg iron dose equivalents, while all-fatal-event reporting rates were 1.4, 0.6 and 0.0 reports per million 100 mg dose equivalents for iron dextran, sodium ferric gluconate and iron sucrose, respectively [12].
Ferric carboxymaltose [FCM; Ferinject®; Vifor (International) Inc., St Gallen, Switzerland] is a next-generation parenteral, dextran-free iron formulation designed to overcome the limitations of existing i.v. iron preparations. The FCM complex is composed of a polynuclear iron(III) hydroxide complexed to carboxymaltose [13]. As FCM is a strong and robust iron complex, it can be administered in high doses, does not release large amounts of reactive (‘free’) iron into the circulation and does not trigger dextran-associated immunogenic reactions [13].
In a pharmacokinetics study of six patients by Beshara et al., no adverse events (AEs) were experienced by any of the patients following the administration of 100 mg of iron as FCM [14]. A Phase I pharmacokinetics and dose-escalation study of 32 patients with mild IDA demonstrated that up to 1000 mg iron administered as an i.v. infusion of FCM over 15 min is well-tolerated [15].
Here, we report the results of a multi-centre, open-label, single-arm, Phase II study designed to assess the safety and efficacy of multiple doses of i.v. iron as FCM, given as single, bolus–push injections in the correction of IDA in patients undergoing HD.
Materials and methods
Patients
Patients (aged 18 to 65 years) with IDA (Hb ≤11.0 g/dl and either serum ferritin ≤200 µg/l or TSAT <20%), undergoing maintenance HD two to three times per week, were recruited from 24 study centres across three countries (11 centres in South Africa, eight in Romania and five in Lithuania). Patients' iron status (serum ferritin, TSAT, serum iron and serum transferrin) was assessed up to 7 days before study commencement to justify inclusion. Patients had to be clinically stable without a hospital admission due to renal decompensation during the 4 weeks prior to study inclusion, and a permanent vascular access appropriate for HD was required. Concomitant administration of ESAs was permitted during the study, provided that the patients had started treatment at least 1 month prior to study inclusion and remained on stable doses throughout the trial. Women of childbearing age had to use reliable forms of contraception throughout the study and for up to 1 month after the final dose of study medication. All patients provided written, informed consent before participating in the study.
Exclusion criteria were: a Hb level <6.5 g/dl, serum ferritin level >500 µg/l, TSAT >50% or serum albumin <2.5 g/dl; known hypersensitivity to iron polysaccharide complexes or to FCM; vitamin B12 or folic acid deficiency; any other type of anaemia; evidence of iron overload conditions (e.g. haemochromatosis); significant cardiovascular disease (including myocardial infarction during the 6 months prior to study inclusion, congestive heart failure New York Heart Association III or IV and poorly controlled hypertension); uncontrolled endocrinological or metabolic disorders; active infection, malignancy, active liver disease, active peptic ulcer, asthma or rheumatoid arthritis; pregnancy or lactation; a history of alcohol or drug abuse; a positive hepatitis B surface antigen or antibody anti-hepatitis C virus test; the need for blood transfusion within 2 months of the start of the study, surgery (with the exception of surgery related to vascular access), treatment with oral or i.v. iron preparations or any investigational drug within 4 weeks prior to study enrolment.
Study design and treatment
The primary objective of this trial was to assess the safety of i.v. iron supplementation as FCM in anaemic patients undergoing HD. The secondary objective was to evaluate the efficacy of FCM in correcting iron deficiency and Hb levels in this patient population. This study was conducted in compliance with the guidelines for Good Clinical Practice and other regulatory requirements.
Eligible patients received 100–200 mg of iron as FCM administered as an i.v. bolus–push injection into the HD venous line 1 h after the start of each dialysis session (two to three sessions per week) for a maximum of 6 weeks. This dose has been found to be well-tolerated when administered as an i.v. infusion of iron sucrose to patients with renal anaemia [16]. The total cumulative dose of iron given to a patient depended on the patient's individual iron deficit (Ganzoni formula: Iron deficit [mg] = b.w. [kg] × (target Hb − actual Hb) [g/dl] × 2.4 + depot iron [mg] where b.w. is body weight, Hb is haemoglobin, target Hb = 15 g/dl, factor 2.4 = 0.0034 (iron content Hb ≅ 0.34%) × 0.07 (blood volume ≅ 7% of b.w.) × 1000 (conversion grammes to milligrammes) × 10 (conversion litre to decilitre), and depot iron is 500 mg for patients ≥35 kg body weight. In patients with a body mass index (weight [kg]/(height [m] × height [m])) >25, a normalized weight was used to calculate the iron deficit. For patients <66 kg, the calculated cumulative dose is rounded down to the nearest 100 mg. For patients >66 kg, the calculated cumulative dose is rounded up to the nearest 100 mg. Normalized weight [kg] = 25 × height [m] × height [m].) [17]. A maximum of 600 mg iron was administered weekly; the cumulative maximum dose of FCM did not exceed 2400 mg of iron. Study medication was halted if serum ferritin levels exceeded 500 µg/l or TSAT was >50%. Following the final administration of study medication, patients entered an observation period for up to 1 month. Patient assessments took place every 2 weeks from baseline, with a final follow-up assessment at the end of the observation period. For some patients who withdrew or discontinued early, the follow-up visit took place as soon as possible afterwards, irrespective of the week of final medication.
Assessments
Safety and tolerability were assessed by the incidence, severity and relation to study medication of AEs, recorded by the investigator from the first dose of FCM. Also monitored were routine clinical laboratory safety parameters, vital signs and physical parameters [including 12-lead electrocardiogram (ECG) measurements], CRP and pre- and post-dialysis serum urea values.
Efficacy was assessed by correction of patients' iron stores and Hb levels; no primary efficacy endpoint was defined. Treatment responders were defined as patients who exhibited an increase of ≥1.0 g/dl in Hb from baseline at any point during the study. All laboratory parameters were determined at local laboratories in each country, and all iron parameters were analysed again at a single central laboratory at the end of the study. Only results from the central laboratory were included in the statistical analyses.
Statistical analyses
This was a single-arm, safety study with no specific hypotheses defined, so no formal sample size calculation was made. However, the probability of at least one patient experiencing a specific AE with an incidence rate of 5% is >99% with 120 patients and the probability with an incidence rate of only 1% is 70.1%. A planned sample size of 150 patients was, therefore, deemed to be adequate to evaluate the safety of FCM in this study.
Safety and efficacy were analysed using descriptive statistics, and for specific variables, 95% confidence intervals (95% CIs) were also used. These 95% CIs were calculated according to the method of Wilson as recommended by Altman et al. [18] for binary data, and as mean ± SE × t for continuous data where SE is the standard error of the mean and t is the upper 97.5 percentile of the appropriate t-distribution. Analyses were performed on observed values only. Analyses of safety data were performed using the safety population (all enrolled patients); efficacy results are described using the intent-to-treat (ITT) population (all enrolled patients who received at least one dose of study medication).
Results
Study patients
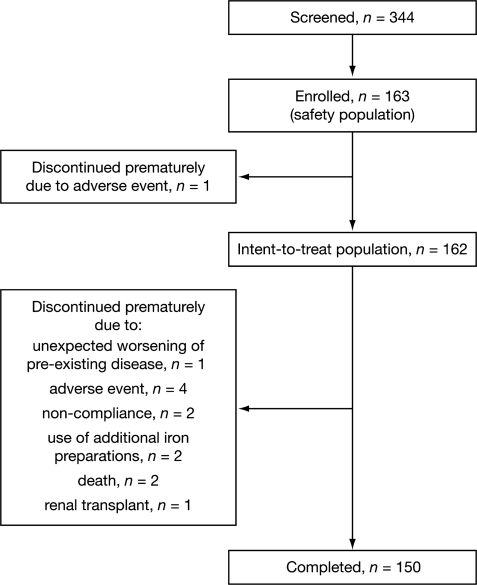
Patient recruitment began in July 2003; the final follow-up assessment visit took place in May 2004. A total of 344 patients were screened, of whom 163 (47.4%) comprised the safety population and 162 were included in the ITT population. In total, 150 out of 163 (92.0%) patients completed the study (Figure 1).
Fig. 1.
Flow of patients through the study.
The majority of patients were Caucasian, with more men (56.4%) than women enrolled to the study (safety population; Table 1). The most common primary renal disease was glomerulopathy [77 out of 163 (47.2%) patients]. Over three-quarters of the patients were undergoing three HD sessions per week [134 out of 163 (82.2%)], the mean duration of HD was 3.2 years [range, 1–23 years]. Nearly three-quarters of the patients [120 out of 163 (73.6%)] were receiving a stable dose of concomitant ESAs at baseline and for the duration of the study. Individual calculated iron deficits ranged from 933 to 2169 mg.
Table 1.
Patient demographics and baseline characteristics (safety population; n = 163)
| Safety population | |
|---|---|
| Age, years | 44.9 ± 12.7 |
| Sex | |
| Female | 71 (43.6%) |
| Male | 92 (56.4%) |
| Race | |
| Caucasian | 113 (69.3%) |
| Asian | 5 (3.1%) |
| Black | 20 (12.3%) |
| Mixed race | 25 (15.3%) |
| Height, cm | 168.3 ± 9.1 |
| Weight, kg | 70.8 ± 15.8 |
| Primary renal disease | |
| Glomerulopathy | 77 (47.2%) |
| Vascular nephropathy | 9 (5.5%) |
| Interstitial nephropathy | 19 (11.7%) |
| Other | 58 (35.6%) |
| Number of weekly HD sessions | |
| 2 | 29 (17.8%) |
| 3 | 134 (82.2%) |
| Patients receiving ESAs | 120 (73.6%) |
| Duration of HD, years | 3.2 ± 3.0 |
| Hb,a g/dl | 9.1 ± 1.3 |
| Serum ferritin,a µg/l | 67.3 ± 106.7 |
| TSAT,a % | 17.4 ± 9.1 |
Values are expressed as mean ± standard deviation or number (percent)
ESAs, erythropoiesis-stimulating agents; Hb, haemoglobin; TSAT, transferrin saturation.
ITT population, Hb, n = 159; serum ferritin and TSAT, n = 145.
Treatment
At all HD sessions, 100 or 200 mg of iron as FCM was administered, to a mean total cumulative dose of 2133.3 ± 57.7 mg at HD Session 11. The mean dose of FCM administered was 200 mg iron for the first four HD sessions, with a mean for subsequent infusions ranging between 196.2 mg (HD Session 5) and 160.0 mg (HD Sessions 9 and 10). Five patients cumulatively received more iron [9, 41, 100 (two patients) and 600 mg, respectively) than their calculated requirements. None of these patients developed any adverse clinical symptoms and only one patient with a serum ferritin level of 620 µg/l was subsequently withdrawn from the study.
The mean overall duration of the therapeutic period was 15.9 ± 4.5 days (minimum duration, 3 days; maximum duration, 31 days). The highest cumulative total iron dose administered was 2200 mg in one patient who underwent 11 HD sessions at ∼3-day intervals.
All patients who were receiving ESAs at baseline (ranging from 2000 IU once weekly to 10 000 IU twice weekly) maintained a stable dose throughout the study.
Safety
Adverse events
Intravenous iron as FCM was well-tolerated by patients in this study. In total, 193 AEs were reported in 89 out of 163 patients (54.6%; safety population; Table 2). This incidence of AEs is as expected for a population of chronically ill, multi-morbid patients. The most commonly reported AEs are detailed in Table 3. At least one serious AE was reported in 12 out of 163 (7.4%) patients (Table 4). This number includes two patients who died (one from lung tuberculosis and one from acute heart failure); none of the serious AEs were considered by the investigators to be related to the study medication. Three patients underwent renal transplantation during the study. AEs that were considered by the investigators to be possibly related to the study medication were reported in 13 out of 163 (8.0%) patients, these were nausea, abdominal pain, constipation, eructation, white blood cell count increase, headache, night sweats, pigmentation disorder, hypertension and hypotension; other AEs related to laboratory parameters and included two reports of increased alanine aminotransferase levels and one each of aspartate aminotransferase increase, liver function test abnormality and CRP increase. Only three out of 163 (1.8%) patients reported AEs (nausea, abnormal liver function test and headache) that were considered by the investigators as probably related to study medication.
Table 2.
Overview of AEs (safety population; n = 163)
| Number (%) of patients experiencing event | |
|---|---|
| At least one AE | 89 (54.6) |
| AEs by worst severity | |
| Mild | 54 (33.1) |
| Moderate | 26 (16.0) |
| Severe | 8 (4.9) |
| Unknown | 1 (0.6) |
| At least one serious AE | 12 (7.4) |
| AEs by relation to study drug | |
| Unrelated or unlikely to be related | 73 (44.8) |
| Possibly or probably related | 16 (9.8) |
| Certainly related | 0 |
| Discontinuation of study medication | 1 (0.6) |
| Unexpected worsening of pre-existing disease | 4 (2.4) |
| AE | |
| Death | 2 (1.2) |
AE, adverse event.
Two cases of overdose not reported as AEs and not included in the analysis.
Table 3.
Incidence of AEs reported in ≥5 patients (safety population; n = 163)
| Body system/preferred term | Number (%) of patients experiencing event |
|---|---|
| At least one AE | 89 (54.6) |
| Infections and infestations | 24 (14.7) |
| Respiratory tract viral infection | 6 (3.7) |
| Vascular disorders | 21 (12.9) |
| Hypertension | 13 (8.0) |
| Hypotension | 8 (4.9) |
| Gastrointestinal disorders | 18 (11.0) |
| Nausea | 5 (3.1) |
| Investigations (laboratory and ECG abnormalities) | 15 (9.2) |
| Nervous system disorders | 15 (9.2) |
| Headache | 13 (8.0) |
| Musculoskeletal and connective tissue disorders | 13 (8.0) |
| Muscle cramp | 8 (4.9) |
| Respiratory, thoracic and mediastinal disorders | 8 (4.9) |
| Injury, poisoning and procedural complications | 5 (3.1) |
| General disorders and administration-site conditions | 7 (4.3) |
| Skin and subcutaneous tissue disorders | 5 (3.1) |
| Surgical and medical procedures | 5 (3.1) |
AE, adverse event; ECG, electrocardiogram.
Table 4.
Summary of serious AEs (safety population; n = 163)
| Body system/preferred term | Number (%) of patients experiencing event |
|---|---|
| At least one serious AE | 12 (7.4) |
| Infections and infestations | 4 (2.5) |
| Gastrointestinal disorders | 3 (1.8) |
| Surgical and medical procedures | 3 (1.8) |
| Renal transplantation | 3 (1.8) |
| Vascular disorders | 2 (1.2) |
| Cardiac disorders | 1 (0.6) |
| General disorders and administration-site conditions | 1 (0.6) |
| Psychiatric disorders | 1 (0.6) |
| Respiratory, thoracic and mediastinal disorders | 1 (0.6) |
AE, adverse event.
Five patients (3.1%) were withdrawn from the study prematurely because of an AE (Table 2), these AEs were bronchopneumonia (n = 1), respiratory tract infection with hypertensive episode (n = 1), increased transaminases (n = 1) and renal transplant (n = 2).
There were five cases of accidental overdose, two of which, in patients who received 100 mg iron as FCM, were considered as certainly related to study treatment, but were not reported as AEs and were not part of the AE statistical analysis. The other three cases were reported as AEs and included in the statistical analysis. None of the five patients developed clinical symptoms.
Clinical evaluations
No clinically relevant changes from baseline or during HD sessions in vital signs (blood pressure, heart rate and axillary temperature), physical examinations or ECGs were observed.
Red blood cell and haematocrit values demonstrated an improvement during the study; there were no clinically relevant changes from baseline in reticulocytes. In addition, no clinically relevant changes from baseline in liver function-related clinical chemistry parameters were observed.
Mean CRP values were above the normal range (0–5 mg/l) at all visits, except at screening and the 4-week post-baseline visit. Mildly increased CRP values have frequently been described in patients undergoing HD in the absence of infection or inflammation. CRP increases were recorded in four patients. In one patient, the high CRP value was not due to any underlying clinical condition. The CRP value at the local laboratory was 4 mg/l at baseline, 20 mg/l at 2 weeks following last administration, 16 mg/l at 4 weeks following last administration and 7 mg/l at the post-treatment follow-up assessment. The event was considered by the investigator to be possibly related to study medication and mild in intensity. For the other three patients, the events were considered by the investigator to be unrelated to the study medication.
Iron status safety thresholds
Iron status safety thresholds of TSAT >50% or serum ferritin >500 µg/l were exceeded by 28 out of 125 (22.4%) and 55 out of 125 (44.0%) patients, respectively, 2 weeks after the first administration of study medication (safety population, only patients with non-missing measurements), decreasing to seven out of 141 (5.0%) and 22 out of 141 (15.6%) patients, respectively, by the post-treatment follow-up assessment. Of these, treatment with i.v. iron as FCM was halted in only 10 out of 163 (6.1%) patients as their serum ferritin levels exceeded 500 µg/l. One patient, who was given an accidental overdose (600 mg iron as FCM), reached a serum ferritin level of 620 µg/l during the treatment period (and TSAT of 54%) and was withdrawn from the study, although no clinical symptoms were reported. The investigator attributed the surpassed iron safety threshold to an overestimation of iron deficit at baseline. Two patients with serum ferritin levels of 634.0 and 678.2 µg/l, respectively, did not halt treatment with study medication as the stopping rules were not followed. Transitory elevations in iron parameters (ferritin levels >800 µg/l) occurred in five out of 163 (3.1%) patients at some point during the study. However, only one patient was withdrawn due to a ferritin level of 1649 µg/l; this was not associated with any clinical symptoms.
Efficacy
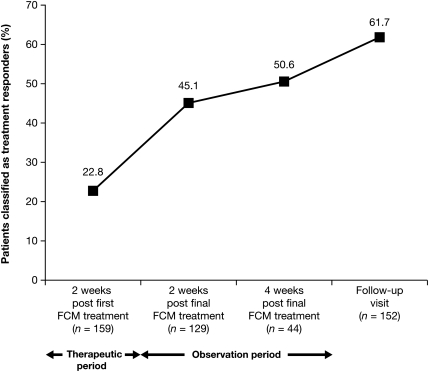
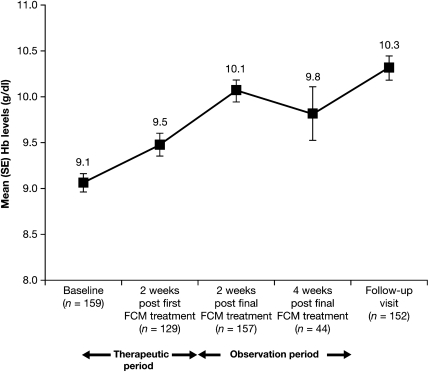
The number of responders, measured by an increase from baseline in Hb of ≥1.0 g/dl, increased at each visit and was achieved by 100 out of 162 (61.7%) patients by the time of the follow-up visit (ITT population; Figure 2). Mean Hb levels increased from 9.1 ± 1.30 g/dl (95% CI = 8.86; 9.26) at baseline to 9.5 ± 1.34 g/dl (95% CI = 9.25; 9.72) at 2 weeks after the first administration of study medication and continued to increase through the observation period to reach 10.3 ± 1.63 g/dl (95% CI = 10.06; 10.58) at follow-up (ITT population; Figure 3). The mean change in Hb 2 weeks following the first administration of study medication was 0.53 ± 0.75 g/dl (95% CI = 0.40; 0.67).
Fig. 2.
The cumulative proportion of treatment responders patients attaining an increase in Hb = 1.0 g/dl and classified as treatment responders over time (ITT population, N = 162).
Fig. 3.
Mean (SE) levels of Hb (in grammes per decilitre) over time (ITT population, N = 162).
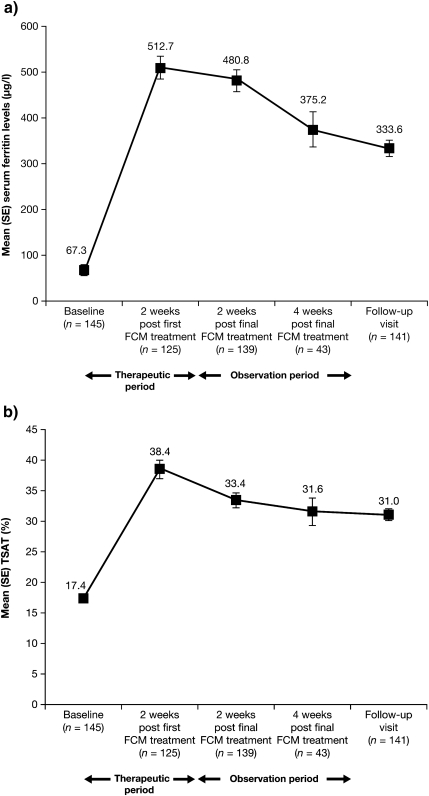
Both serum ferritin levels and TSAT were suboptimal at baseline (67.3 ± 106.73 µg/l and 17.4 ± 9.10%, respectively), as defined by international recommendations [1,19]. However, within the 2 weeks following the first administration of FCM, serum ferritin levels and TSAT had increased to within their respective target ranges (200–500 µg/l serum ferritin and >20% TSAT) [19] for patients undergoing HD (512.7 ± 241.36 µg/l and 38.4 ± 17.23%, respectively) and, although decreasing slightly, remained in the target range until the final follow-up visit (333.6 ± 209.05 µg/l and 31.0 ± 11.00%, respectively; ITT population; Figure 4). Although no formal statistical analysis was planned, the results were considered to be clinically relevant. These data suggest that FCM was successful in increasing iron stores.
Fig. 4.
Mean (SE) (a) serum ferritin (in microgrammes per litre) and (b) TSAT (in percent) values over time (ITT population, N = 162).
Discussion
Patients undergoing HD are unable to maintain their iron balance because of chronic blood (and, therefore, iron) loss at each dialysis session. The initial levels of Hb would indicate that, despite the widespread use of ESAs, although in relatively low doses, reduced iron levels and baseline inflammatory status contributed to the incomplete response to ESA therapy. The initial levels were below the target set for patients with CKD [1]. However, according to the Kidney Disease Outcomes Quality Initiative (KDOQI) 2007 guidelines [19], in patients undergoing HD receiving ESA therapy with a Hb target in the range of 11.0–12.0 g/dl, the proportion of patients who achieve this target in a single month may be 30% or less [19]. Using the National Health and Nutritional Examination Survey, it was found that low levels of iron (serum ferritin <100 µg/l or TSAT <20%) were present in the majority of patients with CKD not undergoing dialysis with reduced creatinine clearance [20] and was associated with absent bone marrow iron in ∼80% of patients [21].
HD induces an inflammatory state that accounts for the associated increased serum hepcidin levels [22]. Hepcidin blocks iron absorption from the duodenum and iron release from the liver (the main iron store) and macrophages, thereby interrupting iron recycling [4,23]. The decreased availability of iron for erythropoiesis aggravates the existing anaemia of chronic disease (functional iron deficiency) in HD patients. Oral iron therapy does not lead to normalization of iron stores, as it is not well-absorbed, is poorly tolerated and is associated with low compliance [1,24]. Therefore, in order to maintain an optimal iron status, it is strongly recommended that patients with CKD who are undergoing HD receive supplemental iron intravenously rather than orally [1,19]. This study demonstrates that 200 mg i.v. iron as FCM, administered by bolus–push injection into the HD venous line, is both well-tolerated and effective in increasing Hb levels and iron stores for patients undergoing HD who require treatment for IDA. The majority of patients (61.7%) were treatment responders, achieving a clinically relevant increase in Hb levels of ≥1.0 g/dl at any time during the study after receiving iron as FCM. The observed clinically relevant increases in Hb levels (from 9.1 g/dl at baseline to 10.3 g/dl by the follow-up visit) in the majority of patients can be solely attributed to the administration of i.v. iron as FCM, as changes in anaemia treatment such as increases in the dosage of ESAs were not permitted during the trial.
There is concern, however, among medical personnel regarding the safety of earlier formulations of parenteral iron. These concerns primarily relate to the potential for excess iron to produce reactive oxygen species that can increase oxidative tissue damage in patients with chronic inflammatory disease: vasoactive reactions that may occur when larger doses of i.v. iron are administered quickly and the incidence of anaphylactic reactions (occasionally fatal) that occur as a consequence of pre-formed anti-dextran antibodies to i.v. iron dextran [1].
In this study, over 90% of participating patients completed iron supplementation with FCM. As expected for a population of chronically ill, multi-morbid patients, more than half of the patients (54.6%) reported an AE. However, <10% of the patients [16 out of 163 (9.8%)] reported an AE considered by the investigators to be possibly or probably related to study medication, and neither of the two deaths that occurred during the study was considered to be related to study medication. The most frequently reported AEs were respiratory tract viral infection, hypertension, hypotension, muscle cramps, nausea and headache. There were no clinically relevant changes in vital signs or physical examinations.
Another recently developed i.v. iron formulation, ferumoxytol, may be also applied by rapid i.v. injections (two doses of 510 mg iron may be given 3–8 days apart), but in contrast to FCM, it contains dextran derivatives [25]. Significantly increased Hb levels in comparison to oral iron were reported in Phase III trials involving patients with CKD requiring HD [26] as well as patients not on dialysis [27].
The main limitation of this study was its open-label, single-arm design. However, as the primary aim was to evaluate safety, and efficacy was assessed by laboratory parameters, no comparator was necessary. Nevertheless, it may be difficult to conclude the extent to which the reported AEs are related to the study treatment rather than the effects of HD.
In a Phase I dose-escalation study, up to 1000 mg iron as FCM, given as an i.v. infusion over 15 min for doses of over 100 mg iron, was well-tolerated in patients with mild IDA [15]. Recently presented clinical data also support the favourable safety and efficacy profile of i.v. iron as FCM [28,29]. Studies have demonstrated that, compared with oral (FeSO4) or i.v. iron as iron sucrose, significant improvements in Hb levels can be achieved with i.v. iron as FCM in both anaemic patients with non-dialysis-dependent CKD [28] and in HD patients with IDA [29]. Furthermore, treatment with iron as FCM resulted in a lower incidence of drug-related AEs during treatment compared with the earlier supplemental iron preparations [30].
According to Geisser et al. [13], the toxic effects of an iron complex can be forecast by the physiochemical properties (molecular mass, kinetic and thermodynamic stability) of the compound. FCM is a strong and robust iron complex and, as such, demonstrates the properties of an ideal iron compound. Owing to the high structural homogeneity and slow degradation kinetics of FCM, i.v. administration results in a low toxicity and targeted delivery of iron to the reticuloendothelial system and its distribution to the bone marrow, liver and spleen [4,14]. In a second step, the complex is metabolized and the iron is slowly and competitively delivered to endogenous iron-binding proteins. The reticuloendothelial uptake is thought to illustrate, indirectly, the safety of i.v. iron polysaccharide complexes with regard to the long-term effects on the parenchyma of different organs.
Conclusion
In conclusion, i.v. iron as FCM administered by bolus–push injection was well-tolerated in this safety study with a population of anaemic patients undergoing HD. The incidence of AEs reported during the study was as expected for a chronically ill patient population, and no new safety concerns were raised. None of the serious AEs and none of the AEs that led to discontinuation of the study medication were considered related to FCM. Furthermore, FCM was effective in correcting anaemia and refilling the iron stores of this population, which translated into a clinically meaningful increase in Hb, TSAT and serum ferritin levels. This study supports FCM as a new treatment for IDA, offering patients an effective supplemental iron formulation with a good safety profile.
Acknowledgments
The authors take full responsibility for the content of the paper but thank Laura Giles DPhil (Caudex Medical; supported by Vifor International Inc.) for her assistance in preparing the initial draft of the manuscript and collating the comments of the authors and other named contributors. The study was sponsored by Vifor International Inc.
Conflict of interest statement. Adrian Covic has participated in a speaker bureau for Amgen and Roche and has acted as a scientific consultant for FMC. Gabriel Mircescu had no involvement that might raise the question of bias in the work reported or in the conclusions, implications or opinions stated. The results presented in this paper have not been published previously in whole or part.
References
- 1.Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19:ii1–47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 2.Hörl WH. Iron therapy in patients with chronic kidney disease: taking the high road? Port J Nephrol Hypert. 2009;23:5–10. [Google Scholar]
- 3.Royal College of Physicians (London). National Collaborating Centre for Chronic Conditions Anaemia management in chronic kidney disease: national clinical guideline for management in adults and children. 2006. Available from: http://www.nice.org.uk/ [PubMed]
- 4.Funk F, Ryle P, Canclini C, Neiser S, Geisser P. The new generation of intravenous iron: chemistry, pharmacology and toxicology of ferric carboxymaltose. Arzneimittelforschung. 2010 doi: 10.1055/s-0031-1296299. Supplement: In press. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Iron Deficiency Anemia: Assessment, Prevention and Control. Report of a Joint WHO/UNICEF/UNU Consultation. 1998. [Google Scholar]
- 6.US National Kidney Foundation. 2008. Available from: http://www.kidney.org/professionals/KDOQI/
- 7.Hudson JQ, Comstock TJ. Considerations for optimal iron use for anemia due to chronic kidney disease. Clin Ther. 2001;23:1637–1671. doi: 10.1016/s0149-2918(01)80135-1. [DOI] [PubMed] [Google Scholar]
- 8.Fishbane S. Safety in iron management. Am J Kidney Dis. 2003;41:18–26. doi: 10.1016/s0272-6386(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 9.Bregman D. Important Drug Warning for Dexferrum® (iron dextran injection, USP) Shirley New York: American Regent Inc; 2009. [Google Scholar]
- 10.INFeD Prescribing Information . Corona: Watson Pharmaceuticals, Inc.; 2009. [Google Scholar]
- 11.CosmoFer Summary of Product Characteristics. Holbaek: Pharmacosmos A/S; 2009. [Google Scholar]
- 12.Bailie GR, Clark JA, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–1449. doi: 10.1093/ndt/gfh820. [DOI] [PubMed] [Google Scholar]
- 13.Geisser P, Baer M, Schaub E. Structure/histotoxicity relationship of parenteral iron preparations. Arzneimittelforschung. 1992;42:1439–1452. [PubMed] [Google Scholar]
- 14.Beshara S, Sorensen J, Lubberink M, et al. Pharmacokinetics and red cell utilization of 52Fe/59Fe-labelled iron polymaltose in anaemic patients using positron emission tomography. Br J Haematol. 2003;120:853–859. doi: 10.1046/j.1365-2141.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- 15.Geisser P, Banké-Bochita J. Pharmacokinetics, safety and tolerability of intravenous ferric carboxymaltose: a dose-escalation study in volunteers with mild iron deficiency anaemia. Arzneimittelforschung. 2010 doi: 10.1055/s-0031-1296301. Supplement: In press. [DOI] [PubMed] [Google Scholar]
- 16.Chandler G, Harchowal J, Macdougall IC. Intravenous iron sucrose: establishing a safe dose. Am J Kidney Dis. 2001;38:988–991. doi: 10.1053/ajkd.2001.28587. [DOI] [PubMed] [Google Scholar]
- 17.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100:301–303. [PubMed] [Google Scholar]
- 18.Altman DG, Machin D, Bryant TN, Gardner MS. Statistics with Confidence. 2nd edition. BMJ Books; 2000. [Google Scholar]
- 19.KDOQI KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Fishbane S, Pollack S, Feldman HI, et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4:57–61. doi: 10.2215/CJN.01670408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Rodriguez AM, Guindeo-Casasus MC, Molero-Labarta T, et al. Diagnosis of iron deficiency in chronic renal failure. Am J Kidney Dis. 1999;34:508–513. doi: 10.1016/s0272-6386(99)70079-x. [DOI] [PubMed] [Google Scholar]
- 22.Eleftheriadis T, Liakopoulos V, Antoniadi G, Kartsios C, Stefanidis I. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial. 2009;22:70–77. doi: 10.1111/j.1525-139X.2008.00532.x. [DOI] [PubMed] [Google Scholar]
- 23.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 24.Maslovsky I. Intravenous iron in a primary-care clinic. Am J Hematol. 2005;78:261–264. doi: 10.1002/ajh.20271. [DOI] [PubMed] [Google Scholar]
- 25.Groman E, Kenneth GP, Frigo TB, Bengele H, Lewis JM. Heat stable colloidal iron oxides coated with reduced carbohydrates and carbohydrate derivatives. 2009 US patent no. US 6,599,498 B1; 29 July 2003.
- 26.Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qunibi W, Martinez CO, Smith M, Benjamin J, Dinh Q. A randomized controlled trial comparing IV ferric carboxymaltose (FCM) to oral iron in anemic patients with non-dialysis-dependent CKD. Poster presented at the 40th Annual Meeting of the American Society of Nephrology, 31 Oct–5 Nov 2007, San Francisco, CA, USA. ASN 2007; SU-PO1030.
- 29.Schaefer RM, Khasabov NN, Todorov NG, Evenepoel P. Intravenous ferric carboxymaltose or iron sucrose to treat iron deficiency anaemia in haemodialysis patients. 2008. Poster presented at the XLV ERA-EDTA Congress, May 10–13 2008, Stockholm, Sweden. Poster no. MP375.
- 30.Qunibi W, Dinh Q, Benjamin J. Safety and tolerability profile of ferric carboxymaltose (FCM): data from the FCM clinical program. 2008. Poster presented at the XLV ERA-EDTA Congress, May 10–13 2008, Stockholm, Sweden. Abstract book ERA-EDTA 2008.