Abstract
The orphan receptor, bombesin receptor subtype-3(BRS-3) is a G-protein-coupled receptor classified in the bombesin(Bn)receptor family because of its high homology (47–51%) with other members of this family[gastrin-releasing peptide receptor[GRPR] and neuromedin B receptor [NMBR]. There is increasing interest in BRS-3, because primarily from receptor knockout studies, it seems important in energy metabolism, glucose control, insulin secretion, motility and tumor-growth. Pharmacological tools to study the role of BRS-3 in physiology/pathophysiology are limited because the natural ligand is unknown and BRS-3 has low affinity for all naturally-occurring Bn-related peptides. However, a few years ago a synthetic high-affinity agonist [DTyr6,, βAla11,Phe13,Nle14]Bn-(6–14) was described but was nonselective for BRS-3 over other Bn-receptors. Based on this peptide, in various studies a number of putative selective, high-potency hBRS-3 agonists were described, however the results on their selectivity are conflicting in a number of cases. The purpose of the present study was to thoroughly study the pharmacology of four of the most select/potent putative hBRS-3 agonists(#2–4,16a). Each was studied in multiple well-characterized Bn-receptor-transfected cells and native Bn-receptor-bearing cells, using binding studies, alterations in cellular signaling (PLC,PKD) and changes in cellular function(growth). Two peptides(#2,#3) had nM affinities/potencies for hBRS-3, peptide #4 had low affinity/potency, and peptide #16a very low (>3000nM). Peptide#3 had the highest selectivity for hBRS-3(100-fold), whereas #2,4 had lower selectivity. Peptide #16a’s selectivity could not be determined because of it low affinity/potencies for all hBn-receptors. These results show that peptide#3 is the preferred hBRS-3 agonist for studies at present, although its selectivity of only 100-fold may limit its utility in some cases. This study underscores the importance of full pharmacological characterization of newly reported selective agonists.
Keywords: bombesin, gastrin-releasing peptide, neuromedin B, agonist, receptor
1. Introduction
Bombesin receptor subtype-3 (BRS-3) is an orphan G protein-coupled receptor, but because of its 51% and 47% amino acid homology with the human bombesin (Bn) receptors [gastrin-releasing peptide receptor (GRPR) and neuromedin B receptor (NMBR)], it is classified in the Bombesin (Bn) receptor family [7,15]. BRS-3 is widespread found in both the central nervous system and peripheral tissues [7,15,31,32,39], but its roles in either normal physiology or pathological conditions is largely unknown. BRS-3 is receiving increased attention [6,11,56] because its targeted disruption leads to obesity, diabetes and hypertension [19,31]. More recent studies demonstrate BRS-3 receptors are present on pancreatic islets [8] and BRS-3 knockout animals have altered plasma insulin levels and altered glucose transporter 4 function [28,30]. Furthermore, from target receptor deletion (knockout) and expression studies, it has been reported that the BRS-3-receptor is frequently over-expressed by various tumors [7,18,34,40], its presence plays a role in tumor invasiveness [13,15,18,57], a role in lung development, bronchial epithelial cell proliferation and lung injury [15,41–43,53], a role in taste preference [63], social response/anxiety [61,62], and may play an important role in regulating gastrointestinal motility [32].
Except for information from receptor knockout studies, little is known about BRS-3’s role in normal physiology or pathophysiology because the natural ligand remains unknown, which has limited the development of selective agonists or antagonist that can be used for physiological/pharmacological studies. Furthermore, studies demonstrate the BRS-3 receptor has low affinity for all occurring natural Bn-related peptides as well as most synthetic bombesin-family member analogues [15,27] with NMB and GRP only interacting with BRS-3 in the micromolar concentrations [7,15,27]. However, it has been possible to perform some pharmacological studies subsequently, because recently a synthetic Bn analogue [DTyr6, βAla11, Phe13, Nle14]Bn(6-14) [peptide#1, Table 1] was discovered to have high affinity for both human [15,27,33] and monkey [39] BRS-3 receptors. Unfortunately, subsequent studies demonstrated that this synthetic Bn peptide was a universal agonist for most Bn receptors, having a unique pharmacology because it had high affinity not only for human/monkey BRS-3-receptors, but also for human GRPR and NMBR as well as GRPR and NMBR in most species and the frog BB4 receptor [7,15,33]. Subsequent studies using this synthetic Bn analogue or its DPhe6-derivative demonstrated hBRS-3 activation, stimulated of phospholipase C, phospholipase D and tyrosine kinase cascades, but did not stimulate adenylate cyclase activation [15,37].
Table 1.
Peptide number and structure of peptides studied.
| PEPTIDE NAME/NUMBER |
STRUCTURE |
|---|---|
| 16a | Phenylacetyl-Ala, DTrp-phenthylamide |
| 1 | [DTyr6, βAla11, Phe13, Nle14]Bn(6-14) |
| 2 | [DTyr6, (R)- Apa11, Phe13, Nle14]Bn(6-14) |
| 3 | [DTyr6, (R)-Apa11-4Cl, Phe13, Nle14]Bn(6-14) |
| 4 | Ac-Phe, Trp, Ala, His(tBzl), Nip, Gly, Arg-NH2 |
| GRP(14–27) | Met, Tyr, Pro, Arg, Gly, Asn, His, Trp, Ala, Val, Gly, His, Leu, Met-NH2 |
| NMB | pGlu, Leu, Trp, Ala, Thr, Gly, His, Phe, Met-NH2 |
| Bn | pGlu, Gln, Arg, Leu, Gly, Asn, Gln, Trp, Ala, Val, Gly, His, Leu, Met-NH2 |
Peptide 16a was reported in 2003 as 16a [54]; peptide #1 was described in 1997[27]; peptide 2 was reported in 2001 as compound #14 in [25]; peptide #3 was originally reported in 2004 as compound #7 in [24]; and peptide #4 was reported as compound #34 in 2005 [5].
Abbreviations: βAla, βAlanine; Nle, Norleucine; Apa, 3-amino, propionic acid; Apa-4Cl, 4 chloro, 3-amino, propionic acid; His(tBzl), histidine(tBenzl); Ac, acetyl; Nip, piperidine-3 carboxylic acid; pGlu, pyroglutamic acid
Because receptor knockout studies have demonstrated the potential importance of BRS-3 in a number of physiological and pathological processes, recently a number of studies have attempted to develop BRS-3-receptor selective agonists, using as the prototype either [DPhe6, βAla11,Phe13, Nle14]Bn(6-14) or [DTyr6, βAla11, Phe13, Nle14]Bn(6-14) as a starting point. In different studies a number of peptides have been reported to have selectivity with high agonist potencies for the hBRS-3 over hGRPR or hNMBR [5,15,24–26,54,55,64]. One analogue, peptide #16a (Table 1) has received particular attention because of its reported high potency/selectivity for hBRS-3 [54,64]. Unfortunately with a number of these putative hRS-3 selective agonists, studies report conflicting results with some showing high potencies/selectivity for hBRS-3 and other not [5,9,15,24–26,54,55,64]. These conflicting results are due to the fact the pharmacological characterization of the different peptides has been very limited, frequently with no binding studies allowing determination of receptor affinity, reported potencies were based on a single assay usually using a calcium FLIDR study. Furthermore, studies were performed in only a single transfected cell system which was uncharacterized, no studies were performed using native Bn receptor-expressing cells and no assessment of effect on cellular function, rather than signaling, was performed. This confusion makes it difficult to critically assess which of these putative hBRS-3, potent, selective agonists could be useful for a given study.
The purpose of the present study was to address these differences, by studying the pharmacology of four of the most potent, selective putative hBRS-3 agonists (peptides # 16a, peptides #2, #3, #4, Table 1) described. This was accomplished by studying their interaction with each of the human Bn receptors (hBRS-3, hNMBR, hGRPR) in multiple well-characterized transfected cells and native receptor bearing cells. Furthermore, both binding studies as well as assessment on difference signaling cascades and cell function (growth) were assessed.
2. Material and Methods
2.1. Materials
The following cells and materials were obtained from the sources indicated: BALB 3T3, HuTu-80 (human duodenal cancer cell line), NCI-N417 and NCI-H1299 cells were from American Type culture Collection (ATCC) (Manassas, VA); Dulbecco’s minimum essential medium (DMEM), phosphate-buffered saline (PBS), Roswell Park Memorial Institute (RPMI-1640), trypsin-EDTA, fetal bovine serum (FBS), G418 sulfate from Invitrogen (Carlsbad, CA); Na125I (2200 Ci/mmol) and myo-[2-3H]Inositol (20 Ci/mmol) were from Perkin Elmer (Boston, MA); formic acid, ammonium formate, disodium tetraborate, soybean trypsin inhibitor, bacitracin, sodium vanadate, triton X-100, deoxycholate, Tween® 20, phenylmethylsulfonyl fluoride (PMSF), ethylene glycol tetra-acetic acid (EGTA), ethylene diamine tetra-acetic acid (EDTA) and sodium azide were from Sigma-Aldrich, St. Louis, MO; 1,2,4,6-tetrachloro-3a,6a-diphenylglycouril (IOD-GEN) from Pierce Chemical Co. (Rockford, IL); AG 1-X8 resin from Bio-Rad, Richmond, CA. Gastrin releasing peptide (GRP), neuromedin B (NMB) and [Tyr4]Bombesin ([Tyr4]Bn) were from Bachem (Torrence, CA); Protease inhibitor Tablet was from Roche (Basel, Switzerland); α/β tubulin and phospho-Protein kinase D (PKD) (Ser744/748) antibodies and non fat dry milk were from Cell Signaling Technology, Inc. (Beverly, MA) and Horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit) and Supersignal Western Pico/Dura were from Thermo Scientific (Rockford, IL).
2.2 Cell Culture
NCI-N417and H1299 cells were cultured in Roswell Park Memorial Institute (RPMI-1640) medium containing 10% heat-inactivated fetal bovine serum. NCI-H1299 cells stably transfected with human BRS-3 (hBRS-3/H1299 cells) or human NMBR (hNMBR/H1299 cells) [10,27,37] were grown in RPMI supplemented with 10% FBS and 300 mg/liter of G418 sulfate. The NCI-N417 cells which contain native hBRS-3 receptors [38] grew as floating aggregates, whereas the NCI-H1299 cells were adherent. NCI-H1299 cells were split twice weekly 1/5 with trypsin-EDTA. NCI-N417 cells were diluted 1/3 into new media. Balb 3T3 cells stably expressing human BRS-3 receptor (hBRS-3/Balb cells), human NMB receptor (hNMBR/Balb cells), or human GRP receptors (hGRPR/Balb cells), were made as described previously [2,10,27,38] and grown in Dulbelcco’s modified Eagle’s cell medium (DMEM) supplemented with 300 mg/liter of G418 sulfate and 10% FBS. HuTu 80 cells which contain native hGRP-receptors [10], were grown in DMEM supplemented with 10% FBS. The cells were mycoplasma free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2/95% air.
2.3. Preparation of peptides
The peptides were synthesized using standard solid-phase methods as described previously [10,26,27]. In brief, solid-phase syntheses of peptide amides were carried out using Boc chemistry on methylbenzhydrylamine resin (Advanced ChemTech, Louisville, KY) followed by hydrogen fluoride-cleavage of free peptide amides or oxime resin (Advanced ChemTech, Louisville, KY) for cleavage as peptide phenethylamides with phenethylamine which is only weakly nucleophilic. The crude peptides were purified by preparative high-performance liquid chromatography on columns (2.5 × 50 cm) of Vydac C18 silica (10 μm), which was eluted, with linear gradients of acetonitrile in 0.1% (v/v) trifluoroacetic acid. Homogeneity of the peptides was assessed by analytical reverse-phase high-performance liquid chromatography, and the purity was usually 97% or higher. Amino acid analysis (only amino acids with primary amino acid groups were quantitated) gave the expected amino acid ratios. Peptide molecular masses were obtained by matrix-assisted laser desorption mass spectrometry (Applied Biosystems, Foster City, CA), and all corresponded well with calculated values.
2.4. Preparation of 125I-[DTyr6, βAla11, Phe13, Nle14]Bn-(6-14)
This radioligand, which has high affinity for all human Bn receptor subtypes, with specific activity of 2200 Ci/mmol, was prepared as previously described [23,27,33,38]. Briefly, 0.8 mg of IOD-GEN solution (0.01 mg/ml in chloroform) was added to a 5 ml plastic test tube, dried under nitrogen, and washed with 100 ml of 0.5 M potassium phosphate solution (pH 7.4). To this tube 20 ml of potassium phosphate solution of (pH 7.4), 8 ug of peptide in 4 uL of water, 2 mCi (20 ml) of Na125I were added and incubated for 6 min at room temperature. The incubation was stopped with 300 ml of water. The radiolabeled peptide was separated using a Sep-Pak (Waters Associates, Milford, MA) and further purified by reverse–phase high performance liquid chromatography on a C18 column. The fractions with the highest radioactivity and receptor binding capacity were neutralized with 0.2 M Tris buffer (pH 9.5) and stored with 0.5% bovine serum albumin (w/v) at 20°C.
2.5. Binding of 125I-[DTyr6, βAla11, Phe13, Nle14]Bn-(6-14) to various cells
Binding studies were performed as described previous [10,24,29]. Briefly, the standard binding buffer contained 24.5 mM HEPES (pH 7.4), 98 mM NaCl, 6 mM KCl, 5 mM MgCl2, 2.5 mM NaH2PO4, 5 mM sodium pyruvate, 5 mM sodium fumarate, 0.01% (w/v) soybean trypsin inhibitor, 1% amino acid mixture, 0.2% (w/v) bovine serum albumin, and 0.05% (w/v) bacitracin. Hutu 80 cells (1 × 106); NCI-N417 cells (1.5 × 106); Balb 3T3 cells stably expressing hGRPR (0.3 × 106), hNMBR (0.03 × 106), or hBRS-3 (0.3 × 106); NCI -H1299 cells stably expressing hBRS-3 (0.5 × 106) or hNMBR (0.4 ×106) were incubated with 50 pM 125I-[DTyr6, βAla11,Phe13,Nle14]Bn-(6-14), with or without the indicated concentration of unlabeled peptides, at 22 °C for 60 min. 2 aliquots (100 ml) were removed and each centrifuged through 300 ml of washing buffer (1% w/v BSA in PBS) in 400 ml microfuge tubes at 10,000 × g for 1 min using a Beckman micro-centrifuge B. The pellets were washed twice with washing buffer and counted for radioactivity in a gamma counter. The nonsaturable binding was the amount of radioactivity associated with cells in incubations containing 50 pM radioligand (2200 Ci/mmol) and 1 μM unlabeled ligand. Nonsaturable binding was <10% of total binding in all the experiments. Receptor affinities were determined using the curve-fitting program KaleidaGraph (Synergy Software).
2.6. Measurement of [3H]IP
Changes in total [3H]inositol phosphates ([3H]IP) were measured as described previously [1,3,35,37]. Briefly, hBRS-3-, hGRPR- or hNMBR-transfected Balb 3T3 cells; hBRS-3- and hNMBR-transfected H1299 cells and HuTu 80 cells were subcultured into 24-well plates (5 × 104 cells/well) in regular propagation media and then incubated for 24 hr at 37 ° C in a 5% CO2 atmosphere. The cells were then incubated with 3 uCi/ml of myo-[2-3H] inositol in growth media supplemented with 2% FBS for an additional 24 hr. Before assay, the 24-well plates were washed by incubating for 30 min at 37°C with 1 ml/well of PBS (pH 7.0) containing 20 mM lithium chloride. The wash buffer was aspirated and replaced with 500 ml of IP assay buffer containing 135 mM sodium chloride, 20 mM HEPES (pH 7.4), 2 mM calcium chloride, 1.2 mM magnesium sulfate, 1 mM EGTA, 20 mM lithium chloride, 11.1 mM glucose, 0.05% BSA (w/v) and incubated with or without different concentrations of the peptides studied. After 60 min of incubation at 37°C, the incubations were terminated by the addition of 1 ml of ice cold 1% (v/v) hydrochloric acid in methanol. Floating cancer cells, NCI-N417 (1 × 106 cells/ml) were sub-cultured into a 75-cm2 tissue culture flask containing 15 ml of RPMI-160 supplemented with 2% (v/v) FBS and 3 uCi/ml myo-[2-3H]inositol and incubated for 24 hr at 37°C. The cells were then washed and incubated for 10 min at 37°C with equivalent volume of PBS (pH 7.0) containing 20 mM lithium chloride. The cells were then re-suspended in IP assay buffer. 300 ml of the cell suspension was added to test tubes containing the peptides to be tested and incubated at 37°C for 60 min after which the incubation was terminated with 1 ml ice-cold HCl/methanol (1% v/v). Total [3H]IP was isolated by anion exchange chromatography as described previously [1,3,35,37]. Briefly, samples were loaded onto Dowex AG1-X8 anion exchange resin columns, washed with 5 ml of distilled water to remove free [3H]inositol, then washed with 2 ml of 5 mM disodium tetraborate/60 mM sodium formate solution to remove [3H]glycerophosphorylinositol. Two ml of 1 mM ammonium formate/100 mM formic acid solution were added to the columns to elute total [3H]IP. Each eluate was mixed with 10 ml of scintillation cocktail and measured for radioactivity in a scintillation counter.
2.7. Determination of protein kinase D activation (PKD)
PKD activation was determined using Western blotting and specific Phospho-PKD (Ser744/748) antibodies as described previously[4]. Briefly, 5 ×105 cells/well, in the case of adherent cells (hNMBR/H1299 and Hutu-80), or 2.5 × 106 cell/ml, in that of floating cells (NCI-N417) were subjected to starvation (culture medium containing 0% FBS overnight or 2% FBS for 3 hours, respectively) before the incubation with the peptides. Whole cells lysates were obtained from cells after 5 min incubation in the absence (control) or presence of peptide #1 or 16a (Table 1) at different concentrations. Then cells were washed once with PBS containing 0.2 mM Na3VO4 and lysed with lysis buffer (50 mM Tris/HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium azide, 1 mM EGTA, 0.4 mM EDTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and one protease inhibitor tablet per 10 ml). After sonication, lysates were centrifuged at 10,000x g for 15 min at 4 C and protein concentration was measured using the Bio-Rad protein assay reagent.
Western blotting were performed as described previously [4]. Equal amounts of protein from whole cell lysates were subjected to SDS-PAGE using 4–20% Tris-Glycine gels. After electrophoresis, protein was transferred to nitrocellulose membranes. Membranes were blocked in blocking buffer (50 mM Tris/HCl pH 8.0, 2 mM CaCl2, 80 mM NaCl, 0.05% Tween® 20, 5% non fat dry milk) at room temperature for one hour. Membranes were then incubated with primary antibody (Phospho-PKD (Ser744/748) or tubulin, as loading control) overnight at 4°C under constant agitation at antibody dilutions suggested by the antibody supplier. After primary antibody incubation membranes were washed twice in blocking buffer for 4 minutes and then incubated with HRP-conjugated secondary antibody (anti-rabbit) for 1 hour at room temperature under constant agitation. Membranes were then washed again twice in blocking buffer for 4 minutes, twice in washing buffer (50 mM Tris/HCl pH 8.0, 2 mM CaCl2, 80 mM NaCl, 0.05% Tween® 20) for 4 minutes, incubated for 4 minutes with chemiluminescense detection reagents and finally exposed to Kodak Biomax film (XS, XR). The intensity of the protein bands was measured using Kodak ID Image Analysis which were assessed in the linear detection range.
2.8. [3H]-Thymidine uptake
Balb 3T3 cells transfected with BRS-3 were trypsinized and 50,000 cells placed in 24 well plates containing DMEM with 10% fetal bovine serum and 0.3 mg/ml G418 sulfate. After 3 days, the confluent cells were place in DMEM containing 30 nM sodium selenite, 5 μg/ml insulin and 10 g/ml transferrin and varying concentrations of Peptide #1 or 16A. After 24 hr the cells were incubated with [3H]thymidine (106 cpm/ml) for 3 hr. The 24 well plates were washed 3 times with 1 ml PBS. The cells were treated with 0.2 N HCl (0.25 ml) and the solution placed in a scintillation vial. The cells were treated with 0.2 N NaOH (0.25 ml) and the solution placed to the scintillation vial. Then 10 ml of scintillation fluid were added to the scintillation vial and after shaking the vial, it was counted in a scintillation counter.
2.9. Statistical analysis
All results are expressed as mean± SEM from at least 3 experiments, and results were considered significant if p<0.05. IC50 and EC50 were calculated using the curve-fitting program, KaleidaGraph (Synegy Software). The Mann-Whitney U test was used to determine the statistical significance of differences.
3. Results
3.1. Ability of GRP, NMB, Peptide #1 and various putative BRS-3 selective agonists to inhibit binding in various cells expressing native or transfected human bombesin receptor subtypes
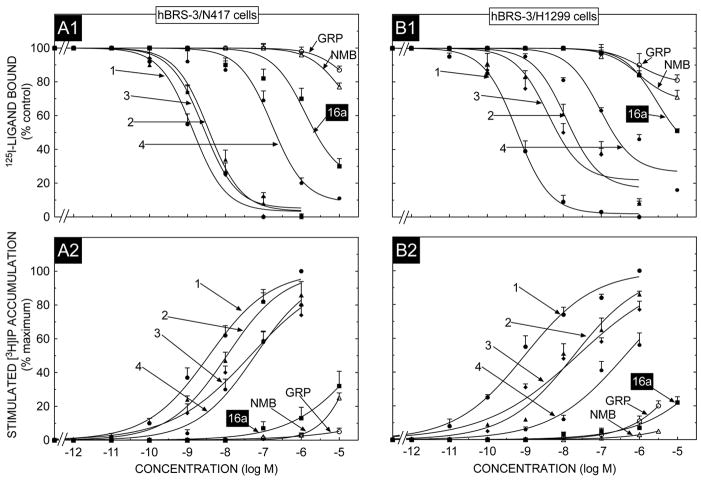
With the native hBRS-3 containing cell line, NCI-H417, the universal ligand (peptide #1, Table 1) had high affinity (Fig. 1, panel A1)(IC50-1.5 nM, Table 2), with the natural occurring GRPR agonist, GRP and NMBR ligand, NMB, having very low affinities (>10,000 nM, Table 2, Fig. 1, panel A1). The putative hBRS-3 selective ligand #3 had a similar high affinity to the universal ligand, peptide #1, whereas peptide #2 had a 3-fold lower affinity and peptide #4 a 157-fold lower affinity than the universal ligand, peptide #1 (Fig. 1 panel A1, Table 2). Peptide 16a, which is reported in some studies to be both a potent and selective hBRS-3 agonist [54,64], had low affinity (IC50 -3162 nM) for the hBRS-3 receptor on the native NCI-N417 cells, with its affinity being 2000-fold lower than the universal ligand, peptide #1 (Fig. 1 panel A1, Table 2).
Fig. 1.
The ability of GRP, NMB, [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) and various putative BRS-3 selective agonists including peptide #16a, to inhibit binding and to stimulate an increase in [3H]IP formation in N417 lung cancer cells natively expressing hBRS-3 or in H1299 lung cancer cells over-expressing hBRS-3. For binding (top panel) N417 cells (1.5 × 106 cell/ml or hBRS-3/H1299 cells (0.5 × 106 cells/ml) cells were incubated for 60 min at at 22°C with 50 pM I125- [DTyr6, βAla11,Phe13, Nle14]Bn(6-14), with or without the indicated concentrations of the various peptides added. Results are expressed as the percentage of saturable binding without unlabeled peptide added (percent control). Bottom panel, N417 cells or hBRS-3/H1299 cells were subcultured and preincubated for 24 h at 37°C with 3 uCi/ml myo-[2-3H]inositol. The cells were then incubated with the ligands at the concentrations indicated for 60 min at 37°C. Values expressed are a percentage of total [3H]IP release stimulated by 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14). Control and 1 μM [DTyr6, βAla11, Phe13,Nle14]Bn(6-14)-stimulated values for N417 lung cancer cells were 1362 ± 230 and 4952 ± 405 dpm, respectively and for H1299/BRS-3 cells were 1988 ± 220 and 10891 ± 540 dpm, respectively. Results are the mean ± SEM from at least four experiments, and in each experiment the data points were determined in duplicate. Numbers refer to the peptide number in Table 1. Abbreviations: See Table 1 legend.
Table 2.
Affinities and potencies of peptides #1 to #4, #16a, GRP and NMB for hBRS-3 expressed in N417 and H1299 cells.
| IC50 (nM) |
EC50 (nM) |
|||
|---|---|---|---|---|
| PEPTIDE NAME/NUMBER |
hBRS-3/N417 |
hBRS-3/H1299 |
hBRS-3/N417 |
hBRS-3/H1299 |
| 16a | 3162±124 | >10,000 | >10,000 | >10,000 |
| 1 | 1.5±0.1 | 0.60±0.03 | 3.3±0.2 | 0.69±0.03 |
| 2 | 4.3±0.2 | 29.5±2.3 | 12.3±0.9 | 10.0±0.9 |
| 3 | 1.4±0.2 | 10.0±0.8 | 33.1±1.9 | 16.6±0.8 |
| 4 | 250±12 | 572±63 | 52.5±3.2 | 398±21 |
| GRP | >10,000 | >10,000 | >10,000 | >10,000 |
| NMB | >10,000 | >10,000 | >10,000 | >10,000 |
Results are calculated from dose-response curves in Fig. 1 as described in Methods. Results are expressed as the peptide concentration causing half-maximal inhibition of binding (IC50) or the peptide concentration causing half maximal stimulation of [3H]IP (EC50). Results are means ± SEM from at least three experiments. Abbreviations: see Table 1.
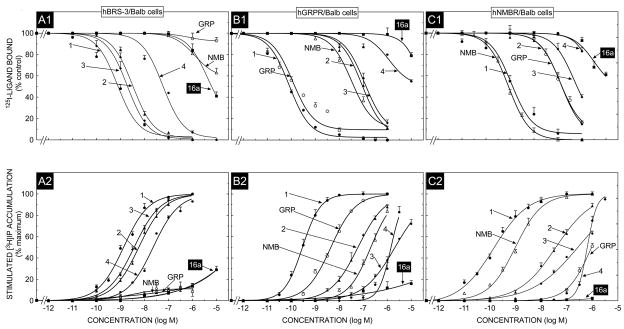
Similar results were obtained in the relative order of potencies of these peptides with hBRS-3 over-expressed in NCI-H1299 cells (Fig. 1 panel B1, Table 2) and with hBRS-3 expressed in Balb cells (Fig 2, panel A1, Table 3). Specifically, in each of these cell lines, the universal agonist (peptide # 1) bound with high affinity (0.85–1.6 nM) and peptide # 1 had 2.5–17 fold higher affinity than peptide #3, 4–49 fold higher than peptide #2 and 74–157 fold higher than peptide #4 (Fig. 1, panel B1, Fig. 2 panel A1, Tables 2 and 3). For each of these hBRS-3 containing cells, peptide #16a had >7000-fold lower affinity (IC50- 6026->10,000 nM) than the universal agonist, peptide #1 (Fig. 1, panel B1, Fig. 2 panel A1, Tables 2 and 3). Similarly, GRP and NMB had very low affinities (>10,000 nM) in both hBRS-3/H1299 cells and hBRS-3/Balb cells (Fig. 1, panel B1, Fig. 2 panel A1, Tables 2 and 3).
Fig. 2.
The ability of GRP, NMB, [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) and various putative BRS-3 selective agonists including peptide #16a, to inhibit binding and to stimulate an increase in [3H]IP formation at the hGRPR, hNMBR and hBRS-3 expressed in Balb 3T3 cells. In binding (top panel) Balb 3T3 cells stably transfected with hGRPR (0.3 × 106 cell/ml), hNMBR (0.03 × 106 cells/ml) or hBRS-3 (0.5 × 106 cells/ml) cells were incubated for 60 min at 22°C with 50 pM I125- [DTyr6, βAla11,Phe13, Nle14]Bn(6-14), with or without the indicated concentrations of the various peptides added. Results are expressed as the percentage of saturable binding without unlabeled peptide added (percent control). Bottom panel, Balb 3T3 cells transfected with hGRPR, hNMBR or hBRS-3 were subcultured and preincubated for 24 h at 37°C with 3 uCi/ml myo-[2-3H]inositol. The cells were then incubated with the ligands at the concentrations indicated for 60 min at 37°C. Values expressed are a percentage of total [3H]IP release stimulated by 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14). Control and 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14)-stimulated values for hGRPR were 3654 ± 560 and 16320 ± 4365 dpm, respectively; for hNMBR 2608 ± 450 and 80862 ± 4450 dpm, respectively; and for hBRS-3 1163 ± 230 and 5079 ±276 dpm, respectively. Results are the mean ± SEM from at least four experiments, and in each experiment the data points were determined in duplicate. Numbers refer to the peptide number in Table 1. Abbreviations: See Table 1 legend.
Table 3.
Affinities and potencies of peptides peptides #1 to #4, #16a, GRP and NMB for hBRS-3, hGRPR and hNMBR expressed in Balb cells.
| IC50 (nM) | EC50 (nM) | |||||
|---|---|---|---|---|---|---|
| PEPTIDE NAME/NUMBER |
hBRS-3/Balb |
hGRPR/Balb |
hNMBR/Balb |
hBRS-3/Balb |
hGRPR/Balb |
hNMBR/Balb |
| 16a | 6026±148 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 |
| 1 | 0.85±0.05 | 0.07±0.01 | 1.48±0.3 | 1.1 ± 0.1 | 0.45±0.02 | 0.16±0.01 |
| 2 | 3.39±0.15 | 110±5 | 158±8 | 5.9 ± 0.2 | 58±2.0 | 38±1.2 |
| 3 | 2.09±0.06 | 258±6 | 589±32 | 3.6 ± 0.1 | 977±56 | 219±12 |
| 4 | 63.1±3.1 | >10,000 | >10,000 | 21 ± 1 | 1480±89 | 661±27 |
| GRP | >10,000 | 0.17±0.01 | 155±9 | >10,000 | 4.8±0.2 | 933±74 |
| NMB | >10,000 | 45.2±2.8 | 0.79±0.21 | >10,000 | 209±14 | 1.2±0.1 |
Results are calculated from dose-response curves in Fig. 2 as described in Methods. Results are expressed as the peptide concentration causing half-maximal inhibition of binding (IC50) or the peptide concentration causing half maximal stimulation of [3H]IP (EC50). Results are means ± SEM from at least three experiments. Abbreviations: see Table 1.
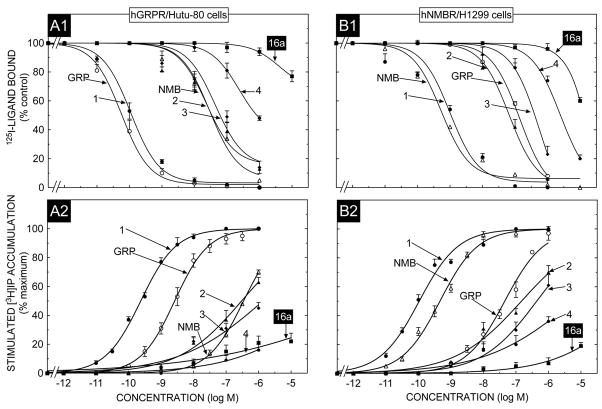
To investigate the selectivity in affinity of these peptides for hBRS-3 receptors over the human Bn receptors, binding studies were performed with both hGRPR- and hNMBR-containing cells using the same radioligand under similar binding conditions to that used in the above studies on hBRS-3 cells (Fig. 2, panels B1, C1; Fig. 3, panels A1, B1). In HuTu-80 cells which contain native hGRPRs [10,58], the natural ligand GRP and the universal agonist, peptide #1, bound with high affinity (IC50-0.05–0.1 nM); and were more than 300-times more potent than NMB. Each of the putative hBRS-3 selective ligands had much lower affinities than GRP or peptide #1, with peptide #2 having a 475-fold lower affinity, peptide #3 a 750-fold lower affinity, peptide #4 a 7700 fold lower affinity and peptide 16a >80,000-fold lower affinity (IC50>10,000 nM) (Fig. 3, panel A1, Table 4). With both the hNMBR/H1299 cells (Fig. 3, panel B1) and hNMBR/Balb cells (Fig. 2, panel C1) the natural ligand NMB and the universal ligand, peptide #1 had high affinities (IC50’s-0. 1–1.4 nM), whereas GRP had >100-fold lower affinity (IC 50’s-140–150 nM) (Table 3 and 4). Similarly each of the four putative hBRS-3 selective ligands had lower affinities than NMB with peptide #2 having a 200–1100 fold lower affinity, peptide #3 a 745–5900 fold lower, peptide #4 and #16 both having a >10,000 fold lower affinity (IC50>3000 nM) (Fig. 2, panel C1; Fig. 3, panel B1; Table 3 and 4).
Fig. 3.
The ability [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) and the putative BRS-3 selective agonist peptide #16a, to inhibit binding and to stimulate an increase in [3H]IP formation HuTu-80 cancer cells which natively express hGRPR or H1299 lung cancer cells in which hNMBR is over-expressed. In binding (top panel) HuTu-80 cancer cells (1.0 × 106 cell/ml), or hNMBR/H1299 lung cancer cells (0.4 × 106 cells/ml) cells were incubated for 60 min at 22°C with 50 pM I125- [DTyr6, βAla11,Phe13,Nle14]Bn(6-14), with or without the indicated concentrations of the various peptides added. Results are expressed as the percentage of saturable binding without unlabeled peptide added (percent control). Bottom panel, HuTu-80 cancer cells or hNMBR/H1299 lung cancer cells were subcultured and preincubated for 24 h at 37°C with 3 uCi/ml myo-[2-3H]inositol. The cells were then incubated with the ligands at the concentrations indicated for 60 min at 37°C. Values expressed are a percentage of total [3H]IP release stimulated by 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14). Control and 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14)-stimulated values for HuTu-80 cancer cells were 1195 ± 122 and 5150 ± 530 dpm, respectively and for hNMBR/H1299 cells. 1950 ± 82 and 20270 ± 2320 dpm, respectively. Results are the mean ± SEM from at least four experiments, and in each experiment the data points were determined in duplicate. Numbers refer to the peptide number in Table 1. Abbreviations: See Table 1 legend..
Table 4.
Affinities and potencies of peptides #1 to #4, #16a, GRP and NMB for hGRPR and hNMBR expressed in Hutu-80 and H1299 cells.
| IC50 (nM) |
EC50 (nM) |
|||
|---|---|---|---|---|
| PEPTIDE NAME/NUMBER |
hGRPR/Hutu-80 |
hNMBR/H1299 |
hGRPR/Hutu-80 |
hNMBR/H1299 |
| 16a | >10,000 | >10,000 | >10,000 | >10,000 |
| 1 | 0.12±0.01 | 1.3±0.1 | 0.26±0.01 | 0.093±0.006 |
| 2 | 57.5±2.8 | 66.1±3.4 | 318±25 | 195±11 |
| 3 | 91.2±4.4 | 355±23 | 4074±350 | 445±36 |
| 4 | 933±15 | 2951±105 | >10,000 | 4786±354 |
| GRP | 0.049±.002 | 141±8.7 | 2.8±0.2 | 602±42 |
| NMB | 37.2±5.2 | 0.063±0.004 | 348±18 | 0.490±0.028 |
Results are calculated from dose-response curves in Fig. 3 as described in Methods. Results are expressed as the peptide concentration causing half-maximal inhibition of binding (IC50) or the peptide concentration causing half maximal stimulation of [3H]IP (EC50). Results are means ± SEM from at least three experiments. Abbreviations: see Table 1.
The least selective ligand was the universal agonist, peptide #1, which had high affinity for all hBRS-3-, hGRPR- and hNMBR-containing cells and demonstrated in comparison to hBRS-3 a small selectivity for hGRPR (12–13 fold), but equal affinity for hNMBR (Tables 2–4). In contrast, in comparison with their affinities for hBRS-3, the natural ligands GRP and NMBR were highly selective (>3,000-fold) for their natural receptors, hGRPR and hNMBR, respectively (Tables 2–4). Peptides #2, #3, and #4 had a 13-, 65- and 4-fold selectivity, respectively for hBRS-3 in NCI-N417 cell over hGRPR natively expressed in Hutu cells, and a 32-, 123-, and >158-fold selectivity in hBRS-3 expressed in Balb cells over hGRPR in the same cells (Table 2 and 3). In comparison with hNMBR containing cells, peptides #2, #3, and #4 had a 47-, 282- and >158-fold selectivity for the hBRS-3 expressed in Balb cells than hNMBR in the same cells and a 15-, 254-, 12-fold selectivity for hBRS-3 natively expressed in NCI-N417 cells than the hNMBR expressed in NCI-H1299 cells (Tables 2–4). The selectivity of peptide #16a did not exceed >3.2 fold for hBRS-3 over hGRPR or hNMBR in any of the cells (Tables 2–4). Because peptide #16a had such low affinity for hBRS-3, hGRPR, and hNMBR in all cells examined, and despite including 16a concentrations as high as 10,000 nM in the assays (Fig. 1–3) (Table 2–4), the exact selectivity, if any, at very high peptide concentrations (i.e. >10 μM), could not be determined.
3.2. Ability of GRP, NMB, Peptide #1 and various putative BRS-3 selective agonists to activate phospholipase C (PLC) and stimulate an increase in [3H]IP generation in various cells expressing native or transfected human bombesin receptor subtypes
To assess the potencies of the various putative hBRS-3 selective agonists, we utilized the fact that each of the human Bn receptor subtypes is coupled to PLC activation [2,15,16,37,38], and the agonist’s abilities to stimulate [3H]IP generation in each of the hBRS-3-, hGRPR-, and hNMBR- containing cells (Fig. 1-panels A2, B2; Fig. 2-panels A2, B2, C2; Fig. 3-panels A2, B2) and compared to the results with the natural ligands (GRP, NMB) and the hBn receptor universal agonist ligand, peptide #1 (Table 1). For the hBRS-3 receptor containing cells, the universal agonist, peptide #1 caused a 4.4- fold increase in [3H]IP generation in hBRS-3/Balb cells, a 5.4- fold increase in hBRS-3/H1299 cells and an 3.6- fold increase in hBRS-3 containing NCI-N417 cells (Fig. 1-panel A2, B2 and Fig. 2-panel A2). In each of these hBRS-3 containing cells, peptides #2, #3, #4, and #16a were either full agonists or even at concentrations up to 10,000 nM, maximal effects were not reached, resulting in none functioning as a partial agonist (Fig. 1 and 2). A similar result was seen with the putative hBRS-3 selective ligands (#2,3,4,16a) in hGRPR- or hNMBR-containing cells, in which the natural ligands (GRP or NMB) or the universal ligand, peptide #1 were maximally effective (Fig. 2 and 3). The maximally effective ligands caused a 4.3- fold increase in [3H]IP generation in hGRPR containing HuTu-80 cells, 4.5-fold increase in hGRPR/Balb cells, 10-fold increase in hNMBR/H1299 cells and 31-fold in hNMBR/Balb cells. (Fig. 2 and 3).
With each of the three hBRS-3 containing cells (hBRS-3 Balb, H1299 NCI-417 cells), the universal agonist, peptide #1 was the most potent at activating PLC(EC50 -0.69–3.3 nM) and was 3.3–10 fold more potent than peptide #3, 4–14-fold more than peptide #2, 16–100-fold more potent than peptide #4 and >3000-fold more potent than peptide #16a (Fig. 1 and 2; Tables 2 and 3). With the hGRPR containing HuTu-80 and Balb cells, GRP and the universal agonist, peptide #1 were both potent (EC50-0.25–4.8 nM), with peptide #1 being 10–25, 123–270, 70–190 and >2000-fold more potent that peptide #2, #3, #4, and #16a (Fig. 2 panel-B2; Fig. 3 panel A2; Tables 3 and 4). Similarly, with the hNMBR containing H1299 and Balb cells, NMB and the universal agonist, peptide #1 were both potent (EC50-0.09–1.2 nM), with peptide #1 being >250-, >1000, >4000 and >6000-fold more potent that peptide #2, #3, #4, and #16a (Fig. 2 panel-C2; Fig. 3 panel B2; Tables 3 and 4). In terms of selectivity for hBRS-3 cells, similar to its binding affinities, the universal ligand, peptide #1, had high affinity for all Bn receptor containing cells and showed a 2–12-fold selectivity for hGRPR containing cells over hBRS-3 cells and a 7–35-fold selectivity for hNMBR containing cells over hBRS-3 cells (Tables 2–4). In contrast, peptides #2, #3, and #4 showed 10 to 26-, 123 to 271-, and 70 to >190-fold selectivity for hBRS-3 cells over hGRPR containing cells and a 6 to 16-, 13 to 61-, 32 to 91-fold selectivity for hBRS-3 containing cells over the hNMBR containing cells (Tables 2–4). Peptide 16a’s potencies were so low for activating PLC in each of the Bn receptor containing cells, that even when concentrations up 10 uM were used in the assays, it was not possible to obtain selectivity data based on its potencies (Fig. 1–3, Tables 2–4).
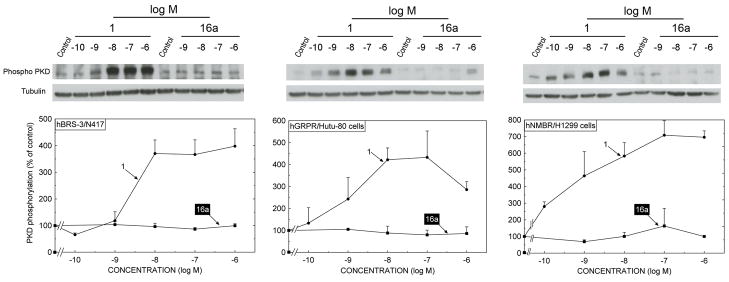
3.3. Ability of Peptides #1 and 16a to activate protein kinase D (PKD) in various cells expressing native or transfected human bombesin receptor subtypes
Because of the marked difference in our data showing peptide #16a had a very low potency for activating PLC in hBRS-3 native and transfected cells, whereas in some studies it was reported to have high potency for activating hBRS-transfected cells and causing other cell signaling changes [54,64], we decided to examine the ability of compound 16a to alter cellular function in more detail (Fig. 4 and 5). We did this by first examining its ability, in comparison to the universal agonist, peptide #1, to activate an important more, distal cellular signaling pathway than PLC, activation of protein kinase D (PKD) (Fig. 4) and determining its ability to cause a change in cellular behavior, by examining its ability to stimulate cell growth and thymidine incorporation (Fig. 5). PKD was chosen because it is a family of serine/threonine kinases which are activated by a large number of cellular stimulants including growth factors, bioactive lipids and some G protein coupled receptors, including members of the Bn receptor family [4,36,47,48]. PKD frequently is partially activated by stimulation of PLC mediated PKC activation and is involved in mediating numerous cellular processes including secretion, proliferation, invasions and apoptosis [4,36,47,48]. The universal agonist, peptide #1 stimulated greater than a 4-fold activation of hBRS-3 natively expressed in NCI-N417 cells (Fig. 4, left panel), hGRPR natively expressed in Hutu-80 cells (Fig. 4, middle panel) and hNMBR over-expressed in H1299 cells (Fig. 4, right panel), assessed by determining the phosphorylation of PKD serine residues 744/748 whose phosphorylation correlates with activation of the PKD [4,50]. Peptide #1 caused detectable activation at 0.1 to 1 nM in the three different hBn receptor-containing cells and maximal activation by 10 nM (Fig. 4). In contrast, in neither hBRS-3 containing NCI-N417 cells nor in hGRPR/HuTu-80 cells or hNMBR/H1299 cells, did peptide #16a, even at concentrations up to 1 μM stimulate PKD activation (Fig. 4). These results demonstrate compound #16a was >1000 fold less potent that peptide #1 for activating PKD, and did not establish it had any agonist activity for activating PKD over the concentration range used.
Fig. 4.
The ability [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) and the putative BRS-3 selective agonist peptide #16a, to stimulate activation of protein kinase D in hBRS-3/N417 cells, hGRPR/HuTu-80 cells and hNMBR/1299 cells. Each of the cells was incubated with the indicated concentration of [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) or peptide #16a for 5 minutes and the activation of PKD was assessed by determining the extent of formation of phospho-PKD (Ser744/748) and the gel loading assessed by determining tubulin. A representative result from three experiments is shown in the top panel. The average result from densitometric analysis of at least 3 experiments (mean ± SEM) is shown in the bottom panel. Results are expressed as the percentage of the control value.
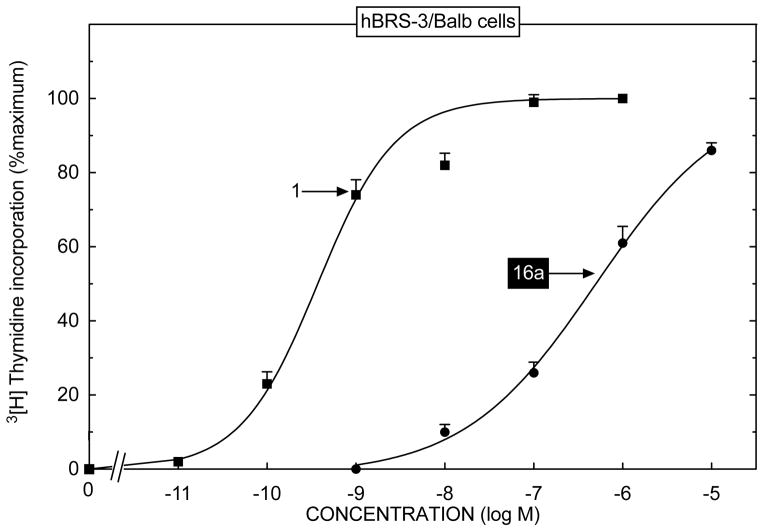
Fig. 5.
The ability [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) and the putative BRS-3 selective agonist peptide #16a, to stimulate activation of growth of hBRS-3/Balb cells. hBRS-3/Balb cells were incubated with the indicated concentration of [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1) or peptide #16a and the affect on [3H]thymidine incorporation determined as described in Methods. Results are means ± SEM from four experiments and are expressed as the percentage of the increase caused by 1 μM [DTyr6, βAla11,Phe13,Nle14]Bn(6-14) (Peptide # 1). Mean control and 1 μM [DTyr6, βAla11,Phe13, Nle14]Bn(6-14)-stimulated values hBRS-3/Balb cells were 2371 ± 92 and 3244 ± 62 cpm, respectively.
3.4. Ability of Peptides #1 and 16a stimulate growth of hBRS-3/BALB cells
In hBRS-3/Balb cells peptide #1 caused detectable [3H]thymidine uptake at 0.1 nM, maximal stimulation at 10 nM and had a half-maximal effect (EC 50) at 0.8 ± 1 nM (Fig. 5). In contrast, peptide #16a had a >800-fold lower potency with an EC50 of 650 ± 117 nM (Fig. 5).
4. Discussion
The purpose of this study was to assess the affinities, potencies and selectivities of recently described putative potent, selective agonists for the human orphan receptor, hBRS-3, compared to other members of the human bombesin (Bn) receptor family. This study was performed because of the recent increasing interest in the hBRS-3 receptor. This is occurring because of pharmacological studies, as well as studies of hBRS-3 knockout mice [15,56], providing strong support for the importance of this receptor in regulating glucose homeostasis and insulin secretion [15,30,31], energy metabolism and obesity [12,15,30,31], tumor growth [15,18,57], bronchial epithelial cell proliferation, lung development and lung injury [15,41–43,53], and in regulating gastrointestinal motility [32]. Because the natural ligand has not yet been identified, the availability of pharmacological tools (selective agonists, antagonists) will be particularly important in studying this receptor. A significant advance was made when it was discovered that the bombesin synthetic analogue, [DTyr6, βAla11, Phe13, Nle14]Bn(6-14) (peptide # 1, Table 1), functions as a high affinity ligand and agonist at the hBRS-3 receptor [27,37,38]. Unfortunately, subsequently this peptide and its related DPhe6 analogue were found to be universal ligands/agonists with high affinity for all subtypes of human Bn receptors (hGRPR, hNMBR, hBRS-3) [27,33]. Subsequently, a number of studies [5,9,24–26,54,55,64] using this compound as a prototype, have described a number of peptides having selectivity and moderate to high affinity/potency for the hBRS-3 receptor over the other hBn receptors. Unfortunately, there is no general agreement on the selectivity and potency of a number of these putatively potent and selective hBRS-3 peptides with some studies finding particular analogues are potent and selective for the hBRS-3 receptor, whereas others report they have very low hBRS-3 receptor selectivity and/or potency [5,9,24–26,54,55,64]. At present it is not clear why this variability is occurring and it makes it difficult to determine which, if any selective ligand should be used in a given study. Possible sources of this variation among studies include different hBRS-3 transfected cells are used, different assay methods and different experimental conditions. In many studies affinities from binding studies have not been performed and only potencies from various signaling cascades were assessed. Also, no studies were performed using non-BRS-3 transfected cells that natively possess the hBRS-3. The fact that all studies used hBRS-3 transfected cells can be a particular source of variation, because a number of studies demonstrate that receptor expression density of various G-protein coupled receptors can have marked effects on receptor affinities, potencies and efficacies for intracellular signaling cascades as well as cellular responses [20,21,44,46,59,65]. Furthermore, varying G-protein coupled receptor expression densities can have different affects on different intracellular cascades or cellular responses, and therefore can be an important variable in determining the absolute and relative potency of an agonist [44]. The assessment of potencies from assessing different intracellular signaling cascades rather than by assessing affinities from binding studies can also lead to marked variation in the relative and/or absolute affinities of different agonists, because differing degrees of receptor spareness for various signaling cascades (activation of PLC, calcium mobilization, tyrosine kinase stimulation) have been described for a given Bn receptor, as well as other G-protein coupled receptors and the magnitude of spareness can also vary from one Bn receptor to the next [14,17,45]. Lastly, with Bn receptors some peptides can behave as biased agonists causing partial or full activation of some signaling cascades with no stimulation of others and this also can contribute to variation in potencies for a give cascade for a given agonist [15,22].
The present study was designed in a number of ways to overcome the limitations listed above. First, the study was done by assessing the abilities of these peptides to interact with and activate the different Bn receptors in a number of carefully selected hBn receptor-containing cells. This was accomplished by using Bn receptor bearing cells that had been produced/identified by three different strategies, so their pharmacology would reflect native Bn receptor cells. For the first time interaction with the hBRS-3 receptor was assessed using NCI-N417 cells which possess native hBRS-3 receptors [38] and with the hGRPR using HuTu cells which natively express this receptor [10,58]. hGRPR -, hBRS-3, and hNMBR expressed in Balb 3T3 cells were studied also, because previous studies show Bn receptors transfected into these cells behave in a similar fashion to native Bn receptor bearing cells [1–3,27,37,38]. Lastly, hBRS-3 and hNMBR natively occur in such low numbers in NCI-H1299 cells that binding studies cannot be performed. Accordingly, each receptor was over-expressed at low levels equal to those that are seen in other native Bn receptor containing cells and sufficient to perform binding studies [10,27]. Second, binding studies were performed on all cells to directly assess affinities under identical conditions using the same ligand for all cells. Third, two different principal intracellular cascades, which are activated by all Bn receptors were studied (PLC, and protein kinase D activation)[4,36,47,48]. Fourth, a cellular response to hBRS-3 was examined by measuring the growth response assessed by measuring [3H]thymidine incorporation.
In the present study, four putative, selective HBRS-3 agonists were examined with particular attention paid to peptide #16a (Table 1). Peptide #16a was examined in more detail because it is reported to have particularly high potency and selectivity for hBRS-3 [54,64]. In studies using hBRS-3 expressed in CHO K1 cells or HEK293 cells, peptide #16a was reported to have a potency of 2.1–14 nM as assessed using a calcium FLIPR assay and >1000 fold selectivity for hBRS-3 over hGRPR or hNMBR [54,64]. In the present study two of the putative hBRS-3 agonists, peptides #2 and #3 (Table 1), had nM affinities from binding studies for the hBRS-3 receptor in each of the 3 hBRS-3 cell lines studied (NC-N417, Balb, H1299), with peptide #4 having moderate-low affinities (63–570 nM) and peptide #16a having very low affinities (3000->10,000). Similar potencies were also found for the abilities of these putative hBRS-3 selective agonists to activate the hBRS-3 receptors and stimulate phospholipase C activation, which is the principal intracellular signaling cascade for this receptor, as it is for the other human Bn receptors [15,37,38,60]. These results demonstrated that peptide #16a had low affinity and potency for activating hBRS-3 receptors on all three hBRS-3 containing cells examined. We further investigated the agonist potencies of peptide #16a in altering other cellular functions its ability to activate PKD, another more distal major signaling cascade involved in mediating hBRS-3 and other Bn receptor’s effects on cellular function [4,36,47,48], as well as its ability to stimulate growth and [3H]thymidine incorporation in hBRS-3/Balb cells. A result similar to its ability to activate PLC was found for peptide 16#a to stimulate PKD activation in hBRS-3/N417 cells where it was inactive up to concentrations of 1 μM. It was therefore greater than 1000-fold less potent than the universal agonist, peptide #1, at activating PKD in these cells. Similarly, peptide #16a had only weak agonist activity on stimulating [3H]thymidine incorporation in hBRS-3/Balb cells where it did not cause significant stimulation until concentrations >100 nM and was >800-fold less potent than the universal agonist, peptide #1 (Table 1). The above results demonstrate that peptides #2, #3 and possibly #4, but not peptide #16a (Table 1) have sufficient affinities and potencies for hBRS-3 receptors to be potentially generally useful.
The other major determinant of usefulness for these hBRS-3 ligands is their degree of selectivity for hBRS-3 over other human Bn receptors (hGRPR, hNMBR). This is an important point because in many cells in both the CNS as well as peripheral tissues, the different subtypes of Bn receptors are often expressed together, as is the case in a number of different tumors [7,15,18,39,49]. The relative order of selectivity for hBRS-3 over hGRPR in the different cells based on binding affinities was peptide #3 (65 to120-fold selective)>peptide #4, (4 to150-fold), peptide #2 (13–32-fold) and with peptide #16a, its affinities were too low to allow a precise determination of its hBRS-3/NMBR selectivity. The relative order of selectivity for hBRS-3 over the hNMBR was peptide #3(250-fold)>peptide #2, (15 to 46 fold) >peptide #4 (12 to 150-fold). With peptide #16a, affinities were too low to allow a precise determination of its hBRS-3/NMBR selectivity to be made. Similar results were found for their relative potencies for activating PLC in the different Bn receptor containing cells. These results support the conclusion that peptide #3 is likely the most useful hBRS-3 agonist available at present. Its general usefulness however, still may be limited by its selectivity, which is relatively modest compared to most generally widely used selective agonists.
5. Conclusion
This study evaluated in detail the ability of existing putative hBRS-3 potent and selective agonists to interact with hBRS-3 and other human Bn receptors (hGRPR, hNMBR) using a number of different hBn receptor-bearing native and transfected cell lines to perform both bind studies and evaluate potencies for activating intracellular signaling cascades and causing cell growth. The results demonstrate demonstrating some of the putative hBRS-3 selective peptides (peptide 16a, #4) have such low affinity that they will not be generally useful. It also demonstrates that peptide #3 (Table 1) is the most selective, potent hBRS-3 agonist available at present, however, its moderate selectivity (100-fold), may limit its usefulness, compared to a number of classes of hGRPR and hNMBR antagonists which are highly selective (>1000-fold) [10,15,51,52]. This study demonstrates the importance of the need for full characterization of putative selective agonist with assessments of both binding affinities as well as potencies for altering cellular signaling/and/or biologic activity on a number of different cells and/or cell lines to critically assess their potential usefulness.
Acknowledgments
This work was partially supported by intramural funds of NIDDK and NCI, NIH, and Tulane University Peptide Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benya RV, Fathi Z, Pradhan T, Battey JF, Kusui T, Jensen RT. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization and growth: Possible role of cAMP. Mol Pharmacol. 1994;46(2):235–45. [PubMed] [Google Scholar]
- 2.Benya RV, Kusui T, Pradhan TK, Battey JF, Jensen RT. Expression and characterization of cloned human bombesin receptors. Mol Pharmacol. 1995;47:10–20. [PubMed] [Google Scholar]
- 3.Benya RV, Wada E, Battey JF, Fathi Z, Wang LH, Mantey SA, et al. Neuromedin B receptors retain functional expression when transfected into BALB 3T3 fibroblasts: analysis of binding, kinetics, stoichiometry, modulation by guanine nucleotide-binding proteins, and signal transduction and comparison with natively expressed receptors. Mol Pharmacol. 1992;42(6):1058–68. [PubMed] [Google Scholar]
- 4.Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, et al. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta. 2007;1773:483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle RG, Humphries J, Mitchell T, Showell GA, Apaya R, Iijima H, et al. The design of a new potent and selective ligand for the orphan bombesin receptor subtype 3 (BRS3) J Pept Sci. 2005;11:136–41. doi: 10.1002/psc.599. [DOI] [PubMed] [Google Scholar]
- 6.Coll AP. Treating Obesity? It’s in the Bag! Cell Metab. 2010;11:95–6. doi: 10.1016/j.cmet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, et al. BRS-3: novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268(8):5979–84. [PubMed] [Google Scholar]
- 8.Fleischmann A, Laderach U, Friess H, Buechler MW, Reubi JC. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Lab Invest. 2000;80:1807–17. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez N, Hocart SJ, Portal-Nunez S, Mantey SA, Nakagawa T, Zudaire E, et al. Molecular basis for agonist selectivity and activation of the orphan bombesin receptor subtype 3 receptor. J Pharmacol Exp Ther. 2008;324:463–74. doi: 10.1124/jpet.107.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez N, Mantey SA, Pradhan TK, Sancho V, Moody TW, Coy DH, et al. Characterization of putative GRP- and NMB-receptor antagonist’s interaction with human receptors. Peptides. 2009;30:1473–86. doi: 10.1016/j.peptides.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan XM, Chen H, Dobbelaar PH, Dong Y, Fong TM, Gagen K, et al. Regulation of Energy Homeostasis by Bombesin Receptor Subtype-3: Selective Receptor Agonists for the Treatment of Obesity. Cell Metab. 2010;11:101–12. doi: 10.1016/j.cmet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Hou X, Wei L, Harada A, Tatamoto K. Activation of bombesin receptor subtype-3 stimulates adhesion of lung cancer cells. Lung Cancer. 2006;54:143–8. doi: 10.1016/j.lungcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Jensen RT. Receptors on pancreatic acinar cells. In: Johnson LR, Jacobson ED, Christensen J, Alpers DH, Walsh JH, editors. Physiology of the Gastrointestinal Tract. 3. Vol. 2. New York: Raven Press; 1994. pp. 1377–446. [Google Scholar]
- 15.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen RT, Coy DH, Saeed ZA, Heinz-Erian P, Mantey S, Gardner JD. Interaction of bombesin and related peptides with receptors on pancreatic acini. Ann N Y Acad Sci. 1988;547:138–49. doi: 10.1111/j.1749-6632.1988.tb23882.x. [DOI] [PubMed] [Google Scholar]
- 17.Jensen RT, Moody T, Pert C, Rivier JE, Gardner JD. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1978;75:6139–43. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: effects on cancer growth/proliferation and cellular signaling in cancer. In: Kastin AJ, editor. Handbook of Biologically active peptides. Amsterdam: Elsevier; 2006. pp. 429–34. [Google Scholar]
- 19.Ladenheim EE, Hamilton NL, Behles RR, Bi S, Hampton LL, Battey JF, et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149:971–8. doi: 10.1210/en.2007-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesage AS, Wouters R, Van Gompel P, Heylen L, Vanhoenacker P, Haegeman G, et al. Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines. Br J Pharmacol. 1998;123:1655–65. doi: 10.1038/sj.bjp.0701766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacEwan DJ, Kim GD, Milligan G. Analysis of the role of receptor number in defining the intrinsic activity and potency of partial agonists in neuroblastoma X glioma hybrid NG108-15 cells transfected to express differing levels of the human β2-adrenoceptor. Mol Pharmacol. 1995;48:316–25. [PubMed] [Google Scholar]
- 22.MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J Biol Chem. 2001;276:28083–91. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- 23.Mantey S, Frucht H, Coy DH, Jensen RT. Characterization of bombesin receptors using a novel, potent, radiolabeled antagonist that distinguishes bombesin receptor subtypes. Mol Pharmacol. 1993;45:762–74. [PubMed] [Google Scholar]
- 24.Mantey SA, Coy DH, Entsuah LK, Jensen RT. Development of bombesin analogs with conformationally restricted amino acid substitutions with enhanced selectivity for the orphan receptor human bombesin receptor subtype 3. J Pharmacol Exp Ther. 2004;310:1161–70. doi: 10.1124/jpet.104.066761. [DOI] [PubMed] [Google Scholar]
- 25.Mantey SA, Coy DH, Pradhan TK, Igarashi H, Rizo IM, Shen L, et al. Rational design of a peptide agonist that interacts selectively with the orphan receptor, bombesin receptor subtype 3. J Biol Chem. 2001;276:9219–29. doi: 10.1074/jbc.M008737200. [DOI] [PubMed] [Google Scholar]
- 26.Mantey SA, Gonzalez N, Schumann M, Pradhan TK, Shen L, Coy DH, et al. Identification of bombesin receptor subtype-specific ligands: effect of N-methyl scanning, truncation, substitution, and evaluation of putative reported selective ligands. J Pharmacol Exp Ther. 2006;319:980–9. doi: 10.1124/jpet.106.107011. [DOI] [PubMed] [Google Scholar]
- 27.Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, et al. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. J Biol Chem. 1997;272(41):26062–71. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Yamada K, Wada E, Hasegawa T, Usui Y, Wada K. Bombesin receptor subtype-3 modulates plasma insulin concentration. Peptides. 2003;24:83–90. doi: 10.1016/s0196-9781(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 29.Moody TW, Mantey SA, Pradhan TK, Schumann M, Nakagawa T, Martinez A, et al. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. J Biol Chem. 2004;279:23580–9. doi: 10.1074/jbc.M401938200. [DOI] [PubMed] [Google Scholar]
- 30.Nakamichi Y, Wada E, Aoki K, Ohara-Imaizumi M, Kikuta T, Nishiwaki C, et al. Functions of pancreatic beta cells and adipocytes in bombesin receptor subtype-3-deficient mice. Biochem Biophys Res Commun. 2004;318:698–703. doi: 10.1016/j.bbrc.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 31.Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997;390(6656):165–9. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- 32.Porcher C, Juhem A, Peinnequin A, Bonaz B. Bombesin receptor subtype-3 is expressed by the enteric nervous system and by interstitial cells of Cajal in the rat gastrointestinal tract. Cell Tissue Res. 2005;320:21–31. doi: 10.1007/s00441-004-1032-1. [DOI] [PubMed] [Google Scholar]
- 33.Pradhan TK, Katsuno T, Taylor JE, Kim SH, Ryan RR, Mantey SA, et al. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. Eur J Pharmacol. 1998;343:275–87. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- 34.Reubi JC, Wenger S, Schumuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radoligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–46. [PubMed] [Google Scholar]
- 35.Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang LH, et al. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol. 1990;259:G655–G665. doi: 10.1152/ajpgi.1990.259.4.G655. [DOI] [PubMed] [Google Scholar]
- 36.Rozengurt E, Guha S, Sinnett-Smith J. Gastrointestinal peptide signalling in health and disease. Eur J Surg Suppl. 2002:23–38. [PubMed] [Google Scholar]
- 37.Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, Battey JF, et al. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J Biol Chem. 1998;273:13613–24. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- 38.Ryan RR, Weber HC, Mantey SA, Hou W, Hilburger ME, Pradhan TK, et al. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther. 1998;287:366–80. [PubMed] [Google Scholar]
- 39.Sano H, Feighner SD, Hreniuk DL, Iwaasa H, Sailer AW, Pan J, et al. Characterization of the bombesin-like peptide receptor family in primates. Genomics. 2004;84:139–46. doi: 10.1016/j.ygeno.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Schulz S, Rocken C, Schulz S. Immunohistochemical detection of bombesin receptor subtypes GRP-R and BRS-3 in human tumors using novel antipeptide antibodies. Virchows Arch. 2006;449:421–7. doi: 10.1007/s00428-006-0265-7. [DOI] [PubMed] [Google Scholar]
- 41.Shan L, Emanuel RL, Dewald D, Torday JS, Asokanathan N, Wada K, et al. Bombesin-like peptide receptor gene expression, regulation, and function in fetal murine lung. Am J Physiol (Lung Cell Mol Physiol) 2004;286:L165–L173. doi: 10.1152/ajplung.00436.2002. [DOI] [PubMed] [Google Scholar]
- 42.Tan YR, Qi MM, Qin XQ, Xiang Y, Li X, Wang Y, et al. Wound repair and proliferation of bronchial epithelial cells enhanced by bombesin receptor subtype 3 activation. Peptides. 2006;27:1852–8. doi: 10.1016/j.peptides.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Tan YR, Qin XQ, Xiang Y, Yang T, Qu F, Wang Y, et al. PPARalpha and AP-2alpha regulate bombesin receptor subtype 3 expression in ozone-stressed bronchial epithelial cells. Biochem J. 2007 doi: 10.1042/BJ20061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997;138:4109–22. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda T, Jensen RT. Neuromedin B causes tyrosine phosphorylation (TYR-P) of p125 focal adhesion kinase (p125FAK) through a Ca2+- and PKC-independent mechanism which is partially mediated by the small GTP-binding protein rho. Gastroenterology. 1996;110:A1127. Ref Type: Abstract. [Google Scholar]
- 46.Tsuda T, Kusui T, Hou W, Benya RV, Akeson MA, Kroog GS, et al. Effect of gastrin-releasing peptide receptor number on receptor affinity, coupling, degradation and receptor modulation. Mol Pharmacol. 1997;51(5):721–32. doi: 10.1124/mol.51.5.721. [DOI] [PubMed] [Google Scholar]
- 47.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A. 1994;91:8572–6. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, et al. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- 49.Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci. 1990;10:2917–30. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem. 2001;276:32606–15. doi: 10.1074/jbc.M101648200. [DOI] [PubMed] [Google Scholar]
- 51.Wang LH, Coy DH, Taylor JE, Jiang NY, Kim SH, Moreau JP, et al. Desmethionine alkylamide bombesin analogues: a new class of bombesin receptor antagonists with a potent antisecretory activity in pancreatic acini and antimitotic activity in Swiss 3T3 cells. Biochemistry (Mosc) 1990;29(3):616–22. doi: 10.1021/bi00455a004. [DOI] [PubMed] [Google Scholar]
- 52.Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, et al. Des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem. 1990;265(26):15695–703. [PubMed] [Google Scholar]
- 53.Wang Y, Zhang M, Tan Y, Xiang Y, Liu H, Qu F, et al. BRS-3 activation transforms the effect of human bronchial epithelial cells from PGE2 mediated inhibition to TGF-beta1 dependent promotion on proliferation and collagen synthesis of lung fibroblasts. Cell Biol Int. 2007;31:1495–500. doi: 10.1016/j.cellbi.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Weber D, Berger C, Eickelmann P, Antel J, Kessler H. Design of selective peptidomimetic agonists for the human orphan receptor BRS-3. J Med Chem. 2003;46:1918–30. doi: 10.1021/jm0210921. [DOI] [PubMed] [Google Scholar]
- 55.Weber D, Berger C, Heinrich T, Eickelmann P, Antel J, Kessler H. Systematic optimization of a lead-structure identities for a selective short peptide agonist for the human orphan receptor BRS-3. J Pept Sci. 2002;8:461–75. doi: 10.1002/psc.407. [DOI] [PubMed] [Google Scholar]
- 56.Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16:66–71. doi: 10.1097/med.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- 57.Weber HC, Walters J, Leyton J, Casibang M, Purdom S, Jensen RT, et al. A bombesin receptor subtype-3 peptide increases nuclear oncogene expression in a MEK-1 dependent manner in human lung cancer cells. Europ J Pharmacol. 2001;412:13–20. doi: 10.1016/s0014-2999(00)00941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams BY, Schonbrunn A. Bombesin receptors in a human duodenal tumor cell line: binding properties and function. Cancer Res. 1994;54(3):818–24. [PubMed] [Google Scholar]
- 59.Wu JM, Hoang DO, Feldman RI. Differential activation of human gastrin-releasing peptide receptor-mediated responses by bombesin analogs. Mol Pharmacol. 1995;47(4):871–81. [PubMed] [Google Scholar]
- 60.Wu JM, Nitecki DE, Biancalana S, Feldman RI. Discovery of high affinity bombesin receptor subtype 3 agonists. Mol Pharmacol. 1996;50(5):1355–63. [PubMed] [Google Scholar]
- 61.Yamada K, Ohki-Hamazaki H, Wada K. Differential effects of social isolation upon body weight, food consumption, and responsiveness to novel and social environment in bombesin receptor subtype-3 (BRS-3) deficient mice. Physiol Behav. 2000;68:555–61. doi: 10.1016/s0031-9384(99)00214-0. [DOI] [PubMed] [Google Scholar]
- 62.Yamada K, Santo-Yamada Y, Wada E, Wada K. Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry. 2002;7:113–7. 6. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- 63.Yamada K, Wada E, Imaki J, Ohki-Hamazaki H, Wada K. Hyperresponsiveness to palatable and aversive taste stimuli in genetically obese (bombesin receptor subtype-3-deficient) mice. Physiol Behav. 1999;66:863–7. doi: 10.1016/s0031-9384(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Nothacker HP, Wang Z, Bohn LM, Civelli O. Pharmacological characterization of a selective agonist for bombesin receptor subtype-3. Biochem Biophys Res Commun. 2009;387:283–8. doi: 10.1016/j.bbrc.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994;46(3):460–9. [PubMed] [Google Scholar]