Abstract
Mutations of the FLT3 receptor tyrosine kinase consisting of internal tandem duplications (ITD) have been detected in blasts from 20–30% of patients with acute myeloid leukemia (AML) and are associated with a poor prognosis. FLT3/ITD results in constitutive auto-phosphorylation of the receptor and factor-independent survival in leukemia cell lines. The C-28 methyl ester of the oleane triterpenoid (CDDO-Me) is a multifunctional molecule that induces apoptosis of human myeloid leukemia cells. Here we report that CDDO-Me blocks targeting of NFκB to the nucleus by inhibiting IKKβ-mediated phosphorylation of IκBα. Moreover, CDDO-Me blocked constitutive activation of signal transducer and activator of transcription 3 (STAT3). We report the potent and selective anti-proliferative effects of CDDO-Me on FLT3/ITD-positive myeloid leukemia cell lines and primary AML cells. The present studies demonstrate that CDDO-Me treatment results in caspase-3-mediated induction of apoptosis of FLT3/ITD expressing cells and its anti-proliferative effects are synergistic with PKC412, a FLT3-tyrosine kinase inhibitor currently in clinical trials. Taken together, our studies indicate that CDDO-Me greatly enhanced the efficacy of the FLT3 inhibitor PKC412, suggesting that combining two separate pathway inhibitors may be a viable therapeutic strategy for AML associated with a FLT3/ITD mutation.
Keywords: FLT3-ITD, AML, STAT3, apoptosis
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by the accumulation of immature hematapoietic stem cells in the marrow with resultant bone marrow failure (1,2). The accumulation of such immature cells is presumed to be in part due to disordered hematopoietic differentiation mechanisms, overabundant proliferation, and/or failure to undergo apoptosis. Many potentially leukemogenic pathogenetic alterations have been described, some of which provide therapeutic targets (3). One important genetic alteration that leads to accelerated proliferation in hematopoietic stem cells is an activating mutation of the FLT3 receptor tyrosine kinase, present in blasts from approximately 35% of patients with AML (4). Two distinct types of FLT3 mutations have been identified in AML patients. Internal Tandem Duplication (ITD) mutations, characterized by a repeat of between 3 and more than 100 amino acids within the juxtamembrane domain, are found in about 25–30% of AML patients (5,6). This extra sequence appears to be responsible for disruption of the auto-inhibitory activity of the juxtamembrane domain resulting in the constitutive activation of FLT3 and an adverse prognosis (particularly if present in homozygous form (7)) in patients with AML (8). Blasts from another 10% of patients with AML harbor a point mutation in the tyrosine kinase domain, typically a D835Y mutation (9). The prognostic impact of tyrosine kinase domain activating mutations is unclear (10,11). FLT3 inhibitors (12), including PKC412 (midostaurin) (13), CEP-701 (14) and sorafenib (15,16) are being developed as potential therapeutic agents in mutant FLT3 AML.
In normal myeloid cells, ligand binding to wild-type FLT3 activates multiple signals including the PI3K/AKT, RAF/MAPK and STAT pathways. In leukemic cells with FLT3-ITD, these pathways are constitutively activated (17). The mechanism of FLT3/ITD induced proliferation is due in part to the activation via phosphorylation of the PI3K/AKT pathway, noted in both transformed BaF3 human AML cell lines and cells from AML patients (18–20).
The STAT family of transcription factors and NFκB are activated by separate upstream signals and are implicated in multiple processes required for neoplasia including transformation, tumor cell survival and invasion/metastasis (21,22). STAT3 activation has also been thought to be pleiotropically important in carcinogenesis (23). STAT3 is a transcription factor originally identified as a mediator of the acute phase of the inflammatory response (24). Moreover, constitutive STAT activity has been observed in approximately 50% of patients with AML (25) and is associated with adverse treatment outcomes (26). Upon activation STAT3 dimerizes and translocates to the nucleus to activate target genes, including regulators of cell cycle progression and inhibitors of apoptosis (27). Inhibiting downstream pathways such as the JAK family has been suggested to be a strategy that could enhance the effects of tyrosine kinase inhibition.
A potential inhibitor of STAT activation is CDDO-Me, belonging to a new class of agents that have antiproliferative and pro-apoptotic activity (28). CDDO-Me is being developed for use in a wide variety of human neoplasms and is currently in phase I and phase II clinical trials. CDDO-Me induces apoptosis in AML, myeloma, and diverse solid tumors (29). One mechanism of resistance to apoptosis in malignant cells may be related to inhibition of reactive oxygen species which normally accumulate during stress. However, when cells are treated with high concentrations of CDDO-Me, apoptosis is triggered by an increase in reactive oxygen species (30). CDDO-Me is also associated with a direct or indirect inhibition of IκB kinase β, thereby preventing the phosphorylation of the inhibitory IκBα moiety. Non-phosphorylated IkBα directly interacts with NFκB causing inhibition of NFκB translocation to nucleus, interfering with the pro-proliferatory function of this moiety (31). Previous studies have also shown that treatment of U937 myelomonocytic leukemia cells with CDDO-Me is associated with significant inhibition of STAT3 activity and NFκB function (32).
The present studies have examined whether dual pathway inhibition with a FLT3/ITD inhibitor, such as PKC412, in combination with the STAT3 inhibitor CDDO-Me, might result in a synergistic level of growth arrest and apoptosis. Synergy has already been demonstrated between protein tyrosine kinase inhibitors and downstream inhibitors such as RAD001 or rapamycin which inhibit mTOR, a critical member of the PI3K/AKT pathway (33–35). Therefore targeting both the mutant oncogene and the critical downstream pathway responsible for enhancing the viability of the leukemic cells may be synergistic. Our results show that BaF3/FLT3/ITD cells possess constitutive STAT3 activity and nuclear NFκB expression. Treatment of leukemic cells with CDDO-Me results in significant inhibition of STAT3 activation, loss of nuclear translocation of NFκB, and an inhibition of proliferation. Combining CDDO-Me with the FLT3/ITD inhibitor results in a synergistic loss of growth potential and induction of apoptosis in human leukemic cell lines and primary patient cells.
Materials and Methods
Cell culture and reagents
The murine IL-3-dependent hematopoietic cell line, BaF3/FLT3-wt, the IL-3-independent BaF3/FLT3/ITD, Mv4-11 and MOLM-14 cell lines were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 2 ng/ml murine IL-3 (BaF3/FLT3-wt cells only; R&D Systems, Inc., Minneapolis, MN), 100 units/ml penicillin, 100 mg/ml streptomycin and 2 mM L-Glutamine. The FLT3 inhibitor PKC412 (N-benzoyl-staurosporine) (36), was obtained from Novartis and CDDO-Me (Methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate) (37), was provided by Reata Pharmaceuticals. BaF3/FLT3-wt, Mv4-11, MOLM-14 or BAF3/FLT3/ITD cells were cultured at a starting density of 2 × 105 cells/ml in RPMI 1640 with 20 ng/ml IL-3 (BAF3/FLT3) for 24 hours before treatments. To determine the effects of the inhibitors of FLT3/ITD or downstream pathways on proliferation or apoptosis, FLT3/ITD inhibitor PKC412 (5 nM and 10 nM) and 1–5 μM CDDO-Me were added 24 hours later for different time intervals.
Cell viability and apoptosis assays
The cells were grown and treated with different inhibitors for varying intervals of time as described above. Cell counts for proliferation studies were determined using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega), according to manufacturer’s instructions. The cells were stained with Annexin V-FITC and propidium iodide (PI) before flow cytometry analysis was performed. The analysis of protein using flow cytometry included cell fixing, permeabilization and immunostaining. The fix buffer, permeabilizing buffer and washing/staining buffer were purchased from SantaCruz Biotechnology and the process is performed according to the manufacturer’s recommendations.
AML patient cells
Patient one was an 89 year old man with AML (FLT3 wt, complex karyotype) whose marrow showed background dysplasia; patient two a 74 year old male with M1 AML (FLT3 wt, normal karyotype) and patient three a 54 year old male with M4 AML (FLT3-ITD 165 bp, normal karyotype). The patient’s blood or bone marrow samples were diluted 2:1 with PBS or with basic media without serum, gently mixed it by inverting the tubes 8 to 10 times, carefully layered on ficoll-paque and spun down at room temperature for 20 minutes at 2000 rpm. The layer of cells was removed using a sterile transfer pipette and transferred to a 15 ml tube. The cells were mixed with 50 ml ice cold PBS and spun down at 1000 rpm for 5 minutes. Following washing 2 times with PBS or media, cells were counted using hemocytometer. Cells were then cultured in RPMI 1640 media with 15 % FBS, 100 units/ml penicillin, 100 mg/ml streptomycin and 2 mM Glutamine. Cell survival for patient sample studies was determined using the trypan blue exclusion assay.
Colony assays
Plates of 5 × 104 BaF3/FLT3/ITD, Mv4-11 or MOLM-14 cells in methylcellulose medium in RPMI1640 containing fetal bovine serum were prepared. These plates also contained CDDO-Me with or without PKC412 at different concentrations. The plates were incubated for more than one week at 37°C in 5% CO2 and colonies were then counted on an inverted microscope.
Isolation of nuclear and cytoplasmic fractions
Subcellular fractionation was performed as described (38). In brief, BaF3/FLT-wt or BaF3/FLT3/ITD cells were washed twice with ice cold PBS and resuspended in 1 ml fractionation lysis buffer (1 mM EGTA, 1 mM EDTA, 10 mM β-glycerophosphate, 0.5 mM sodium orthovanadate, 2 mM MgCl2, 10 mM KCl, 1 mM DTT, 40 mg/ml PMSF, 10 mg/ml leupeptin and 10 mg/ml aprotinin. pH 7.2). Following incubation on ice for 1 h, the cells were disrupted by Dounce homogenizer using 20 strokes. The homogenate was layered onto 1 ml of 1 M sucrose in lysis buffer and centrifuged at 1600 × g for 15 minutes to pellet the nuclei. The supernatant above the sucrose cushion was collected and centrifuged at 150,000 × g for 30 minutes at 4°C to collect the soluble or cytoplasmic fraction. Purity of the fractions was monitored by immunoblott analysis with anti-IκBα or anti-Lamin B antibodies.
Immunoblot analysis
Cells were harvested and rinsed with ice-cold phosphate buffered saline (PBS). Ice-cold lysis buffer [0.5 ml; 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 mg/ml leupeptin, 1 mmol/PMSF] was added to 1 × 107 cells and sonicated on ice four times for 5 seconds each followed by microcentrifugation for 10 minutes at 4\C. The soluble proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blotted with primary antibodies and then with secondary antibodies as described (38). The protein bands were then detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). The same blots were stripped and re-probed with desired antibodies to confirm equal loading of proteins in various lanes.
Statistical analysis
Isobologram analysis was performed using the CalCuSyn Software Program (Biosoft, Fergusan, MO and Cambridge, United Kingdom). A combination index (CI) less than 1.0 indicates synergism and a CI of 1 indicates additive activity (19).
Results
CDDO-Me inhibits NFκB activation by blocking IκBα phosphorylation in FLT3/ITD myeloid leukemic cells
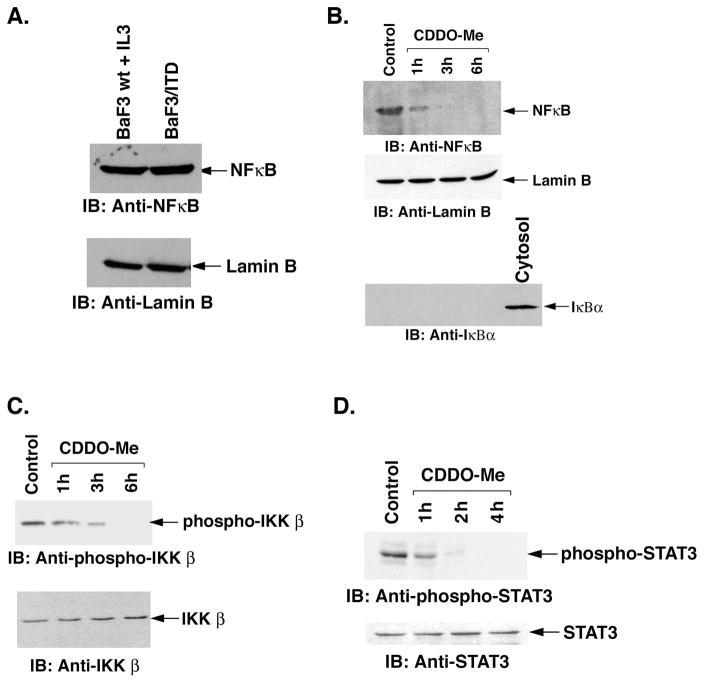
NFκB activates transcription of diverse genes that regulate cell proliferation and survival. In the absence of growth stimulation, NFκB localizes in the cytoplasm in complex with members of the IκB family of inhibitor proteins (39, 40). Phosphorylation of IκBα by IκB kinase β (IKKβ) in the presence of growth factor stimuli induces degradation of IκBα which results in the release of free NFκB to the nucleus which then promotes proliferation (39, 40). To assess localization of NFκB in BaF3-wt cells grown in the presence of IL-3, we stimulated BaF3-wt cells with this growth factor and analyzed nuclear lysates by immunoblotting with anti-NFκB antibody. Nuclear lysates from BaF3/FLT3/ITD cells were also analyzed by immunoblotting with anti-NFκB. Our results demonstrated that NFκB is present in the nucleus of BaF3/FLT3/ITD cells or in IL3-induced BaF3-wt cells (Fig. 1A). Equal loading was confirmed by immunoblots with nuclear Lamin B (Fig. 1A). To determine the localization of NFκB in response to CDDO-Me, we treated BaF3/FLT3/ITD cells with 1 μM CDDO-Me for different time intervals. Nuclear and cytosolic lysates were analyzed by immunoblotting with anti-NFκB antibody. The results demonstrate significant inhibition of the nuclear levels of NFκB in cells treated with CDDO-Me (Fig. 1B). For equal loading and to assess the purity of nuclear lysates, the immunoblots were also analyzed with anti-Lamin B and anti-IκBα antibodies (Fig. 1B). To ensure that the nuclear fractions were pure, we showed that IκBα (limited to cytosol) was not present (Fig 1B, bottom panel).
Figure 1.
A. BaF3-wt cells were treated with IL-3 for 24 hours. Nuclear lysates from BaF3/FLT3/ITD and BaF3-wt cells treated with IL-3 were analyzed by immunoblotting with anti-NFκB and anti-Lamin B antibodies. B. BaF3/FLT3/ITD cells were treated with 1 μM CDDO-Me for the indicated times. Nuclear lysates were prepared and analyzed by immunoblotting with anti-NFκB, anti-Lamin B and anti-IκBα antibodies. Cytosolic lysate (last lane) was also immunoblotted as a positive control for cytosol. C. BaF3/FLT3/ITD cells were treated with 1 μM CDDO-Me for the indicated times. Total cell lysates were analyzed by immunoblotting with anti-phospho-IKKβ and anti-IKKβ antibodies. D. BaF3/FLT3/ITD cells were treated with 1 μM CDDO-Me for the indicated times. Total cell lysates were then analyzed by immunoblotting with anti-phospho-STAT3 and anti-STAT3 antibodies.
NFκB is released from cytosolic IκBα and targeted to the nucleus in response to phosphorylation by IKKβ kinase and subsequent ubiquitination of phospho-IκBα. To determine whether CDDO-Me affects IKKβ phosphorylation, whole cell lysates from control and CDDO-Me-treated BaF3/FLT3/ITD cells were analyzed by immunoblotting with anti-phospho-IKKβ. The results demonstrate that CDDO-Me inhibits constitutive phosphorylation of IKKβ (Fig. 1C) and thereby inhibits the phosphorylation of IκBα. There was no change in the levels of total IKKβ protein (Fig. 1C). In concert with these results, CDDO-Me also inhibited degradation of IκBα (data not shown) and thereby translocation of NFκB to the nucleus (Fig. 1B).
CDDO-Me inhibits constitutive activation of STAT3 in cells expressing FLT3/ITD
Studies have shown that the JAK1-STAT3 pathway is constitutively activated in multiple leukemia cell lines (41). Constitutive activation of STAT3 contributes to tumor cell proliferation and inhibits apoptosis (41). To determine whether CDDO-Me-induced apoptosis is mediated by inhibiting constitutive activation of STAT3, we analyzed lysates of BaF3/FLT3-ITD cells growing in the absence of IL-3 with anti-phospho STAT3 antibodies and found that STAT3 is constitutively phosphorylated (Fig 1D, control lane). CDDO-Me significantly inhibits the constitutive phosphorylation of STAT3 within 4 hours (Fig. 1D). There was no change in the levels of non-phosphorylated STAT3 protein (Fig. 1D). Taken together, these findings suggest that CDDO-Me inhibits both STAT3 phosphorylation and blocks nuclear translocation of NFκB.
CDDO-Me and PKC412 inhibit proliferation of FLT3/ITD-positive AML cells
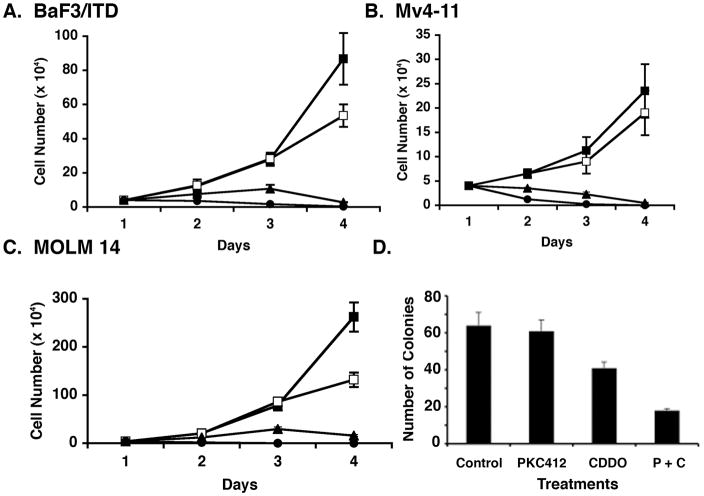
To confirm that CDDO-Me would inhibit proliferation in our model cell line we treated the murine BaF3/FLT3/ITD cells with different concentrations of CDDO-Me for varying time intervals. BaF3/FLT3/ITD cells were sensitive, in a dose dependent manner to CDDO-Me, with an IC50 of 1 μM at 3 days of treatment (Fig. 2A). Furthermore, the human myeloid leukemia cell lines which have FLT3 ITD mutations, Mv4-11 and MOLM-14, were treated with different concentrations of CDDO-Me and analyzed for proliferation. CDDO-Me inhibited the proliferation of these cell lines at 1–5 μM (Fig 2B and C). We had previously shown that the proliferation of cells made growth factor independent by transduction of a FLT3-ITD tyrosine kinase (19), is inhibited by the FLT3 inhibitor, PKC412. We next assessed the effect of PKC412 and CDDO-Me alone and in combination on colony formation of BaF3/FLT3-ITD cells. Results demonstrate that CDDO-Me and PKC412 synergistically inhibited colony formation of murine BAF3/FLT3-ITD cells (Fig. 2D). Isobologram analysis was performed and the combination index was 0.56 at doses of 10 nM and 100 nM of PKC412 and CDDO-Me respectively. Similar results were obtained when human Mv4-11 and MOLM-14 (FLT3-ITD expressing cell lines) were treated with both agents.
Figure 2.
A. BaF3/FLT3/ITD cells were passaged a day before and treated with different concentrations (1 μM to 5 μM) of CDDO-Me for the indicated times. The cells were assessed for viability using trypan blue exclusion method. B and C. Mv4-11 and MOLM-14 cells were treated with varying concentrations of CDDO-Me for the indicated times. The cells were assessed for viability using trypan blue exclusion. A, B and C: [Closed squares: untreated; open squares: 1 μM CDDO-Me; closed triangles: 2.5 μM CDDO; closed circles: 5.0 μM CDDO] D. BaF3/FLT3/ITD cells were treated with 1 μM CDDO-Me and 5 nM PKC412 for 24 hours, either alone or in combination (P + C) and the cells were seeded for colony formation. After 10 days, the number of colonies were counted and compared to that with untreated (control) cells. Isobologram analysis was performed to determine additive versus synergistic effects. Independent experiments were performed at least three times; error bars indicate the standard deviations.
CDDO-Me in combination with PKC412 induces apoptosis in myeloid leukemia cells expressing FLT3-ITD
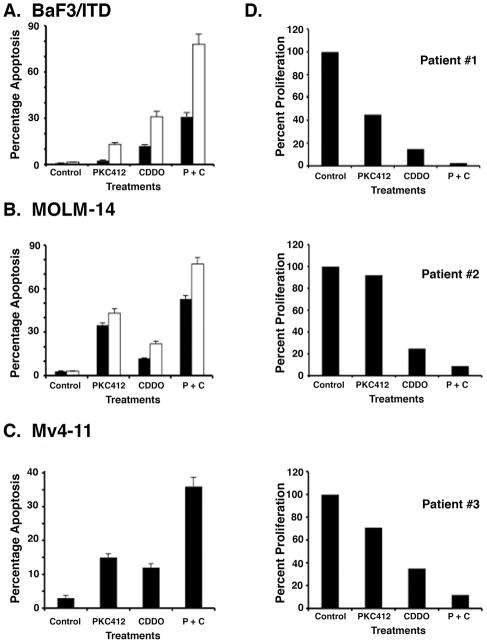
To determine the combinatorial activity of CDDO-Me and PKC412, we treated BaF3/FLT3-ITD cells with low doses of both agents alone and in combination for two or three days and assessed apoptosis by flow cytometry. The data demonstrated that treatment of cells with 5 nM PKC412 + 1 μM CDDO-Me was associated with over 80% induction of apoptosis (Fig. 3A). The results from combined treatment were also compared with cells treated with PKC412 or CDDO-Me separately. Isobologram analysis demonstrated moderate synergism in apoptosis in cells treated with PKC412 + CDDO-Me compared to either agent alone (Fig. 3A). These results were also duplicated in the human leukemic cell lines, Mv4-11 and MOLM-14 (Fig. 3B and 3C). To extend these findings, we treated cells from three different AML patients with diverse concentrations of CDDO-Me and PKC412 and analyzed proliferation. Patient one was an 89 year old man with AML with background dysplasia (FLT3 wt, complex karyotype); patient two a 74 year old male with M1 AML (FLT3 wt, normal karyotype) and patient three a 54 year old male with M4 AML (FLT3-ITD 165 bp, normal karyotype). In concert with the leukemia cell lines, treatment of patient cells with CDDO-Me and PKC412 is also associated with significant inhibition of cell survival (Fig. 3D).
Figure 3.
A. BaF3/FLT3/ITD cells were treated with 1 μM CDDO-Me and 5 nM PKC412, alone or in combination (P + C), for 2 (closed bars) and 3 (open bars) days and analyzed for apoptosis by flow cytometry. Assessment of cells by annexin V/PI staining was used as a measure for apoptosis. B. MOLM-14 cells were treated with 1 μM CDDO-Me and 5 nM PKC412, alone or in combination (P + C), for 2 (closed bars) and 3 (open bars) days and analyzed for apoptosis by Flow cytometry. C. Mv4-11 cells were treated with 1 μM CDDO-Me and 5 nM PKC412, alone or in combination (P + C), for 3 days and analyzed for apoptosis by Flow cytometry. D. Cells from three different AML patients were treated with 1 μM CDDO-Me and 5 nM PKC412, either alone or in combination (P + C) for three days. The cells were assessed for viability using trypan blue exclusion and showed as percent survival of cells.
CDDO-Me in combination with PKC412 synergistically promotes cleavage of PARP and Caspase-3
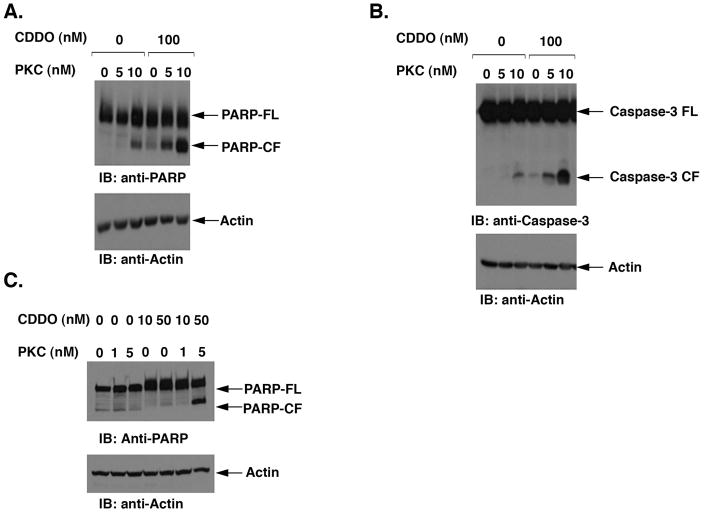
To determine the mechanism of apoptosis mediated by both of these drugs we asked whether the combination of low concentrations of CDDO-Me and PKC412 would induce the intrinsic apoptosis pathway by measuring cleavage of PARP and caspase-3. There was little cleavage of PARP in BaF3/FLT3/ITD cells treated with low concentrations of CDDO-Me (100 nM) or PKC412 (5 nM) alone (Fig. 4A). However, there was a significant induction in PARP cleavage in cells treated with CDDO-Me plus PKC412 (Fig. 4A). Based on densitometric scanning of the immunoblots, we noted a 4–5-fold induction of cleaved PARP fragment in cells treated with the combination of PKC412 and CDDO-Me to that compared with either agent alone. Similar results were obtained when BaF3/FLT3-ITD cells were treated with PKC412 and CDDO-Me and analyzed by immunoblotting with anti-caspase-3 (8–9-fold induction) (Fig. 4B). We also demonstrated that treatment of Mv4-11 cells with CDDO-Me alone or in combination with PKC412 was associated with significant cleavage of PARP (Fig. 4C). Taken together, our results therefore showed that combining CDDO-Me with PKC412 is associated with highly significant activation of Caspase-3 and PARP.
Figure 4.
A. BaF3/FLT3/ITD cells were treated with CDDO-Me and PKC412 at various concentrations, alone or in combination, for the indicated times. Total cell lysates were analyzed by immunoblotting with anti-PARP or anti-Actin antibodies. B. BaF3/FLT3/ITD cells were treated with CDDO-Me and PKC412 and total cell lysates were analyzed by immunoblotting with anti-Caspase-3 or anti-Actin antibodies. C. MOLM-14 cells were treated with CDDO-Me and PKC412 at various concentrations, alone or in combination, for 48 hours. Total cell lysates were analyzed by immunoblotting with anti-PARP or anti-Actin antibodies.
Discussion
Previous studies with FLT3 inhibitors as single agents in patients with mutant FLT3 AML showed biological activity but few clinical responses (14,15,42). For example, in a 20 patient proof of concept trial in which patients with abnormal FLT3 mutant AML were given the multi targeted kinase inhibitor PKC412, 70% experienced a reduction in the peripheral blood blast count but no complete remissions were noted (14). Many reasons have been postulated to explain the lack of more pronounced clinical activity, including insufficiently prolonged inhibitory drug levels, elaboration of survival factors in a protected leukemic stem cell niche, or activation of alternative/downstream pathways.
It has been suggested that it may be possible to counteract resistance to FLT3 inhibitors by inhibiting more than one pathway. Inhibiting tyrosine kinase activation in conjunction with AKT/mTOR inhibition may represent one such approach (43). Our results show that inhibiting both FLT3 activation and the JAK-STAT pathway, which in neoplastic cells results in activation of NFκB, via simultaneous treatment with FLT3 inhibitor PKC412 plus CDDO-Me, results in synergistic cessation of cell growth. Such results were seen in both murine leukemia lines transfected with FLT3/ITD, human leukemic mutant FLT3 cell lines as well as primary patient cells. Additionally, other studies have shown that CDDO-Me induces ROS generation from both non-mitochondrial or mitochondrial sources, which is associated with the induction of apoptosis (44). Therefore, there is also the possibility that simultaneous FLT3 inhibition increases oxidative injury. Since both CDDO-Me and PKC412 are being developed independently as potential anti-neoplastic agents, these studies suggest that combination clinical trials with these two agents are indicated.
Following demonstrating that the FLT3 inhibitor PKC412 and the STAT3 inhibitor CDDO-Me synergistically kill FLT3 mutant leukemia cells, we attempted to determine the mechanism of this synergy. NFκB is commonly involved in maintaining survival of cancer cells in general (44) and AML specifically (45–47). Our earlier studies have shown that CDDO-Me blocks tumor necrosis factor α-induced targeting of NFκB p65 to the nucleus (48,49). The ability of NFκB to activate pro-neoplastic genes depends on it being free of the inhibitor IκBα, which in turn is negatively regulated by phosphorylation by IKKβ kinase. Therefore agents which inhibit phosphorylation of IκBα and thereby prevent NFκB activation, might potentially be anti-neoplastic. STAT3 activation activates IKKβ kinase which indirectly allows NFκB translocation to the nucleus thereby promoting survival and proliferation. We showed that interrupting the STAT3 pathway with CDDO-Me in conjunction with inhibiting FLT3 by PKC412 might be useful as a synergistic anti-leukemic approach. Our preclinical experiments detail a potentially useful combination which could have relevance in patients whose myeloblasts have activating mutations in FLT3. The mechanisms of this synergy between CDDO-Me and PKC412 may be due to an enhancement of NFκB inactivation.
Acknowledgments
This study was supported in part by NCI grant PO1CA5PO1CA669 and the Kristen Sesselman Amico Fund.
Contributor Information
Rehan Ahmad, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Suiyang Liu, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Ellen Weisberg, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Erik Nelson, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Ilene Galinsky, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Colin Meyer, Reata Pharmaceuticals Inc., Dallas, Texas.
Donald Kufe, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Surender Kharbanda, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
Richard Stone, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115.
References
- 1.Haferlach T. Molecular genetic pathways as therapeutic targets in acute leukemia. Hematology (Am Soc Hematol Educ Program) 2008:400–411. doi: 10.1182/asheducation-2008.1.400. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell MR, Appelbaum FR, Coutre SE, et al. Acute myeloid leukemia. JNCCN. 2008;6:962–993. doi: 10.6004/jnccn.2008.0074. [DOI] [PubMed] [Google Scholar]
- 3.Scholl C, Gilliland DG, Frohling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol. 2008;35:326–335. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Stirewalt DL, Radich JP. The role of FLT3 in hematopoetic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 5.Nakao M, Yokoto S, Nakao M, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 6.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogeneous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 7.Whitman S, Archer K, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 8.Kottaridis P, Gale R, Linch D. Flt3 mutations and leukemia. Br J Haematol. 2003;122:523–538. doi: 10.1046/j.1365-2141.2003.04500.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 10.Whitman S, Ruppert A, Radmacher M, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheikhha M, Awan A, Tobal K, et al. Prognostic significance of FLT3 ITD and D835 mutations in AML patients. Hematol J. 2003;4:41–46. doi: 10.1038/sj.thj.6200224. [DOI] [PubMed] [Google Scholar]
- 12.Small D. Targeting Flt3 for the treatment of leukemia. Seminars Hematology. 2008;45:17–21. doi: 10.1053/j.seminhematol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone R, DeAngelo D, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 14.Smith B, Lewis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Konopleva M, Shi Y, et al. Mutant FLT3: a direct target of sorafenib in acute myeloid leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Cortes J, Kantarjian H, et al. Potential role of Sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–2255. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheijen B, Ngo HT, Kang H, Griffin JD. FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene. 2004;23:3338–3349. doi: 10.1038/sj.onc.1207456. [DOI] [PubMed] [Google Scholar]
- 18.Piloto O, Wright M, Brown P, et al. Prolonged exposure to FLT3 inhibitors leads resistance via activation of parallel signaling pathways. Blood. 2007;109:1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg E, Banerji L, Wright R, et al. Potentiation of antileukemic therapies by the dual PIsK/PDK-1 inhibitor, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells. Blood. 2008;111:3723–3734. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Z, Samudio I, Zhang W, et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase-3 signaling by a small molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myeloid leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 21.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 23.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 24.Hodge D, Hurt E, Farrar W. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;16:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Ghoshal S, Baumann H, Wetzler M. Epigenetic regulation of signal transducer and activator of transcription 3 in acute myeloid leukemia. Leukemia Research. 2008;32:1005–1014. doi: 10.1016/j.leukres.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain D, Frank D. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez J, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 2005;65:5054–5062. doi: 10.1158/0008-5472.CAN-04-4281. [DOI] [PubMed] [Google Scholar]
- 28.Liby KT, Yore MM, Sporn MB. Triterpenoids and retinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 29.Ito Y, Pandey P, Place A, et al. The novel triperpenoid CDDO induces apoptosis of human myeloid leukemia cells by a caspase-8 dependent mechanism. Cell Growth Differentiation. 2000;11:261–267. [PubMed] [Google Scholar]
- 30.Konopleva M, Tsao T, Ruvolo P, et al. Novel triperpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–335. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 31.Stadheirm TA, Suh N, Ganju N, Sporn MB, Eastman A. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) potently enhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem. 2002;277:16448–16455. doi: 10.1074/jbc.M108974200. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda T, Sporn MB, Honda T, Gribble GW, Kufe D. Triterpenoid CDDO-Me down regulates PML/RARα expression in acute promyelocytic leukemia cells. Cell Growth Differentiation. 2005;12:523–531. doi: 10.1038/sj.cdd.4401574. [DOI] [PubMed] [Google Scholar]
- 33.Bianco R, Garofalo S, Rosa R, et al. Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumors sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008;98:923–930. doi: 10.1038/sj.bjc.6604269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 35.Johnson B, Jackman D, Janne P. Rationale for a phase I trial of erlotinib and the mammalian target of rapamycin (mTOR) inhibitor everolimus (RAD001) for patients with relapsed non small cell lung cancer. Clin Cancer Res. 2007;13:4628–4631. doi: 10.1158/1078-0432.CCR-07-0717. [DOI] [PubMed] [Google Scholar]
- 36.Monnerat C, Henriksson R, Chevalier TL, et al. Phase I study of PKC412 (N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor, combined with gemcitabine and cisplatin in patients with non-small cell lung cancer. Ann of Oncol. 2004;15:316–323. doi: 10.1093/annonc/mdh052. [DOI] [PubMed] [Google Scholar]
- 37.Samudio I, Konopleva M, Pelicano H, et al. A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate: direct permeabilization of the inner mitochondrial membrane to inihibit electron transport and induce apoptosis. Mol Pharm. 2006;69:1182–1193. doi: 10.1124/mol.105.018051. [DOI] [PubMed] [Google Scholar]
- 38.Kharbanda S, Saleem A, Yuan ZM, et al. Nuclear signaling induced by ionizing radiation involves colocalization of the activated p56/p53 Lyn tyrosine kinase with p34 CDC2. Cancer Res. 1996;56:3617–3621. [PubMed] [Google Scholar]
- 39.Ghosh S, Hayden M. New regulators of NF-kappaB in inflammation. Nature Review Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 40.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor-kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nature Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 42.DeAngelo D, Stone R, Heaney M, et al. Phase I clinical results with tandutinib (MLN518), a novel FLT3 antagonist in patients with acute myelogeneous leukemia or high risk myelodysplastic syndrome: safety, pharmacokinetics and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohi MG, Boulton C, Gu TL, et al. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogene PTKs. PNAS USA. 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeb D, Xiaohua G, Jiang H, et al. Oleanane triterpinoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol. 2010;79:350–360. doi: 10.1016/j.bcp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagemann T, Biswas S, Lawrence T, et al. Regulation of macrophage function in tumors: the multifaceted role of NF-κB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strair R, Gharibo M, Schaar D, et al. Nuclear factor-kappaB modulation in patients undergoing induction chemotherapy for acute myeloid leukemia. Clin Cancer Res. 2008;14:7564–7568. doi: 10.1158/1078-0432.CCR-08-1390. [DOI] [PubMed] [Google Scholar]
- 47.Frelin C, Imbert V, Griessinger E, et al. Targeting NF-κB activation via pharmacologic inhibition of IKK-induced apoptosis of human acute myeloid leukemia cells. Blood. 2005;105:804–811. doi: 10.1182/blood-2004-04-1463. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad R, Raina D, Meyer C, et al. Triterpenoid CDDO-Me blocks the NFκB pathway by direct inhibition of IKKβ on Cys-179. J Biol Chem. 2006;281:35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-Me ester inhibits the Janus-Activated Kinase-1 (JAK1) →Signal Transducer and activator of Transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68:2920–2926. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]