Abstract
Prostate stem cell antigen (PSCA) is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein. Although PSCA is thought to be involved in intracellular signaling, much remain unknown regarding its physiological function and regulatory mechanism in normal and cancer cells. It is up-regulated in several major cancers including prostate, bladder and pancreatic cancers. The expression of PSCA is positively correlated with advanced clinical stage and metastasis in prostate cancers and is also associated with malignant progression of pre-malignant prostate lesions. Therefore, PSCA has been proposed as a biomarker of diagnosis and prognosis, as well as a target of therapy for these cancers. In addition, PSCA has also shown clinical potential in immunotherapy as a prostate specific antigen which, when presented by dendritic cells, may elicit strong tumor specific immunity. In contrast, PSCA is down-regulated in esophageal and gastric cancer and may have tumor-suppressing function in the gastric epithelium. Recent exciting findings that genetic variations of PSCA conferred increased risks of gastric cancer and bladder cancer have opened up a new avenue of research regarding the pathological function of PSCA. PSCA appears to be a Jekyll and Hyde molecule that plays differential roles, tumor promoting or suppressing, depending on the cellular context.
Background
Prostate stem cell antigen (PSCA) is a small, glycosylphosphatidylinositol (GPI)-anchored cell surface protein belonging to the Thy-1/Ly-6 family. It shares 30% homology with stem cell antigen type 2 (SCA-2), a surface marker of immature lymphocytes (1). In human, the PSCA is expressed in the epithelial cells of prostate, urinary bladder, kidney, skin, esophagus, stomach and placenta (1–4). Although it was originally designated as a “stem cell antigen” for similarity to SCA-2, PSCA is now known to be expressed mainly in differentiating cells rather than stem cells, which was demonstrated by studies on prostate and gastric epithelial cells (5, 6). Other than the expression patterns, the physiological functions of the PSCA remain an enigma. PSCA knockout mice were viable and showed no gross abnormal phenotype (7). The Thy-1/Ly-6 family to which PSCA belongs does not seem to offer much clue, because the family members show a remarkable functional diversity ranging from T cell activation (8) to apoptosis regulation in the nervous system (9).
Initially PSCA was identified and isolated as a tumor antigen over-expressed in prostate cancer (1), and subsequent investigations have revealed that it is also up-regulated in urinary bladder cancer, renal cell carcinoma, pancreatic cancer, hydatidiform mole and ovarian mucinous tumor (10–14). Remarkably, it is down-regulated in esophageal and gastric cancers (2, 6).
Although little is known about the regulatory mechanism of PSCA expression, it is certain that androgen is involved in the PSCA regulation, at least in prostate epithelium, because an androgen responsive element was identified in its promoter region (15). Transgenic mice introduced with PSCA promoter-driven GFP constructs showed that the GFP expression was influenced by puberty, castration and androgen restoration (16). In human, complete androgen ablation suppresses PSCA mRNA expression in human prostate carcinoma in vivo (17). In the bladder carcinoma cell line RT112, PSCA expression was stimulated by a culture dish surface that causes aggregation of cells, and by phorbol ester in a cycloheximide- and actinomycin-inhibitable manner, indicating that its expression is regulated by mechanisms related to the adhesion of epithelial cells and by some pathways involving protein kinase C and newly synthesized protein(s) (18). PSCA was recently reported to be down-regulated in telomerase-transduced urothelial cells (19), suggesting that PSCA may be regulated by some telomerase-related mechanism.
Although members of the GPI-anchor proteins have the GPI-moiety, a common feature for the family members, they have diverse structures and functions (20). In mammals, GPI-anchored proteins lacking a transmembrane domain are believed to be located in lipid raft (Fig. 1), which is still a somewhat hypothetic microdomain on the surface of the outer cell membrane; however, several pieces of biological evidence support its existence and propose that it is detergent-insoluble and enriched for sphingolipids and cholesterol (20). The structure of PSCA suggests at least two distinct mechanisms of its potential function. The first possibility is that PSCA may form a complex with another protein that has a transmembrane domain and intracellular domain to activate downstream target. In this regard, it is interesting to note that through a protein motif scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan), PSCA contains an activin types I and II extracellular receptor domain, which binds to the transforming growth factor beta (TGF-β) superfamily of ligands and plays important roles in many cellular functions (21). Evolutionally, Ly-6 family and activin receptor family are closely related and cluster together at the family level (22). It would be interesting to test whether TGF-β family ligand binds to PSCA and whether there are transmembrane proteins on cell surface that partner with PSCA and transmit signals. The second potential mechanism of PSCA function is through the cleavage of the GPI anchor by phospholipase C, which will release PSCA from membrane into secretion and act through a receptor-mediated signaling pathway. There have been no reports of any proteins that bind to PSCA and the identification of such proteins would shed significant light into the biologic function of PSCA.
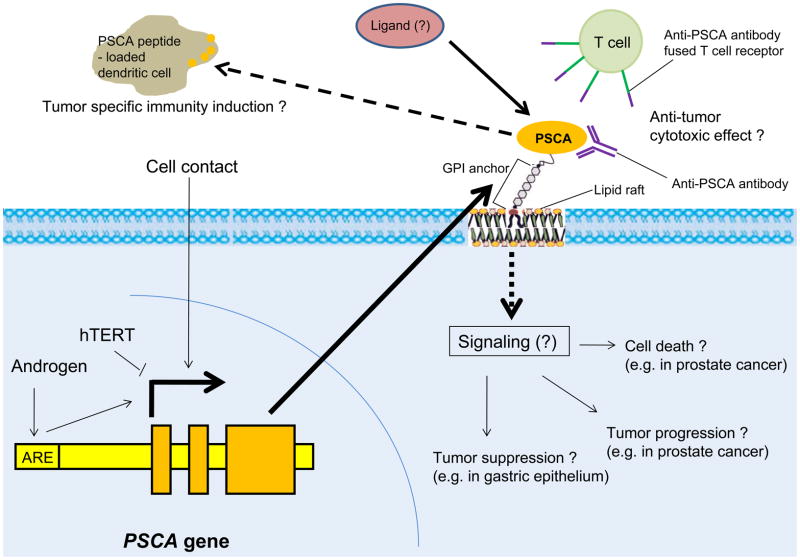
Fig. 1.
Presumptive PSCA signaling pathway and PSCA-targeted immunotherapy.
In the prostate epithelium, PSCA expression is induced by androgen through the binding of androgen-androgen receptor complex to androgen responsive element (ARE) located at the upstream of the gene. Its expression is also up-regulated by cell-cell contact in vitro. It was reported that introduction of human telomerase reverse transcriptase (hTERT) to urothelial cells suppresses PSCA expression. After translation, PSCA is sorted to endoplasmic reticulum, in which it is attached with glycosylphosphatidylinositol (GPI) and transported to the outer surface of the cell membrane where it is anchored to outer lipid layer via fatty-acid chain of the GPI-moiety. As other GPI-anchored proteins, PSCA is believed to be located to a specialized micro-domain, so-called lipid raft, in the cell membrane. PSCA may play multiple roles in cell-death induction, tumor progression, and tumor suppression. However, the ligand of PSCA is not yet identified and the downstream signaling pathway and the physiological function are unknown. PSCA targeted immunotherapy could be a new therapeutic option for hormone therapy-refractory prostate cancer. Possible strategies include direct cytotoxic effects by anti-PSCA antibody or by T cells with T cell receptor genetically fused to anti-PSCA antibody, and induction of tumor specific immunity by dendritic cells loaded with PSCA peptide.
A mouse monoclonal anti-PSCA antibody 1G8 inhibited tumor growth, prevented metastasis, and prolonged the survival of mice harboring inoculated human prostate cancer xenografts (23), which is probably through inducing caspase-independent cell death by cross-linking the PSCA proteins (24). On the other hand, PSCA was found to prevent subpopulation of choroid cells in chicken brain from cell death by modulating a signaling pathway involving α7-containing nicotinic acetylcholine receptors (9). In addition, PSCA showed cell growth inhibition activity for a gastric cancer cell line without a significant induction of cell death (6). These paradoxical observations, together with the up- and down-regulation of PSCA in different human tissues, suggest that PSCA may be oncogenic for some epithelial cells and also be a tumor suppressor for others. This kind of Jekyll and Hyde molecule is not unprecedented. For example, in normal epithelium, Mucin1 (MUC1) is protective against potentially tumorigenic environmental insults, but after significant epithelial damage that results in the loss of cell polarity and change in the membrane proteins’ distribution, it becomes promotive for cancer cell growth and survival (25). Likewise, the Wilms’ tumor 1 (WT1) (26) and NOTCH gene (27) may function either as an oncogene or a tumor suppressor depending on the cellular context and the crosstalk with other cellular molecules and pathways.
In sum, although mechanistic details are unknown, the accumulated findings suggest that PSCA has a functional diversity depending on both tissue types and cell status, normal or malignant.
Clinical-Translational Advances
Clinical application of PSCA in prostate cancer
Since PSCA was originally isolated as a tumor antigen over-expressed in prostate cancer, investigation for its clinical application has mainly focused on prostate cancer. PSCA expression was detected in about 90% of primary prostate cancers, and the expression level is positively correlated with advanced clinical stage, invasion to seminal vesicle and prostate capsule, and progression to androgen-independence (1, 3, 28, 29). PSCA expression was also detected in the metastatic sites at bone, lymph node, and liver (65–100%, dependent on the organs and reports) (3, 30). One study reported increased PSCA-gene copy number in 71% (5 of 7 cases) of prostate cancer with MYC gene amplification, and both genes were located in the same Chr. 8q amplicon, suggesting that gene amplification is the major cause of the over-expression of PSCA in prostate cancer (31). PSCA is approximately 15 Mb distal to Myc oncogene on 8q24, which is one of the most frequently amplified regions in human cancers (32). It is conceivable that increased copy number of PSCA is the major cause of the observed PSCA overexpression in some tumors. A study on 117 prostate biopsy specimens of prostate intraepithelial neoplasia (PIN) revealed that PSCA expression was higher in high-grade PIN (HGPIN), a premalignant condition, than in low-grade PIN. Moreover, the expression levels were elevated in the PIN lesions that subsequently progressed to cancer compared to those that did not progress (33). This value of PSCA expression in predicting cancer progression was also observed in patients with benign prostatic hyperplasia (34, 35).
PSCA also seems to be a useful marker for a detection of metastasis and circulating tumor cells (CTC). More than 90% of lymph node and bone specimens with metastasis were positive for PSCA expression (36). PSCA expression was not detectable in peripheral blood samples of 71 nonmalignant controls and 41 cases of prostate cancer confined to the organ, but detectable in 8 of 17 prostate cancers of extraprostate invasion (37). However, another study on blood samples showed a lower sensitivity for the castration-refractory prostate cancer cases (38). Combined use of other molecules, e.g. prostate-specific antigen (PSA), may overcome this limitation. The presence of PSCA transcripts in the peripheral blood was also a significant predictor of biochemical recurrence after radical prostatectomy in high-risk prostate cancer (39).
The PSCA expression also could be utilized as an index in the evaluation of therapeutic effect. After treatment of HGPIN with flutamide, an androgen receptor antagonist, 66 patients who showed reduction of PSCA mRNA in the prostate tissue did not develop cancer on follow-up, while 11 of 13 cases with increased PSCA expression levels developed cancer afterward (40). The reduction of PSCA expression in the prostate tissue was also demonstrated in localized prostate cancer cases after external beam radiotherapy (41).
In addition to its potential applications as a diagnosis and prognosis biomarker, PSCA has been suggested as a therapeutic target. Current standard treatments for patients with localized prostate cancer are radiation, hormonal therapy, and radical prostatectomy. For patients with metastases, an androgen-ablation therapy is the first choice, which has shown a benefit in 70–80% of the cases (42). When the prostate cancer becomes refractory to the hormone therapy, PSCA gene based strategies may offer a new therapeutic option.
The first step of mounting an immune response is the antigen presentation by dendritic cells (DC). Vaccination with tumor antigen-loaded DC is hypothesized to be a powerful therapeutic strategy to potentiate a tumor-specific immunity induction, and several studies have been conducted to identify a prostate cancer-specific tumor antigen suitable for the loading (43). Effect of PSCA peptide-loaded DC has already been evaluated in phase I/II trial for patients with hormone- and chemotherapy-refractory prostate cancer (44). Of 12 patients entered into the trial, 5 patients developed delayed-type hypersensitivity (DTH) reaction, indicating that the patients had obtained the tumor antigen-specific immunity. The patients tended to be free of disease progression and showed superior overall survival (median survival; 22 months) than the remaining patients (8 months). In another phase I/II study in advanced patients with hormone-refractory prostate cancer, DC loaded with a mixture of peptides from 4 prostate-specific antigens, PSCA, PSA, PSMA and PAP, were shown to elicit strong cytotoxic T cell response against all these tested tumor antigens. Clinically, the long-term DC vaccination was associated with an increase in PSA doubling time (45).
Some other strategies for immunotherapy involving PSCA gene have been tested preclinically. Introduction of a PSCA-expression plasmid into mice harboring transplanted prostate cancer cells inhibited tumor growth via generating PSCA-specific CD8+ T-cell immune response (DNA vaccination) (46–48). T cell containing chimeric T-cell receptor (TCR), which was generated by fusing an anti-PSCA antibody single-chain fragment to the β-chain of TCR, showed potent cytotoxicity against PSCA-positive tumor cells (49).
Cytotoxic therapy targeting PSCA is another potential clinical application. As described before, anti-PSCA monoclonal antibody 1G8 exhibited tumor growth inhibition, metastasis prevention, and prolongation of the survival of mice inoculated with human prostate cancer xenografts (23, 24). Humanized 1G8 was generated by grafting complementarity determining regions to anti-p185 4D5va (trastuzumab) framework and the radioiodinated antibody was shown to have specificity for PSCA. The localization of 1G8 to prostate cancer xenograft was demonstrated in mouse bodies with high-contrast microPET imaging (50–52).
Clinical application in other cancers
PSCA has also been investigated as a biomarker for other cancers. In urinary bladder cancer, immunocytochemical study of PSCA was applied to voided urine specimens and the sensitivity of transitional cell carcinoma detection was 80% for PSCA immunocytochemistry alone compared to 46.7% for cytology alone. Combining cytology with PSCA staining increased the sensitivity of to 84% without decreasing the specificity significantly, suggesting that immunocytochemical analysis of PSCA on voided urine samples may provide a simple and quantitative adjunct marker for cytological diagnosis of urothelial bladder cancer (53). On the other hand, despite its up-regulation in urinary bladder cancer over normal urothelium, PSCA had significantly higher expression in superficial than in invasive bladder cancer (p<0.001) and there was a significant inverse relationship between PSCA expression and recurrence in superficial bladder cancer (54). Pancreatic cancer has aberrantly up-regulated PSCA in nearly 60% of cases, while the gene is not expressed in normal pancreatic duct (12). PSCA was proposed as a specific biomarker of pancreatic adenocarcinoma cells in cytologic examination of fine-needle aspiration specimens (55) and as a biomarker for the detection of CTCs in peripheral blood of pancreatic cancer patients (56). Elevation of IgG reactive to PSCA-derived peptides was demonstrated in 80% of pancreatic cancer, but only in 18% of subjects without the cancer (57). Radiolabeled and bioconjugated anti-PSCA antibodies have been tested for diagnostic imaging of pancreatic cancer (58, 59). As in prostate cancer, an anti-PSCA antibody 1G8 showed an inhibitory effect on tumor growth and progression in a mouse model of pancreatic cancer xenograft (60). It should be noted, however, that PSCA may have a dual function, oncogenic and tumor suppressive, depending on the tissue types and malignant status of the cells. A close evaluation of a potential adverse effect on various organs may be required in the development of a PSCA-targeting systemic therapy.
PSCA as a cancer susceptibility gene: genome-wide association study
Although PSCA has been identified for over a decade, the investigations have been largely superficial and focusing on its potential application as a biomarker and therapeutic target, as described above. However, a couple of unexpected, exciting findings from a totally different field of research, genome-wide association studies (GWAS), have injected fresh life into the research of PSCA. We found a significant association of a functional SNP in the PSCA gene, rs2294008, with the risk of gastric and bladder cancers in two separate GWAS (6, 61). Two recent case control studies confirmed the association of this SNP with gastric and bladder cancer in Chinese population (62, 63). The rs2294008 SNP is a missense SNP that alter the start codon of PSCA. In addition, in vitro reporter assays showed that the risk allele reduced the transcriptional activity of the PSCA promoter in both gastric and bladder cell lines (6, 61). Analysis of PSCA mRNA expression in 16 adjacent normal bladder tissues showed a significant correlation of the risk allele with low expression, consistent with in vitro data (63). These findings are congruous with the notion that PSCA may act as a tumor suppressor (6). No cases of somatic or germline mutations in the PSCA gene have been reported in any cancers to date. It is perplexing that the risk allele is the same and the reduced transcriptional activity is consistent in both gastric and bladder cancers, even though the PSCA gene is down-regulated in gastric cancer but up-regulated in bladder cancer. Nevertheless, these findings have opened up a new avenue of research regarding the pathological role of PSCA and created a new application of PSCA in cancer risk prediction.
Future Directions
PSCA is up-regulated in prostate, urinary bladder, and pancreatic cancers and its clinical utility has been shown in the diagnosis and prognosis of these cancers. PSCA has also been investigated as a potential target for tumor-targeted immunity and for direct suppression by anti-PSCA antibody. Moreover, the PSCA germline genetic variation has been associated with the risk of specific cancers including gastric and urinary bladder cancers. However, it should be stressed that our knowledge of the physiological and pathological functions of PSCA is still quite limited. It appears that PSCA is a Jekyll and Hyde molecule with dual functions depending on the context of tissue specificity and pathophysiological conditions. There are many challenges and opportunities ahead. The priority of research is to find the physiological functions of PSCA, it ligand, and its downstream signaling cascade. To determine the regulatory mechanisms of its expression in different normal and tumor tissues is essential to decipher its dual role of tumor promoting and suppressing functions. The functional consequence of the risk allele of rs2294008 on PSCA protein function and cellular trafficking and the biological mechanisms for the increased cancer risks associated with rs2294008 are a completely new territory. It is interesting to know whether genetic variations in PSCS are associated with other cancers and whether there are somatic mutations of PSCA gene in certain cancers. The clinical applications of PSCA need to be further validated and explored. The next decade of research on PSCA will undoubtedly be exciting and fruitful.
Acknowledgments
NIH R01CA131335 (J. Gu), The program for promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (T. Yoshida), NIH U01CA127615, R01CA74880 and P50CA91846 (X.Wu).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrenberg G, Brauers A, Joost HG, et al. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem Biophys Res Commun. 2000;275:783–8. doi: 10.1006/bbrc.2000.3393. [DOI] [PubMed] [Google Scholar]
- 3.Gu Z, Thomas G, Yamashiro J, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–96. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 4.de Nooij-van Dalen AG, van Dongen GA, Smeets SJ, et al. Characterization of the human Ly-6 antigens, the newly annotated member Ly-6K included, as molecular markers for head-and-neck squamous cell carcinoma. Int J Cancer. 2003;103:768–74. doi: 10.1002/ijc.10903. [DOI] [PubMed] [Google Scholar]
- 5.Tran CP, Lin C, Yamashiro J, et al. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res. 2002;1:113–21. [PubMed] [Google Scholar]
- 6.Sakamoto H, Yoshimura K, Saeki N, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–40. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 7.Moore ML, Teitell MA, Kim Y, et al. Deletion of PSCA increases metastasis of TRAMP-induced prostate tumors without altering primary tumor formation. Prostate. 2008;68:139–51. doi: 10.1002/pros.20686. [DOI] [PubMed] [Google Scholar]
- 8.Bamezai A. Mouse Ly-6 proteins and their extended family: markers of cell differentiation and regulators of cell signaling. Arch Immunol Ther Exp. 2004;52:255–66. [PubMed] [Google Scholar]
- 9.Hruska M, Keefe J, Wert D, et al. Prostate stem cell antigen is an endogenous lynx1-like prototoxin that antagonizes α7-containing nicotinic receptors and prevents programmed cell death of parasympathetic neurons. J Neurosci. 2009;29:14847–54. doi: 10.1523/JNEUROSCI.2271-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amara N, Palapattu GS, Schrage M, et al. Prostate stem cell antigen is overexpressed in human transitional cell carcinoma. Cancer Res. 2001;61:4660–5. [PubMed] [Google Scholar]
- 11.Elsamman EM, Fukumori T, Tanimoto S, et al. The expression of prostate stem cell antigen in human clear cell renal cell carcinoma: a quantitative reverse transcriptase-polymerase chain reaction analysis. BJU Int. 2006;98:668–73. doi: 10.1111/j.1464-410X.2006.06350.x. [DOI] [PubMed] [Google Scholar]
- 12.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4. [PubMed] [Google Scholar]
- 13.Feng HC, Tsao SW, Ngan HY, et al. Overexpression of prostate stem cell antigen is associated with gestational trophoblastic neoplasia. Histopathology. 2008;52:167–74. doi: 10.1111/j.1365-2559.2007.02925.x. [DOI] [PubMed] [Google Scholar]
- 14.Cao D, Ji H, Ronnett BM. Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int J Gynecol Pathol. 2005;24:67–72. [PubMed] [Google Scholar]
- 15.Jain A, Lam A, Vivanco I, et al. Identification of an androgen-dependent enhancer within the prostate stem cell antigen gene. Mol Endocrinol. 2002;16:2323–37. doi: 10.1210/me.2002-0004. [DOI] [PubMed] [Google Scholar]
- 16.Watabe T, Lin M, Ide H, et al. Growth, regeneration, and tumorigenesis of the prostate activates the PSCA promoter. Proc Natl Acad Sci USA. 2002;99:401–6. doi: 10.1073/pnas.012574899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhigang Z, Wenlu S. Complete androgen ablation suppresses prostate stem cell antigen (PSCA) mRNA expression in human prostate carcinoma. Prostate. 2005;65:299–305. doi: 10.1002/pros.20290. [DOI] [PubMed] [Google Scholar]
- 18.Bahrenberg G, Brauers A, Joost HG, et al. PSCA expression is regulated by phorbol ester and cell adhesion in the bladder carcinoma cell line RT112. Cancer Lett. 2001;168:37–43. doi: 10.1016/s0304-3835(01)00497-9. [DOI] [PubMed] [Google Scholar]
- 19.Chapman EJ, Kelly G, Knowles MA. Genes involved in differentiation, stem cell renewal, and tumorigenesis are modulated in telomerase-immortalized human urothelial cells. Mol Cancer Res. 2008;6:1154–68. doi: 10.1158/1541-7786.MCR-07-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee S, Mayor S. The GPI-anchor and protein sorting. Cell Mol Life Sci. 2001;58:1969–87. doi: 10.1007/PL00000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal. 2009;7:15. doi: 10.1186/1478-811X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Van Vlijmen HW, Singh J. A classification of disulfide patterns and its relationship to protein structure and function. Protein Sci. 2004;13:2045–58. doi: 10.1110/ps.04613004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saffran DC, Raitano AB, Hubert RS, et al. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc Natl Acad Sci U S A. 2001;8:2658–63. doi: 10.1073/pnas.051624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Yamashiro J, Kono E, et al. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res. 2005;65:9495–500. doi: 10.1158/0008-5472.CAN-05-2086. [DOI] [PubMed] [Google Scholar]
- 25.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Han Y, Suarez Saiz F, et al. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–76. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 27.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 28.Han KR, Seligson DB, Liu X, et al. Prostate stem cell antigen expression is associated with gleason score, seminal vesicle invasion and capsular invasion in prostate cancer. J Urol. 2004;171:1117–21. doi: 10.1097/01.ju.0000109982.60619.93. [DOI] [PubMed] [Google Scholar]
- 29.Zhigang Z, Wenlv S. Prostate stem cell antigen (PSCA) expression in human prostate cancer tissues and its potential role in prostate carcinogenesis and progression of prostate cancer. World J Surg Oncol. 2004;2:13. doi: 10.1186/1477-7819-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam JS, Yamashiro J, Shintaku IP, et al. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clin Cancer Res. 2005;11:2591–6. doi: 10.1158/1078-0432.CCR-04-1842. [DOI] [PubMed] [Google Scholar]
- 31.Reiter RE, Sato I, Thomas G, et al. Coamplification of prostate stem cell antigen (PSCA) and MYC in locally advanced prostate cancer. Genes Chromosomes Cancer. 2000;27:95–103. doi: 10.1002/(sici)1098-2264(200001)27:1<95::aid-gcc12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 33.Zhigang Z, Wenlu S. Prostate stem cell antigen (PSCA) mRNA expression in prostatic intraepithelial neoplasia: implications for the development of prostate cancer. Prostate. 2007;67:1143–51. doi: 10.1002/pros.20610. [DOI] [PubMed] [Google Scholar]
- 34.Zhigang Z, Wenlu S. The association of prostate stem cell antigen (PSCA) mRNA expression and subsequent prostate cancer risk in men with benign prostatic hyperplasia following transurethral resection of the prostate. Prostate. 2008;68:190–9. doi: 10.1002/pros.20701. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Z, Liu J, Li S, et al. Prostate stem cell antigen mRNA expression in preoperatively negative biopsy specimens predicts subsequent cancer after transurethral resection of the prostate for benign prostatic hyperplasia. Prostate. 2009;69:1292–302. doi: 10.1002/pros.20973. [DOI] [PubMed] [Google Scholar]
- 36.Ananias HJ, van den Heuvel MC, Helfrich W, et al. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–8. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 37.Hara N, Kasahara T, Kawasaki T, et al. Reverse transcription-polymerase chain reaction detection of prostate-specific antigen, prostate-specific membrane antigen, and prostate stem cell antigen in one milliliter of peripheral blood: value for the staging of prostate cancer. Clin Cancer Res. 2002;8:1794–9. [PubMed] [Google Scholar]
- 38.Helo P, Cronin AM, Danila DC, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55:765–73. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joung JY, Cho KS, Kim JE, et al. Prostate stem cell antigen mRNA in peripheral blood as a potential predictor of biochemical recurrence in high-risk prostate cancer. J Surg Oncol. 2010;101:145–8. doi: 10.1002/jso.21445. [DOI] [PubMed] [Google Scholar]
- 40.Zhigang Z, Wenlu S. Flutamide reduced prostate cancer development and prostate stem cell antigen mRNA expression in high grade prostatic intraepithelial neoplasia. Int J Cancer. 2008;122:864–70. doi: 10.1002/ijc.23150. [DOI] [PubMed] [Google Scholar]
- 41.Zhigang Z, Wenlu S. External beam radiotherapy (EBRT) suppressed prostate stem cell antigen (PSCA) mRNA expression in clinically localized prostate cancer. Prostate. 2007;67:653–60. doi: 10.1002/pros.20536. [DOI] [PubMed] [Google Scholar]
- 42.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–21. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 43.Matera L. The choice of the antigen in the dendritic cell-based vaccine therapy for prostate cancer. Cancer Treat Rev. 2009 doi: 10.1016/j.ctrv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Thomas-Kaskel AK, Zeiser R, Jochim R, et al. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119:2428–34. doi: 10.1002/ijc.22097. [DOI] [PubMed] [Google Scholar]
- 45.Waeckerle-Men Y, Uetz-von Allmen E, Fopp M, et al. Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma. Cancer Immunol Immunother. 2006;55:1524–33. doi: 10.1007/s00262-006-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Yu C, Zhao J, et al. Vaccination with a DNA vaccine based on human PSCA and HSP70 adjuvant enhances the antigen-specific CD8+ T-cell response and inhibits the PSCA+ tumors growth in mice. J Gene Med. 2007;9:715–26. doi: 10.1002/jgm.1067. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Hernandez Mde L, Gray A, Hubby B, et al. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–9. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad S, Casey G, Sweeney P, et al. Prostate stem cell antigen DNA vaccination breaks tolerance to self-antigen and inhibits prostate cancer growth. Mol Ther. 2009;17:1101–8. doi: 10.1038/mt.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgenroth A, Cartellieri M, Schmitz M, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007;67:1121–31. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 50.Olafsen T, Gu Z, Sherman MA, et al. Targeting, imaging, and therapy using a humanized antiprostate stem cell antigen (PSCA) antibody. J Immunother. 2007;30(4):396–405. doi: 10.1097/CJI.0b013e318031b53b. [DOI] [PubMed] [Google Scholar]
- 51.Leyton JV, Olafsen T, Lepin EJ, et al. Humanized radioiodinated minibody for imaging of prostate stem cell antigen-expressing tumors. Clin Cancer Res. 2008;14:7488–96. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leyton JV, Olafsen T, Sherman MA, et al. Engineered humanized diabodies for microPET imaging of prostate stem cell antigen-expressing tumors. Protein Eng Des Sel. 2009;22:209–16. doi: 10.1093/protein/gzn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng L, Reiter RE, Jin Y, et al. Immunocytochemical analysis of prostate stem cell antigen as adjunct marker for detection of urothelial transitional cell carcinoma in voided urine specimens. J Urol. 2003;169:2094–100. doi: 10.1097/01.ju.0000064929.43602.17. [DOI] [PubMed] [Google Scholar]
- 54.Elsamman E, Fukumori T, Kasai T, et al. Prostate stem cell antigen predicts tumour recurrence in superficial transitional cell carcinoma of the urinary bladder. BJU Int. 2006;97:1202–7. doi: 10.1111/j.1464-410X.2006.06153.x. [DOI] [PubMed] [Google Scholar]
- 55.McCarthy DM, Maitra A, Argani P, et al. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11(3):238–43. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Lukyanchuk VV, Friess H, Kleeff J, et al. Detection of circulating tumor cells by cytokeratin 20 and prostate stem cell antigen RT-PCR in blood of patients with gastrointestinal cancers. Anticancer Res. 2003;23:2711–6. [PubMed] [Google Scholar]
- 57.Tanaka M, Komatsu N, Terakawa N, et al. Increased levels of IgG antibodies against peptides of the prostate stem cell antigen in the plasma of pancreatic cancer patients. Oncol Rep. 2007;18:161–6. [PubMed] [Google Scholar]
- 58.Foss CA, Fox JJ, Feldmann G, et al. Radiolabeled anti-claudin 4 and anti-prostate stem cell antigen: initial imaging in experimental models of pancreatic cancer. Mol Imaging. 2007;6:131–9. [PubMed] [Google Scholar]
- 59.Yong KT, Ding H, Roy I, et al. Imaging pancreatic cancer using bioconjugated InP quantum dots. ACS Nano. 2009;3:502–10. doi: 10.1021/nn8008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wente MN, Jain A, Kono E, et al. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119–25. doi: 10.1097/01.mpa.0000173459.81193.4d. [DOI] [PubMed] [Google Scholar]
- 61.Wu X, Ye Y, Kiemeney LA, et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat Genet. 2009;41:991–995. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Chen J, Ding Y, et al. Genetic variation of PSCA gene is associated with the risk of both diffuse- and intestinal-gastric cancer in a Chinese population. Int J Cancer. 2010 Feb 3; doi: 10.1002/ijc.25228. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Tang J, Wang M, Yuan L, Zhang Z. Genetic variation in PSCA and bladder cancer susceptibility in a Chinese population. Carcinogenesis. 2010;31:621–4. doi: 10.1093/carcin/bgp323. [DOI] [PubMed] [Google Scholar]