Abstract
Introduction
We sought to determine the effect of peritoneal fluid from a novel animal model of abdominal compartment syndrome (ACS) on the pro-inflammatory status of PMNs (polymorphonuclear leukocytes) and monocytes. We hypothesize that peritoneal fluid is a potential priming and/or activating agent for PMNs/monocytes.
Materials and Methods
ACS was induced in female Yorkshire swine and peritoneal fluid was collected at the time of decompressive laparotomy. Naïve PMN’s/monocytes were primed and/or activated with peritoneal fluid, PAF (phosphatidylcholine) plus peritoneal fluid, peritoneal fluid plus fMLP (n-formyl-met-leu-phe), and peritoneal fluid plus PMA (phorbol 12-myristate 13-acetate). Activation was determined by surface marker expression of integrins (CD11b, CD18) and selectins (CD62L). Additionally, pro-inflammatory cytokines in peritoneal fluid were analyzed.
Results
Peritoneal fluid did not activate PMNs but increased CD11b expression on monocytes. When used as a primer for fMLP or PMA induced activation, peritoneal fluid significantly increased CD11b and CD18 expression on PMN’s and monocytes. Peritoneal fluid collected at 6 and 12 hours post decompressive laparotomy had similar effects. IL-6 and TNF-alpha levels were increased in peritoneal fluid.
Discussion
Peritoneal fluid represents a primer for PMN’s/monocytes and appears to act through receptor dependent and independent pathways. Strategies to reduce the amount of peritoneal fluid may decrease the locoregional and systemic inflammatory response by reducing priming and activation of neutrophils/monocytes.
Keywords: Trauma, Resuscitation, Abdomen, White blood cells, Neutrophil activation
Introduction
Translocation of fluid into the peritoneal cavity is a consequence of massive resuscitation and is compounded by decreased plasma protein (hemodilution) and factors affecting capillary permeability (i.e., hemorrhagic shock/resuscitation: global ischemia/reperfusion injury). Peritoneal fluid is typically a plasma ultrafiltrate and may be considered representative of the biologic profile of plasma.
Kowal-Vern et. al. demonstrated peritoneal fluid in burned patients undergoing resuscitation contains increased levels of pro-inflammatory cytokines. (1) Sagara et. al. reported in preliminary data that trans-serosally derived peritoneal fluid accumulated from the small intestine during repair of abdominal aortic aneurysms increased polymorphonuclear (PMN) expression of CD11b. (2) The question of whether the pro-inflammatory components of peritoneal fluid in abdominal compartment syndrome (ACS) serve as a primer and/or activator of neutrophils and/or monocytes has not been evaluated.
We and others have shown that gut-derived mesenteric lymph serves as a priming agent for PMN’s after gut ischemia/reperfusion, hemorrhagic shock, and trauma. (3-7) As lymph represents interstitial fluid, peritoneal fluid accumulates as both a cause and effect of resuscitation and is thought to be of similar composition. Indeed, the concepts of filtration secretion popularized by Granger et. al. demonstrated the re-distribution of gut interstitial fluid into the gut lumen and peritoneum. (8)
Negative pressure therapy (NPT) is widely utilized in the treatment of open abdominal wounds, resulting from damage control surgical procedures, intra-abdominal sepsis, and decompression from abdominal compartment syndrome (ACS). Common to all negative pressure therapies is the augmented removal of peritoneal fluid. However, the biological behavior of peritoneal fluid is not well characterized. As investigators have shown peritoneal fluid to be a potential reservoir of pro-inflammatory substances, we hypothesized that peritoneal fluid is pro-inflammatory by serving as a priming and/or activating agent of neutrophils and/or monocytes. To that end, we interrogated the polymorphonuclear cell (PMN) and monocyte response to peritoneal fluid both in its potential as a priming and as an activating stimulus for the neutrophil/monocyte activation cascade using a novel non-infectious large animal model of ACS. We additionally sought to evaluate the effect of peritoneal fluid collected after decompressive laparotomy on PMN/monocyte activation.
Materials and Methods
Abdominal Compartment Syndrome Model
All procedures were approved by the University of Texas Animal Welfare Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. (HSC-AWC-07-156) ACS was induced without the artificial instillation of air, fluid, or other space occupying substances based on a novel large animal model developed by our group. (9) Female Yorkshire swine were fasted overnight (12-16 hours) with free access to water. General anesthesia was induced and maintained by intravenous infusion of pentobarbital (starting dose 6 mg/hr/kg) and ketamine (starting dose 7.2 mg/hr/kg). The animals were intubated and ventilated in volume control mode, with a starting FiO2 of 21%, PEEP of 5 mmHg, and tidal volume of 10cc/kg; these values were adjusted based on results of arterial blood gas analysis performed at various time points. Invasive instrumentation of these animals was performed as described in previous, published work. (9)
After instrumentation, hemorrhagic shock was induced by bleeding to a mean arterial pressure (MAP) of approximately 35 mmHg. The pigs were allowed to stabilize for a period of one hour. After stabilization, the collected blood (anti-coagulated with 100 units/kilogram heparin) plus two additional equal volumes of Lactated Ringer’s (Baxter, Deerfield, Il) were reinfused. To mimic the effects of abdominal packing, mesenteric venous pressure was increased to approximately 30 mmHg by tightening the previously placed portal vein snare. We have shown previously that this technique does not result in complete occlusion of the portal vein. (9) Lactated Ringer’s solution was infused to maintain hemodynamic status. Development of ACS was considered achieved when intra-abdominal pressures reached 30 mmHg with the concurrent development of new-organ dysfunction in at least one organ system. (10) Abdominal decompression was performed immediately upon the development of ACS (intra-abdominal pressure of 30 mmHg and new organ dysfunction). At the time of decompression, a Bogota bag was placed as temporary abdominal closure. Peritoneal fluid was collected at baseline (prior to any instrumentation and immediately after opening of the peritoneal cavity), at the time of decompression, and at 6 and 12 hours after decompression.
Experimental Design
Detailed information regarding materials and vendors can be found in previously published work. (11, 12) Human neutrophils were isolated as previously described. (11, 12) Peritoneal fluid was evaluated both as a potential priming agent for PMNs/monocytes, an activator of PMNs/monocytes, or as a primer and activator of PMNs/monocytes. To accomplish these objectives we undertook the following experiments. Peritoneal fluid collected at baseline was utilized as a control.
A: Determination of the activation and/or priming capability of peritoneal fluid
The ability of peritoneal fluid to activate PMNs/monocytes independently was determined. We next determined whether peritoneal fluid was a primer (i.e., receptor) dependent activator of PMNs/monocytes by examining activation after priming with phosphatidylcholine (PAF), a known receptor dependent primer for n-formyl-met-leu-phe (fMLP) induced activation.
Finally, peritoneal fluid was evaluated as a primer for fMLP (receptor dependent activator of neutrophils) and phorbol 12-myristate 13-acetate (PMA (receptor independent activator of neutrophils)) by pretreatment of PMNs/monocytes with peritoneal fluid prior to treatment with these agents. Activation was determined by surface marker expression of integrins (CD11b and CD18) and selectins (CD62L).
B. Determination of whether the priming potential of peritoneal fluid changes after abdominal decompressive laparotomy
Peritoneal fluid was collected at 6 and 12 hours after decompression from ACS to determine whether decompressive laparotomy alters the biological activity of the fluid. Naïve PMNs/monocytes were primed with peritoneal fluid collected at one of three timepoints (time of decompression, 6 hours after decompression, and 12 hours after decompression) followed by activation with fMLP and PMA. Activation was determined by surface marker expression of integrins (CD11b and CD18) and selectins (CD62L).
Neutrophil Activation Assay
All reactions were done in a 96 well plate with a total reaction volume of 200 μl. Each well (reaction) contained approximately 1 × 106 cells. PAF (final concentration 2 μM) or peritoneal fluid (10 μl, end concentration 5% total assay volume) was added to select wells to test the priming capabilities of PAF (one well) and peritoneal fluid (two wells). The samples were allowed to incubate for 5 minutes in the dark at 37°C. Subsequently, fMLP (final concentration 1 μM) or PMA (final concentration 200 ng/ml) were added to wells incubated only with peritoneal fluid. At this time, peritoneal fluid was added to the wells treated with PAF to determine whether peritoneal fluid is a receptor/primer dependent activator of PMN’s. Additionally, peritoneal fluid was added alone (one well) to test the activation potential of it alone. The samples were allowed to incubate for 20 minutes in the dark at 37°C. At the conclusion of the incubation period, the plate was immediately removed and placed on ice for 10 minutes in the dark to stop the reaction. Subsequently, APC conjugated CD11b, PE conjugated CD18, and FITC conjugated CD62L were added to all experimental wells as per manufacturers suggested protocol. APC, PE, and FITC isotype controls were added to designated isotype control wells. The samples were then incubated for 30 minutes protected from light prior to analysis by flow cytometry. An n of 5 or 6 (i.e., peritoneal fluid from 5 or 6 different animals) was used for all experiments. Of note, the doses for fMLP, PAF, and PMA are based on previous, published literature. (4, 12, 13)
Flow Cytometry Analysis
All flow cytometry was performed on an LSR II flow cytometer (BD Biosciences, San Jose, CA). The PMN and monocyte populations were gated using the forward scatter and side scatter plot and the mean fluorescent intensity (MFI) for each sample was recorded. For each sample, 5,000 events were collected for analysis. Analysis was performed using FACSDiva software (BD Biosciences, San Jose, CA).
IL-6/TNF-α ELISA
Levels of IL-6 and TNF-α were measured in peritoneal fluid at baseline and at time of decompression using a commercially available ELISA kit as per manufacturers suggested protocol (R&D Systems, Minneapolis, MN).
Statistical Analysis
Unless otherwise mentioned, all values are represented as mean ± SEM. Statistical comparisons were made using either a paired two tailed t-test or repeated measures analysis of variance (ANOVA) with post hoc Tukey testing, as indicated. A p-value of <0.05 was used to denote statistical significance.
Results
Abdominal Compartment Syndrome Model
The weight of the animals was 34.7 ± 0.84 kg. The baseline MAP was 94 ± 5 mmHg and the baseline mesenteric venous pressure was 21 ± 1 mmHg. At the end of the hemorrhage period, the MAP was 35 ± 0 mmHg. The amount of blood withdrawn to achieve the desired MAP was 560 ± 53 ml. At the conclusion of resuscitation and non-occlusive elevation of the mesenteric venous pressure, the mesenteric venous pressure was increased significantly to 32 ± 1 mmHg. The animals received 89.0 ± 3.8 cc/kg/hr of Lactated Ringer’s from the time of resuscitation until the development of ACS. ACS developed in 2.9 ± 0.2 hours. Intra-abdominal pressure increased from 6 ± 1 mmHg at baseline to 32 ± 0 mmHg at the time of ACS. New organ dysfunction was manifested by several parameters, including mean arterial pressure, central venous pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, peak airway pressures, serum lactate, anion gap, base excess, and plasma protein. The results are summarized in Table 1.
Table 1.
Effect of development of abdominal compartment syndrome (ACS) on various physiological/serum variables
| Variable | Baseline | Time of ACS | Effect of ACS development on variable |
|---|---|---|---|
| Mean arterial pressure (mmHg) |
94 ± 5 | 75 ± 5* | Decrease |
| Central venous pressure (mmHg) |
12 ± 1 | 16 ± 1* | Increase |
| Pulmonary capillary wedge pressure (mmHg) |
12 ± 1 | 16 ± 1* | Increase |
| Pulmonary artery pressure (mmHg) |
22 ± 2 | 33 ± 2* | Increase |
| Peak airway pressure (mmHg) |
19 ± 0 | 37 ± 2* | Increase |
| Serum lactate (mmol/L) |
3.6 ±0.5 | 6.0 ± 0.5* | Increase |
| Anion gap (mmol/L) | 10.6 ± 0.8 | 14.3 ± 0.5* | Increase |
| Base excess (mmol/L) | 3.2 ± 0.8 | -1.7 ± 0.9* | Decrease |
| Plasma protein (g/dl) | 4.6 ± 0.1 | 3.4 ± 0.1* | Decrease |
p<0.05; 2 tailed paired t-test
Neutrophil Activation Assay
For all sets of results, unless otherwise indicated, the baseline value is presented first followed by the value at time of decompression. The values are presented as mean fluorescent intensity and represents the ratio of sample intensity to isotype intensity (S:I).
Baseline Activation Potential of Peritoneal Fluid
When PMNs were incubated with peritoneal fluid alone, there was no significant difference in CD62L (8.11 ± 0.27 versus 7.95 ± 0.34), CD18 (5.50 ± 0.40 versus 4.88 ± 0.27), or CD11b (12.73 ± 1.42 versus 11.58 ± 0.40) expression. With regards to monocytes, peritoneal fluid did cause a significant increase in CD11b expression (16.12 ± 0.67 versus 19.71 ± 1.24, p=0.01). However, there was no increase in CD18 expression (13.06 ± 0.38 versus 13.97 ± 0.41) or decrease in CD62L expression (3.12 ± 0.12 versus 3.61 ± 0.16).
PAF Primed Peritoneal Fluid Induced Activation
We examined whether peritoneal fluid was a receptor dependent activator of PMNs and/or monocytes by incubating the cells with a known primer (PAF) prior to treatment with peritoneal fluid. There was no difference noted in CD62L (3.52 ± 0.20 versus 3.24 ± 0.14), CD18 (7.55 ± 0.30 versus 6.89 ± 0.25), or CD11b (20.03 ± 1.71 versus (20.03 ± 1.71 versus 20.07 ± 0.39) expression. Similarly, for monocytes, there was no difference noted for CD62L (2.08 ± 0.08 versus 2.16 ± 0.11) or CD18 (13.02 ± 0.07 versus 14.54 ± 0.70) expression; however CD11b was significantly increased by peritoneal fluid collected at the time of decompression (21.86 ± 0.69) versus that collected at baseline (18.07 ± 0.97) (p=0.01). Of note, however, there was no difference in monocyte CD11b expression when comparing monocytes with peritoneal fluid alone versus monocytes with PAF and peritoneal fluid, suggesting that PAF is not priming monocytes.
Peritoneal Fluid as a Primer for Receptor Dependent (fMLP) Pathways
When peritoneal fluid was used as a primer for fMLP induced PMN activation, there was a significant increase in CD18 (7.10 ± 0.22 versus 8.41 ± 0.21, p=0.002) and CD11b (19.77 ± 0.82 versus 24.24 ± 0.33, p=0.0004) expression when peritoneal fluid at baseline was compared to peritoneal fluid at time of decompression. Peritoneal fluid at time of decompression did not cause a decrease in CD62L expression. Similarly for monocytes, there was a significant increase observed for CD18 (15.33 ± 0.50 versus 18.25 ± 0.43, p=0.01) and CD11b (18.81 ± 1.01 versus 27.83 ± 0.77, p=0.0002) expression and a significant decrease in CD62L (2.66 ± 0.15 versus 2.05 ± 0.11, p=0.03) expression. The results are summarized in Figure 1.
Figure 1.
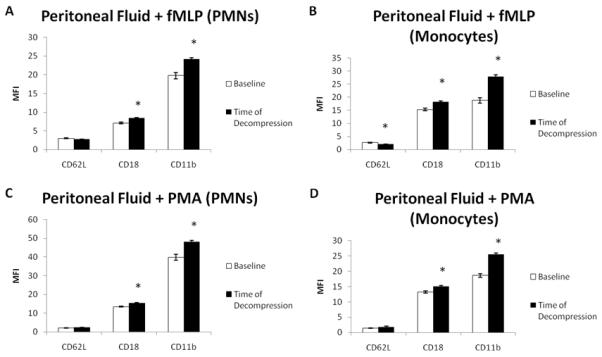
Peritoneal fluid appears to prime PMNs and monocytes in both receptor dependent (fMLP) and receptor independent (PMA) manners. The is evidenced by the increased CD18 and CD11b expression in both PMNs and monocytes when peritoneal fluid collected at time of decompression is compared to control peritoneal fluid (collected at baseline) (A&B (PMNs), C&D (monocytes), n=6; *p<0.05 by paired 2 tailed t-test)
Peritoneal Fluid as a Primer for Receptor Independent (PMA) Pathways
When peritoneal fluid was used as a primer for PMA induced PMN activation, there was a significant increase in CD18 (13.54 ± 0.26 versus 15.30 ± 0.41, p=0.01) and CD11b (39.94 ± 1.55 versus 48.06 ± 0.85, p=0.0006) expression when peritoneal fluid at baseline was compared to peritoneal fluid at time of decompression. Similarly for monocytes, there was a significant increase observed for CD18 (13.21 ± 0.34 versus 15.12 ± 0.29, p=0.01) and CD11b (18.60 ± 0.63 versus 25.48 ± 0.47, p=0.0005) expression. There was no decrease observed in CD62L expression in either PMNs or monocytes. The results are summarized in Figure 1.
Effect of Decompression on Biological Activity of Peritoneal Fluid
As we demonstrated peritoneal fluid to be a primer of PMNs/monocytes, we next evaluated the effect of decompressive laparotomy and temporary abdominal closure on the biological effect of peritoneal fluid. When peritoneal fluid collected at 6 and 12 hours post decompression was compared to peritoneal fluid collected at the time of decompression, there was no significant difference in priming capability for receptor dependent or independent pathways when CD62L, CD18, or CD11b expression were used as markers for PMN activation. (Figure 2) There was no difference on CD62L and CD11b expression on monocytes primed with peritoneal fluid at the post decompression timepoints. However, for fMLP stimulated monocytes, there was a significant decrease (p=0.02) in CD18 expression at six (14.55 ± 0.41) and twelve (14.84 ± 0.36) hours post decompression when compared to time of decompression (16.49 ± 0.24). For PMA stimulated monocytes, there was a significant increase (p=0.008) in CD18 expression at twelve (16.21 ± 0.24) hours post decompression when compared to six (14.98 ± 0.52) hours post decompression and time of decompression (14.48 ± 0.30). (Figure 2)
Figure 2.
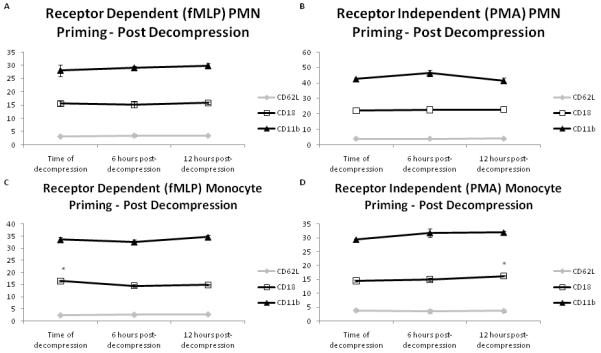
Peritoneal fluid continues to prime naïve PMNs and monocytes in both receptor dependent (fMLP) and receptor independent (PMA) manners when measured up to 12 hours post decompression. No change was observed in CD62L, CD11b, or CD18 expression on PMNs. No change was observed in CD62L or CD11b expression on monocytes; CD18 expression decreased over time for fMLP stimulated monocytes and increased over time for PMA stimulated monocytes. This suggests that peritoneal fluid continues to remain a priming stimulus even after decompression. (A&B (PMNs), C&D (monocytes), n=5/timepoint; *p<0.05 by repeated measures analysis of variance (ANOVA) with post hoc Tukey testing)
IL-6/TNF-α Cytokine Levels
Levels of IL-6 (0.0391 ± 0 versus 4.872 ± 0.968, p=0.008) and TNF-α (0.023 ± 0 versus 0.124 ± 0.032, p=0.04) were increased in peritoneal fluid at time of decompression when compared to baseline. The baseline value is presented first followed by the value at decompression. IL-6 values continued to be elevated at 6 hours (8.439 ± 2.147) and 12 hours (7.480 ± 2.031) (p=0.0008). Similarly, TNF-α levels were elevated at 6 hours (0.093 ± 0.012, p=0.005) and 12 hours (0.089 ± 0.016, p=NS). Values are presented as ng/ml.
Discussion
Our data demonstrate that peritoneal fluid, in a non-infectious model of ACS, serves as a priming agent for receptor dependent and independent pathways in naïve PMN’s and monocytes. Additionally, peritoneal fluid collected post decompressive laparotomy continues to function as a priming agent for PMN’s and monocytes. This is likely secondary to increased levels of pro-inflammatory cytokines (IL-6 and TNF-α). A significant body of literature has been developed regarding the role of mesenteric lymph in the priming and activation of neutrophils in hemorrhagic shock and ischemia/reperfusion. A major component of mesenteric lymph that is common to peritoneal fluid is interstitial compartment fluid which is believed to be the major harbinger of pro-inflammatory gut derived mediators after hemorrhagic shock and/or ischemia/reperfusion injury. (14, 15) We therefore extrapolated from that information to hypothesize that peritoneal fluid may have a similar effect on PMN’s as has been demonstrated with mesenteric lymph.
In this study, changes in cell surface markers were utilized as markers of activation. Changes in surface markers are one of the early steps in the activation cascade and are necessary for adhesion and subsequent induction of an inflammatory response. (16) Additionally, we have demonstrated in previous work that changes in cell surface markers correlates well with intracellular markers of activation, specifically superoxide production, when receptor dependent (fMLP) and independent (PMA) pathways of activation are examined. (11, 12, 16)
We tested the effect of peritoneal fluid derived from a swine model of ACS on human PMN’s. A valid critique of our work is whether or not the behavior of PMN’s derived from the same species (i.e., swine) exposed to peritoneal fluid would have been different and whether at least some of the activation seen is secondary to using human neutrophils for our assay. Our decision for the use of human neutrophils is based on several factors. Most importantly, Sarin et. al. demonstrated in previous work, that the reactivity of PMN’s derived from swine or humans behave similarly when exposed to potential stimulants (i.e., mesenteric lymph) from swine. Given the similar characteristics of peritoneal fluid and mesenteric lymph, there is an argument to be made for the translatability of this work. Assays for the evaluation of superoxide production in human neutrophils has been extensively studied and characterized. (4) In addition, as an internal experimental control, we utilized peritoneal fluid collected at baseline to exclude any potential effect secondary to the differing species used.
In addition to examining the effect of peritoneal fluid collected at decompression, we sought to determine whether decompressive laparotomy altered the biological behavior of peritoneal fluid with regards to neutrophil priming. A Bogota bag was chosen for temporary abdominal closure to exclude any confounding factors that negative pressure therapy (vacuum pack or Vacuum Assisted Closure (VAC, Kinetic Concepts, Inc., San Antonio, TX) may have on the peritoneal cavity. Globally, peritoneal fluid collected at 6 and 12 hours post decompressive laparotomy remained a priming stimulus for PMNs/monocytes. These results are not entirely surprising given that pro-inflammatory cytokines remain elevated in the peritoneal fluid post decompression. Decompressive laparotomy after ACS may be associated with a reperfusion like syndrome, subsequently leading to release of pro-inflammatory mediators and propagating the inflammatory response. (17)
Increased levels of pro-inflammatory cytokines may account for the observed effect of peritoneal fluid on neutrophil priming. Peritoneal fluid has been shown to contain increased levels of pro-inflammatory cytokines with injury in human studies and in animal models. Increased concentrations of pro-inflammatory cytokines have been demonstrated in burn patients with intra-abdominal hypertension/ACS, with increasing concentrations correlating with poorer outcome. (1) Increased levels of pro-inflammatory cytokines (TNF-α and IL-6) were associated with death in an animal model of peritonitis. (18) Although the concentration of IL-6 and TNF-α were small (ng) in our model, other investigators have demonstrated alterations in neutrophil biology at similar concentrations. (19)
Bokesch et. al. demonstrated that peritoneal fluid after cardiopulmonary bypass to contain increased levels of pro-inflammatory cytokines and that peritoneal drainage was associated with decreased serum levels. (20) It has been demonstrated that peritoneal fluid can be reabsorbed into the circulation via both the capillary and lymphatic system. (21) Given the data we present regarding priming and activation of neutrophils, this could offer a potential mechanism for systemic effects of pro-inflammatory peritoneal fluid. This pathway likely becomes even more important when inflammatory ascites may be a significant stimulator for an inflammatory response as in intra-abdominal sources of sepsis. Common to all negative pressure assisted forms of temporary abdominal closure is the augmented drainage of peritoneal fluid. Therefore, negative pressure abdominal pressure therapy systems may exert additional benefit by removal of a potential priming and/or activating stimulus for neutrophils which may contribute to a loco-regional inflammatory response.
Increased CD11b and CD18 expression on monocytes are indicative of activation. (22, 23) While few investigators have directly evaluated monocyte activation in the setting of trauma and/or other potential reservoirs of pro-inflammatory substances (i.e., mesenteric lymph), published literature offers some insight as to the potential role of monocytes. IL-6 is a soluble product of monocyte activation. (24) Increased serum levels of IL-6 in the setting of multiple trauma may be predictive of the development of multiple organ dysfunction and mortality. (25-27) We purport that pro-inflammatory stimuli (i.e., peritoneal fluid) may affect and contribute to continued systemic activation of monocytes.
It has been demonstrated that patients who have had significant trauma have circulating primed and activated neutrophils. (28) It is also believed and has been shown in animal work that primed neutrophils may make patients more susceptible to second hit injury. (28)The source of the activating/priming agent is not completely known. Multiple investigators have noted that post-shock mesenteric lymph and it’s components serve as priming and activating agents for neutrophils. (4, 29) We have demonstrated that peritoneal fluid may additionally serve as a continued priming and activating stimulus for neutrophils and monocytes. Whether this means that early removal may downreguate the inflammatory response in ACS is unknown. Future experiments would involve determining whether augmented removal of peritoneal fluid affects the priming ability of circulating PMN’s and/or monocytes in vivo, or PMN/monocyte mediated secondary organ injury.
Acknowledgments
SOURCES OF SUPPORT: Kinetic Concepts, Inc; NIH Grants T32 GM 0879201, K01 DK 070758, RO1 HL 36115, and P50 GM 38529; Children’s Memorial Hermann Hospital Foundation; Texas Higher Education Coordination Board
Conflict statement: Funding for this project was provided, in part, by a grant from Kinetic Concepts, Inc. (Grant to Charles S. Cox, Jr, MD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kowal-Vern A, Ortegel J, Bourdon P, et al. Elevated cytokine levels in peritoneal fluid from burned patients with intra-abdominal hypertension and abdominal compartment syndrome. Burns. 2006;32:563–569. doi: 10.1016/j.burns.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Sagara D, Unno N, Mitsuoka H, Yamamoto N, Ishimaru K, Konno H. Transerosaly leaked gut-derived fluid activates neutrophils via phospholipase-A2 during open abdomina (sp) aortic aneurysm repair. Shock. 2009;31:43. [Google Scholar]
- 3.Hassoun HT, Fischer UM, Attuwaybi BO, et al. Regional hypothermia reduces mucosal NF-kappaB and PMN priming via gut lymph during canine mesenteric ischemia/reperfusion. J Surg Res. 2003;115:121–126. doi: 10.1016/s0022-4804(03)00298-1. [DOI] [PubMed] [Google Scholar]
- 4.Sarin EL, Moore EE, Moore JB, et al. Systemic neutrophil priming by lipid mediators in post-shock mesenteric lymph exists across species. J Trauma. 2004;57:950–954. doi: 10.1097/01.ta.0000149493.95859.6c. [DOI] [PubMed] [Google Scholar]
- 5.Zallen G, Moore EE, Johnson JL, et al. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–88. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez RJ, Moore EE, Ciesla DJ, et al. Mesenteric lymph is responsible for posthemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51:1069–1072. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Adams CA, Jr., Hauser CJ, Adams JM, et al. Trauma-hemorrhage-induced neutrophil priming is prevented by mesenteric lymph duct ligation. Shock. 2002;18:513–517. doi: 10.1097/00024382-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Granger DN, Barrowman JA. Microcirculation of the alimentary tract I. Physiology of transcapillary fluid and solute exchange. Gastroenterology. 1983;84:846–868. [PubMed] [Google Scholar]
- 9.Shah SK, Jimenez F, Walker PA, Xue H, Uray KS, Aroom KR, Fischer UM, Laine GA, Stewart RH, Norbury KC, Cox CS. A novel physiologic model for the study of abdominal compartment syndrome (ACS) J Trauma. 2009 doi: 10.1097/TA.0b013e3181c453cb. In press. [DOI] [PubMed] [Google Scholar]
- 10.Balogh Z, McKinley BA, Cox CS, Jr, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock. 2003;20:483–492. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 11.Harting MT, Jimenez F, Adams SD, et al. Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harting MT, Jimenez F, Kozar RA, et al. Effects of poloxamer 188 on human PMN cells. Surgery. 2008;144:198–203. doi: 10.1016/j.surg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuno T, Moore EE, Cheng AM, et al. Bioactivity of postshock mesenteric lymph depends on the depth and duration of hemorrhagic shock. Shock. 2006;26:285–289. doi: 10.1097/01.shk.0000223132.72135.52. [DOI] [PubMed] [Google Scholar]
- 14.Matheson PJ, Mays CJ, Hurt RT, et al. Modulation of mesenteric lymph flow and composition by direct peritoneal resuscitation from hemorrhagic shock. Arch Surg. 2009;144:625–634. doi: 10.1001/archsurg.2009.125. [DOI] [PubMed] [Google Scholar]
- 15.Deitch EA, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Waele JJ, Hoste EA, Malbrain ML. Decompressive laparotomy for abdominal compartment syndrome--a critical analysis. Crit Care. 2006;10:R51. doi: 10.1186/cc4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martineau L, Shek PN. Peritoneal cytokine concentrations and survival outcome in an experimental bacterial infusion model of peritonitis. Crit Care Med. 2000;28:788–794. doi: 10.1097/00003246-200003000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Shelton JL, Wang L, Cepinskas G, et al. Human neutrophil-pulmonary microvascular endothelial cell interactions in vitro: differential effects of nitric oxide vs. peroxynitrite. Microvasc Res. 2008;76:80–88. doi: 10.1016/j.mvr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Bokesch PM, Kapural MB, Mossad EB, et al. Do peritoneal catheters remove proinflammatory cytokines after cardiopulmonary bypass in neonates? Ann Thorac Surg. 2000;70:639–643. doi: 10.1016/s0003-4975(00)01453-3. [DOI] [PubMed] [Google Scholar]
- 21.Tarpila E, Nystrom PO, Ihse I. The resorption of FITC-dextran 10,000 from the peritoneum in different modifications of bile-induced acute pancreatitis and in bacterial peritonitis. Int J Pancreatol. 1991;10:229–236. doi: 10.1007/BF02924160. [DOI] [PubMed] [Google Scholar]
- 22.Ono S, Aosasa S, Tsujimoto H, et al. Increased monocyte activation in elderly patients after surgical stress. Eur Surg Res. 2001;33:33–38. doi: 10.1159/000049690. [DOI] [PubMed] [Google Scholar]
- 23.Fink R, Al-Obaidi M, Grewal S, et al. Monocyte activation markers during cardiopulmonary bypass. Perfusion. 2003;18:83–86. doi: 10.1191/0267659103pf645oa. [DOI] [PubMed] [Google Scholar]
- 24.Muller Kobold AC, Kallenberg CG, Tervaert JW. Monocyte activation in patients with Wegener’s granulomatosis. Ann Rheum Dis. 1999;58:237–245. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frink M, van Griensven M, Kobbe P, et al. The Prognostic Value of IL-6 for Organ Dysfunction and Mortality in Patients With Multiple Injuries. Scand J Trauma Resusc Emerg Med. 2009;17:49. doi: 10.1186/1757-7241-17-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jastrow KM, 3rd, Gonzalez EA, McGuire MF, et al. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Stensballe J, Christiansen M, Tonnesen E, et al. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53:515–521. doi: 10.1111/j.1399-6576.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 28.Zallen G, Moore EE, Johnson JL, et al. Circulating postinjury neutrophils are primed for the release of proinflammatory cytokines. J Trauma. 1999;46:42–48. doi: 10.1097/00005373-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez RJ, Moore EE, Ciesla DJ, et al. Phospholipase A(2)--derived neutral lipids from posthemorrhagic shock mesenteric lymph prime the neutrophil oxidative burst. Surgery. 2001;130:198–203. doi: 10.1067/msy.2001.115824. [DOI] [PubMed] [Google Scholar]


