Abstract
Epitope selection is an important consideration in the design of cancer vaccines, but factors impacting selection are not fully understood. We compared the immune response to peptides and glycopeptides from the common human tumor antigen MUC1, a mucin that is coated with O-linked carbohydrates in its variable number of tandem repeats (VNTR) region. MUC1 expressed on tumor cells is characteristically underglycosylated , creating peptide and glycopeptide neoepitopes that are recognized by the immune system. The response to VNTR peptides is weaker in MUC1 transgenic mice (MUC1-Tg mice) than in wild type (WT) mice, whereas the response to VNTR glycopeptides is equally strong in both strains. Thus, glycopeptides appear to be recognized as foreign, while peptides, although immunogenic, are perceived as self. To explore this further, we generated MUC1 peptide and glycopeptide-specific TCR transgenic mice and studied the function of their CD4 T cells when adoptively transferred into MUC1-Tg or WT mice. Peptide-specific T cell precursors were not centrally deleted in MUC1-Tg mice and did not acquire a T regulatory (Treg) phenotype. However, their response to the cognate peptide was reduced in MUC1-Tg mice compared to WT mice. In contrast, glycopeptide-specific CD4 T cells responded equally well in both hosts, and when simultaneously activated also enhanced the peptide-specific T cell responses. Our data show that the immune system differentially recognizes various epitopes of tumor-associated antigens either as self or as foreign, and this controls the strength of anti-tumor immunity. This represents an important consideration for designing safe and effective cancer vaccines.
Introduction
Transformed cells express many self-derived tumor-associated antigens (1) that can elicit antibody and T cell responses in cancer patients. However, studies in transgenic and knockout mice indicate that anti-tumor immunity may be hindered by central and/or peripheral self-tolerance to the form of the self-tumor antigen expressed on normal tissues (2-4). This may explain why attempts to boost these responses have been met with limited success.
Abnormal expression of many self molecules, via pre- and post-translational modifications, generates a spectrum of tumor-specific epitopes. These provide potential targets for eliciting tumor immunity without the risk of autoimmunity that is associated with breaking tolerance to self (5). The transmembrane glycoprotein Mucin 1 (MUC1) is over-expressed by most human adenocarcinomas in an aberrantly glycosylated form containing characteristic short O-linked sugar chains and exposed non-glycosylated protein backbone in the VNTR. Each VNTR tandem repeat is a 20 amino acid sequence, HGVTSAPDTRPAPGSTAPPA, which can vary in number from fewer than 25 to more than 125 repeats per allele, effectively dominating the extracellular domain of MUC1 (6). MUC1 VNTR peptide-specific CD8+ cytotoxic T lymphocytes (CTL) are found in patients with MUC1+ tumors, indicating that an immune response can be generated against them (7-8). However, clinical trials using MUC1 VNTR peptide-based vaccines to boost this immunity in cancer patients have resulted in only marginal increases in CTL activity and ineffective anti-MUC1 antibody class switching (9-10). The MUC1-transgenic (MUC1-Tg) mouse, which displays the same tissue-specific expression of human MUC1 seen in healthy and diseased human tissues (11-12), mounts only low antibody and CTL responses to MUC1 VNTR peptides, due in part to reduced responses of MUC1 peptide-specific CD4 T cells compared to those in transgene-negative (wild type; WT) littermates (13-15). This highlights the critical role of CD4 T helper (Th) cells in promoting anti-tumor immunity, effective B cell priming, antibody isotype switching, CTL expansion, and CD8 T memory cell responses (16).
Tumor-associated glycoprotein antigens that do not show mutations in their peptide sequence, could also be targeted via aberrantly glycosylated and thus tumor-specific glycopeptide epitopes. For MUC1, those are peptides carrying Tn (GalNAc-O-S/T) and T (Gal-GalNAc-O-S/T) glycans (17). On normal cells, these core carbohydrates are further glycosylated to form complex oligosaccharides and thus are not exposed. Abberant glycosylation in ~90% of adenocarcinomas reveals the Tn and T antigens (18) and allows tumor-associated MUC1 glycopeptides carrying these core glycans to be processed by antigen presenting cells (APC) and presented on Class I and Class II MHC, making them targets for T cell recognition and anti-tumor immunity (19-21). Indeed, responses to MUC1 glycopeptide in the MUC1-Tg mouse are stronger than those obtained against the MUC1 peptide (22).
To study the mechanisms that cause this disparity in CD4 T cell immunity to MUC1 peptide versus glycopeptide epitopes, we generated two new MUC1-specific T cell receptor (TCR) transgenic mice on the WT background. One mouse (RFT) expresses a TCR that preferentially recognizes MUC1 glycopeptide carrying the tumor-associated Tn glycan (TnMUC1); the other (VFT) is specific for unglycosylated MUC1 peptide (MUC1p). We show that MUC1p-specific VFT CD4 T cells are not deleted during thymic development or in the periphery of MUC1-Tg mice. However, upon antigen-specific stimulation, their proliferation in MUC1-Tg mice was attenuated compared to proliferation in WT mice. TnMUC1-specific RFT CD4 T cells, however, respond equally well in WT and MUC1-Tg mice, thereby mimicking the behavior of OTII CD4 T cells specific for the foreign antigen ovalbumin (ova). Furthermore, co-activation of TnMUC1-specific T cells in the MUC1-Tg mouse confers “help” for MUC1p-specific T cells, raising their activation to levels obtained in WT mice.
Materials and Methods
Mice and cell lines
Mice were bred and maintained in specific pathogen-free conditions at the University of Pittsburgh and treated under IACUC-approved guidelines in accordance with approved protocols. C57BL/6, B6.PL-Thy1a/Cy, B6.SJL-PtprcaPepcb/BoyJ, and BALBc mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and MUC1-Tg mice (12) from Dr. S. Gendler (Mayo Clinic, Scottsdale, AZ). MUC1-transgene positive and transgene negative (wild type; WT) mice from heterozygous breeding were identified by PCR analysis. VFT and RFT TCR transgenic mice were generated at the University of Pittsburgh Transgenic Mouse Facility.
VF5 and RF6 T cell hybridomas were generated as previously described (20, 22). Briefly, wild type C57BL/6 (WT) mice were immunized three times with dendritic cells (DC) loaded either with MUC1p or TnMUC1. Seven days after the final immunization, splenocytes and lymph node cells were re-stimulated in vitro and fused with the BW5147 lymphoma using polyethylene glycol 1500 (Roche, Mannheim, Germany). CD3+CD4+ hybridomas were screened by FACS, then selected for IL-2 production in response to TnMUC1 or MUC1p. Hybridomas were subsequently cloned by limiting dilution.
TCR Cloning
TnMUC1-specific TCRα/β cDNAs from the RF6 hybridoma (22) were amplified by 5′ RACE using a Generacer Kit (Invitrogen, Carlsbad, CA) in combination with reverse primers specific for TCR-Cα and TCR-Cβ constant regions. MUC1p- specific TCRα cDNA from the VF5 hybridoma (20) was similarly amplified by 5′ RACE. VF5 TCRβ cDNA was amplified using degenerate Vβ primers (23) and reverse primers specific for TCR-Cβ. The amplified TCR chains were sequenced at the University of Pittsburgh Sequencing Facility. pMUC1-specific VF5 TCR contains Vα2.5-Jα49 and Vβ6-Jβ2.5. TnMUC1-specific RF6 TCR contains Vα4.1-Jα16 and Vβ15-Jβ1.3. These rearranged TCR gene segments (, including ~150bp (VF5) or ~100bp (RF6) of intron downstream of the J gene segments, were cloned from genomic DNA of the VF5 and RF6 hybridomas into pcDNA3.1/V5-His (Invitrogen)(VF5) or TOPO TA (Invitrogen)(RF6), then sub-cloned into the TCR cassette vectors pTα or pTβ (a generous gift from Drs. Diane Mathis and Christophe Benoist, Joslin Diabetes Institute) (24). TCR expression constructs were re-sequenced and tested for functional expression by transfection into DO11.10 or 58a-b-(25) hybridomas.
Generation of TCR transgenic mice
Linear pTα-VF5α and pTβ-VF5β constructs were microinjected into B6.PL-Thy1a/Cy (CD90.1+) embryos. Linear pTα-RF6α and pTβ-RF6β constructs were microinjected into B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) embryos. VF5α and VF5β founder mice were identified by PCR of tail tissue DNA using primers specific for the Vα2-Jα49 and Vβ6-Jβ2.5 rearrangements. RF6α and RF6β founder mice were identified by PCR for the Vα4.1-Jα16 and Vβ15-Jβ1.3 rearrangements. Founder mice were crossbred to produce double-transgenic CD90.1+VFT and CD45.1+RFT mice.
VFT and RFT TCR transgene expression is controlled by the natural TCRα and TCRβ promoter/enhancer elements included in the cassette vectors (24). Antigen recognition in VFT-Tg and RFT-Tg mice is mediated by CD4+ T cells (Fig. S1). Figure S2 shows preferential recognition of the MUC1 peptide by VFT-Tg CD4 T cells and MUC1 glycopeptide by RFT-Tg CD4 T cells.
Flow cytometry
Cells were labeled with indicated antibodies at a 1:50 dilution in FACS buffer (PBS, 5% FBS, .01% sodium azide) for 30 minutes on ice. Intracellular labeling was performed with the BD Cytofix/Cytoperm Solution Kit (BD Biosciences, San Jose, CA). Labeled cells were analyzed on a LSR II Flow Cytometer using FACSDiva software (BD Biosciences).
Bone marrow transplantation
Lineage negative (Linneg) bone marrow precursors were purified from VFT-Tg mice using a Lineage Cell Depletion Kit (Miltenyi Biotech, Auburn, CA). WT or MUC1-Tg recipient mice were irradiated (900Rad) 4 hours prior to i.v. injection with 105 Linneg cells, plus 2×105 host-type whole BM cells to ensure recipient survival (26). The presence of VFT T cells and intracellular Foxp3 expression in spleens from recipient mice 5-6 weeks post transfer were determined by flow cytometry.
T cell adoptive transfer
T cells were purified from spleens of VFT-Tg, RFT-Tg, or OTII-Tg donor mice by CD3 negative selection using magnetic antibody cell sorting (MACS) microbeads (Miltenyi). Where indicated, T cells were labeled with 5μM CFSE (Invitrogen, Carlsbad, CA) prior to transfer. 3-5 × 106 T cells were transferred to recipient mice via lateral tail vein. For experiments involving in vivo stimulation of donor T cells, recipient mice were vaccinated with antigen-loaded or control (no antigen) DCs, administered by lateral tail vein (i.v.) injection.
MUC1 peptides and glycopeptides
100mer peptide (MUC1p) represents 5 repeats of the 20 amino acid sequence HGVTSAPDTRPAPGSTAPPA from the MUC1 VNTR region and was synthesized as described previously (13). GalNAc-100mer (Tn100mer/TnMUC1) was prepared by enzymatic addition of GalNAc to synthetic peptide substrate using recombinant human UDP-GalNAc:polypeptide N-acetyl-galactosaminyltransferase rGalNAc-T1 as previously described (20, 27). The final reaction product contained a heterogeneous mixture of 9 to 15 GalNAc residues per 100mer peptide molecule, incorporated within the threonine of the VTSA region and adjoining serine and threonine within the GSTA region as defined previously (20, 27). MUC1p and TnMUC1 were synthesized and analyzed at the University of Pittsburgh Genomics and Proteomics Core Laboratories.
Generation of bone marrow derived DC and vaccination
DCs were generated as previously described (20) with a few modifications. Briefly, RBC lysed bone marrow cells from C57Bl/6 mice were plated at 1×106 cells/ml in serum-free AIM-V medium (Invitrogen) containing sodium pyruvate, nonessential amino acids, and 2-ME; supplemented with 10ng/ml GM-CSF (R&D Systems, Minneapolis, MN). Cells were fed on Day 3 by replacing -half the medium with fresh AIM-V plus 10ng/ml GM-CSF. DCs were purified on Day 6 of culture using Nycoprep 1.068 (Accurate Chemical, Westbury, NY) gradient. For vaccinations, DCs were loaded with peptides and/or glycopeptides overnight at 37°C in the presence of 10ng/ml GM-CSF, then washed with PBS and injected i.v. (3-5 × 105 cells/mouse) with soluble MUC1p or TnMUC1.
Results
MUC1 peptide-specific CD4 T cells are not deleted in the thymus of MUC1-Tg mice but their response to antigen is inhibited in the periphery
Previous work suggested that MUC1-specific tolerance in the MUC1-Tg mouse might be a reason for hypo-responsiveness of MUC1-specific CD4 T cells in vivo (12-13, 28). More recent studies in MUC1-Tg mice revealed the existence of MUC1p-specific CD4 T cells ex vivo using alternative vaccination strategies (14-15) and in vitro using T cell cloning techniques (our unpublished data), suggesting that anti-MUC1p CD4 T cells have not simply been deleted. Similarly, although MUC1p-specific CD4 T cells have been difficult to detect directly ex vivo from cancer patients, they have been detected in vitro using T cell cloning techniques (29). To better address the function of MUC1p-specific T cells in an environment where MUC1 is present as a self molecule, we generated MHC class II (I-Ab)–restricted, MUC1 peptide (MUC1p)-specific VFT TCR (Vα2.5-Vβ6) transgenic (VFT-Tg) mice. The VFT TCR recognizes a peptide epitope HGVTSAPDTRPAP (MUC1p) and was cloned from the VF5 CD4+ hybridoma derived from WT mice that were immunized with MUC1p (20). Although not on a RAG−/− background, greater than 60% of VFT CD4 T cells are Vα2+ (unpublished data). The VFT-Tg mice were generated on the congenic C57BL/6 CD90.1+ background, allowing us for the first time to follow the fate of MUC1p-specific CD4 T cells (VFT) in MUC1-Tg mice (CD90.2+) where MUC1 is a self-antigen.
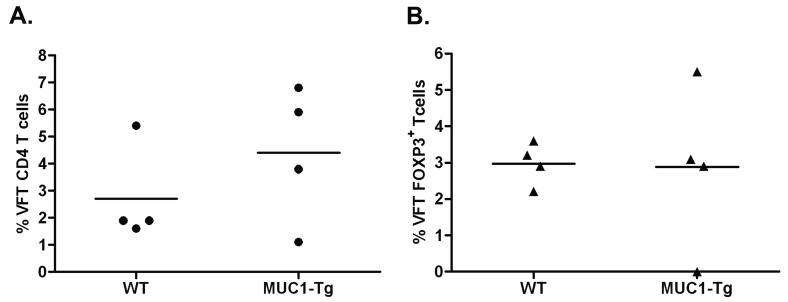
We transferred lineage negative VFT-Tg bone marrow precursors to lethally irradiated WT and MUC1-Tg mice, along with bulk syngeneic bone marrow to ensure survival of the irradiated recipients (26). Five weeks later we found no significant difference between WT and MUC1-Tg recipient mice in the percentage of VFT CD4 T cells that had matured and migrated to secondary lymphoid organs (Fig. 1A, Fig. S3A). This indicated that MUC1p-specific CD4 T cells were not deleted in the thymus of MUC1-Tg mice and, at this time after reconstitution (5 weeks), were not subject to peripheral deletion.
Figure 1. VFT precursors develop through the thymus and enter the periphery at equal levels in WT and MUC1-Tg mice.
Recipient mice (WT, n=4 and MUC1-Tg, n=4) were lethally irradiated prior to bone marrow transfer. Five weeks post VFT bone marrow transfer, the presence of mature donor VFT CD4 T cells in the spleens of recipient mice was assessed by flow cytometry. A) Percent of donor cells (Vα2+CD90.1+) in the CD3+CD4+ gated population of each recipient mouse. B) Intracellular Foxp3 expression in donor cells. Mean values are shown (—). These data are representative of two independent experiments.
To address the possibility that transferred MUC1p-specific CD4 T cell precursors might encounter MUC1p in the thymus and develop into natural T regulatory (Treg) cells, we examined Foxp3 expression in the VFT CD4 T cell thymic emigrants. While Foxp3+ VFT CD4 T cells were present, there was no significant difference in their percentage in WT versus MUC1-Tg recipient mice (Fig. 1B, Fig. S3B, representative analysis in Fig. S4).
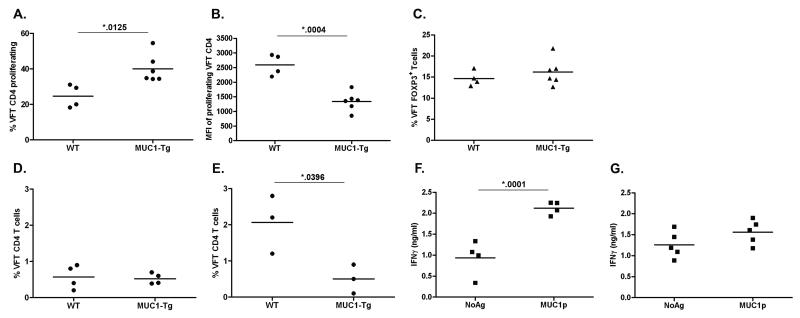
VFT T cells provided an excellent tool to answer the longstanding question of whether endogenous MUC1p epitopes are presented to the immune system in MUC1-Tg mice. Naive CFSE-labeled VFT CD4 T cells were transferred into WT and MUC1-Tg mice. 5 to 7 days later, recipient mice were sacrificed and examined for evidence of T cell activation. CD90.1+TCRVα2+ VFT T cells were readily detected in both WT and MUC1-Tg recipients. However, a significantly higher percentage of VFT CD4 T cells had proliferated in MUC1-Tg recipients compared to WT recipients (Fig. 2A, Fig. S5, representative analysis in Fig. S6). Proliferating VFT CD4 T cells in MUC1-Tg mice had lower CFSE mean fluorescence intensities (MFI) than those in WT mice, indicating that they had gone through more cell divisions (Fig. 2B). The proliferating cells in the MUC1-Tg mice did not appear to have converted into Foxp3+ Treg cells as the percentage of these cells at 5-7 days post transfer was comparable in WT and MUC1-Tg mice (Fig. 2C). Furthermore, although the percentage of Foxp3+ cells in proliferating vs. nonproliferating cells was elevated, as previously reported (30-31), Foxp3 expression in proliferating and non-proliferating VFT CD4 T cells was the same in WT and MUC1-Tg mice (Fig. S7). There was also no significant expansion of VFT CD4 T cells in the MUC1-Tg mice as shown by the same overall percentage of transferred cells in WT and MUC1-Tg recipients (Fig. 2D).
Figure 2. MUC1p-specific VFT CD4 T cells proliferate in the periphery to endogenous stimulation but are hyporesponsive to MUC1p vaccination in MUC1-Tg mice.
3-5×106 CFSE-labeled VFT T cells were transferred (i.v.) to recipient mice. Five to 7 days following adoptive transfer, some recipient mice (WT, n=4; MUC1-Tg, n=6) were sacrificed and the presence of donor VFT CD4 T cells (Vα2+CD4+CD90.1+) in the spleen was determined by flow cytometry. T cell proliferation is shown as A) percentage of VFT CD4 T cells with decreased CFSE fluorescence and B) CFSE mean fluorescence intensity (MFI) of proliferating cells, calculated using the gating strategy in panel A. C) Percent VFT CD4 T cells that are Foxp3+. D) Percent of VFT CD4 T cells with respect to the recipient splenic CD4 T cell population prior to vaccination, 5-7 days post adoptive transfer (WT, n=4; MUC1-Tg, n=4), and E) following two doses of the DC-MUC1p vaccine (i.v.) separated by a two week interval (WT, n=3; MUC1-Tg, n=3). Following the primary DC-MUC1p dose some recipient mice F) WT (n=4) and G) MUC1-Tg (n=5) were sacrificed and splenocyte IFNγ production was measured by ELISA after a 72 hr in vitro MUC1p stimulation (NoAg = No antigen control). Each IFNγ production data point is the mean of triplicate wells per condition for individual mice. The mean values for each group of data points are shown (—). Data is representative of two independent experiments. The p values were calculated using an unpaired t test.
In the next set of experiments, WT and MUC1-Tg mice receiving VFT T cells were vaccinated twice with MUC1p-loaded DC plus soluble MUC1p (DC-MUC1p) as previously described (28). Significant expansion of VFT CD4 T cells was observed in WT recipients, indicating effective T cell stimulation (Fig. 2E). In contrast, VFT CD4 T cells failed to expand in response to the vaccine in MUC1-Tg recipients (Fig. 2E). Several recipient mice from each group were sacrificed 5 days after the primary DC-MUC1p vaccination and their splenocytes re-stimulated with DC-MUC1p ex vivo to assess the recall responses. In vivo primed T cells from WT recipients produced significantly higher levels of IFNγ in response to secondary MUC1p stimulation (Fig. 2F) compared to those primed in the MUC1-Tg recipients (Fig. 2G).
MUC1 glycopeptide-specific CD4 T cells respond equally well in WT and in MUC1-Tg mice
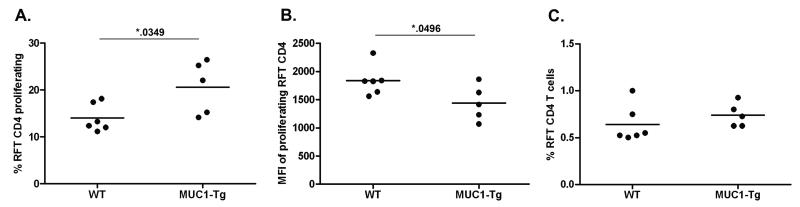
To compare MUC1p specific T cell responses with tumor-associated MUC1 glycopeptide-specific CD4 T cell responses, we generated MHC class II (I-Ab)–restricted (Fig. S8), TnMUC1-specific TCR transgenic mice (RFT-Tg) on the congenic C57BL/6 CD45.1+ background. The RFT TCR (Vα4.1-Vβ15) recognizes TnMUC1 (HGVTSAPDTRPAPGS(GalNAc-O-)TAPPA glycopeptide epitope and was cloned from the RF6 hybridoma derived from CD4 T cells isolated from TnMUC1-immunized WT mice (22). CFSE-labeled RFT CD4 T cells were transferred into WT and MUC1-Tg recipients (CD45.2+). At 5-7 days post transfer we detected a slight, but nonetheless statistically significant increase in RFT CD4 T cell proliferation in MUC1-Tg recipients compared to WT recipients (Fig. 3A,B; representative analysis in Fig. S9), yet there was no significant difference in the overall percentage of RFT CD4 T cells in WT and MUC1-Tg recipients (Fig. 3C). The minimal proliferation of RFT CD4 T cells observed in unvaccinated MUC1-Tg mice was expected because of their low level cross-reactivity with MUC1p originally seen with the RF6 hybridoma (22).
Figure 3. Glycopeptide specific RFT CD4 T cells proliferate to endogenous stimulation in the periphery of MUC1-Tg mice.
3-5×106 CFSE-labeled T cells were transferred (i.v.) to recipient mice (WT, n=6; MUC1-Tg, n=5). 5-7 days following adoptive transfer, recipient mice splenocytes were analyzed by flow cytometry for the presence of RFT CD4 T cells (CD3+CD4+CD45.1+). T cell proliferation is shown as A) percent of RFT CD4 T cells with decreased CFSE fluorescence and B) CFSE mean fluorescent intensity (MFI) of proliferating cells, calculated using the gating strategy in panel A. C) Percent of RFT CD4 T cells with respect to the recipient mouse CD4 T cell population. Mean values are shown (—). Data is representative of two independent experiments. The p values were calculated using an unpaired t test.
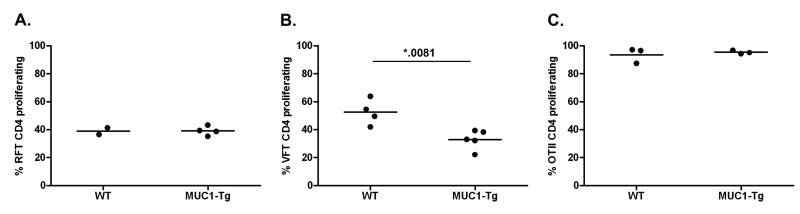
We next addressed if there was an in vivo functional difference between RFT and VFT CD4 cells in the WT and MUC1-Tg environments, especially the possibility that glycopeptide specific RFT cells might respond normally in MUC1-Tg mice. Unlike VFT CD4 T cells that recognize a normal (self) peptide, RFT CD4 T cells recognize a tumor-specific glycopeptide that should not be viewed as self antigen by the immune system. We transferred CFSE-labeled RFT or VFT T cells into WT and MUC1-Tg recipients one day prior to a single vaccination with DC loaded with MUC1p or TnMUC1 and analyzed in vivo proliferation 4-5 days later (representative analysis in Fig. S10). In response to TnMUC1, RFT T cells proliferated to the same extent in both the WT and the MUC1-Tg environment (Fig. 4A). In contrast, VFT CD4 T cell responses to MUC1p were again significantly inhibited in MUC1-Tg mice (Fig. 4B), as previously shown in Figure 2.
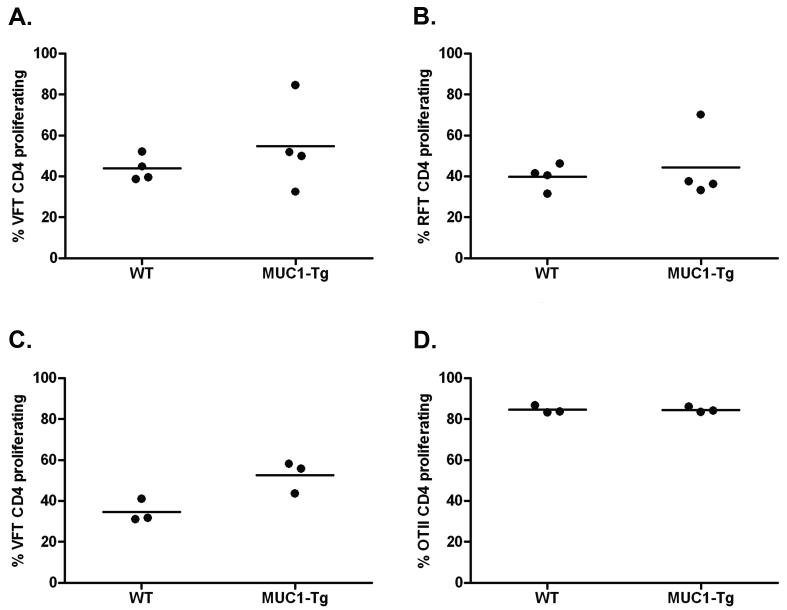
Figure 4. MUC1p-specifc hyporesponsiveness of VFT CD4 T cells, but not RFT or OTII CD4 T cells.
3-5×106 CFSE-labeled RFT, VFT and OTII T cells were transferred (i.v.) to WT and MUC1-Tg recipient mice. One day later, mice were vaccinated (i.v.) using DC-TnMUC1, DC-MUC1p, or DC-ova, respectively. 4-5 days following the vaccination, the spleens of recipient mice were analyzed by flow cytometry for the presence of donor A) RFT CD4 T cells (CD45.1+) (WT, n=2; MUC1-Tg, n=4) or B) VFT CD4 T cells (Vα2+CD90.1+) (WT, n=4; MUC1-Tg, n=5) or C) OTII CD4 T cells (CFSE+) (WT, n=3; MUC1-Tg, n=3) as a percentage of total CD3+CD4+ cells. Proliferation of donor cells was determined by a decrease of CFSE fluorescent intensity. Mean values are shown (—). The p value was calculated using an unpaired t test.
As a control for T cell responses to an antigen foreign to both WT and MUC1-Tg mice, we used CD4 T cells from OTII TCR-Tg mice specific for chicken ovalbumin (ova) (32). Mice receiving CFSE-labeled OTII CD4 T cells were vaccinated with ova peptide-loaded DC. Similar to the RFT CD4 T cells, there was no difference in OTII T cell responses between WT and MUC1-Tg mice (Fig. 4C). Additional control groups comprising WT recipient mice that received unloaded DCs alone showed no T cell proliferation (unpublished data).
Hyporesponsive peptide-specific T cells are rescued by simultaneous activation of glycopeptides-specific T cells
Peptide-specific VFT and glycopeptide-specific RFT CD4 T cells were mixed at a 1:1 ratio, labeled with CFSE, and transferred to WT and MUC1-Tg recipients. One day later, recipient mice were vaccinated with DCs loaded with both MUC1p and TnMUC1 with the expectation that at least some of the DC would process and present both glycopeptide and peptide epitopes simultaneously. When RFT and VFT T cells were concurrently activated in vivo, we saw for the first time equal responses of VFT CD4 T cells in MUC1-Tg and WT recipients (Fig. 5A). Importantly, RFT CD4 T cells were themselves not negatively affected by the presence of VFT cells (Fig. 5B). Hyporesponsiveness of VFT CD4 T cells in MUC1-Tg recipients was also overcome by co-activation of (ova)-specific OTII CD4 T cells (Fig. 5C). Like RFT T cells, OTII CD4 T cells responded at similar levels in both environments (Fig. 5D).
Figure 5. Stimulation of foreign antigen-specific CD4 T cells can help break endogenous MUC1p-specific CD4 T cell tolerance.
3-5×106 CFSE-labeled A,B) VFT and RFT T cells (WT, n=4; MUC1-Tg, n=4) or C,D) VFT and OTII T cells (WT, n=3; MUC1-Tg, n=3) were co-transferred (i.v.) to recipient mice (WT and MUC1-Tg). One day later, mice were vaccinated (i.v.) using co-loaded A,B) DC-(MUC1p+TnMUC1) or C,D) DC-(MUC1p+ova). 4-5 days following the vaccination, the spleen of each recipient mouse was analyzed by flow cytometry for the presence of either A) VFT CD4 T cells (Vα2+CD90.1+) and B) RFT CD4 T cells (CD45.1+) or C) VFT CD4 T cells and D) OTII CD4 T cells (CFSE+) as a percentage of total CD3+CD4+ cells. The proliferation of donor cells was determined by a decrease of CFSE fluorescent intensity. Mean values are shown (—).
Discussion
Past and present studies in the MUC1-Tg mouse model have indicated that in the context of that “self” environment, immune responses to unglycosylated MUC1 VNTR peptides are greatly reduced, thus compromising anti-MUC1 tumor immunity (4, 13-15, 28). As a strategy to increase the potency of MUC1 vaccines, we added tumor-associated Tn glycans to the peptide immunogen to more closely represent epitopes that are displayed on all MUC1+ tumors and on APCs that cross-present tumor MUC1 to T cells in patients.
To study the potential differences in T cell responses to the MUC1 peptide (“self”) and the glycopeptide (“foreign”) epitopes, we generated two TCR-Tg mice, one (VFT) bearing a peptide-specific TCR and the other (RFT) a glycopeptide-specific TCR. By adoptively transferring TCR transgenic T cell precursors or mature T cells into WT and MUC1-Tg mice we found that reduced responses of MUC1 peptide-specific CD4 T cells were not due to their deletion during thymic development in MUC1-Tg mice. Rather, their activation was inhibited in the periphery at the time of antigen stimulation. On the other hand, MUC1 glycopeptide-specific CD4 T cell stimulation did not appear to be subject to that same inhibition, behaving more like the CD4 T cells specific for the foreign antigen ova. We further showed that the hyporesponsiveness of VFT T cells in MUC1-Tg mice can be overcome in the presence of activated RFT or OTII T cells.
The best known mechanism of self-tolerance is thymic deletion of self-reactive T cells (negative selection) (33). More recently, it was shown that developing thymocytes could also differentiate into CD4 T regulatory (Treg) cells due to positive signals received by self-antigen recognition in the thymus (34-35). Normal, fully glycosylated MUC1 is expressed by human medullary thymic epithelial cells (mTEC) (36) and could theoretically influence the MUC1-specific T cell repertoire. Ectopic expression of other peripheral-tissue antigens by mTECs has been reported to result in central tolerance [reviewed in (37)], including that to tumor-associated carcinoembryonic antigen (CEA) (2). However, we previously published that complete glycosylation of the MUC1 VNTR region as expressed in healthy tissues prevents processing and presentation of peptide epitopes by DC (38). Thus, the extent to which the MUC1p-specific T cell repertoire might be affected by self-tolerance was not clear. By comparing MUC1p-specific T cell development in WT versus MUC1-Tg mice, we did not detect any difference in the efficiency of emigration from the thymus, suggesting that the MUC1-HGVTSAPDTRPAP peptide epitope is either not presented to developing thymocytes, or is presented at levels insufficient to induce clonal deletion or conversion to nTreg.
Although peripheral tolerance is not well understood, studies have shown that auto-reactive T cells specific for model self-antigens (39) and tumor-associated antigens (3, 40) that are expressed in peripheral tissues can be tolerized at peripheral sites. We observed proliferation of transferred VFT CD4 T cells in unvaccinated MUC1-Tg mice and not in WT mice, suggesting that peptide epitopes were being presented in the periphery. Lowering the level of T cell responses to those epitopes in vaccinations might prevent autoimmunity. Similar levels of Foxp3+ VFT CD4 cells in the periphery of WT and MUC1-Tg mice, as well as the lack of suppression of RFT T cell responses when co-activated with VFT T cells, imply that this is not mediated by induced Treg, although a more detailed analysis is needed to rule out or reveal preexisting endogenous MUC1-specific Treg.
There have been no reports of MUC1p vaccine-induced autoimmunity in MUC1-Tg mice (4, 15, 28), primates (41), or human clinical trials (9-10). Thus, it is likely that MUC1p epitopes are presented at very low levels on normal tissues in the absence of co-stimulation, which could induce anergy or peripheral deletion of high affinity T cells. Recently, populations of nonhematopoietic cells that express and present PTAs have been characterized in secondary lymphoid organs (42-43). These cells could be the source of MUC1 peptides being presented to CD4 T cells in the periphery, rather than the non-lymphoid ductal epithelial tissue (non-MHC class II expressing) where MUC1 is normally expressed. In addition, secondary lymphoid tissues may contain circulating DCs that have captured peripheral tissue antigens, such as MUC1, from dying cells and are presenting the antigens in the steady state to maintain self tolerance (44-45).
Gerloni et al. (14) previously reported that, by providing a non-self determinant (heterologous help) together with MUC1p, they could activate previously hypo-responsive MUC1p-specific CD4 T cells. We have confirmed that by simultaneously activating ova-specific OTII T cells and showing that they provide heterologous help to VFT T cells. More importantly, however, we show that TnMUC1 can provide similar help by activating glycopeptide-specific T cells thus leading to improved responses of MUC1p specific T cells. While both ova and TnMUC1 can serve the helper function and improve responses at the time of priming, only TnMUC1 would be present and available to perform that function during recall responses when the tumor recurs or a new MUC1+ tumor arises. Even though this was not directly tested in our experiments, the ability of VFT T cells to respond in MUC1-Tg mice under some circumstances as robustly as RFT T cells, shows that their hyporesponsivness is not simply due to a potentially lower affinity TCR but rather the environment in which their TCR is activated.
Beyond traditional peptide-specific T cell responses, recent studies have shown that T cells (via the TCR) can respond to MHC class I and II restricted peptides that contain post-translational modifications, such as phosphorylation and glycosylation (46). Abnormal glycosylation has been related to autoimmune diseases where abnormally glycosylated proteins efficiently activate effector T cells resulting in autoimmune cytotoxicity (47-48). In the case of tumor immunity, the ability to direct such cytotoxic responses against abnormal molecules on malignant cells would be beneficial. The fact that the majority of cell proteins are glycosylated and that protein glycosylation is known to be dysregulated in cancer cells (49-50) should encourage more effort on targeting of tumor-specific glycopeptides (19-20, 22), a viable alternative to non-mutated peptide targets that have been shown to face self tolerance.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health grants T32CA82084 (SR, MT), RO1CA56103 and PO1CA73743 (OF) and a grant from the Canadian Breast Cancer Research Alliance in association with the Canadian Cancer Society (JG). The authors gratefully acknowledge expert help of Dr. Jaspal S. Khillan, Mr. Kazi Islam and Ms. Joyce M. Dawes.
Abbreviations used
- CTL
Cytotoxic T lymphocyte
- DC
dendritic cell
- MUC1
Mucin 1
- MUC1p
MUC1 peptide
- TnMUC1
MUC1 glycopeptide
- TAA
Tumor-associated antigen
- TCR
T cell receptor
- Tg
Transgenic
- Th
T helper
- Treg
T regulatory
- VNTR
Variable number of tandem repeats
- WT
Wild type
References
- 1.Ryan SO, Gantt KR, Finn OJ. Tumor antigen-based immunotherapy and immunoprevention of cancer. Int Arch Allergy Immunol. 2007;142:179–89. doi: 10.1159/000097020. [DOI] [PubMed] [Google Scholar]
- 2.Bos R, van Duikeren S, van Hall T, et al. Expression of a natural tumor antigen by thymic epithelial cells impairs the tumor-protective CD4+ T-cell repertoire. Cancer Res. 2005;65:6443–9. doi: 10.1158/0008-5472.CAN-05-0666. [DOI] [PubMed] [Google Scholar]
- 3.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 4.Tempero RM, VanLith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ Lymphocytes Provide MUC1-Specific Tumor Immunity In Vivo That Is Undetectable In Vitro and Is Absent in MUC1 Transgenic Mice. J Immunol. 1998;161:5500–6. [PubMed] [Google Scholar]
- 5.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–38. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 6.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. Advances in Immunology. Academic Press; 2004. MUC1 Immunobiology: From Discovery to Clinical Applications; pp. 249–93. [DOI] [PubMed] [Google Scholar]
- 7.Jerome KR, Barnd DL, Bendt KM, et al. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–16. [PubMed] [Google Scholar]
- 8.Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993;151:1654–62. [PubMed] [Google Scholar]
- 9.Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: Transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–9. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 12.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 13.Turner MS, Cohen PA, Finn OJ. Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. J Immunol. 2007;178:2787–93. doi: 10.4049/jimmunol.178.5.2787. [DOI] [PubMed] [Google Scholar]
- 14.Gerloni M, Castiglioni P, Zanetti M. The cooperation between two CD4 T cells induces tumor protective immunity in MUC.1 transgenic mice. J Immunol. 2005;175:6551–9. doi: 10.4049/jimmunol.175.10.6551. [DOI] [PubMed] [Google Scholar]
- 15.Ding C, Wang L, Marroquin J, Yan J. Targeting of antigens to B cells augments antigen-specific T-cell responses and breaks immune tolerance to tumor-associated antigen MUC1. Blood. 2008;112:2817–25. doi: 10.1182/blood-2008-05-157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 17.Vlad AM, Finn OJ. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast Disease. 2004;20:73–9. doi: 10.3233/bd-2004-20109. [DOI] [PubMed] [Google Scholar]
- 18.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 19.Ninkovic T, Kinarsky L, Engelmann K, et al. Identification of O-glycosylated decapeptides within the MUC1 repeat domain as potential MHC class I (A2) binding epitopes. Mol Immunol. 2009;47:131–40. doi: 10.1016/j.molimm.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlad AM, Muller S, Cudic M, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–46. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Sette A, Sidney J, Gendler SJ, Franco A. Tumor-associated carbohydrate antigens: A possible avenue for cancer prevention. Immunol Cell Biol. 2005;83:440–8. doi: 10.1111/j.1440-1711.2005.01347.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryan SO, Vlad AM, Islam K, Gariépy J, Finn OJ. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol Chem. 2009;390:611–8. doi: 10.1515/BC.2009.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broeren CP, Verjans GM, Van Eden W, Kusters JG, Lenstra JA, Logtenberg T. Conserved nucleotide sequences at the 5′ end of T cell receptor variable genes facilitate polymerase chain reaction amplification. Eur J Immunol. 1991;21:569–75. doi: 10.1002/eji.1830210306. [DOI] [PubMed] [Google Scholar]
- 24.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180:273–80. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 25.Letourneur F, Malissen B. Derivation of a T cell hybridoma variant deprived of functional T cell receptor alpha and beta chain transcripts reveals a nonfunctional alpha-mRNA of BW5147 origin. Eur J Immunol. 1989;19:2269–74. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- 26.Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–74. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 27.Brokx RD, Revers L, Zhang Q, et al. Nuclear magnetic resonance-based dissection of a glycosyltransferase specificity for the mucin MUC1 tandem repeat. Biochemistry. 2003;42:13817–25. doi: 10.1021/bi0353070. [DOI] [PubMed] [Google Scholar]
- 28.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–63. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 29.Hiltbold EM, Ciborowski P, Finn OJ. Naturally processed class II epitope from the tumor antigen MUC1 primes human CD4+ T cells. Cancer Res. 1998;58:5066–70. [PubMed] [Google Scholar]
- 30.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: A state attained by all activated human T-cells. Clinical Immunology. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 33.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 34.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 35.Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre M-L. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol. 2009;182:6736–43. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cloosen S, Arnold J, Thio M, Bos GMJ, Kyewski B, Germeraad WTV. Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer Res. 2007;67:3919–26. doi: 10.1158/0008-5472.CAN-06-2112. [DOI] [PubMed] [Google Scholar]
- 37.Tykocinski L-O, Sinemus A, Kyewski B. The thymus medulla slowly yields its secrets. Ann N Y Acad Sci. 2008;1143:105–22. doi: 10.1196/annals.1443.018. [DOI] [PubMed] [Google Scholar]
- 38.Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol. 2000;165:3730–41. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher AL, Lukacs-Kornek V, Reynoso ED, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bos R, van Duikeren S, Morreau H, et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 2008;68:8446–55. doi: 10.1158/0008-5472.CAN-08-1864. [DOI] [PubMed] [Google Scholar]
- 41.Barratt-Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T-cell responses. Clin Cancer Res. 1999;5:1918–24. [PubMed] [Google Scholar]
- 42.Gardner JM, DeVoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic aire-expressing cells. Science. 2008;321:843–7. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen JN, Guidi CJ, Tewalt EF, et al. Lymph node–resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–8. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinman RM, Hawiger D, Liu K, et al. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 45.Parish IA, Heath WR. Too dangerous to ignore: self-tolerance and the control of ignorant autoreactive T cells. Immunol Cell Biol. 2008;86:146–52. doi: 10.1038/sj.icb.7100161. [DOI] [PubMed] [Google Scholar]
- 46.Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL. Post-translational modifications of naturally processed MHC-binding epitopes. Curr Opin Immunol. 2006;18:92–7. doi: 10.1016/j.coi.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Ju T, Cummings RD. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 48.Bäcklund J, Carlsen S, Höger T, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263-270) in humanized transgenic mice and in rheumatoid arthritis. PNAS. 2002;99:9960–5. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanisch F-G. O-glycosylation of the mucin type. Biol Chem. 2001;382:143–9. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 50.Schietinger A, Philip M, Yoshida BA, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–8. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.