Abstract
Purpose
Inhibitors of TORC1 have been shown to be active in patients with metastatic renal cell carcinoma (RCC). As the PI3-K pathway activates numerous other kinases, transcription factors and proteins associated with cell growth and survival besides mTOR, disruption of this pathway upstream of mTOR may be more effective than inhibition of TORC1 alone.
Experimental Design
To investigate this possibility, the dual PI3-K/mTOR inhibitor NVP-BEZ235 was compared with rapamycin in RCC cell lines and xenografts generated from 786-O and A498 cells.
Results
Treatment of RCC cell lines with NVP-BEZ235 in vitro resulted in the nuclear translocation of p27, greater reduction in tumor cell proliferation, and more complete suppression of Akt, Mnk-1, eIF4E, and 4EBP-1 phosphorylation and Cyclin D1 and HIF2α expression than that achieved with rapamycin. The reduction of HIF2α levels correlated with reduced HIF activity as determined by luciferase assay. NVP-BEZ235 induced growth arrest in both the 786-O and A498 xenografts that was associated with inhibition of Akt and S6 phosphorylation as well as the induction of apoptosis and reduction in markers of tumor cell proliferation. In contrast, rapamycin induced only minimal growth retardation.
Conclusion
Dual inhibition of PI3-K/mTOR with NVP-BEZ235 induced growth arrest in RCC cell lines both in vitro and in vivo more effectively than inhibition of TORC1 alone. These results provide the rationale for the clinical assessment of agents such as NVP-BEZ235 in patients with advanced RCC.
Keywords: Renal Cancer, PI3-Kinase, Akt, mTOR, HIF
INTRODUCTION
The kinase mTOR (mammalian Target Of Rapamycin) exists in two functionally distinct multiprotein complexes TORC1 and TORC2. TORC1, a complex including mTOR and raptor (regulatory-associated protein of mTOR), regulates protein translation and many aspects of metabolism (1). TORC2, a complex including mTOR and rictor (rapamycin-insensitive companion of TOR), regulates the activity of the kinase Akt (PKB) (1). Inhibitors of TORC1 have significant activity in patients with renal cell carcinoma (RCC) and two such agents, temsirolimus and everolimus, are now FDA-approved for the therapy of advanced RCC (2, 3). However, responses to TORC1 inhibitors are infrequent and typically short-lived and all patients treated with these drugs eventually develop progressive disease.
One of the proposed shortcomings of TORC1 inhibitors is the feedback activation of the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway. Treatment with TORC1 inhibitors has been shown in some cases to result in the activation of PI3-K through a feedback loop involving the IGF-1 receptor (4). TORC1 inhibitors also can activate Akt directly through the derepression of TORC2, which results in TORC2-mediated phosphorylation of Akt on Ser473 (5). The primary mechanism of action of TORC1 inhibitors is thought to be the dephosphorylation and activation of eukaryotic translation initiation factor (eIF4E) binding proteins (4EBPs), which function to sequester and block eIF4E from carrying out cap-dependent translation of certain “difficult to translate” mRNAs such as those of VEGF, cyclin D, c-Myc, and survivin (6, 7). The feedback activation of PI3-K may directly undermine the efficacy of TORC1 inhibitors by promoting the phosphorylation of eIF4E by Mnk1, thereby enhancing its affinity for the mRNA cap structure and activating cap-dependent translation (8). Not surprisingly, inhibition of PI3-K and/or TORC2 simultaneously with TORC1 has emerged as a therapeutic strategy that may negate activation of this feedback loop and more effectively suppress the translation of these critical mRNAs.
The ability to avoid the feedback activation of PI3-K is not the only theoretical advantage of PI3-kinase inhibitors over TORC1 inhibitors. PI3-K/Akt signaling activates an array of kinases, transcription factors and other proteins besides mTOR which promote cell growth and survival (9, 10). These prosurvival effects include the phosphorylation and nuclear export of FOXO3a, which reduces the expression of fas ligand, Bim, and other pro-apoptotic proteins. PI3-kinase activation also results in the downstream activation of NF-κB and the inactivation of pro-apoptotic proteins such as BAD and procaspase 9. Disruption of any of these pro-survival signals may have therapeutic benefits that complement the effects of TORC1 inhibition and enhance antitumor activity.
In addition to PI3-K inhibition, attenuation of TORC2 is yet another potential strategy in the treatment of RCC, particularly the clear cell variant. The majority of these tumors possess bi-allelic alterations in the von Hippel Lindau (VHL) gene (11, 12) resulting in the accumulation of Hypoxia-Inducible Factors (HIF)-1 and -2 and the subsequent activation of their target genes, including VEGF, PDGF, TGF-α, and CXCR4 (13–15). Although the two HIFs have overlapping effects on gene expression, HIF-2α is thought to be the more relevant HIF with respect to the development and progression of RCC (16–19). Recent studies have demonstrated that the translation of HIF-2α is completely dependent upon the activity of TORC2 (19). These data suggest the potential value of TORC2 inhibition in the treatment of RCC.
A new generation of agents with activity against PI3-K and mTOR is currently in clinical development. NVP-BEZ235 is a novel, orally bioavailable imidazoquinoline which potently and reversibly inhibits class 1 PI3-K activity by binding to its ATP-binding domain (20). NVP-BEZ235 also binds directly to the mTOR ATP-binding domain and directly inhibits its catalytic activity, thereby inhibiting both TORC1 and TORC2. NVP-BEZ235 has been demonstrated to inhibit PI3-K and mTOR activity and tumor growth in numerous pre-clinical models including prostate, breast, and pancreatic carcinoma, glioblastoma, and multiple myeloma, and is currently in Phase I testing in patients with solid tumors (21–23).
Although RCC is the only malignancy for which TORC1 inhibitors have gained FDA approval, the benefit derived from the use of these drugs in RCC patients is modest. Agents that block PI3-kinase or TORC2 in addition to TORC1 have numerous theoretical advantages over TORC1 inhibitors that may translate into superior antitumor activity. In this report, we have compared the effects of the novel dual PI3-K/mTOR inhibitor NVP-BEZ235 with those of the selective TORC1 inhibitor rapamycin on intracellular signaling in vitro and in vivo and on tumor growth in RCC xenografts. We show that NVP-BEZ235 downmodulates cyclin D, survivin and HIF-2α to a far great extent than does rapamycin. It induces the nuclear translocation of p27, blocks the phosphorylation of eIF4E and Mnk1, and inhibits tumor cell proliferation both in vitro and in vivo better than rapamycin. Our data reinforce the view that the concurrent suppression of PI3-kinase and/or TORC2 in addition to TORC1 is likely to be a more effective strategy in the treatment of RCC than the inhibition of TORC1 alone.
METHODS
Cell Lines and Reagents
The human RCC cell lines (786-O, 769-P, A498, Caki-1, and Caki-2) were obtained from the American Type Culture Collection (Manassas, VA) and passaged for less than 6 months following receipt. 786-0 and 769-P lines were maintained in RPMI 1640; A498 in Minimal Essential Media; Caki-1 and Caki-2 in McCoys 5A. All media contained 10% fetal bovine serum, 4mM glutamine, and 50μM gentamycin. Cells were incubated at 37°C at 5% CO2. NVP-BEZ235 (Novartis Phamaceuticals, Basel, Switzerland) and Rapamycin (Santa Cruz Biotechnology, Santa Cruz, CA) were solubilized for in vitro assays in DMSO.
Western Blots
Cells were treated as described in Results and then lysed in Lysis Solution (Cell Signaling, Beverly, MA) supplemented with sodium fluoride (10μM) and phenylmethylsulfonyl fluoride (100μg/mL). Lysates were fractionated on polyacrylamide gels and transferred to nitrocellulose. The blots were probed with specific antibodies followed by a second antibody-horseradish peroxidase conjugate and then incubated with SuperSignal substrate (Pierce, Rockford, IL). Phospho- and total Akt, S6, eIF4E, Mnk1, 4EBP, GSK3β and phospho-ERK, and cyclin D1 antibodies were purchased from Cell Signaling. The total ERK and c-Myc antibody was purchased from Santa Cruz. The survivin, vinculin, and HIF-2α antibodies were purchased from R&D Systems (Minneapolis, MN), Sigma (St. Louis, MO), and Novus Biologicals (Littleton, CO), respectively.
Cell Proliferation Assays
MTS proliferation studies were performed using the Promega CellTiter 96® AQueous Cell Proliferation Assay (Promega, Madison, WI). RCC cell lines were grown in tissue-culture coated 96-well plates and treated as described in Results. Cells were then treated with the MTS/PMS solution for 1 hour at 37°C. Absorbance at 490nM for each condition was determined using an ELISA plate reader. Data is reported as percent viable tumor cells in each condition as compared with cells treated with DMSO alone.
Cell Death Assay
Cells were treated as described in Results. In each assay, the adherent cells were detached by treatment with trypsin and combined with the non-adherent cells. Propidium iodide (5ng/ml) was added to the cells and after 20 minutes at room temperature, the cells were analyzed by flow cytometry with a Coulter FC 500 cytometer. The percentage of cells staining positive was recorded.
HIF Reporter Assay
The HIF luciferase reporter construct, encoding Firefly luciferase under control of a CMV promoter and three tandem copies of the VEGF hypoxia response element, was provided by Andrew Kung (Children’s Hospital, Boston, MA). RCC cell lines were transiently co-transfected with the reporter and a construct encoding Renilla luciferase using the TransIT-TKO® system. After 24 hours, the transfected cells were treated with DMSO, rapamycin (100nM), or BEZ235 (250nM) for 24 hours. Renilla and Firefly activities were then determined by luminometry using the Dual-Luciferase Reporter Assay System (Promega) and the ratio calculated. Results were expressed as the ratio of Firefly to Renilla luciferase activity.
Localization of p27
Nuclei were isolated from the cytosolic fraction in 786-O and A498 cells treated with DMSO, rapamycin (100nM), or NVP-BEZ235 (250nM) for 6 hours using the Nuclei EZ Prep Nuclei Isolation Kit (Sigma). Lysates from nuclear and cytosolic fractions were analyzed for p27 by western blot. To determine localization of p27 by immunoflourescence, 786-O and A498 cells were grown on tissue culture treated slides, treated as above, and fixed with 0.5% zinc chloride and 0.5% zinc acetate for 15 minutes. Cells were then treated with rabbit anti-p27 (1:100, Cell Signaling), followed by goat-anti-rabbit coupled to Alexa Fluor 488 (1:100, Cell Signaling). Nuclei were detected with Hoechst 33342 (Sigma). Slides were then mounted and analyzed by fluorescence microscopy.
Xenograft Model
Eight-week old, female, nude/beige mice were purchased from Charles River Laboratories (Wilmington, MA). Approximately 5×106 786-0 or A498 cells were injected into the flanks and tumors allowed to reach 10mm in maximal diameter. Mice were then treated once daily by gavage with either vehicle, rapamycin (3.5mg/kg), or NVP-BEZ235 (40mg/kg). Bi-dimensional tumor measurements were taken every other day and mice weighed once weekly. Tumor volume was estimated using the standard formula: (length × width2)/2. Mice were sacrificed after 3 or 21 days of treatment and tumors excised. Tumors were divided and either flash frozen in liquid nitrogen or placed in 10% buffered formalin. Rapamycin was initially solubilized as a stock solution of 20mg/ml in ethanol. Prior to gavage, rapamycin was brought up to volume (0.2ml) in PBS with 0.5% TWEEN 80 and 2.5% N, N-Dimethylacetamide. Prior to gavage, NVP-BEZ235 was initially solubilized in one part N-Methylpyrrolidone and brought up to volume (0.2ml) in nine parts PEG 300.
Immunohistochemistry (IHC)
All specimens were kept in 10% buffered formalin for 48 hours after which they were embedded in paraffin and 4μm thick slides prepared and stained with hematoxylin and eosin. Sections were dewaxed, soaked in alcohol, and after pressure cooker treatment in antigen unmasking solution at 125°C for 30 seconds, incubated in 3% hydrogen peroxide for 5 minutes. Sections were incubated with the appropriate antibody and epitopes detected using the DAKO EnVision™+ horseradish peroxidase detection kit (Dako, Carpinteria, CA). Slides immunostained for CD34, Ki67, and caspase 3 were scanned using the ScanScope® slide scanning system (Aperio Technologies, Vista, CA) and analyzed using the Microvessel Analysis, Nuclear Image Analysis, and Pixel Analysis Algorithms (Aperio), respectively. The Caspase 3, Ki67, and CD34 antibodies were purchased from Dako, Cell Signaling, and Abcam (Cambridge, MA), respectively. Human tonsilar tissue was used as a positive control for caspase 3 staining.
Statistical Analysis
Results for proliferation and reporter assays were reported as mean +/− standard deviation. Statistical comparisons for mean final tumor volumes in the xenograft studies were made using a one-way ANOVA. P values of pairwise comparisons were adjusted using Tukey’s method. Comparison of luciferase activity between treatment conditions as well as IHC staining quantification between tumors from various treatment groups were made using two-sample t-tests. P<0.05 was considered significant.
RESULTS
Concentration-dependent effects of NVP-BEZ235 on intracellular signaling and proliferation in RCC cell lines
To assess the effects of NVP-BEZ235 on intracellular signaling in RCC, two human RCC cell lines (786-O [VHL(−/−), PTEN-null] and 769-P [VHL(−/−), PTEN-WT]) were exposed to increasing concentrations of NVP-BEZ235 in vitro for 6 hours, lysed, and analyzed by western blot. As shown in Figure 1A, NVP-BEZ235 inhibited TORC1/2 activity at low nanomolar (<25 nM) concentrations as evidenced by the dephosphorylation of S6, 4E-BP, and Akt (Ser473). A higher concentration of NVP-BEZ235 was required to suppress the phosphorylation of Akt (Thr308) and GSK3β (Ser9), suggesting that the IC50 for NVP-BEZ235 for PI3-K may be higher than that for either TORC1 or TORC2 in RCC cell lines. NVP-BEZ235 also induced the activation of MAP-kinase as indicated by enhanced ERK phosporylation. Both the hierarchical suppression of PI3-K and TORC1/2 and the activation of MAP kinase by NVP-BEZ235 have been previously reported in other tumor types (21, 24).
Figure 1.
A) Intracellular signaling events induced by increasing concentrations of NVP-BEZ235 in vitro in the RCC cell lines 786-0 and 769-P. B) Inhibition of cell proliferation by increasing concentrations of NVP-BEZ235 in vitro as determined by MTS Assay. Proliferation is expressed as the percent viable cells in NVP-BEZ235-treated culture compared with that of cells treated with DMSO. Each bar represents the mean of three independent experiments (error bar = 1 S.D.)
In order to assess the activity of NVP-BEZ235 on tumor cell proliferation, the RCC cell lines were exposed to increasing concentrations of the drug in vitro for 48 hours and then analyzed by MTS assay. As shown in Figure 1B, treatment with NVP-BEZ235 resulted in a concentration-dependent reduction in viable tumor cells. To determine if this effect might be due to the induction of apoptosis, the treated cells were stained with propidium iodide and analyzed by flow cytometry. Drug exposure failed to increase the percentage of propidium iodide-staining (apoptotic) cells (data not shown) suggesting the reduction in viable tumor cells in vitro was due to suppression of proliferation rather than the induction of apoptosis.
Comparison of the effects of NVP-BEZ235 versus Rapamycin in RCC cell lines
To determine if NVP-BEZ235 might downmodulate the cap-dependent translation of “difficult to translate” mRNAs such as those for cyclin D1, c-Myc, and survivin more effectively than a TORC1 inhibitor, 5 different RCC cell lines (786-0, 769-P, A498 [VHL−/−, PTEN-WT], Caki-1 [VHL+/+, PTEN-WT], and Caki-2 [VHL+/+, PTEN-WT]) were treated with either DMSO, rapamycin (100 nM), or NVP-BEZ235 (250 nM) for 24 hours and subsequently analyzed by western blot. The concentration of rapamycin was chosen based on similar studies in the literature and, as shown in Supplemental Figure 1, was at least 10X greater than that required to suppress the phosphorylation of S6 in 786-O cells (25). The NVP-BEZ235 concentration was chosen based on the complete inhibition of Akt (Thr308) phosphorylation as well as maximal suppression of tumor cell proliferation achieved at this dose. As shown in Figure 2A, both drugs completely suppressed S6 phosphorylation, but only NVP-BEZ235 consistently blocked both Akt and 4E-BP1 phosphorylation and reduced cyclin D1 and survivin expression. The expression of c-Myc was unaffected by either drug. NVP-BEZ235 also reduced the phosphorylation of eIF4E (Ser209) and Mnk1 (Thr197/204), neither of which were appreciably affected by rapamycin.
Figure 2.
A) Differential effects of rapamycin versus NVP-BEZ235 on intracellular signaling in RCC cells treated with DMSO, rapamycin (100nM), or NVP-BEZ235 (250nM) for 24 hours and then lysed and analyzed by Western blot. B) Superior inhibition of RCC cell proliferation by NVP-BEZ235 as compared with rapamycin. Proliferation is expressed as the percent viable cells in the NVP-BEZ235 (B) or rapamycin (R) treated cultures as compared with DMSO (0) treated cells. Each bar represents the mean of three independent experiments (error bar = 1 S.D.).
In order to compare the effects of rapamycin and NVP-BEZ235 on proliferation, the same 5 RCC cell lines were treated with either DMSO, rapamycin (100 nM), or NVP-BEZ235 (250 nM) for 48 hours and analyzed for viability by MTS assay. As shown in Figure 2B, treatment with NVP-BEZ235 resulted in significantly greater reduction in proliferation than rapamycin in all RCC cell lines.
Effects of NVP-BEZ235 and Rapamycin on HIF-2α expression and HIF transcriptional activity
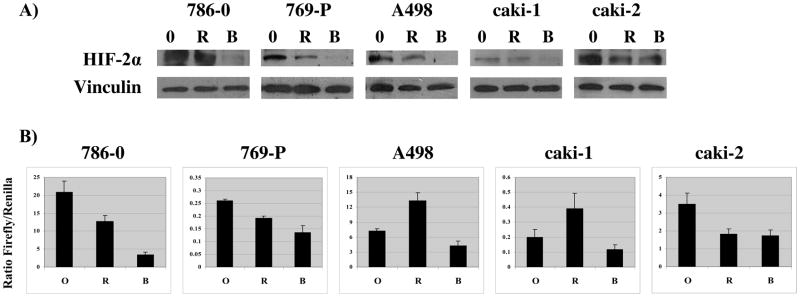
To investigate the hypothesis that an agent with activity against both TORC1 and TORC2 would be more effective than a TORC1 inhibitor at suppressing HIF-2α expression, lysates from RCC cell lines treated with DMSO, rapamycin (100nM), or NVP-BEZ235 (250nM) for 24 hours were analyzed by western blot for HIF-2α expression. As shown in Figure 3A, NVP-BEZ235 was significantly more effective than rapamycin in reducing HIF-2α expression in all five RCC cell lines. To assess the effect of NVP-BEZ235 on overall HIF activity in RCC cell lines (many of which express only HIF-2α) the five cell lines were transiently transfected with a HIF-luciferase reporter construct, treated with drug, and analyzed by luminometry for luciferase activity. As shown in Figure 3B, NVP-BEZ235 was significantly more effective than rapamycin (p<0.05) in inhibiting overall HIF activity in all of the RCC cell lines except Caki-2, in which the two drugs were equally effective.
Figure 3.
A) Effect of NVP-BEZ235 compared with rapamycin on HIF-2α expression in RCC cells. B) Effect of NVP-BEZ235 compared with rapamycin on HIF reporter activity. Data are reported as the ratio of firefly to renilla luciferase activities, as described in Methods. Each bar represents the mean of three independent experiments (error bar = 1 S.D.).
Effect of NVP-BEZ235 and Rapamycin on p27 Localization
One mechanism by which PI3-K blockade results in cell cycle arrest is induction of p27 nuclear translocation (25). To investigate whether NVP-BEZ235 and Rapamycin may have differential effects on p27 localization, the intracellular distribution of p27 in RCC cells treated with DMSO, rapamycin, or NVP-BEZ235 was assayed by both western blot and immunofluorescence. As shown in Figure 4A, treatment of both 786-O and A498 cells with NVP-BEZ235 resulted in the translocation of p27 to the nucleus. This finding was corroborated in intact cells by immunofluorescence (Figure 4B).
Figure 4.
A) Expression of p27 in nuclear and cystolic fractions of 786-O and A498 cells treated with DMSO (0), rapamycin (R), or NVP-BEZ235 (B). B) Immunoflourescence imaging of p27:Alexa488, Bisbenzimide H33342, and overlays in 786-O cells treated with DMSO, rapamycin, or NVP-BEZ235.
Antitumor activity of NVP-BEZ235 compared with that of rapamycin in 786-0 and A498 RCC xenografts
To assess the in vivo efficacy of NVP-BEZ235, nude/beige mice bearing either 786-0 or A498 xenografts were treated daily with vehicle, rapamycin, or NVP-BEZ235 as described in Methods for 21 consecutive days. As shown in Figure 5A, in both xenografts models, NVP-BEZ235 treatment resulted in growth arrest whereas rapamycin had only a modest retarding effect on tumor growth. In both xenograft models, there was a significant difference in the average tumor volume among the three-groups on day 21 (p value<0.01 for each pair-wise comparison). The NVP-BEZ235-treated group had the lowest tumor volume in both 786-O and A498 xenograft models (418.8 ± 108.1mm3 and 550.5 ± 96.3 mm3, respectively), which was significantly different from both the rapamycin (1394.9 ± 192.5 mm3; 1263.9 ± 141.2mm3) and vehicle (1858.2 ± 239.4mm3; 1840.8 ± 292.0mm3) treated groups.
Figure 5.

Antitumor activity of of NVP-BEZ235 compared with rapamycin in 786-O and A498 xenografts. A) Growth curves of 786-O and A498 xenografts in nude beige mice treated with saline, rapamycin, or NVP-BEZ235 daily by gavage. Each treatment group was comprised of 8 mice in 786-O model (6 mice in A498), and each data point represents the mean estimated tumor volume of the tumors (error bar = 1 S.D.). B) Intracellular signaling events in 786-O and A498 xenografts from mice treated with saline (S), rapamycin (R), or NVP-BEZ235 (B) for 3 days.
To evaluate the in vivo effects of NVP-BEZ235 and rapamycin on intracellular signaling, 3 mice per treatment group were sacrificed after 3 days of treatment and tumors excised for analysis by western blot and IHC. S6 phosphorylation was suppressed in tumors from mice treated with either drug (Figure 5B and 6). Akt phosphorylation was inhibited only in the tumors from mice treated with NVP-BEZ235. In both xenograft models, tumors from mice treated with NVP-BEZ235 also demonstrated significantly lower expression of phospho-eIF4E, phospho-4E-BP1, cyclin D1, and HIF-2α than tumors from saline- or rapamycin-treated mice. These in vivo data recapitulate the in vitro results obtained with drug-treated RCC cell lines (Figures 2 and 3). In both xenograft models, IHC analyses revealed reduced Ki-67 staining and increased cleaved caspase 3 staining in the tumors from NVP-BEZ235-treated mice relative to the saline controls. Quantification of Ki67 staining demonstrated a significant decrease in mean percent cells positive for nuclear staining in tumors from NVP-BEZ235-treated mice compared with saline controls in the A498 model (17.1 vs. 26.3; n=3 per group; p=0.026) and a nearly significant decrease in the 786-O model (31.3 vs. 44.7; n=3 per group; p=0.059). Quantification of cleaved caspase 3 staining demonstrated modest but significantly higher staining in tumors from NVP-BEZ235 treated mice compared to saline controls in both xenograft models (29.0 vs. 15.3 pixels/μM2; p=0.017 in 786-O and 9.75 vs 3.57 pixels/μM2; p=0.024 in A498). Microvessel density determined by quantification of CD34 staining revealed no significant difference in NVP-BEZ235-treated mice compared with saline controls (2.41×10−4 vs. 2.53×10−4 vessels/μM2; p=0.85) in the 786-O xenografts and a slight decrease in A498 xenografts which did not meet criteria for significance (4.5×10−4 vs. 6.0×10−4 vessels/μM2; p=0.17). These results suggest that the failure of the tumors in the NVP-BEZ235-treated mice to grow was due to a combination of antiproliferative and pro-apoptotic effects of the drug on tumor cells rather than through inhibition of angiogenesis.
Figure 6.
Immunohistochemical analysis of representative 786-O tumors from mice treated with saline, rapamycin, or NVP-BEZ235 for 3 days.
DISCUSSION
Although both temsirolimus and everolimus are FDA-approved for the treatment of RCC, neither agent induces substantial tumor regression except in a minority of patients. There are numerous factors that could account for the limited efficacy of these selective TORC1 inhibitors, among which is the feedback activation of the PI3-K pathway. Our data, however, do not support the hypothesis that this is a frequent limiting factor in RCC since rapamycin treatment resulted in an unequivocal increase in Akt phosphorylation (Ser473) in only one of the 5 RCC cell lines examined (Figure 2A) and the drug had no effect on Akt phosphorylation in 786-0 or A498 xenografts (Figure 5B). These data suggest that rapalogue-induced PI3-K or Akt activation may be uncommon in RCC and therefore not a dominant mechanism underlying treatment failure.
One clear distinction between rapamycin and NVP-BEZ235 is the differential effects of the two drugs on 4E-BP1 phosphorylation. As shown in Figure 2A, rapamycin had virtually no effect on this parameter in any of the RCC cell lines tested. The basis for the failure of rapamycin to suppress 4E-BP1 phosphorylation in the RCC cell lines is unclear but cannot be attributed to an inadequate drug concentration used in the study as the concentration tested (100 nM) is at least 10-fold higher than that required to inhibit S6 phosphorylation (Supplemental Figure 1). Although 4E-BP1 is a canonical TORC1 substrate, its phosphoryation has long been recognized to be less responsive to rapalogues than that of S6 (27). In fact, Choo et al have recently shown that the suppression of 4E-BP1 phosphorylation by rapamycin is reversed within a few hours of drug exposure (28). Regardless of the mechanism, the failure of rapamycin to block 4E-BP1 phosphorylation is likely to limit the extent to which the drug suppresses eIF4E function and cap-dependent translation and to contribute to the apparent inferiority of rapalogues to agents such as NVP-BEZ235.
In addition to 4E-BP1, the phosphorylation of Mnk1 and its substrate eIF4E is differentially affected by NVP-BEZ235 and rapamycin (Figure 2A). The combined effect of reduced phosphorylation of both eIF4E and its binding partner (4E-BP) resulting from exposure to NVP-BEZ235 would be predicted to have major consequences for the translation of certain “difficult to translate” mRNAs that by virtue of their extended, complex 5′UTR, are ignored by the ribosome except when eIF4E is available and functional. The translation of the survivin and cyclin D transcripts are cap-dependent and as shown in Figure 2A, the levels of these proteins are profoundly downmodulated in NVP-BEZ235-treated but not rapamycin-treated RCC cells. In addition to its role in cap-dependent translation, eIF4E regulates the nuclear export of certain mRNAs based on a sequence present in the transcript 3′UTR. The cyclin D mRNA has such a 4E-sensitivity element (4ESE) and it is possible that the nuclear retention of the transcript in response to eIF4E dephosphorylation contributes to the cyclin D downmodulation achieved with NVP-BEZ235 (29). Although there are numerous possible mechanisms for the reduction of tumor cell proliferation induced by NVP-BEZ235, reduction of cyclin D levels and the induced nuclear translocation of p27 may be the dominant mechanisms for the antiproliferative effects of this drug. Our data suggest that the failure of rapamycin to suppress both 4E-BP and eIF4E phosphorylation or to translocate p27 to the nucleus may be responsible for its limited effect on tumor cell proliferation and tumor growth.
Despite the inhibition of 4EBP and eIF4E phosphorylation and suppression of survivin and Cyclin D1 levels, NVP-BEZ235 had no effect on c-Myc levels. The persistence of this gene product in circumstances in which cap-dependent translation should be blocked raises the possibility that the c-Myc mRNA may be translated through a cap-independent mechanism involving an internal ribosome entry-site (IRES). Indeed, the utilization of IRES to translate this mRNA as well as that of Cyclin D1 has been described in the setting of TORC1 inhibition (30). Furthermore, utilization of IRES was shown to be promoted by low basal Akt activity, a condition induced pharmacologically by NVP-BEZ235. Whether this mechanism can account for the failure of NVP-BEZ235 to suppress c-Myc levels as well as the apparent enhancement of HIF activity induced by rapamycin in some cell lines requires further study.
Our data suggest that the predominant antitumor effect of NVP-BEZ235 is a combination of slowed tumor proliferation and the induction of apoptosis. Akt inhibition would be expected to induce the expression of fas ligand and numerous pro-apoptotic BH3-only Bcl-2 family members (e.g. Bim, BNIP) as well as suppress the translation of IAP family members such as survivin, all of which should predispose tumor cells to undergo apoptosis. Although we did not observe significant induction of cell death in vitro by NVP-BEZ235 as determined by PI staining, IHC analyses of the xenografts from NVP-BEZ235-treated mice demonstrated a modest but significantly greater activation of caspase 3 compared with those from saline-treated mice.
The various components of the PI3-kinase signaling pathway can exert either pro- or anti-angiogenic effects, depending on the experimental circumstances. As a result, the consequences of inhibiting the pathway on the tumor vasculature are likely to be complex and variable from one model to the next. As an illustration of this point, mice in which the dominant endothelial Akt isoform (i.e. Akt1) has been knocked out are hypersensitive to exogenous VEGF, possibly due to an impaired ability to produce thrombospondin (31). Tumors implanted into these mice grow more rapidly than similar implants in control animals and have an exaggerated vasculature. This suggests that treatment with a PI3-kinase inhibitor, which should block endothelial Akt1 activity, might actually result in an increase in tumor angiogenesis, a prediction in fact validated in a 786-0 xenograft study using the PI3-kinase inhibitor LY294002 (32). On the other hand, treatment of mice bearing murine breast carcinomas or rat C6 gliomas with rapamycin at doses sufficient to block Akt in the tumor endothelium resulted in vascular normalization (33, 34). Treatment of rats bearing orthotopic BN472 mammary carcinomas with NVP-BEZ235 resulted in marked reduction in tumor vascular permeability and interstitial pressure, further illustrating the variability in vascular response to PI3-K inhibition (35). Although we demonstrated a marked reduction in HIF-2α in RCC cell lines exposed to NVP-BEZ235 and xenografts from mice treated with NVP-BEZ235, we were unable to demonstrate any reduction in tumor angiogenesis, suggesting that the pro-angiogenic effects of Akt inhibition may have been able to compensate for the reduced HIF activity in the tumor cells in this model.
The results of our investigation into the mechanism of action of NVP-BEZ235 suggest that the antitumor activity of the drug is due primarily to suppression of tumor cell proliferation as well as the induction of apoptosis rather than through the disruption of tumor angiogenesis. Our data support the view that agents that block PI3-K and/or TORC2 in addition to TORC1 are likely to outperform selective TORC1 inhibitors in the treatment of RCC.
Statement of Translational Relevance.
Although two inhibitors of TORC1, temsirolimus and everolimus, are FDA-approved for the treatment of renal cell carcinoma (RCC), these agents induce only modest tumor regression and extend progression-free survival only a few months in most patients. Our data suggest that agents that inhibit either PI3-Kinase/Akt and/or TORC2 simultaneously with TORC1 may have advantages over selective TORC1 inhibitors in the treatment of RCC. The results of our studies with NVP-BEZ235, a dual inhibitor of PI3-K and mTOR, in RCC cell lines support this hypothesis. As several such agents are now in early stage clinical testing, our results provide the rationale to assess these agents in patients with RCC.
Supplementary Material
Effects of increasing concentrations of rapamycin on phosphorylation of S6 (Ser235/236) and 4E-BP1 (Thr37/46) in 786-O cells exposed to drug for 24 hours in vitro.
Acknowledgments
Supported by: NIH Grant 2P50CA101942, 1K08CA142890
References
- 1.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 4.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 6.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 7.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yue P, Chan C-B, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun S-Y. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and mnk-mediated eukaryotic translation initiation factor 4E phosphorylation. Mol Cell Biol. 2007;27:7405–13. doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–57. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 10.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–50. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 11.Kenck C, Wilhelm M, Bugert P, Staehler G, Kovacs G. Mutation of the VHL gene is associated exclusively with the development of non-papillary renal cell carcinomas. J Pathol. 1996;79:157–61. doi: 10.1002/(SICI)1096-9896(199606)179:2<157::AID-PATH557>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Foster K, Prowse A, van den Berg A, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3:2169–73. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 14.Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–6. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 15.de Paulsen N, Brychzy A, Fournier MC, et al. Role of transforming growth factor-alpha in von Hippel--Lindau (VHL)(−/−) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci U S A. 2000;98:1387–92. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordan JD, Lal Priti, Dondeti VR, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–46. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–46. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 18.Kondo K, Kim WY, Lechpammer M, Kaelin WG. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:439–444. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on TORC1 and TORC2. J Biol Chem. 2008;283:34495–9. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maira S-M, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 21.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 22.Baumann P, Mandl-Weber S, Oduncu F, Schmidmaier R. The novel orally bioavailable inhibitor of phosphoinositol-3-kinase and mammalian target of rapamycin, NVP-BEZ235, inhibits growth and proliferation in multiple myeloma. Exp Cell Res. 2009;315:485–97. doi: 10.1016/j.yexcr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Cao P, Maira SM, García-Echeverría C, Hedley DW. Activity of a novel, dual PI3-kinase/mTor inhibitor NVP-BEZ235 against primary human pancreatic cancers grown as orthotopic xenografts. Br J Cancer. 2009;100:1267–76. doi: 10.1038/sj.bjc.6604995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Jonasch E, Alexander A, et al. Cytoplasmic sequestration of p27 by Akt phosphorylation in renal cell carcinoma. Clin Cancer Res. 2009;15:81–90. doi: 10.1158/1078-0432.CCR-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culjkovic B, Toposirovic I, SkraBanek L, Ruiz-Gutierrez M, Borden KLB. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–26. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J. Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J Biol Chem. 2005;280:10964–73. doi: 10.1074/jbc.M407874200. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathologic angiogenesis, vascular maturation and permeability in vivo. Nature Med. 2005;11:1188–96. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, et al. The phosphoinositide 3-kinase/Akt pathway: A new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–42. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- 33.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–70. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phung TL, Eyiah-Mensah G, O’Donnell RK, et al. Endothelial Akt signaling is rate-limiting for rapamycin inhibition of mouse mammary tumor progression. Cancer Res. 2007;67:5070–5. doi: 10.1158/0008-5472.CAN-06-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell CR, Stauffer F, Allegrini PR, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications of clinical imaging. Cancer Res. 2008;68:6598–607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of increasing concentrations of rapamycin on phosphorylation of S6 (Ser235/236) and 4E-BP1 (Thr37/46) in 786-O cells exposed to drug for 24 hours in vitro.