Pavlovian fear conditioning has become an essential behavioral task in a wide array of neuroscience research avenues. Increasingly, researchers from a variety of fields of study are turning to fear conditioning tasks to probe for behavioral and cognitive phenotypes following pharmacological, genetic, and other experimental manipulations. Delay tone fear conditioning has become a widely used assay as it allows for the assessment of “hippocampus-independent” tone fear and “hippocampus-dependent” context fear in the same animal. The main advantage of this procedure is that these two different types of fear can be tested independently. However, accurate assessment of these two types of fear makes the assumption that one does not interact with and confound the measurement of the other.
In a tone fear conditioning procedure, fear is simultaneously acquired for both the tone and contextual cues. In delay tone conditioning, acquisition of tone fear has been shown to depend on synaptic plasticity of auditory inputs into the amygdala (Medina et al., 2002) Contextual fear acquisition depends on synaptic plasticity in the amygdala as well as additional neural substrates such as the hippocampus, which are thought to mediate the integration of multimodal cues into a “contextual representation” that can function as a conditional stimulus (CS) for amygdalar circuits (Kim and Fanselow, 1992; Maren and Fanselow, 1995). Context fear is assessed by returning the animal to the original training context for a “context test.” Tone fear is usually assessed by placing the animal in a distinctly different context where the tone is presented during a “tone test.” The primary behavioral measure of fear is the percentage of time an animal spends “freezing,” which is a species-specific defensive response in the rodent characterized by complete immobility. Differences in the level of freezing during tone presentations are used to infer alterations in the acquisition or expression of tone fear.
A critical unresolved issue in the measurement of tone fear is the extent to which baseline levels of freezing observed prior to tone presentation influences the measurement of tone freezing. Baseline freezing is usually driven by fear of the training context that has generalized to the testing chamber due to similarities between the two apparatuses. For this reason most researchers make the training and testing chambers as different as possible. The shift in context for the tone test attempts to isolate expression of tone fear by eliminating the expression of context fear. However, baseline fear is rarely reduced to zero, especially in mice which tend to show a higher degree of context generalization relative to rats.
Even though the use of fear conditioning has expanded exponentially in recent years, critical assumptions about baseline fear have remained untested and no standardized method of addressing differences in baseline fear has been developed. Researchers have used a variety of ways to report tone fear, making the results of many influential findings very difficult to interpret. A small selection of such examples include researchers that ignore baseline fear responses and do not report them (e.g. Gewirtz and Davis, 1997; Marsicano et al. 2002; Schafe et al. 2005; Han et al. 2007; Gogolla et al. 2009; Monfils et al. 2009), calculate tone fear by subtracting baseline freezing from tone freezing (e.g. Reijmers et al. 2007) or from inter-trial interval freezing (e.g. Huerta et al. 2000), or calculate tone fear using the Annau and Kamin (1961) suppression ratio (e.g. Shors et al. 2002; Bangasser et al. 2006). These concerns apply to virtually all fear conditioning methodologies, including fear potentiated startle, which uses a subtraction score based on the difference between baseline and cued responses (e.g. Gewirtz and Davis, 1997).
The purpose of the current study was to characterize the interaction between baseline and tone fear in mice, which have become an essential tool in current biomedical research. To do this, we conditioned two different levels of tone fear and then used post-training manipulations to produce a range of baseline freezing for the tone test. It should be noted that one assumption of our design, which is supported by the overall pattern of results, is that these post-training manipulations, such as extinction and additional US presentations did not influence the true level of tone fear. This approach provided a data set with quantitative gradations in both tone and baseline fear that could be used to assess the efficacy of four methods of reporting tone fear: absolute freezing level, ratio of tone to baseline freezing, subtraction of baseline freezing, and an adjusted score derived from using baseline freezing as a covariate in an analysis of covariance (ANCOVA).
MATERIALS
Subjects
The subjects were 109 three month old, C57/BL6 male mice (Taconic, Oxnard, CA). Animals were housed with four mice per cage and had access to food and water ad libitum. Animals were kept on a 12 hour regular light-dark cycle.
Fear Conditioning
All behavioral testing was done with MedAssociates VideoFreeze fear conditioning equipment. Mice were placed in a holding room each day 30 min before sessions began and were returned to the vivarium immediately afterwards. Context A had aluminum walls, grid floors with 36 stainless steel rods each with a diameter of 0.32 cm and spaced 0.64 cm apart (center to center), was scented with Simple Green®, and cleaned with 70% isopropyl alcohol in between trials. Context B had rounded walls made from two 47.5 cm by 20.5 cm rectangular sheets of white plastic, had grid floors with 19 stainless steel rods that alternated in diameter from 0.95 cm to 0.35 cm and were spaced 1.46 cm apart (center to center), was scented with Windex® Original Glass Cleaner, and was cleaned with 70% ethanol. Tone conditioning on Day 1 in Context A consisted of a 180 s baseline period followed by either 1 or 3 tones (20 s, 2800 Hz, 85 dB) paired with electric footshock (2 s, 0.5 mA) that began immediately after the offset of each tone presentation, with a 180 s inter-trial interval in between the offset of each shock and the onset of the following tone. The no-extinction (NE) groups were then left in the vivarium until Day 6. All other groups then received 3 extinction trials consisting of 15 min of stimulus free exposure to Context A on days 2–4 in order to extinguish fear of the training context. Baseline manipulation on Day 5 was conducted in Context B and consisted of a 180 s baseline period followed by 0, 1, 2 or 6 unsignaled footshocks (2 s, 0.5 mA) separated by 60 s. On Day 6 levels of baseline and tone fear were assessed in Context B, which included a 180 s baseline period followed by 3 unpaired tone presentations (20 s, 2800 Hz, 85 dB) separated by 180 s intertrial intervals.
Automated Behavioral Scoring
Freezing behavior was scored using MedAssociates VideoFreeze software. Video was recorded from infrared cameras mounted in front of the conditioning chambers at 30 frames per second. VideoFreeze software scored animal behavior as freezing if a subject’s activity was below 19 activity units for 30 or more continuous frames. These parameters were selected to correlate strongly (r >0.9) with highly trained human observers (Jesse Cushman and Michael Fanselow).
Tone Fear Reporting Methods
Four methods of reporting tone fear were used: absolute freezing, baseline covariate, subtraction, and ratio. The absolute freezing method reported percent freezing averaged across all tone presentations; the baseline covariate method used baseline scores as a covariate in an ANCOVA during statistical analysis of absolute freezing measures; the subtraction method subtracted baseline from tone freezing; and the ratio method calculated tone fear by dividing tone freezing by the sum of baseline and tone freezing similar to the Annau and Kamin (1961) suppression ratio.
Statistical Analysis
For all statistical analyses an ANOVA (or ANCOVA for the covariate method) was used with baseline manipulation group as a factor and subsequent a priori planned LSD post-hoc tests were performed for individual comparisons, when justified.
RESULTS
Absolute Freezing During the Tone Test
Figure 2 illustrates absolute levels of freezing (percent time spent freezing) during the baseline period and during the tone presentations. Baseline fear was successfully manipulated for both weak (F(4, 44)=13.44, p<0.001) and strong (F(4, 48)=17.97, p<0.001) tone conditioning groups, where baseline freezing increased as a function of the number of unsignaled shocks (Fig. 1b,c). Freezing to the tone also increased as a function of the number of unsignaled shocks (Fig. 1b, c). Levels of tone freezing remained consistent across all tone presentations as indicated by both a non-significant repeated measures effect (weak tone conditioning F(4, 78) < 1; strong conditioning F(4, 86)=1.883, p > .05;) and a non-significant repeated measure by baseline manipulation group interaction (weak tone conditiong F(8, 78) < 1; strong tone conditioning F(8, 86)=1.447, p > .05; for more details see Supp. Fig. 1).
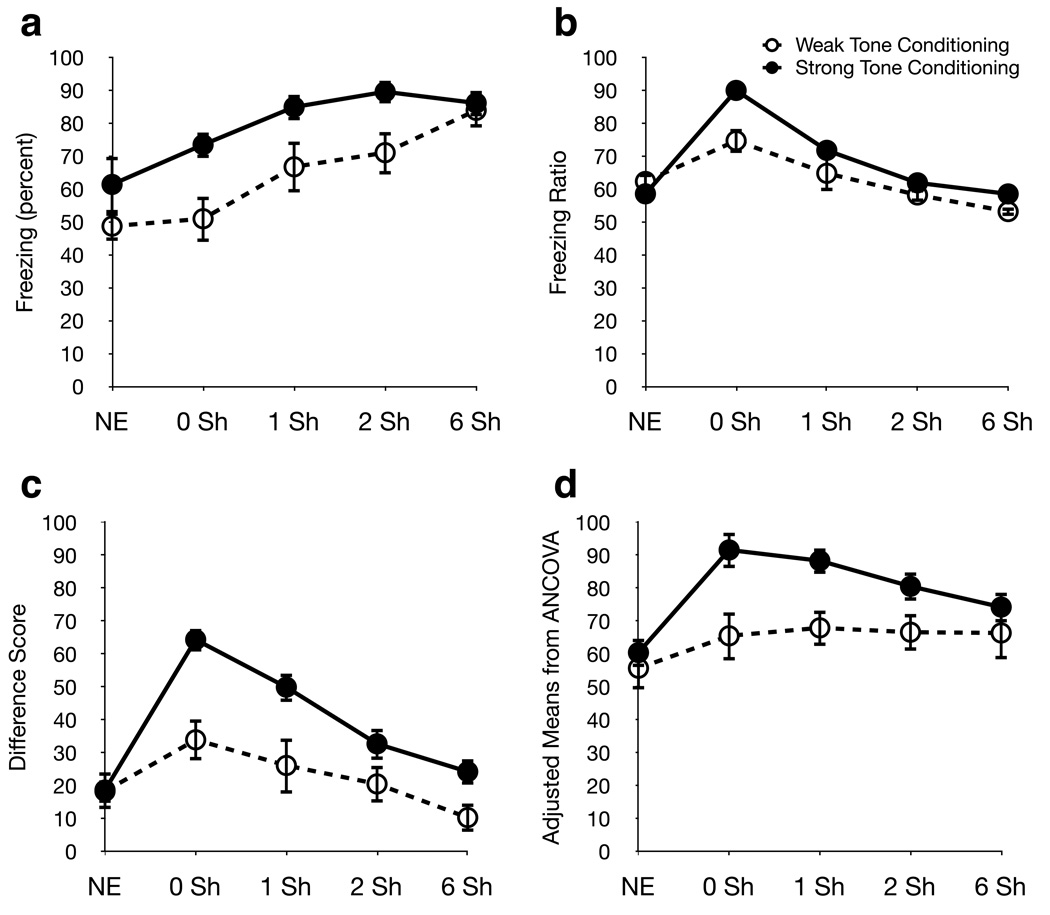
Figure 2.
Common methods of reporting tone fear. (a) Absolute freezing (percentage time spent freezing) averaged over all tone presentations. (b) Freezing ratio of percentage freezing during the CS divided by the sum of percentage freezing during the CS and the percentage freezing during baseline. (c) Subtraction score calculated by subtracting percentage freezing during the 180s baseline period from percentage freezing during the tone. (d) Baseline covariate (adjusted means and standard error from ANCOVA). Open circles, weak tone conditioning groups; closed circles, strong tone conditioning.
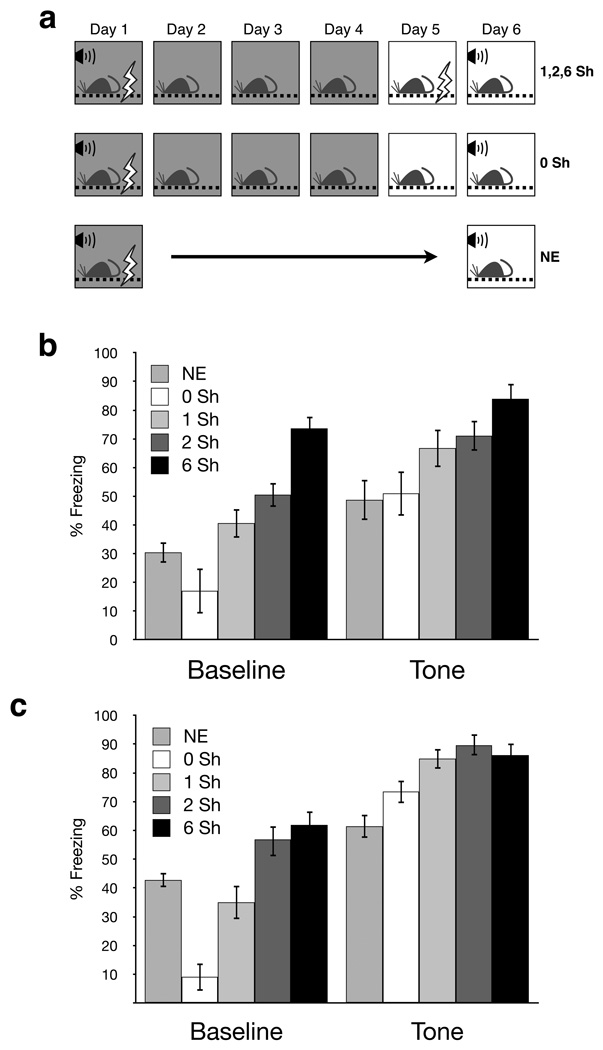
Figure 1.
Percent freezing during the tone test. (a) Experimental design. On Day 1 all animals were trained in Context A (dark boxes) with either 1 (weak) or 3 (strong) tone-shock pairings. Context fear extinction sessions were given in Context A on Days 2–4. No-extinction (NE) groups remained in their homecages for Days 2–5 and were given a tone test on Day 6 with all other groups. All sessions on Day 5 occured in Context B (white boxes), a distinctly different chamber used for the tone test on Day 6, where animals received 1, 2, or 6 unsignaled footshocks (top row, “1, 2, 6 Sh”), were pre-exposed to the context (middle row, “0 Sh”), or were kept in their homecage (bottome row, “NE”). Note that the 0 Sh groups received no aversive foot shocks on Day 5 so these animals were essentially pre-exposed to the testing chamber. (b, c) Percent freezing during the tone test on Day 6. For weak tone conditioning (b), both baseline and tone freezing increased as a function of the number of unsignaled footshocks in the 0, 1, 2, and 6Sh groups. Note that compared to the 0 Sh group, the NE group had higher baseline freezing but equivalent tone freezing. For strong tone conditioning (c), again both baseline and tone freezing increased as a function of the number of unsignaled footshocks given. Note that the NE group had significantly higher baseline freezing and lower tone freezing than the 0Sh group.
Interestingly, the NE groups had similar levels of tone freezing compared to the 0Sh groups despite having lower levels of baseline freezing (strong tone conditioning groups, p<0.05; weak tone conditioning groups, p=0.058; a priori planned LSD post hoc). Thus, in contrast to the pattern of baseline and tone freezing seen in the other groups, the NE groups had a considerable amount of baseline fear that did not lead to increased levels of tone freezing.
Tone Fear Reporting Methods
Tone fear was reported using four methods: absolute freezing, ratio, subtraction, and covariate. Efficacy of reporting methods was based on two criteria: 1) Elimination of the confounding effect of baseline freezing, i.e. reported equivalent levels of tone fear for groups that received identical tone conditioning on Day 1 (either 1 or 3 CS-US pairings) but different baseline manipulations on Day 5 , and 2) Sensitivity to differences between weak and strong tone conditioning, where higher levels of tone fear is reported in the groups that received strong tone conditioning.
Tone fear reported using the Absolute Freezing method (Fig. 2a) was repeated in Figure 3 for the sake of comparison with the other reporting methods. As noted above, this method was significantly affected by baseline manipulation for weak and strong tone conditioning groups. The Ratio method (Fig. 2b) was also significantly affected by baseline manipulation for weak (F(4, 44)= 4.913, p<0.005) and strong (F(4, 48)= 35.79, p<0.001) tone conditioning groups. The Subtraction (Fig. 2c) method did report equivalent levels of tone fear across baseline manipulation groups for weak tone conditioning, but not for strong tone conditioning (F(4, 48)=17.3, p<0.001). Similar to the Subtraction method, the Baseline Covariate method (Fig. 2d) reported equivalent levels of tone fear across baseline manipulation groups for weak but not strong (F(4, 48)= 7.03, p<0.001 ) tone conditioning. None of the four reporting methods satisfied both our criteria. Subtraction and Baseline Covariate were able eliminate differences between baseline manipulation group for the weak, but not the strong group. Figure 2 also clearly illustrates that the 0 Sh condition had the greatest difference in reported tone fear between weak and strong tone conditioning, regardless of which method was used to report the data.
DISCUSSION
These results clearly show that there is a positive interaction between baseline fear and tone fear, where groups with higher levels of baseline freezing had higher levels of tone freezing. Baseline fear, therefore, clearly confounds measures of tone fear and must be dealt with in some way. However, none of the post-hoc data manipulation techniques investigated here were able to successfully correct for confounding differences in baseline freezing while still preserving sensitivity to differences in the level of tone conditioning. This complicates the interpretation of a number of high-profile publications that have employed these techniques (e.g. Gewirtz and Davis 1997; Huerta, Sun et al. 2000; Shors, Townsend et al. 2002; Bangasser, Waxler et al. 2006; Reijmers, Perkins et al. 2007). We propose that a change in the standard fear conditioning methodology, involving extinction of fear to the training context and pre-exposure to the test context, may be the best solution to this problem.
It is important to note that our analysis of each tone fear reporting method was dependent on the assumption that post-acquisition manipulations on Days 2–5, which included context extinction, context preexposure, and additional footshock presentations, did not indirectly alter true levels of tone fear. Some theories of conditioning suggest that CS-US contingency is calculated at the time of testing (Gallistel, 1990; Stout and Miller, 2007) and thus predict that unsignaled US presentations would reduce expression of tone fear whereas extinction of competing CS’s, such as the context, should increase tone fear. Neither of these predicted outcomes were observed in the present study. The groups that received shocks in the tone test context showed enhanced expression of tone fear, opposite of what is predicted by this class of models. This is consistent with findings that US exposure in a context different from that used in training greatly attenuates interference with the CS-US association (Wasserman and Miller, 1997). Furthermore, we found that context extinction in the 0-Sh groups did not alter levels of tone freezing relative to the NE groups (Fig. 1b, c). Thus, the assumption that our post-training manipulations did not indirectly alter the true level of tone fear is supported by the experimental results.
Our results also bring up a few unexpected theoretical issues. First, our results seem to indicate that baseline fear responses may be caused by multiple sources of fear. Although most group comparisons show that increased baseline freezing leads to increased tone freezing, comparisons between the 0 Sh and NE groups did not: the NE groups had higher levels of baseline fear but equivalent levels of tone fear relative to the 0 Sh groups. This implies that in addition to baseline fear that directly interacts with levels of freezing during the tone, it is also possible to have a considerable amount of baseline fear which does not interact with freezing during the tone. If there are indeed multiple components of baseline fear, it also raises the concern that different sources of baseline fear may be differentially affected by certain experimental manipulations. Even equivalent but non-zero levels of baseline fear may therefore significantly confound tone fear data. Second, it has been argued previously that contextual fear and tone fear only interact under specific conditions, such as if the CS has undergone extinction trials (Bouton, 1984). Our results suggest that, under our parameters, baseline and tone fear can interact without this condition being met.
In light of these findings, it is clearly unacceptable to neglect baseline fear when interpreting measures of tone fear in Pavlovian conditioning studies. Thus, reducing baseline fear to a small fraction of cued fear responses may be the only viable option for resolving the baseline issue in fear conditioning studies. Low baseline fear can be partially achieved by using distinctly different training and testing environments, yet in some cases this may not be enough, particularly in studies using C57Bl/6 mice which tend to show a high level of context generalization (Balogh et al, 2002). In situations where high levels of baseline fear persist, we propose a specific methodological solution consisting of multiple days of context extinction to the conditioning chamber followed by at least one day of pre-exposure to the testing chamber, similar to the 0-Sh groups in this study. A potential criticism of this alternate methodology is that it adds additional learning stages prior to the tone test, such as context extinction. The true level of tone fear does not appear to be altered by context extinction and pre-exposure to the tone test context, however, as the 0-Sh and NE groups had equivalent levels of tone fear. The 0-Sh groups also showed the greatest sensitivity to differences in tone conditioning strength as seen in Figure 2. This new methodology also allows for a more complete assessment of fear learning and memory phenotypes: contextual fear (Day 2), extinction learning (Days 3–4), a full assessment of generalized fear to the tone testing chamber (Day 5), and lastly tone fear (Day 6). Thus, the methodology proposed here avoids the potential for uninterpretable tone fear data, increases the sensitivity to differences in tone fear compared to the current standard, and provides additional phenotypic information about fear learning.
Supplementary Material
Supplementary Figure-1 Detailed freezing behavior during the tone test. Percent freezing was calculated in 20 s time intervals for the entire duration of the tone test. Black bars above plots designate when tone presentations were delivered. (a) Weak tone conditioning groups. (b) Strong tone conditioning groups.
ACKNOWLEDGEMENTS
Supported by NIMH R01MH62122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliff RA, Logue SF, Wehner JM. Contextual and Cued Fear Conditioning in C57BL/6J and DBA/2J Mice: Context Discrimination and the Effects of Retention Interval. Behavioral Neuroscience. 2002;116(6):947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26(34):8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. Learning, development, and conceptual change. Cambridge, MA, US: MIT Press; 1993. The organization of learning. [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388(6641):471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Adelaide PY, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316(5823):457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25(2):473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. Journal of Neuroscience. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Herman H, Tang J, Hofmann C, Zieglgansberger W, Marzo VD, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa C, Mauk MD, Ledoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nature Reviews, Neuroscience. 2002;3(2):122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Naoki M, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317(5842):1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bauer EP, Rosis S, Farb CR, Rodrigues SM, LeDoux JE. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur J Neurosci. 2005;22(1):201–211. doi: 10.1111/j.1460-9568.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SC, Miller RR. Sometimes-competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review. 2007;114(3):759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Miller RR. What's elementary about associative learning? Annual Review of Psychology. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure-1 Detailed freezing behavior during the tone test. Percent freezing was calculated in 20 s time intervals for the entire duration of the tone test. Black bars above plots designate when tone presentations were delivered. (a) Weak tone conditioning groups. (b) Strong tone conditioning groups.