Abstract
Germ granules are large, non–membrane-bound, ribonucleoprotein (RNP) organelles found in the germ line cytoplasm of most, if not all, animals. The term germ granule is synonymous with the perinuclear nuage in mouse and human germ cells. These large RNPs are complexed with germ line–specific cytoplasmic structures such as the mitochondrial cloud, intermitochondrial cement, and chromatoid bodies. The widespread presence of germ granules across species and the associated germ line defects when germ granules are compromised suggest that germ granules are key determinants of the identity and special properties of germ cells. The nematode Caenorhabditis elegans has been a very fruitful model system for the study of germ granules, wherein they are referred to as P granules. P granules contain a heterogeneous mixture of RNAs and proteins. To date, most of the known germ granule proteins across species, and all of the known P granule components in C elegans, are associated with RNA metabolism, which suggests that a main function of germ granules is posttranscriptional regulation. Here we review P granule structure and localization, P granule composition, the genetic pathway of P granule assembly, and the consequences in the germ line when P granule components are lost. The findings in C elegans have important implications for the germ granule function during postnatal germ cell differentiation in mammals.
Keywords: Germ granules, nuage, chromatoid bodies
Mammalian germ granules, in their different forms (perinuclear nuage, intermitochondrial cement, chromatoid bodies), are found in the germ line cytoplasm of maturing gametes. As protein components of these germ granules have been identified and subsequently compromised, their crucial role during postnatal male germ cell differentiation has become apparent. To date, mutations in mouse germ granule components result in male sterility (Chuma et al, 2008), so understanding the critical role that germ granules play during spermatogenesis is imperative.
Germ granules are found in the germ line cytoplasm of all animals that have been examined (Eddy, 1975). As a consequence, much of what we now know concerning the composition and function of germ granules has come from work done in invertebrate model systems, such as Drosophila and Caenorhabditis elegans (Strome and Lehmann, 2007). Here, we focus on the key discoveries during the past 30 years of research on germ granules in C elegans and how these findings complement research on mammalian germ granules and their essential role in fertility.
Generation of Primordial Germ Cells in C elegans
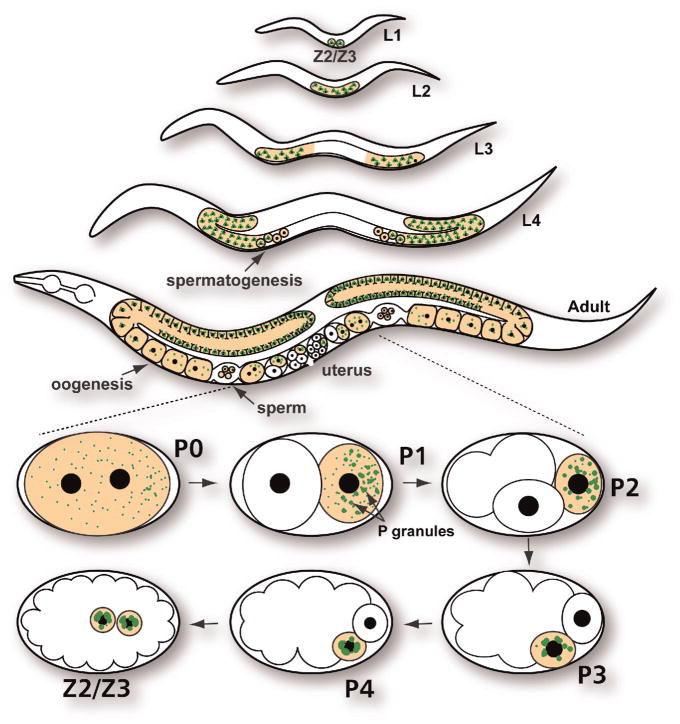
Primordial germ cells (PGCs) are established by very different mechanisms during mammalian and C elegans development. In mammalian development, inductive signals from the extraembryonic ectoderm and the visceral endoderm instruct a small number of proximal epiblast cells to become PGCs (Hayashi et al, 2007). In C elegans, maternally contributed germ granules in the 1-cell zygote (P0) are progressively segregated to the germ line blastomeres, or P cells (P1, P2, P3, and P4), through 4 asymmetric cell divisions, resulting in delivery of germ granules to the P4 cell, which is the C elegans PGC (Figure 1; Strome and Lehmann, 2007). Because of the segregation of germ granules to the P lineage, they were termed P granules. P4 divides only once during embryogenesis, generating the PGC daughter cells Z2 and Z3. At the end of the first larval stage, Z2 and Z3 start dividing symmetrically to eventually produce about 1000 germ cells in the adult gonad.
Figure 1.
C elegans germ line development. The 2 primordial germ cells (Z2/Z3) begin to proliferate at the end of the first larval stage (L1). During the fourth larval stage (L4) approximately 40 meiotic germ cells undergo spermatogenesis. In the adult hermaphrodite germ line, germ cells switch from making sperm to making oocytes. After fertilization (P0 zygote), P granules are segregated to the germ line blastomeres P1 to P4. At the ~100-cell stage, the primordial germ cell P4 divides into the daughter cells (Z2/Z3). Germ line is in orange. P granules are in green. L2 indicates second larval stage; L3, third larval stage.
When P granules were first described, over 25 years ago, it was noted that their segregation to the embryonic cells that become germ line could be used to investigate the establishment of asymmetry or polarity within a cell (Strome and Wood, 1982, 1983). Indeed, much of the focus on P granules over the subsequent decades has been aimed at investigating their segregation during the unequal P cell divisions. Detailed genetic pathways have been worked out to explain cellular asymmetry using P granules as markers (Gonczy and Rose, 2005). Many of the polarity genes involved are conserved during polarization of many mammalian cell types (Gonczy, 2008). Because establishment of mammalian germ line appears not to depend on asymmetric cell divisions, these pathways are not discussed here. Instead we focus on the many similarities between P granules and the mammalian germ granules that arise during gametogenesis.
P Granule Structure and Localization
P granules and mammalian germ granules are conserved in both structure and localization in the germ line cytoplasm. Ultrastructural studies have demonstrated the electron-dense, fibrillar nature of germ granules in mammals and in C elegans (Eddy, 1975; Krieg et al, 1978; Wolf et al, 1983). These studies and others have shown that cytoplasmic germ granules across species also lack a surrounding membrane. It has also been shown that germ granules reside close to organelles such as mitochondria (Eddy and Ito, 1971; Mahowald, 1968). P granules infrequently contain microtubules, centrioles, or mitochondria, and it is thought that these cytoplasmic components might become engulfed randomly as P granules grow or fuse with neighboring P granules (Pitt et al, 2000; Schisa et al, 2001).
Another similarity among germ granules across species is their close proximity to the nuclear periphery (Eddy and Ito, 1971; Mahowald, 1971; Eddy, 1974). P granules are no exception. However, their size and distribution change dramatically as early embryogenesis progresses from the 1-cell zygote to the birth of P4 in the ~16-cell embryo (Strome and Wood, 1982; Wolf et al, 1983; Hird et al, 1996; Pitt et al, 2000; Brangwynne et al, 2009). Prior to and immediately after fertilization, approximately 200 small P granules are dispersed throughout the cytoplasm (Figure 1). During the next 2 cell divisions, these granules begin to coalesce as they are segregated into the germ line blastomeres P1 and P2. The few P granules that are not segregated to the germ line, but remain in the somatic blastomeres, are subsequently degraded (DeRenzo et al, 2003; Spike and Strome, 2003; Zhang et al, 2009). In P2, the cytoplasmic distribution of P granules begins to change as P granules that are dispersed in the cytoplasm begin to attach to the nuclear periphery. The perinuclear association of P granules continues in P3 and P4, resulting in the nucleus of P4 being almost completely surrounded by a handful of large P granules. P granules remain associated with the nuclear envelope throughout germ line proliferation, and detach only during the maturation of male and female gametes. In the C elegans hermaphrodite, gametogenesis starts in the fourth larval stage (L4), when approximately 40 meiotic germ cells near the proximal end of each gonad arm generate sperm (Kuwabara and Perry, 2001). As spermatocytes complete meiosis, P granules are disassembled and remain dispersed in the cytoplasm of the residual body, and spermatids and spermatozoa appear depleted of P granule components (Gruidl et al, 1996; Figure 2). In the adult hermaphrodite, the germ line switches from making sperm to making oocytes, which allows the worm to be self-fertile. During oocyte maturation, P granules detach from the nuclear periphery; they persist as small aggregates during ovulation and fertilization, after which they again coalesce as they are segregated to the germ line blastomeres.
Figure 2.
An oocyte and developing spermatocytes and sperm in C elegans. GLH-1 (green) is localized to P granules in the oocyte and in primary and secondary spermatocytes, dispersed throughout the cytoplasm of residual bodies, and undetectable in mature spermatids. PGL-3 (red) is localized to P granules in the oocyte and in primary spermatocytes, and then drops to undetectable at later stages of spermatogenesis. DAPI (4′,6-diamidino-2-phenylindole)-stained DNA is in blue.
P Granule Composition
RNAs
P granules, like their mammalian counterparts, contain RNA. During C elegans embryogenesis, a class of maternally expressed transcripts (described as class II messenger RNAs [mRNAs]) are selectively degraded in somatic daughter cells after each cell division, but remain protected in the germ line blastomeres (Seydoux and Fire, 1994). Many of the class II transcripts become enriched around the nuclear periphery of P3 and P4 nuclei in a P granule–like distribution. Seydoux and Fire (1994) showed that poly(A)+ and SL1-spliced RNAs are enriched in P granules from the 1-cell zygote to P4, demonstrating that embryonic P granules contain maternal RNAs. In the adult, P granules that surround germ line nuclei also contain RNA (Schisa et al, 2001). The accumulation of RNA in P granules increases when oogenesis is dramatically slowed down by the absence of sperm. This accumulation appears to be primarily mRNA, as probes against multiple ribosomal RNAs showed no enrichment in P granules. Schisa et al (2001) also examined the perinuclear accumulation of specific mRNAs in the adult germ line, and discovered that all 6 of the developmentally regulated transcripts they analyzed because of their role in gonadogenesis or early embryogenesis are enriched in P granules, whereas nondevelopmentally regulated transcripts for actin and tubulin are not enriched. The enrichment of most of the developmentally regulated transcripts with P granule components is observed even after P granules detach from the nuclear periphery in oocytes. In C elegans, transcription is globally repressed during oocyte maturation, and most transcription remains repressed in the germ line throughout embryogenesis (Schaner and Kelly, 2006). One model is that maternal transcripts required in the embryonic germ line are preserved in P granules until transcription resumes during germ line proliferation in hatched larvae.
Proteins
One of the first germ granule protein components identified was the Drosophila DEAD-box RNA helicase Vasa (Hay et al, 1988; Lasko and Ashburner, 1988). The C elegans Vasa homologs were identified as P granule components within the next few years (Gruidl et al, 1996). We now know that Vasa is a conserved and essential contributor to germ line development and fertility in all animals in which it has been examined. In C elegans, there are 4 Vasa paralogs, which are named germ line helicase (GLH)-1, GLH-2, GLH-3, and GLH-4 (Kuznicki et al, 2000; Spike et al, 2008a). Like their mammalian counterpart (eg, mouse MVH), the GLHs contain both DEAD-box (GLH-1 to GLH-4) and Gly-rich (GLH1, GLH-2, GLH-4) domains. Interestingly, the Gly-rich domains of the C elegans GLHs consist of FGG repeats instead of the RGG repeats found in most Vasa orthologs. RGG repeats are an established RNA-binding domain (Kiledjian and Dreyfuss, 1992), whereas FGG repeats are associated with nuclear pore components (Suntharalingam and Wente, 2003). P granules not only localize to the nuclear periphery, but 75% of nuclear pores in the C elegans germ line are associated with P granules (Pitt et al, 2000). The association of P granules at the nuclear periphery can be disrupted by depleting specific nuclear pore components by RNA interference (RNAi; Updike and Strome, 2009). It is likely that the FGG domains of the GLHs contribute to the localization of P granules at the nuclear periphery. In fact, after depletion of glh-1 and glh-2, only very small foci of electron-dense germ granule material persist at the nuclear periphery; they appear to completely lack the fibrillar-granular matrix of wild-type P granules (Schisa et al, 2001). It is possible that the GLHs serve both localization and structural roles in P granules. In other organisms in which Vasa does not contain FG repeats, it is possible that RGG domains or even hydrophobic repeats found in other germ granule components serve these roles.
The list of germ granule components continues to grow, but the majority of germ granule components across species, and all P granule components in C elegans, are associated with RNA metabolism (Strome, 2005). A current list of P granule components is shown in the Table. Some of these components are constitutive, that is, they are found in P granules throughout the life cycle of the worm. Other components show a more transient association with P granules at only specific stages in the life cycle. The list includes factors that have roles in mRNA splicing, translation initiation, poly(A) polymerization, deadenylation, decapping, degradation, slicing, Piwi-interacting RNA (piRNA) and endogenous RNAi (endo-siRNA) synthesis. Included in the list are nematode-specific components like DEPS-1 and the PGL proteins. The PGLs all contain RGG motifs that are thought to bind RNA, much like the RGG motifs in Vasa, but their other domains seem to be nematode specific. Taken together, these components emphasize the role of P granules in RNA metabolism and posttranscriptional regulation.
Table.
C elegans P granule proteins
| Protein | Description | Reference |
|---|---|---|
| CAR-1 | Cytokinesis, apoptosis, and RNA-binding 1 TRAL/Lsm14 | Audhya et al, 2005; Boag et al, 2005; Squirrell et al, 2006 |
| CCF-1 | CCR4/NOT deadenylase complex | Gallo et al, 2008 |
| CDE-1 | Uracil nucleotidyltransferase | van Wolfswinkel et al, 2009 |
| CGH-1 | Dhh1/DDX6 DEAD-box helicase | Navarro et al, 2001 |
| CSR-1 | Argonaute required for endo-siRNA | Claycomb et al, 2009 |
| DCAP-1 | mRNA decapping enzyme | Squirrell et al, 2006 |
| DCAP-2 | mRNA decapping enzyme | Lall et al, 2005 |
| DEPS-1 | Novel defective P granules and sterile | Spike et al, 2008b |
| DRH-3 | Dicer related DEAD-box helicase | Claycomb et al, 2009 |
| EGO-1 | RNA-directed RNA polymerase | Claycomb et al, 2009 |
| GLD-1 | RNA-binding KH domain | Jones et al, 1996 |
| GLD-2 | Poly(A) polymerase | Wang et al, 2002 |
| GLD-3 | RNA-binding KH domain | Eckmann et al, 2002 |
| GLD-4 | Poly(A) polymerase | Schmid et al, 2009 |
| GLH-1 | Vasa DEAD-box helicase | Gruidl et al, 1996 |
| GLH-2 | Vasa DEAD-box helicase | Gruidl et al, 1996 |
| GLH-3 | Vasa DEAD-box helicase | Kuznicki et al, 2000 |
| GLH-4 | Vasa DEAD-box helicase | Kuznicki et al, 2000 |
| GLS-1 | Novel GLD-3/4 interacting protein | Rybarska et al, 2009 |
| IFE-1 | eIF4E mRNA cap-binding | Amiri et al, 2001 |
| LAF-1 | DDX3 DEAD-box helicase | Hubert and Anderson, 2009 |
| MEG-1 | Novel maternal effect germ cell defective | Leacock and Reinke, 2008 |
| MEG-2 | Novel maternal effect germ cell defective | Leacock and Reinke, 2008 |
| MEX-1 | CCCH-type zinc-finger protein | Guedes and Priess, 1997 |
| MEX-3 | RNA-binding KH domain | Draper et al, 1996 |
| OMA-1 | CCCH-type zinc-finger protein | Shimada et al, 2002 |
| OMA-2 | CCCH-type zinc-finger protein | Shimada et al, 2002 |
| PAB-1 | Poly(A)-binding protein 1 | Gallo et al, 2008 |
| PATR-1 | Pat1-decapping cofactor | Gallo et al, 2008 |
| PGL-1 | Novel RGG domain | Kawasaki et al, 1998 |
| PGL-2 | Novel PGL-1 related | Kawasaki et al, 2004 |
| PGL-3 | Novel RGG domain | Kawasaki et al, 2004 |
| PIE-1 | CCCH-type zinc-finger protein | Mello et al, 1996 |
| POS-1 | CCCH-type zinc-finger protein | Tabara et al, 1999 |
| PRG-1 | Argonaute required for piRNA synthesis | Batista et al, 2008 |
| Sm proteins | Splicing factors | Barbee et al, 2002 |
| SPN-2 | eIF4E-binding protein | Li et al, 2009 |
| SPN-4 | RNP-type RNA-binding domain | Ogura et al, 2003 |
| TIA-1 | TIA-1 RNP-type RNA-binding domain | Gallo et al, 2008 |
| VBH-1 | Vasa Belle-like DEAD-box helicase | Salinas et al, 2007 |
| WAGO-1 | Argonaute required for endo-siRNA | Gu et al, 2009 |
Abbreviations: endo-siRNA, endogenous RNAi synthesis; mRNA, messenger RNA; piRNA, Piwi-interacting RNA; RNP, ribonucleoprotein.
P Granule Assembly Pathway
Some P granule components have been placed into a P granule assembly pathway: DEPS-1→GLH-1→PGL-1→IFE-1. At the top of this pathway is DEPS-1, a nematode-specific novel protein with a serine-rich C-terminal domain. A genome-wide analysis of mRNA levels in germ lines from deps-1 mutants identified only 13 genes that are down-regulated, including deps-1 and glh-1 (Spike et al, 2008b). In deps-1 mutants, glh-1 mRNA and protein are reduced 5- to 10- fold. GLH-1 is subsequently required for the localization of the RGG-containing protein PGL-1 to P granules (Kawasaki et al, 1998). So in the absence of either DEPS-1 or GLH-1, PGL-1 (as well as PGL-2 and PGL-3) is dispersed throughout the cytoplasm. At the end of the known pathway is the P granule component IFE-1. IFE-1 is 1 of the 5 C elegans homologs of eIF4E, which is the mRNA cap-binding subunit of the translation initiation complex. IFE-1 was identified as a PGL-1 interacting protein in a yeast 2-hybrid screen (Amiri et al, 2001). In the absence of PGL-1, IFE-1 fails to localize to P granules. The association of IFE-1 with P granules supports the view that these granules are involved in translational regulation. In addition to the current list of known P granule assembly components, other potential components in this pathway continue to be identified through genome-wide screens for disrupted PGL-1 localization (Spike et al, 2008b; Updike and Strome, 2009).
What are the developmental consequences of loss of P granule components? Mutant alleles of glh-2, glh-3, glh-4, pgl-2, and pgl-3 do not cause dramatic phenotypes on their own, but animals with null or strong loss-of-function mutations in deps-1, glh-1, pgl-1, and ife-1 have temperature-sensitive defects in germ line proliferation and gametogenesis (Amiri et al, 2001; Kawasaki et al, 2004; Spike et al, 2008a,b). For glh-1 and pgl-1, the absence of sterility at low temperatures is due to functional redundancy with their paralogs. For example, glh-1;glh-4 and pgl-1;pgl-3 double mutants exhibit sterility even at low temperatures. Currently, a mechanism to explain the temperature sensitivity of sterility observed in deps-1, glh-1, pgl-1, and ife-1 mutants is unknown. Interestingly, elevated temperature enhances the null or strong loss-of-function phenotypes of many germ line–required genes, suggesting that the germ line is inherently sensitive to elevated temperature (Spike et al, 2008a). An intriguing possibility is that the stability and function of P granules and other germ line complexes are compromised at higher temperatures, such that the loss of otherwise redundant components results in sterility. This model could potentially explain the inherent sensitivity of germ line development across multiple species.
The Roles of P Granule Components During Gametogenesis
Analysis of deps-1, glh-1, pgl-1, and ife-1 mutants has revealed the germ line processes that those P granule components participate in regulating. deps-1, glh-1, and pgl-1 mutants have underproliferated germ lines, and the majority of mutants fail to make oocytes and sperm at restrictive temperatures (Kawasaki et al, 2004; Spike et al, 2008a,b). Therefore, these components are involved in germ cell proliferation and gametogenesis. ife-1 mutants make oocytes, but the production of mature sperm is delayed, causing the majority of hermaphrodites to be spermless. Thus, it is thought that IFE-1 is specifically required during spermatogenesis (Amiri et al, 2001; Henderson et al, 2009). After the pachytene stage of spermatogenesis, PGL-1 and PGL-3 disappear, whereas GLH-1 persists and is deposited in the residual body (Figure 2). It has been speculated that the association of IFE-1 with P granules reduces its availability until it is needed during postpachytene stages of spermatogenesis, when PGL-1 is absent (Amiri et al, 2001).
The mutant phenotype of ife-1 is much like that of another constitutive P granule component, prg-1. PRG-1 is an argonaute required for C elegans piRNA synthesis, which, when mutated, results in a temperature-sensitive sterile phenotype (Batista et al, 2008; Das et al, 2008; Wang and Reinke, 2008). The prg-1 phenotype arises, in part, from a defect in late spermatogenesis. prg-1 mutants raised at the restrictive temperature possess differentiated spermatocytes, but relatively few mature spermatids, each of which fails to produce a normal pseudopod (Wang and Reinke, 2008). How PRG-1 regulates genes preferentially expressed during spermatogenesis, most likely through piRNAs, is a matter of debate (Batista et al, 2008; Wang and Reinke, 2008). Perhaps a similar mechanism to that proposed for IFE-1 is being used for PRG-1 to allow it to regulate targets that act relatively late in spermatogenesis, after P granules dissociate.
Conclusions
In C elegans, P granules occupy a significant portion of the germ line cytoplasm, consistent with the critical role these granules play in germ line identity, maintenance, and fertility. We have a much better idea of that role now that more P granule components have been identified. Each of the 41 known P granule–associated factors listed in the Table highlights the importance of RNA metabolism in germ cells. So far, predicted levels of RNA regulation include mRNA splicing, translation initiation, poly(A) polymerization, deadenylation, de-capping, degradation, slicing, and mRNA turnover. Although many of the P granule factors are associated with RNA destabilization and degradation, others, like PAB-1, can potentially serve to protect transcripts. Recent studies investigating live germ cells have demonstrated that P granules and P granule composition are dynamic, showing that P granules have domains that other cytoplasmic ribonucleoproteins, like P bodies, dock or merge with (Boag et al, 2008; Gallo et al, 2008; Noble et al, 2008). Protein composition could vary in each aggregate. Some aggregates may facilitate mRNA storage, whereas others may direct mRNA degradation or promote gene expression.
Although some functions and components of P granules appear to be nematode specific (DEPS-1, the PGLs, and the MEGs), there are many similarities in structure and composition between germ granules in C elegans and mammals. Future studies include investigating the significance of the proximity of germ granules to the nuclear periphery, how germ granules cluster nuclear pores, and how mRNAs are distributed and processed as they exit the nucleus and become enriched in germ granules. Do germ granules extend the nuclear pore environment and act as a scaffold for posttranscriptional activities? Do germ granules activate or inhibit their components and release them at specific times during development when their function is needed? These questions will be best answered by a combinatorial approach using mammalian, C elegans, and other model systems.
References
- Amiri A, Keiper BD, Kawasaki I, Fan Y, Kohara Y, Rhoads RE, Strome S. An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development. 2001;128:3899–3912. doi: 10.1242/dev.128.20.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR, 3rd, Desai A, Oegema K. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol. 2005;171:267–279. doi: 10.1083/jcb.200506124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SA, Lublin AL, Evans TC. A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr Biol. 2002;12:1502–1506. doi: 10.1016/s0960-9822(02)01111-9. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, Conte D, Jr, Luo S, Schroth GP, Carrington JC, Bartel DP, Mello CC. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Atalay A, Robida S, Reinke V, Blackwell TK. Protection of specific maternal messenger RNAs by the P body protein CGH-1 (Dhh1/RCK) during Caenorhabditis elegans oogenesis. J Cell Biol. 2008;182:543–557. doi: 10.1083/jcb.200801183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Nakamura A, Blackwell TK. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development. 2005;132:4975–4986. doi: 10.1242/dev.02060. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Chuma S, Hosokawa M, Tanaka T, Nakatsuji N. Ultrastructural characterization of spermatogenesis and its evolutionary conservation in the germline: germinal granules in mammals. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, Conte D, Jr, Mello CC. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, Matthews N, Berezikov E, Ketting RF, Tavare S, Miska EA. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRenzo C, Reese KJ, Seydoux G. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature. 2003;424:685–689. doi: 10.1038/nature01887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper BW, Mello CC, Bowerman B, Hardin J, Priess JR. MEX-3 is a KH domain protein that regulates blastomere identity in early C. elegans embryos. Cell. 1996;87:205–216. doi: 10.1016/s0092-8674(00)81339-2. [DOI] [PubMed] [Google Scholar]
- Eckmann CR, Kraemer B, Wickens M, Kimble J. GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell. 2002;3:697–710. doi: 10.1016/s1534-5807(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat Rec. 1974;178:731–757. doi: 10.1002/ar.1091780406. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Ito S. Fine structural and radioautographic observations on dense perinuclear cytoplasmic material in tadpole oocytes. J Cell Biol. 1971;49:90–108. doi: 10.1083/jcb.49.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CM, Munro E, Rasoloson D, Merritt C, Seydoux G. Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Rose LS. Asymmetric cell division and axis formation in the embryo. WormBook. 2005:1–20. doi: 10.1895/wormbook.1.30.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Gruidl ME, Smith PA, Kuznicki KA, McCrone JS, Kirchner J, Roussell DL, Strome S, Bennett KL. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1996;93:13837–13842. doi: 10.1073/pnas.93.24.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D, Jr, Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, Chen CC, Chaves DA, Duan S, Kasschau KD, Falgren N, Yates JR, 3rd, Mitani S, Carrington JC, Mello CC. Distinct Argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36(2):231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes S, Priess JR. The C. elegans MEX-1 protein is present in germline blastomeres and is a P granule component. Development. 1997;124:731–739. doi: 10.1242/dev.124.3.731. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- Henderson MA, Cronland E, Dunkelbarger S, Contreras V, Strome S, Keiper BD. A germline-specific isoform of eIF4E (IFE-1) is required for efficient translation of stored mRNAs and maturation of both oocytes and sperm. J Cell Sci. 2009;122:1529–1539. doi: 10.1242/jcs.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird SN, Paulsen JE, Strome S. Segregation of germ granules in living Caenorhabditis elegans embryos: cell-type-specific mechanisms for cytoplasmic localisation. Development. 1996;122:1303–1312. doi: 10.1242/dev.122.4.1303. [DOI] [PubMed] [Google Scholar]
- Hubert A, Anderson P. The C. elegans sex determination gene laf-1 encodes a putative DEAD-box RNA helicase. Dev Biol. 2009;330(2):358–367. doi: 10.1016/j.ydbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AR, Francis R, Schedl T. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol. 1996;180:165–183. doi: 10.1006/dbio.1996.0293. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Amiri A, Fan Y, Meyer N, Dunkelbarger S, Motohashi T, Karashima T, Bossinger O, Strome S. The PGL family proteins associate with germ granules and function redundantly in Caeno-rhabditis elegans germline development. Genetics. 2004;167:645–661. doi: 10.1534/genetics.103.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I, Shim YH, Kirchner J, Kaminker J, Wood WB, Strome S. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Kiledjian M, Dreyfuss G. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 1992;11:2655–2664. doi: 10.1002/j.1460-2075.1992.tb05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Cole T, Deppe U, Schierenberg E, Schmitt D, Yoder B, con Ehrenstein G. The cellular anatomy of embryos of the nematode Caenorhabditis elegans. Analysis and reconstruction of serial section electron micrographs. Dev Biol. 1978;65:193–215. doi: 10.1016/0012-1606(78)90190-2. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Perry MD. It ain’t over till it’s ova: germline sex determination in C. elegans. Bioessays. 2001;23:596–604. doi: 10.1002/bies.1085. [DOI] [PubMed] [Google Scholar]
- Kuznicki KA, Smith PA, Leung-Chiu WM, Estevez AO, Scott HC, Bennett KL. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 2000;127:2907–2916. doi: 10.1242/dev.127.13.2907. [DOI] [PubMed] [Google Scholar]
- Lall S, Piano F, Davis RE. Caenorhabditis elegans decapping proteins: localization and functional analysis of Dcp1, Dcp2, and DcpS during embryogenesis. Mol Biol Cell. 2005;16:5880–5890. doi: 10.1091/mbc.E05-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Leacock SW, Reinke V. MEG-1 and MEG-2 are embryo-specific P-granule components required for germline development in Cae-norhabditis elegans. Genetics. 2008;178:295–306. doi: 10.1534/genetics.107.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeBella LR, Guven-Ozkan T, Lin R, Rose LS. An eIF4E-binding protein regulates katanin protein levels in C. elegans embryos. J Cell Biol. 2009;187:33–42. doi: 10.1083/jcb.200903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP. Polar granules of Drosophila. II. Ultrastructural changes during early embryogenesis. J Exp Zool. 1968;167:237–261. doi: 10.1002/jez.1401670211. [DOI] [PubMed] [Google Scholar]
- Mahowald AP. Polar granules of Drosophila. IV. Cytochemical studies showing loss of RNA from polar granules during early stages of embryogenesis. J Exp Zool. 1971;176:345–352. doi: 10.1002/jez.1401760309. [DOI] [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Navarro RE, Shim EY, Kohara Y, Singson A, Blackwell TK. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development. 2001;128:3221–3232. doi: 10.1242/dev.128.17.3221. [DOI] [PubMed] [Google Scholar]
- Noble SL, Allen BL, Goh LK, Nordick K, Evans TC. Maternal mRNAs are regulated by diverse P body-related mRNP granules during early Caenorhabditis elegans development. J Cell Biol. 2008;182:559–572. doi: 10.1083/jcb.200802128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Kishimoto N, Mitani S, Gengyo-Ando K, Kohara Y. Translational control of maternal glp-1 mRNA by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans. Development. 2003;130:2495–2503. doi: 10.1242/dev.00469. [DOI] [PubMed] [Google Scholar]
- Pitt JN, Schisa JA, Priess JR. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev Biol. 2000;219:315–333. doi: 10.1006/dbio.2000.9607. [DOI] [PubMed] [Google Scholar]
- Rybarska A, Harterink M, Jedamzik B, Kupinski AP, Schmid M, Eckmann CR. GLS-1, a novel P granule component, modulates a network of conserved RNA regulators to influence germ cell fate decisions. PLoS Genet. 2009;5:e1000494. doi: 10.1371/journal.pgen.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas LS, Maldonado E, Macias-Silva M, Blackwell TK, Navarro RE. The DEAD box RNA helicase VBH-1 is required for germ cell function in C. elegans. Genesis. 2007;45:533–546. doi: 10.1002/dvg.20323. [DOI] [PubMed] [Google Scholar]
- Schaner CE, Kelly WG. Germline chromatin. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.73.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Schisa JA, Pitt JN, Priess JR. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development. 2001;128:1287–1298. doi: 10.1242/dev.128.8.1287. [DOI] [PubMed] [Google Scholar]
- Schmid M, Kuchler B, Eckmann CR. Two conserved regulatory cytoplasmic poly(A) polymerases, GLD-4 and GLD-2, regulate meiotic progression in C. elegans. Genes Dev. 2009;23:824–836. doi: 10.1101/gad.494009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G, Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994;120:2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Shimada M, Kawahara H, Doi H. Novel family of CCCH-type zinc-finger proteins, MOE-1, -2 and -3, participates in C. elegans oocyte maturation. Genes Cells. 2002;7:933–947. doi: 10.1046/j.1365-2443.2002.00570.x. [DOI] [PubMed] [Google Scholar]
- Spike C, Meyer N, Racen E, Orsborn A, Kirchner J, Kuznicki K, Yee C, Bennett K, Strome S. Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins. Genetics. 2008a;178:1973–1987. doi: 10.1534/genetics.107.083469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike CA, Bader J, Reinke V, Strome S. DEPS-1 promotes P-granule assembly and RNA interference in C. elegans germ cells. Development. 2008b;135:983–993. doi: 10.1242/dev.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike CA, Strome S. Germ plasm: protein degradation in the soma. Curr Biol. 2003;13:R837–R839. doi: 10.1016/j.cub.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Squirrell JM, Eggers ZT, Luedke N, Saari B, Grimson A, Lyons GE, Anderson P, White JG. CAR-1, a protein that localizes with the mRNA decapping component DCAP-1, is required for cytokinesis and ER organization in Caenorhabditis elegans embryos. Mol Biol Cell. 2006;17:336–344. doi: 10.1091/mbc.E05-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S. Specification of the germ line. WormBook. 2005:1–10. doi: 10.1895/wormbook.1.9.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- Strome S, Wood WB. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1982;79:1558–1562. doi: 10.1073/pnas.79.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Wood WB. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell. 1983;35:15–25. doi: 10.1016/0092-8674(83)90203-9. [DOI] [PubMed] [Google Scholar]
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell. 2003;4:775–789. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Tabara H, Hill RJ, Mello CC, Priess JR, Kohara Y. pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development. 1999;126:1–11. doi: 10.1242/dev.126.1.1. [DOI] [PubMed] [Google Scholar]
- Updike DL, Strome S. A genome-wide RNAi screen for genes that affect the stability, distribution, and function of P granules in Caenorhabditis elegans. Genetics. 2009;183:1397–1419. doi: 10.1534/genetics.109.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wolfswinkel JC, Claycomb JM, Batista PJ, Mello CC, Berezikov E, Ketting RF. CDE-1 affects chromosome segregation through uridylation of CSR-1-bound siRNAs. Cell. 2009;139:135–148. doi: 10.1016/j.cell.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr Biol. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J. A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature. 2002;419:312–316. doi: 10.1038/nature01039. [DOI] [PubMed] [Google Scholar]
- Wolf N, Priess J, Hirsh D. Segregation of germline granules in early embryos of Caenorhabditis elegans: an electron microscopic analysis. J Embryol Exp Morphol. 1983;73:297–306. [PubMed] [Google Scholar]
- Zhang Y, Yan L, Zhou Z, Yang P, Tian E, Zhang K, Zhao Y, Li Z, Song B, Han J, Miao L, Zhang H. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]