Abstract
Hypoxia-inducible factor-1 (HIF-1) represents an important tumor-selective therapeutic target for solid tumors. In search of novel small molecule HIF-1 inhibitors, 5400 natural product-rich extracts from plants, marine organisms, and microbes were examined for HIF-1 inhibitory activities using a cell-based reporter assay. Bioassay-guided fractionation and isolation, followed by structure elucidation, yielded three potent natural product-derived HIF-1 inhibitors and two structurally related inactive compounds. In a T47D cell-based reporter assay, manassantin B1, manassantin A, and 4-O-methylsaucerneol inhibited hypoxia-induced HIF-1 activation with IC50 values of 3, 3, and 20 nM, respectively. All three compounds are relatively hypoxia-specific inhibitors of HIF-1 activation, in comparison to other stimuli. The hypoxic induction of HIF-1 target genes CDKN1A, VEGF and GLUT-1 were also inhibited. These compounds inhibit HIF-1 by blocking hypoxia-induced nuclear HIF-1α protein accumulation without affecting HIF-1α mRNA levels. In addition, preliminary structure-activity studies suggest specific structural requirements for this class of HIF-1 inhibitors.
Keywords: Hypoxia, Breast Cancer, HIF-1 Inhibitor, Natural Product, Small Molecule, Dineolignan, Sesquineolignan, Manassantin, Saururus cernuus
Introduction
Gene regulation (selective activation and inactivation of genes) plays an important role in the etiology and progression of cancer. Hypoxia-inducible factor-1 (HIF-1) is a transcription factor composed of the oxygen-regulated HIF-1α and the constitutively expressed HIF-1β subunits [1]. HIF-1 activates gene expression and promotes tumor cell survival under hypoxic conditions (reduced oxygen tension) [1]. In solid tumors, the degree of tumor hypoxia correlates with advanced disease stages and poor prognosis [2]. Clinical studies indicate that the overexpression of HIF-1α is a negative indicator for tumor treatment outcome [3–5]. Results from preclinical studies support the concept of HIF-1 inhibition as an effective molecular target-based approach for suppression of tumor growth and enhancement of treatment outcomes for radiation and chemotherapy [6–10]. Currently, intense research efforts are directed at the discovery of small molecule HIF-1 inhibitors [1,11].
In order to discover novel small molecule HIF-1 inhibitors, natural product-rich plant, marine invertebrate, and microbe extracts were selected as the source of chemical diversity. The term "natural product" refers to low molecular weight organic compounds that are produced by the host organism, presumably fulfilling specialized biological roles. In contrast to the essential biochemicals produced by all living organisms, these "secondary metabolites" are often unique to a given species (or Genera) and are typically thought to serve a specific ecological role, such as chemical defense. Natural products have been a major source of new drugs for centuries and the range of biochemical diversity offered by natural products is far greater than that readily achieved through synthetic chemistry. Over 60% of currently approved anticancer agents are of natural origin (natural products or synthetic compounds based on natural product models) [12]. A T47D human breast tumor cell-based reporter assay was recently used to examine natural product-rich extracts for HIF-1 inhibitory activities [13]. Bioassay-guided chromatographic fractionation of an active extract from the aquatic plant Saururus cernuus yielded manassantin B and 4-O-demethylmanassantin B, two potent hypoxia-selective HIF-1 inhibitors that blocked HIF-1 activation at submicromolar concentrations [13]. Saururus cernuus L. (Saururaceae) is a perennial plant found throughout the eastern half of the United States and has been used as a medicinal plant by both Native Americans and early colonists [14]. The majority of the compounds isolated from S. cernuus are lignans, sesquineolignans, and dineolignans. In this present study, we describe the identification and characterization of one sesquineolignan and two dineolignan HIF-1 inhibitors from S. cernuus, and examine the structural requirements for S. cernuus lignans to inhibit hypoxia-induced HIF-1 activation in breast tumor cells.
Materials and Methods
General Experimental Procedures for Natural Product Chemistry
Acquisition of 1HNMR, 1H-1H COSY, NOESY, HMBC, 13C NMR, and HMQC spectra; methods used to obtain HRESIMS and ESIMS data, optical rotation, and IR spectrum; equipment and supplies used to perform HPLC and TLC analysis were as previously described [13].
Plant Material Collection, Extraction, and Isolation
Collection, identification, and processing of Saururus cernuus samples were described [13]. Crude extract (16.6 g) was obtained by extracting dried powdered stems and leaves (172 g) with 50% CH2Cl2 in MeOH. A portion of the extract (11.0 g) was subjected to vacuum liquid chromatography (VLC, Si gel, 32–63 µm, hexanes-EtOAc-MeOH, step gradient) to yield eight fractions. The second fraction (230 mg, eluted with CH2Cl2) was purified by NP-HPLC [Prodigy® Silica (3), 5 µm, 250 × 21.2 mm, 2% CH2Cl2 in hexanes, 15.0 mL min−1, photodiode-array detection (PDA) monitored at 232 and 280 nm] to yield austrobailignan-5 (5, 80 mg). The fifth fraction (1.4 g, eluted with 50% hexanes in EtOAc) was separated using a C18 solid phase extraction (SPE) cartridge (MeOH:H2O step gradient) to obtain four subfractions. The first subfraction (440 mg) was subjected to repeated RP-HPLC (Prodigy® ODS-3, 5 µm, 250 × 21.2 mm, 30% CH3CN:30% MeOH:40% H2O, 9.0 mL min−1, PDA detection monitored at 232 and 280 nm) and yielded verrucosin (4, 6 mg) and 4-O-methylsaucerneol (3, 27 mg). Purification of the active constituents from the second subfraction (95 mg) was accomplished by RP-HPLC (Prodigy® ODS-3, 5 µm, 250 × 21.2 mm, 9.0 mL min−1, PDA detection monitored at 232 and 280 nm), successively eluted first with 30% CH3CN:30% MeOH:40% H2O and then with 25% CH3CN:25% MeOH:50% H2O. This effort yielded the dineolignans manassantin B (6, 6.5 mg) and manassantin B1 (1, 3.5 mg). The sixth fraction from the original VLC (862 mg, eluted with 100% EtOAc) was separated on Sephadex LH-20 with 100% MeOH to yield 14 subfractions. The fifth subfraction (150 mg) was first separated by RP-HPLC (Prodigy® ODS-3, 5 µm, 250 × 21.2 mm, 65% CH3CN:35% H2O, 9.0 mL min−1, PDA detection monitored at 232 and 280 nm) and the major partially purified compound then separated by NP-HPLC [Prodigy® Silica (3), 5 µm, 250 × 21.2 mm, 5% MeOH in CH2Cl2, 13.0 mL min−1, PDA detection monitored at 232 and 280 nm] to yield manassantin A (2, 35 mg).
Structure Data
Manassantin B1 (1): white amorphous powder; [α]25D −51.7° (c 0.33, MeOH); UV (MeOH) λmax (log ε) 208 (7.76), 234 (4.27), 282 (3.80) nm; IR (film) νmax 3473, 2962, 2931, 1590, 1511, 1450, 1259, 1139, 1037, 935, 809, 736 cm−1; 1H NMR (C6D6, 400 MHz) δ 0.68 (6H, d, J = 5.5 Hz, H-9', 9"), 1.20 (3H, d, J = 6.2 Hz, H-9), 1.26 (3H, d, J = 6.4 Hz, H-9'"), 2.15 (2H, m, H-8', 8"), 3.38 (3H, s, 3"-OCH3), 3.41 (3H, s, 3-OCH3), 3.42 (3H, s, 4-OCH3), 3.44 (3H, s, 3'-OCH3), 4.30 (1H, m, H-8'"), 4.36 (1H, m, H-8), 4.85 (1H, d, J = 7.9 Hz, H-7), 4.94 (1H, d, J = 7.9 Hz, H-7'"), 5.31 (2H, s, 3'"-OCH2O-4'"), 5.48 (2H, d, J = 5.7 Hz, H- 7', 7"), 6.61 (1H, d, J = 8.1 Hz, H-5), 6.67 (1H, d, J = 8.0 Hz, H-5'"), 6.77 (1H, d, J = 8.0 Hz, H-6'"), 6.89 (2H, d, J = 8.0, H-6', 6"), 6.92 (1H, d, J = 8.0, H-5"), 6.95 (2H, br, s, H-2', 2"), , 6.96 (1H, d, J = 8.0, H-6), 7.04 (1H, d, J = 8.0, H-5'), 7.08 (1H, J = 2.0 Hz, H-2), 7.16 (1H, overlapped with solvent residual peak, H-2'"); 13C NMR (C6D6, 100 MHz) δ (ppm): 14.1 (CH3, C-9'"), 15.5 (CH3, C-9'), 15.5 (CH3, C-9"), 17.6 (CH3, C-9), 45.0 (CH, C-8'), 45.0 (CH, C-8"), 56.0 (4C, CH3, −OCH3, C-3, 4, 4', 4"), 74.5 (CH, C-7'"), 79.2 (CH, C-7), 83.0 (CH, C-8'"), 83.8 (2C, CH, C-7', 7"), 84.9 (CH, C-8), 101.2 (CH2, 3'"-OCH2O-4'"), 107.9 (CH, C-2'"), 108.5 (CH, C-5'"), 111.3 (CH, C-6"), 111.4 (CH, C-6'), 112.1 (CH, C-2), 112.5 (CH, C-5), 119.5 (CH, C-2"), 119.6 (CH, C-2'), 119.8 (CH, C-5"), 120.2 (CH, C-5'), 120.3 (CH, C-6'"), 120.7 (CH, C-6), 134.2 (C, C-1), 135.4 (C, C-1'"), 137.3 (C, C-1'), 137.4 (C, C-1"), 146.8 (C, C-4'), 147.6 (C, C-4"), 147.8 (C, C-3'"), 148.6 (C, C-4'"), 150.4 (C, C-3), 150.6 (C, C-4), 151.9 (C, C-3'), 152.4 (C, C-3"); HR-ESIMS [M+Na]+ m/z 739.3087 (calculated for [C41H48O11 +Na], 739.3094).
Manassantin A (2): clear amorphous solid; [α]25D −98.4° (c 1.0, CHCl3); HRESIMS m/z 755.3488 (calculated for C42H52O11Na, 755.3407). Structure was assigned by 1D and 2D-NMR data, and the IR, UV, [α]D, 1H and 13C NMR spectroscopic data are essentially identical to previously published values [15].
4-O-Methylsaucerneol (3): white amorphous solid; [α]25D −50.9° (c 0.7, CHCl3); HR-ESIMS: (M+Na)+ m/z 575.2615 (calculated for C32H40O8Na, 575.2621). 1H and 13C NMR spectroscopic data are essentially identical to previously published values [15].
Verrucosin (4): white amorphous solid; [α]25D +20.6° (c 0.07, CHCl3); HR-ESIMS: (M-H)− m/z 343.1552 (calculated for C20H23O5, 343.1545). 1H and 13C NMR spectroscopic data are essentially identical to previously published values [16,17].
Austrobailignan-5 (5): light yellow oil; [α]25D −27.2° (c 2.5, CHCl3); HR-ESIMS: (M+Na)+ m/z 349.1389 (calculated for C20H22O4Na, 349.1416) [18].
Manassantin B (6): The isolation and spectroscopic identification of this compound was recently reported [13].
Cell Culture and Compound Treatment
Human breast carcinoma T47D cells (ATCC) were maintained in DMEM/F12 medium (JRH Biosciences) supplemented with 10% (v/v) fetal bovine serum (FBS, Hyclone) and 0.5% penicillin/streptomycin (equivalent to 50 units/mL and 50 µg/mL, respectively, GIBCO). Test compounds were prepared as stock solutions in DMSO (Sigma) and stored at −80°C. The final concentration of DMSO is less than 0.5% (v/v) in all assays. The hypoxic conditions were achieved by purging a humidified modular incubator chamber (Billups-Rothenberg) with a hypoxic gas mixture (5% CO2/1% O2/94% N2). In general, the cells were exposed to test compounds for 30 min under normoxic conditions prior to extended incubation.
T47D Cell-Based Reporter Assay
To monitor HIF-1 activity, a dual luciferase assay employing the pHRE3-TK-Luc reporter and an internal control pRL-TK (Promega) was performed as described [19].
RNA Extraction and Quantitative Real Time RT-PCR
Exponentially grown T47D cells were plated at the density of 0.9 × 106 cells/well of a 6-well plate (Corning) in 3 mL DMEM/F12 medium supplemented with 10% FBS and penicillin/streptomycin. Sixteen hours later, a serum-free DMEM/F12 medium supplemented with penicillin/streptomycin and each specific test compound was used to replace half of the conditioned media. Following incubation (30 min, 37°C), the exposure was prolonged for another 4 or 16 h under hypoxic conditions. Total RNA samples were extracted immediately following treatments with the RNeasy Mini Prep kit (QIAGEN). Synthesis of the first strand cDNAs, gene-specific primer sequences, quantitative real-time PCR reactions, and data analysis were described previously [19].
ELISA Assay for Human VEGF Protein
The plating of T47D cells, compound treatments, ELISA assay for secreted human VEGF proteins, and determination of the number of viable cells were the same as previously described [19].
Nuclear Extract Preparation and Western Blot Analysis
The experimental procedures were previously described in detail [13]. Final concentration of the solvent (DMSO) is 0.1% (v/v).
Mitochondrial Isolation and Respiration Studies
These studies were performed in the same manner as we have previously reported [20].
Statistical Analysis
Data were compared using ANOVA and Fisher's PLSD post hoc analyses (StatView® Software Version 5.01, SAS Institute Inc). Differences were considered significant when p <0.05.
Results and Discussion
Identification and Characterization of Novel HIF-1 Inhibitors from Saururus cernuus
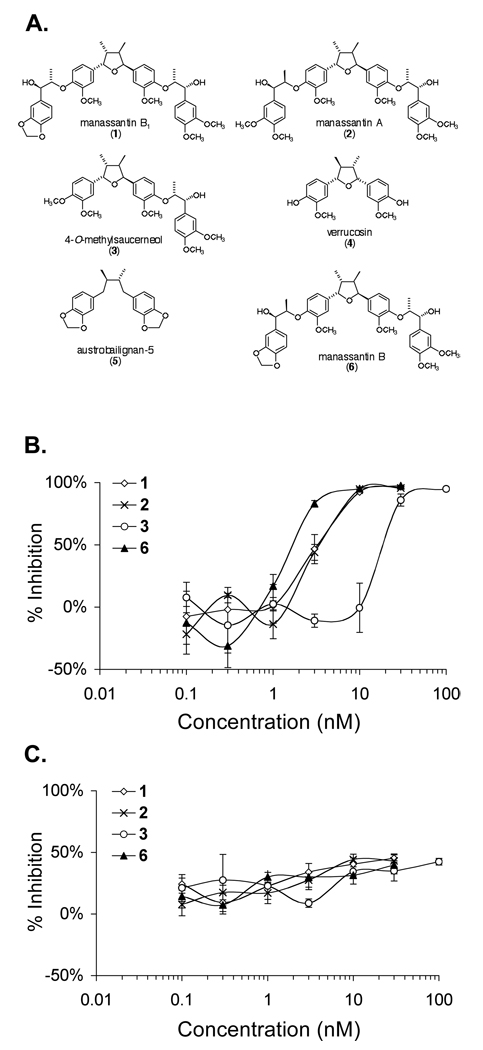
A T47D cell-based reporter assay was used to monitor HIF-1 activity [13]. Bioassay-guided chromatographic fractionation of the S. cernuus lipid extract yielded three new HIF-1 inhibitors (1, 2, 3) and two structurally related inactive compounds (4, 5) (Figure 1A). A combination of spectroscopic and spectrometric methods including Multi-Dimensional Nuclear Magnetic Resonance Spectroscopy (NMR) and High-Resolution Mass Spectrometry (HRMS) were used to elucidate the chemical structures. The absolute configurations were defined by NMR analysis of Mosher ester derivatives. Compound 1 is a previously unreported stereoisomer of manassantin B that we now refer to as manassantin B1. Compound 2 was found to be the S. cernuus dineolignan manassantin A, and compound 3 was determined to be the sesquineolignan 4-O-methylsaucerneol. Both inactive compounds 4 (verrucosin) and 5 (austrobailignan-5) are monolignans. The chemical structures of compounds 1–5 and manassantin B (6), which was used as a positive control, are shown in Figure 1A.
Figure 1.
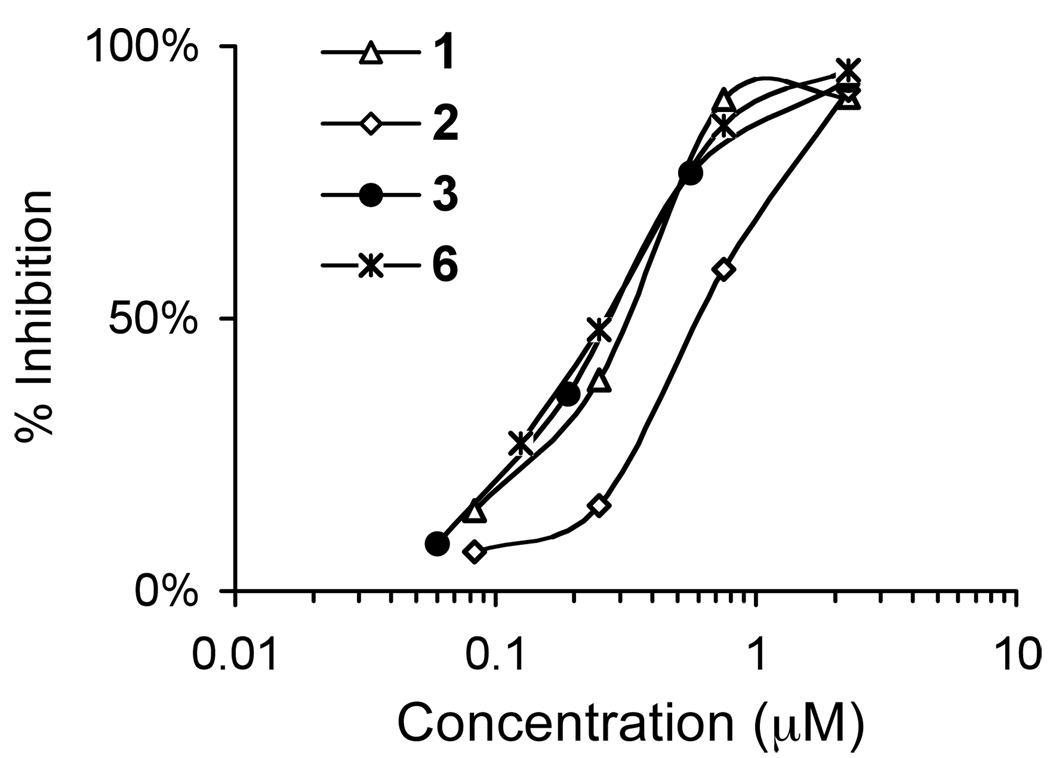
Chemical structures of six Saururus cernuus lignans and their effects on HIF-1 activity in T47D cells. The structures, trivial names, and numbers are shown in (A). Dose response studies were performed in T47D cells using a dual luciferase reporter assay to determine the effects of 1–3 on HIF-1 activation by hypoxia (B) and by 10 µM 1,10-phenanthroline (C). Compound 6 was included as a positive control. The cells were exposed to test compounds for 30 min prior to hypoxic exposure (16 h, 1% O2) or 1,10- phenanthroline treatment under normoxic conditions (16 h, 95% Air). Data are presented as averages from one representative experiment performed in triplicate and the error bars represent standard error.
The effects of 1–5 on HIF-1 activity were examined and manassantin B (6) was used as a positive control. In a T47D cell-based dual luciferase reporter assay, no inhibition of hypoxia-induced HIF-1 activation (1% O2, 16 h) was observed in the presence of either 4 (up to 1 µM) or 5 (up to 1 µM). Dose-response studies were performed to determine the effects of 1–3 on hypoxia-induced HIF-1 activation in T47D cells and the data are shown in Figure 1B. Compounds 1, 2, and the positive control 6, all completely inhibited hypoxic activation of HIF-1 at the concentration of 10 nM. Compound 3 is about an order of magnitude less potent than 1, 2, and 6 at inhibiting hypoxia-induced HIF-1 activation. Since manassantins A (2) and B (6) inhibit hypoxic activation of HIF-1 with similar potency, it appears that the methylenedioxyl moiety in manassantin B (6, IC50 2 nM) can be replaced by a pair of O-methyl groups, as in manassantin A (2, IC50 3 nM) with only a slight change in potency. Similarly, there is only a minor difference in potency between manassantin B (6, IC50 2 nM) and manassantin B1 (1, IC50 3 nM). Therefore, the difference in relative configuration of at least one of the two side chains in manassantin B (threo) and B1 (erythro) has only a minor influence on the potency of HIF-1 inhibition. A more dramatic effect was observed with the absence of one side chain phenylpropyl unit, as in the sesquilignan 4-O-methylsaucerneol (3). This reduced the potency of 3 by about 90% (IC50 20 nM), relative to that of manassantin B (6, IC50 2 nM). The most striking difference was observed with the two monolignans verrucosin (4) and austrobailignan-5 (5) that show virtually no HIF-1 inhibition, even at 1 µM, a concentration 500-times that of the IC50 for manassantin B (6). This suggests that the absence of the second hydroxylated side chain segment not only reduces the HIF-1 inhibitory activity of S. cernuus lignans, but for all practical purposes, renders these compounds essentially inactive.
Two conditions that are commonly used to activate HIF-1 are those of physiological hypoxia [21] and chemical hypoxia [22,13]. To discern the specificity of HIF-1 inhibition, experiments were performed to determine the effects of 1–3 on iron chelator-induced HIF-1 activation in T47D cells (10 µM 1,10-phenanthroline, 16 h). As shown in Figure 1C, none of the compounds exhibited greater than 50% inhibition of 1,10-phenanthroline-induced HIF-1 activation at the concentrations tested. This observation suggests that, like the positive control 6, 1–3 may function as hypoxia-selective HIF-1 inhibitors. It is most likely that these compounds target a process or set of processes specific to the activation of HIF-1 by hypoxia.
Effects of S. cernuus Lignans on HIF-1 Target Gene Expression
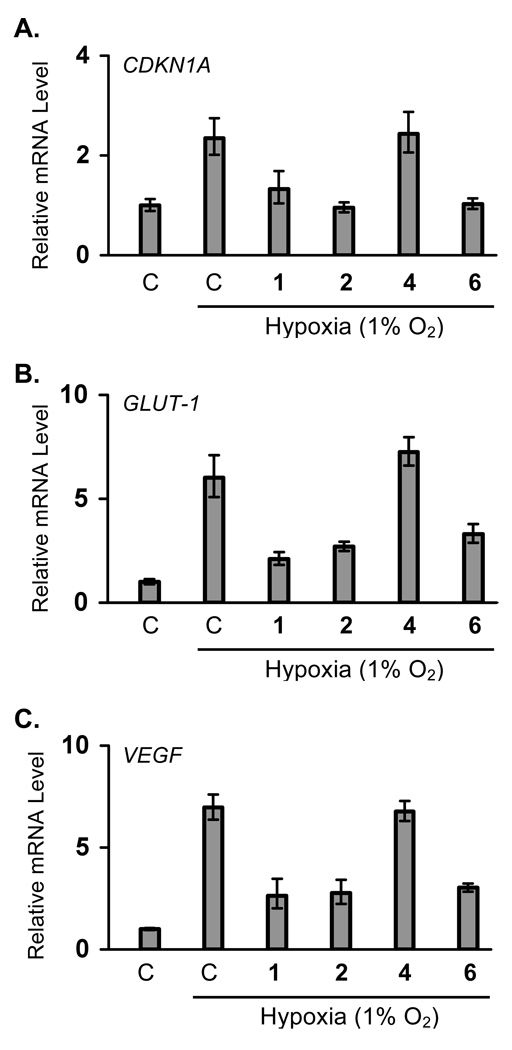
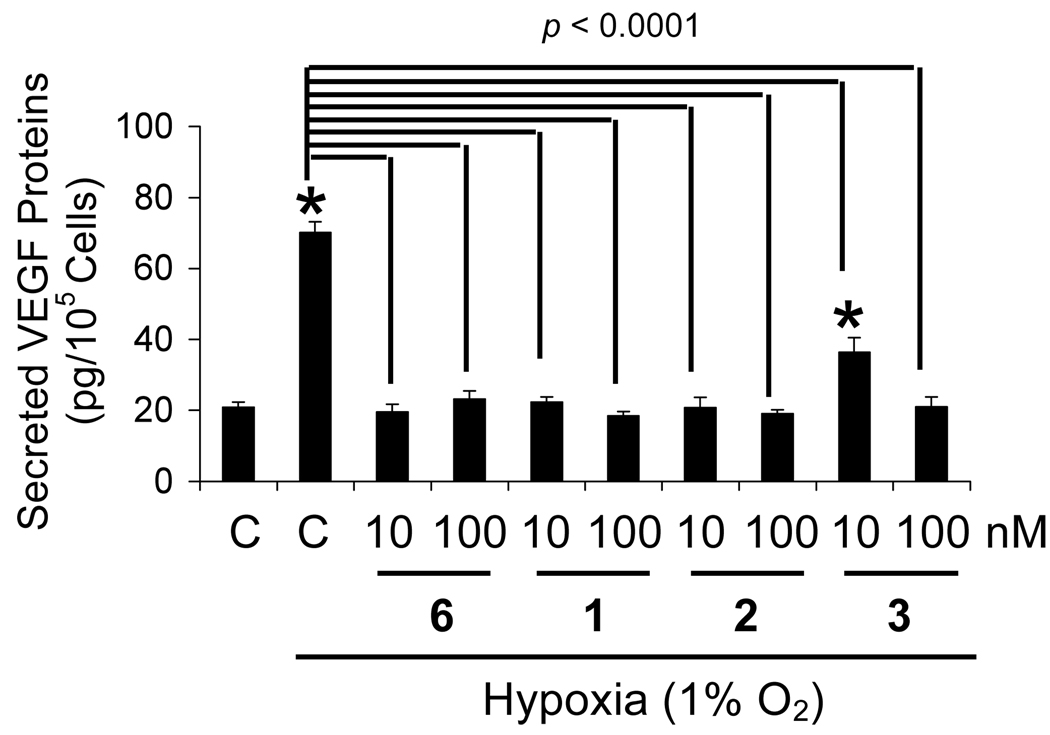
More than seventy genes have been identified to be cellular targets of HIF-1 [1]. Most of these genes are regulated by HIF-1 in a cell type-specific manner, while a few genes are induced in most cell types. The effects of manassantins on hypoxic induction of HIF-1 target genes (CDKN1A, GLUT-1, and VEGF) were examined in T47D cells and these results are shown in Figure 2. All three genes are induced in most cell types upon HIF-1 activation. In T47D cells, hypoxic exposure (1% O2, 16h) increased the expression of CDKN1A, GLUT-1,and VEGF at the mRNA level by 1.4-, 5-, and 6-fold, respectively. Compounds 1, 2, and 6 (at 30 nM each) blocked the hypoxia-induced increase in CDKN1A mRNA, while 4 did not affect the induction (Figure 2A). These compounds exerted similar effects on hypoxia-induced increases in GLUT-1 (Figure 2B) and VEGF (Figure 2C) mRNA levels. Compounds 1, 2, and 6 inhibited the hypoxia-induced increase in GLUT-1 and VEGF mRNA levels, while compound 4 was inactive. Vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors known [23]. Inhibition of VEGF gene expression/function has been pursued as a noncytotoxic approach for the treatment of cancer [23]. Since secreted VEGF proteins are the bioactive forms and hypoxia is one of the major physiological stimuli for VEGF production, the effects of 1–3 on hypoxic induction of secreted VEGF proteins were also examined in T47D cells (Figure 3). Compounds 1, 2 and the positive control 6, blocked the hypoxia-induced increase in secreted VEGF proteins at the lowest concentration tested (10 nM). In the presence of 3, partial inhibition (60%) was observed at the lower concentration (10 nM), while complete inhibition was achieved at the higher concentration (100 nM). When tested at 100 nM, none of the compounds interfered with the ELISA assay using recombinant human VEGF165 protein as the standard (no greater than 15% difference, data not shown).
Figure 2.
Manassantins inhibit hypoxic induction of HIF-1 target genes CDKN1A, GLUT-1, and VEGF in T47D cells. Quantitative real time RT-PCR analysis was performed to determine CDKN1A (A), GLUT-1 (B), and VEGF (C) mRNA levels following treatments with 1, 2, 4, and 6 (30 nM each) under hypoxic conditions (1% O2) for 16 h. "C" represents media only. Data shown are relative expression levels (mean ± SD) of target genes normalized to that of an internal 18S rRNA control, determined from one representative experiment performed in triplicate.
Figure 3.
Manassantins and 4-O-methylsaucerneol block the induction of secreted VEGF proteins (VEGF121 and VEGF165) by hypoxia in T47D cells. Following compound treatment under hypoxic conditions (1% O2) for 16 h, levels of secreted VEGF proteins in the conditioned media were determined by ELISA and normalized to the number of viable cells. The control "C" represents media only. Data shown are mean ± SE from one representative experiment performed in triplicate. An asterisk (*) indicates p <0.05 when compared to the normoxic control. The p values between the hypoxic control and the compound treatments are indicated.
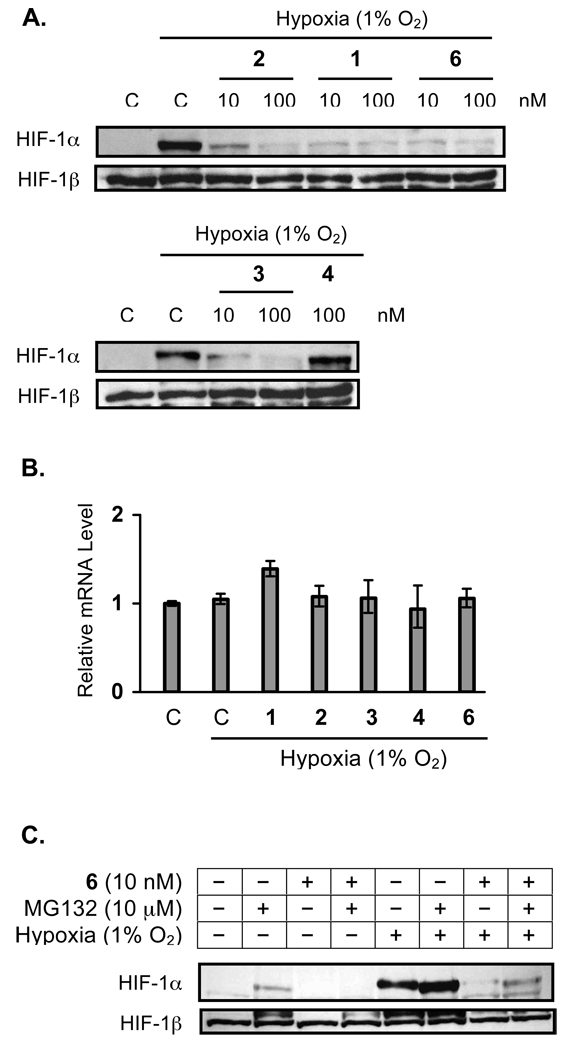
Manassantins and 4-O-Methylsaucerneol Inhibit HIF-1 by Blocking Nuclear HIF-1α Protein Accumulation
In general, the oxygen-regulated HIF-1α subunit determines HIF-1 activity [21]. Under normoxic conditions, HIF-1α protein is post-translationally modified and rapidly degraded by the ubiquitin-proteasome pathway [24–26]. Under hypoxic conditions, stabilization and accumulation of nuclear HIF-1α protein precedes the activation of HIF-1 [24–26]. In T47D cells, hypoxic exposure (1% O2, 4 h) leads to the accumulation of nuclear HIF-1α protein without affecting the constitutively expressed HIF-1β protein (Figure 4A). Compounds 1–3 (and the positive control 6) blocked the hypoxia-induced accumulation of nuclear HIF-1α protein (Figure 4A). The structurally related, yet inactive, compound 4 did not affect the induction of nuclear HIF-1α protein by hypoxia (Figure 4A). No significant changes in nuclear HIF-1β protein levels were observed following treatments (Figure 4A). The effects of test compounds on HIF-1α expression at the mRNA level were also examined. As shown in Figure 4B, none of the compounds (1–4, and 6 at 30 nM each) affected HIF-1α mRNA levels under hypoxic conditions (1% O2, 4 h). Thus, compounds 1–3 most likely inhibit HIF-1 by regulating the availability of HIF-1α protein. There are at least three possible scenarios that can contribute to this outcome: 1) inhibition of HIF-1α protein synthesis; 2) degradation of HIF-1α protein; and 3) blockade of HIF-1α protein nuclear translocation. However, the following two observations suggest that it is unlikely that these HIF-1 inhibitors function by blocking HIF-1α protein synthesis or nuclear translocation: 1) these compounds did not inhibit 1,10-phenanthroline-induced HIF-1 activation with the same potency (Figure 1C); and 2) manassantin B (6) did not inhibit 1,10-phenanthroline-induced accumulation of nuclear HIF-1α protein [13].
Figure 4.
Manassantins and 4-O-methylsaucerneol inhibit HIF-1 activation by preventing the accumulation of nuclear HIF-1α protein. After hypoxic exposure (1% O2, 4 h) in the presence of test compounds, levels of HIF-1α and HIF-1β proteins in the nuclear extract samples from T47D cells were determined by Western blot (A). The control "C" represents media only. Quantitative real time RT-PCR analysis was employed to determine the effects of 1–4 and 6 (30 nM each) on HIF-1α mRNA levels under hypoxic conditions (1% O2, 4 h). Data shown are relative expression levels of HIF-1α mRNA, determined from one representative experiment performed in triplicate (B). Effects of 6 on the stabilization of nuclear HIF-1α protein induced by a proteasome inhibitor (MG-132) under normoxic (95% Air, 4 h) and hypoxic conditions (1% O2, 4 h) were examined by Western blot (C). Levels of nuclear HIF-1β protein detected by Western blot were also shown (C).
To test the hypothesis that HIF-1 inhibitors such as manassantin B (6) can promote HIF-1α protein degradation, the effect of 6 on nuclear HIF-1α protein accumulation was examined in the presence of MG132. The compound MG-132 is a membrane permeable and reversible inhibitor of the proteasome and has been shown to block proteasome-mediated degradation of HIF-1α protein and induce nuclear HIF-1α protein accumulation [27,28]. Under normoxic conditions, MG-132 (10 µM, 4 h) induced the accumulation of nuclear HIF-1α protein in T47D cells, and this induction was blocked by manassantin B (6) (Figure 4C). Under hypoxic conditions, MG132 enhanced the induction of HIF-1α protein by hypoxia, and manassantin B (6) inhibited the induction, even though the inhibition was not complete (Figure 4C). One obvious explanation is that manassantin B can effectively overcome the reversible proteasome inhibition imposed by MG132 under normoxic conditions but not under hypoxic conditions. However, additional studies will be required to confirm this hypothesis.
Effects of S. cernuus Lignans on Mitochondrial Respiration
Among the known HIF-1 inhibitors that we have examined, rotenone exhibited similar effects as those observed for the active S. cernuus neolignans on the induction of nuclear HIF-1α protein by various stimuli. In T47D cells, rotenone (1 µM) inhibited the accumulation of nuclear HIF-1α protein induced by hypoxia (1% O2, 4 h), MG-132 (10 µM, 4 h), MG-132 plus hypoxia, but not by 1,10-phenanthroline (10 µM, 4 h) (data not shown). Rotenone is a potent inhibitor of mitochondrial electron transport and has also been shown to inhibit the activation of HIF-1 [29]. Recent studies have shown that mitochondrial inhibitors block hypoxic activation of HIF-1 by increasing intracellular oxygen levels [30]. To test the hypothesis that these active S. cernuus neolignans block HIF-1 activation via mitochondrial inhibition, the effects of 1–4 and 6 on mitochondrial respiration were examined, and these results are shown in Figure 5. Compounds 1, 2, 3, and 6 inhibited mitochondrial oxygen consumption with IC50 values of 320 nM, 600 nM, 260 nM, and 250 nM, respectively. No effects on oxygen consumption by mitochondria were observed in the presence of 4 (highest concentration tested: 9 µM). Addition of succinate did not relieve the inhibition exerted by 1, 2, 3, and 6 on mitochondria, suggesting that these compounds act as inhibitors of mitochondrial electron transport chain complex I. At present time, it can not be conclusively determined whether the precise mechanism of action for these potent HIF-1 inhibitors is mediated through the inhibition of mitochondrial oxygen consumption. Several other natural product-based mitochondria inhibitors suppress both HIF-1 activation and mitochondrial function with similar potency [20,30].
Figure 5.
Effects of compounds 1–3 and 6 on the consumption of oxygen by mitochondria. The mitochondrial oxygen consumption rate (nmole O2/min/mg protein) was measured in the presence and absence of test compounds in vitro. Data shown are averages from two independent assays, presented as percentage inhibition in comparison to the untreated control.
Previously reported activities of manassantins A and B include a tranquilizer-like activity in mice (manassantin A) [31,32], inhibition of NFκB activation (manassantins A and B) [33,34], inhibition of human acyl-CoA: cholesterol acyltransferase-1 and -2 (manassantins A and B) [35], inhibition of PMA-induced cell aggregation (manassantins A and B) [36], and inhibition of HIF-1 activation (manassantin B) [13]. Most of these activities that have been demonstrated in either cell-based or enzyme-based assays require manassantin concentrations that are in the micromolar range. Manassantins A and B were shown to inhibit PMA-induced aggregation of HL-60 cells with MICs of 1.0 and 5.5 nM, while concentrations at least ten-fold higher are required for each manassantin to block PMA-induced ICAM expression (IC50s: >10 nM, and MICs: 10–100 nM)[36]. However, it was suggested that manassantins A and B inhibit cell aggregation by blocking ICAM-1 expression [36]. In our previous report, isolated samples of both manassantin B (6) and 4-O-demethylmanassantin B were shown to block hypoxia-induced HIF-1 activation in T47D cells with IC50s of 3 and 30 nM, respectively [13]. Inhibitory effects of manassantin B on hypoxia-induced HIF-1α protein accumulation were also observed in other cell lines (Hep3B and MDA-MB-231) with similar potency (data not shown). Therefore, it is unlikely that the potent HIF-1 inhibitory activity of manassantin B is limited to T47D cells.
As mentioned, intense research efforts are currently underway in many laboratories to discover and develop new strategies to inhibit HIF-1. Among the small molecule HIF-1 inhibitors identified, many of these compounds (i.e. 2-methoxyestradiol [37], taxol [37], geldanamycin [27], rapamycin [38], topotecan and camptothecin analogues [39], and PX-478 [40]) are also known to exert their anticancer effects through other mechanisms. Most of these compounds were discovered initially due to their effects on other cellular targets, and their inhibitory effects on HIF-1 were later identified. In the published literature, the sources of chemicals used in screening efforts to discover new HIF-1 inhibitors can be classified into two general categories: 1) libraries of pure compounds [39,41,42]; and 2) libraries of natural product-rich extracts [13]. Among the HIF-1 inhibitors discovered from such screening efforts, the most potent inhibitors of HIF-1 activation (with IC50 values in the nM range) are all natural products or compounds based on natural product scaffolds (e.g. manassantins A, B [13], B1, 4-O-demethylmanassantin B [13], 4-O-methylsaucerneol, chetomin [41], topotecan [39], and camptothecin analogues [39]). The recently identified natural product-based synthetic analog 103D5R, inhibits HIF-1 activation at high concentrations (IC50 of 35 µM), but is also cytostatic [42]. The unique properties of manassantin B and related compounds include their relative selectivity towards hypoxia-induced HIF-1 activation (in contrast to iron chelator-induced HIF-1 activation), and that they exert HIF-1 inhibitory effects at concentrations far below cytotoxic concentrations (supporting information).
Supplementary Material
Acknowledgments
We thank Dr. Steven L. McKnight (University of Texas Southwestern Medical Center at Dallas) for the pTK-HRE3-luc construct and Ms. Melanie Mask (USDA/ARS) for technical assistance with quantitative real-time RT-PCR assays. This work was supported in part by the National Institutes of Health-National Cancer Institute CA-98787-01 (D.G.N.), CA-95471 (R.K.B.), and the DOD/2000-Breast Cancer Research Program DAMB17-01-1-0566 (D.G.N.). R.K.B. is the Michael L. Rosenberg Scholar in Medical Research and is supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences. Additional support was provided by NOAA NURP/NIUST NA16RU1496, and USDA/Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
References
- 1.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 3.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 4.Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1α during breast carcinogenesis. J. Natl. Cancer Inst. 2001;93:309–314. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 5.Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat. Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 9.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. The hypoxia-inducible factor-1α is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 10.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 11.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat. Rev. Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 12.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 13.Hodges TW, Hossain CF, Kim YP, Zhou YD, Nagle DG. Molecular-targeted antitumor agents: the Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J. Nat. Prod. 2004;67:767–771. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- 14.Hartwell JL. Plants used against cancer. A survey. Lloydia. 1971;34:204–255. [PubMed] [Google Scholar]
- 15.Rao KV, Alvarez FM. Manassantins A/B and saucerneol: novel biologically active lignoids from Saururus cernuus. Tetrahedron Lett. 1983;24:4947–4950. [Google Scholar]
- 16.Dias AF, Giesbrecht AM, Gottlieb OR. The chemistry of Brazilian Lauraceae. Part LXVII. Neolignans from Urbanodendron verrucosum. Phytochemistry. 1982;21:1137–1139. [Google Scholar]
- 17.Hattori M, Hada S, Kawata Y, Tezuka Y, Kikuchi T, Namba T. Constituents of mace. Part II. New 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans from the aril of Myristica fragrans. Chem. Pharm. Bull. 1987;35:3315–3322. [Google Scholar]
- 18.Rao KV, Alvarez FM. Chemistry of Saururus cernuus. I. Saucernetin, a new neolignan. J. Nat. Prod. 1982;45:393–397. [Google Scholar]
- 19.Zhou YD, Kim YP, Li XC, Baerson SR, Agarwal AK, Hodges TW, Ferreira D, Nagle DG. Hypoxia-inducible factor-1 activation by (-)-epicatechin gallate: potential adverse effects of cancer chemoprevention with high-dose green tea extracts. J. Nat. Prod. 2004;67:2063–2069. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed KA, Hossain CF, Zhang L, Bruick RK, Zhou YD, Nagle DG. Laurenditerpenol, a new diterpene from the tropical marine alga Laurencia intricata that potently inhibits HIF-1 mediated hypoxic signaling in breast tumor cells. J. Nat. Prod. 2004;67:2002–2007. doi: 10.1021/np049753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 22.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 23.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 25.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIF-1α targeted for VHL-mediated destruction by praline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 26.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-1α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 27.Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, Zhong H. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. [PubMed] [Google Scholar]
- 28.Lee DH, Goldberg AL. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J. Biol. Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 29.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF-1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 31.Rao KV, Puri VN, Diwan PK, Alvarez FM. Preliminary evaluation of manassantin A, a potential neuroleptic agent from Saururus cernuus. Pharmacol. Res. Commun. 1987;19:629–638. doi: 10.1016/0031-6989(87)90117-2. [DOI] [PubMed] [Google Scholar]
- 32.Rao KV, Puri VN, el-Sawaf HA. Further studies on the neuroleptic profile of manassantin A. Eur. J. Pharmacol. 1990;179:367–376. doi: 10.1016/0014-2999(90)90177-8. [DOI] [PubMed] [Google Scholar]
- 33.Hwang BY, Lee JH, Nam JB, Hong YS, Lee JJ. Lignans from Saururus chinensis inhibiting the transcription factor NF-κB. Phytochemistry. 2003;64:765–771. doi: 10.1016/s0031-9422(03)00391-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Hwang BY, Kim KS, Nam JB, Hong YS, Lee JJ. Suppression of RelA/p65 transactivation activity by a lignoid manassantin isolated from Saururus chinensis. Biochem. Pharmacol. 2003;66:1925–1933. doi: 10.1016/s0006-2952(03)00553-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee WS, Lee DW, Baek YI, An SJ, Cho KH, Choi YK, Kim HC, Park HY, Bae KH, Jeong TS. Human ACAT-1 and -2 inhibitory activities of saucerneol B, manassantin A and B isolated from Saururus chinensis. Bioorg. Med. Chem. Lett. 2004;14 doi: 10.1016/j.bmcl.2004.04.023. 3109-3012. [DOI] [PubMed] [Google Scholar]
- 36.Rho MC, Kwon OE, Kim K, Lee SW, Chung MY, Kim YH, Hayashi M, Lee HS, Kim YK. Inhibitory effects of manassantin A and B isolated from the roots of Saururus chinensis on PMA-induced ICAM-1 expression. Planta Med. 2003;69:1147–1149. doi: 10.1055/s-2003-818007. [DOI] [PubMed] [Google Scholar]
- 37.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 38.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 39.Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, Melillo G. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- 40.Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol. Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 41.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.