Abstract
INTRODUCTION
The physiological function of the mastoid air cell system (MACS) with respect to middle ear (ME) pressure-regulation remains controversial because predictive mathematical models and experimental data to formulate and test hypotheses are lacking.
OBJECTIVE
A mathematical description of MACS volume effects on the rate of ME pressure change is presented; the agreement between published data and model prediction is examined for consistency with the hypothesis that the MACS acts as a functional rate-limiter of ME pressure change, and an explanation for the relationship between MACS volume and otitis media is discussed.
METHODS
The mathematical description shows that the value of a single, free parameter, termed the “MACS buffering efficiency” (M) determines if MACS volume affects the rate of ME pressure change caused by diffusive gas exchange. The MACS serves no rate-limiting function for M=0, acts as a gas sink for M>1 and acts as a gas reserve (rate-limiter) for M<1.
RESULTS
Fitting the model equation to published adult human data yielded an estimate for M of 0.2. This implies that larger MACS volumes are associated with lesser rates of change in ME pressure caused by diffusive gas exchange and lesser required frequencies of effective Eustachian tube openings to maintain near ambient ME pressures.
CONCLUSTION
If well-controlled studies confirm M<1 for children and adults, larger MACS volumes will increase the time required to develop sufficient ME underpressures to cause otitis media by hydrops ex vacuo during transient or prolonged periods of Eustachian tube dysfunction.
Keywords: mastoid volume, rate-limiting function, middle ear pressure-buffering, mathematical modeling
INTRODUCTION
The mastoid air cell system (MACS) is a multiply partitioned airspace located within the petrousal bone posterior to and in communication with the airspace of the tympanum [1, 2]. Developmentally, the MACS shows an age-related increase in volume to exceed that of the tympanum by fold magnitudes in adults. Previous studies reported that children with small MACS volume had significantly more episodes of otitis media and otitis media episodes of longer duration when compared to age-matched controls with large MACS volume, and other studies reported that adults with small MACS volume were at higher risk for otitis media during upper respiratory infections when compared to those with large MACS volume [3–7]. This linkage between MACS size and predisposition to middle ear (ME) disease has been interpreted as evidencing directional causality with either small MACS volume predisposing to otitis media or, alternatively, with otitis media stunting the expansion of MACS volume during growth [2, 4, 7–11].
To date, there is no clear resolution as to which, if either, of these interpretation is correct. Experimental studies using animals are not feasible since, with the exception of higher primates that have a prolonged period of growth and development, most species used in ME research have an inflated ME bulla rather than a MACS [12]. Moreover, the physiological function(s), if any, of the MACS is not known though a number of hypotheses have been advanced. These include active gas generation and ME pressure-buffering. While the physiological mechanism underlying MACS gas generation is not well developed [3, 5, 13] and experimental evidences supporting that possibility have alternative explanations [14], ME pressure-buffering is easily demonstrated. There, pressure-buffering can be decomposed into two effects, a magnitude-limiting effect on pressure change under conditions where ME cavity volume is changed (Boyle’s Law) [15] and a rate-limiting effect on pressure change under conditions where the contained volume of ME gas is changed (General Gas Law) [16]. Confusion between the consequences of changing ME volume and changing ME gas volume is common in the published literature and this underlies much of the existing controversies regarding the functional role of the MACS in ME pressure-regulation [4, 17]. ME pressure-regulation refers to those physiological processes that compensate for or minimize the effects of external and internal factors that disturb the ME-ambient pressure equilibrium required for maintaining good hearing levels and an uninflamed ME mucosa.
In this paper, the ME pressure-buffering effects of the MACS as a magnitude and a rate-limiter of pressure change are discriminated, a mathematical description for determining if the MACS acts as a rate-limiter of ME pressure change is developed, the hypothesized rate-limiting function of the MACS is tested against published data [18], and a contextual consideration of the results is used to explain the relationship between MACS volume and otitis media risk.
METHODS
Mathematical Description of MACS Volume Effects on ME Pressure
For any time (t), the general gas law relates the gas pressure (P) in the ME cavity (e) to its extant volume (V), temperature (T) and contained gas moles (N) as:
| EQ1a |
where ΣNeg is the sum of moles over all contained gases (g). Under physiological conditions, ME temperature is constant and ME volume can be decomposed into the sum of tympanum and MACS volumes, such that
| EQ1b |
where Ce is a constant representing the product of the general gas constant R and ME temperature. Two independent factors can effect ME pressure change; changing ME volume (Vm+Vt) and/or changing the contained number of gas moles (ΣNeg). The relevant equations describing these effects are the respective derivatives of EQ1b (Note that throughout, “//” indicates conditions that are extant, and “k” indicates that those conditions are constant),
| EQ2a |
| EQ2b |
In strict sense, EQ2a is applicable only to conditions wherein the volume of the “closed” (i.e. no gas entry, exit or production) ME is changed situationally as, for example, during displacements of the tympanic membrane in ears with a closed Eustachian tube. There, identical volume displacements will cause greater ME pressure changes for those MEs with lesser initial volumes; i.e. MACS volume acts as a magnitude-limiter of ME pressure change [19]. This phenomenon is a valid physical property of all similarly constructed pressure systems but, with few isolated exceptions conditioned by an extremely high ratio of tympanic membrane displacement volume to total ME volume (e.g. hyper-compliant tympanic membrane in a low volume ME can protect from barotitis or delay the onset of ME pressure change caused by diffusive, transmucosal gas exchange [15]), is not relevant to explaining the relationship between MACS volume and otitis media risk. In contrast, EQ2b describes the effect on ME pressure of adding or removing gas to a ME system with fixed volume as for example during diffusive gas exchange between the ME and adjacent compartments (See Below). The time derivative,
| EQ3 |
describes the rate of change in ME pressure as a function of the rate of change in contained gas moles for a fixed volume ME, and the validity of the hypothesis that the MACS acts as a rate-limiter of ME pressure change depends on whether or not this relationship is conditioned on MACS volume. To appreciate this, it is necessary to assign a value to the rate of change in ME gas moles (ΣδNeg/δt) based on a consideration of gas flow (QP) across all pathways linking the ME to its adjacent compartments: i.e. gas exchange with the nasopharynx via the Eustachian tube; the atmosphere via the tympanic membrane, the inner ear via the round window and the local blood via the ME mucosa.
Because the Eustachian tube is usually closed, gas transfers require muscle-assisted openings [20, 21]. For an individual ear, volume gas flow (QET=δN/ET opening) is a function of the extant total pressure gradient and the values of relatively fixed structural and functional parameters of the Eustachian tube [15]. From EQ3, the change in ME pressure effected by each Eustachian tube opening is an inverse function of ME volume and a direct function of volume gas flow that, in turn, depends on the above listed factors. Because the major influences affecting MACS volume are age and disease history and these also relate to the efficiency of trans-Eustachian tube gas exchange (e.g. greater age is associated with both greater MACS volumes and more efficient volume gas transfers), it is difficult to predict the effect of MACS volume on this component of ME pressure-regulation.
In contrast, the pathways linking the ME to the other three compartments are characterized by bidirectional, gas-species dependent, diffusional flows (QPathg). There, for times between Eustachian tube openings, EQ3 can be rewritten for the fixed-volume, temperature stable ME as:
| EQ4a |
where Σpath indicates summing across the three passive exchange routes. This equation can be simplified by noting that gas exchange across the round window and the tympanic membrane at physiological pressure-gradients is extremely slow when compared to gas transfers across the ME mucosa [22, 23]. Consequently, as a first approximation, it is assumed that the total rate of ME pressure change is attributable to the exchange rate across the MACS and tympanum mucosa, and EQ4a reduces to:
| EQ4b |
Also, under physiological conditions and at constant blood O2 and CO2 pressures, the transmucosal partial-pressure gradient for those gases is approximately 0 mmH2O and the ME is saturated with water vapor [24, 25]. Because there is no gradient to drive gas exchange, Qm,tO2 = Qm.tCO2 = Qm,tH2O = 0 moles/min, and EQ4b reduces to:
| EQ4c |
Under EQ4c, the quantity of N2 transferred to the ME must be equal to the extant difference between the arterial N2 moles on entering the ME mucosa and the venous N2 moles exiting the ME mucosa (i.e. δNeN2 = NaN2−NvN2) which can be written as N2 flow rates across the MACS and tympanum mucosa by taking the time derivative of the relationship, or:
| EQ5a |
The number of moles of an inert gas in blood is the product of gas partial-pressure (PbN2), gas solubility in blood (SbN2, equal for arterial and venous blood) and blood volume (Vb), and substituting this relationship into EQ 5 yields:
| EQ5b |
This set of equations can be simplified by recognizing that the arterial N2 pressure is identical for MACS and tympanum mucosa, that the change in blood volume per unit time is blood flow (Qb) and that, by continuity, the venous and arterial blood flows are equal for each compartment (no blood pooling in mucosa of tympanum or mastoid) which yields the paired equations:
| EQ5c |
Substituting this result into EQ4c and simplifying yields:
| EQ6a |
Under physiological conditions, the ME pressure of N2 is at all times greater than or equal to that of the local arterial and venous blood, i.e. PeN2 ≥ PvN2 ≥ PaN2 [25, 26]. From this it follows that PaN2− PvN2 ≥ PaN2− PeN2 and a constant of proportionality (FeN2) can be chosen such that (PaN2 − PvN2) = FeN2(PaN2− PeN2). Also, experimental studies and theoretical calculations show that air-phase gas exchange is extremely rapid vis a vis exchange across the mucosa [6, 27] such that gas partial pressures are approximately equal in the two ME compartments, i.e. PeN2 ~ PmN2 ~ PtN2. Substituting these results into EQ 6a and simplifying yields the final equation for the rate of change in ME pressure caused by diffusive transmucosal gas exchange,
| EQ6b |
Model Application to MACS as a Rate-Limiter of ME Pressure Change
The inclusion of possible differences in regional blood flow as controlling factors governing ME pressure change caused by diffusive gas exchange complicates the simple relationship between ME pressure-regulation and MACS volume suggested by others. To appreciate this, note that total volume blood flow for either the MACS or tympanum compartment (c) can be decomposed as follows: Qcb=(Qcb/Ac)(Ac/Vc)Vc where Ac and Vc are the surface area and volume of the compartment. Then, Qcb/Ac is the average blood flow per unit surface area (RcQ/A) and Ac/Vc is the surface area-volume ratio (RcA/V). For the MACS, these parameters, with volume and the proportionality constant can be written in terms of the respective values for the tympanum such that: FmN2 = UFtN2, Vm = XVt, RmQ/A = YRtQ/A and RmA/V = ZRtA/V, where U, X, Y and Z are scaling constants. Substituting these relationships into EQ 6b and simplifying yields:
| EQ7 |
where M is the product of the scaling constants Y, Z, and U (M=YZU). For the non-inflamed ME, the product CeSbN2(Vm+Vt)−1(VtQtbFtN2) is valued as a constant and, therefore, the rate of ME pressure change is a function of the arterial-ME N2 pressure gradient and the structural geometry of the ME represented by 1+XM, where X is the number of equivalent tympanum volumes summed to equal MACS volume and M is the average effective perfusion rate vis a vis the tympanum over all equivalent volumes. M is referred to as the MACS buffering coefficient and its value is the single determinant whether or not the MACS functions as a rate-limiter for ME pressure change.
Relationship Between the Time-Constant for Transmucosal Gas Exchange and M
A time-constant (KeN2) for transmucosal N2 exchange can be calculated as the ratio of the rate of change in ME pressure to the driving gradient, such that
| EQ8a |
The term (Vm+Vt) can be rewritten as Vt(X+1) and EQ 8a can be simplified as:
| EQ8b |
where Ce’ is the product of the constants Ce, SbN2, Qtb and FtN2. As represented, the time-constant is independent of the units of measure and extant driving gradients since it has the units of time−1 (e.g. mm H2O/sec/mm H2O = /sec). EQ8b shows that the time constant for N2 exchange is dependent only on certain properties of the gas, ME geometry and the value of the MACS buffering coefficient, M. The time-constant is also useful to standardize exchange rates when comparing the volume exchange of different inert gases across the same pathway (See below for N2O to N2 conversion) or of the same inert gas across different pathways [16, 22, 28].
Hypothesis Testing
To test the hypothesis that the MACS is a rate-limiter of ME pressure change (i.e. serves as a gas reserve), it is necessary to measure the value of M for transmucosal N2 exchange. The hypothesis can be rejected for all M ≥ 1 and supported for all M < 1, i.e. for all conditions where the volume rate of gas loss/volume is greater for the tympanum when compared to the MACS. Note that a simple physical model explaining this relationship without the cumbersome mathematics was previously published and the reader is referred to that publication for a more descriptive presentation [29].
Practically, measurement of M is made difficult by the extremely slow transmucosal N2 exchange rate and the subsequent need to maintain a closed ET for long measurement periods, conditions that are not easily maintained in laboratory experiments [28]. However, EQ8b is equally valid for any inert gas (i.e. gases characterized by perfusion limited transmucosal exchange). Previously, a significant increase in ME pressure was reported for anesthetized patients breathing gas mixtures that included N2O [7, 30] and one study reported paired data for pneumatized MACS area and the rate of change in ME pressure during 50% N2O breathing in adult subjects [18]. Like N2, N2O is an inert gas whose rate of exchange across the ME mucosa is primarily perfusion limited [28]. For strict application of the model equation to the N2O data, it is necessary to show that, like the other physiological gases, the rate of N2 exchange is effectively 0 mm H2O/min over the time course of the experiment. This is a reasonable assumption for the conditions of the N2O experiment reported by Elam and colleagues, given the short duration of the measurement period and the results of past studies that showed the transmucosal time-constant for N2O to be orders of magnitude greater than that for N2 (KeN2O ≫ Ke N2) [16, 28].
To model their experiment and provide an estimate of M for adult humans, it is necessary to recover from the presented data paired estimates of the rate of change in ME pressure, the driving N2O gradient and the volume of the ME (tympanum+MACS), other model parameters can be considered to be constant across ears/experiments. For each subject, the rate of change in ME pressure (δPe/δt) and pneumatized MACS area (proportional to MACS volume) were reported. Because all subjects breathed the same gas mixture at a controlled ventilation rate and the initial (physiological) N2O pressure in the ear is 0 mm H2O, the maximum blood-ME N2O gradient at arterial saturation is estimated to approximate 5000 mm H20 [16, 28] and the gradient decay can be estimated as the difference between that value and the total increase in ME pressure during the experiment (<40 mm H2O). This shows that the N2O gradient (GN2O) can be assumed to be constant within and between experiments. EQ 8b can then be rewritten as a simple and predictable relationship between the ratio the rate of change in ME pressure (δPe/δt) at a specified N2O gradient (GN2O) which equals the N2O time-constant, a representation of MACS geometry (the scaling constant, Ce’), the number of equivalent tympanum volumes represented by MACS volume (X) and the MACS buffering coefficient M, or
| EQ8c |
To estimate M, the MACS buffering coefficient for the human ME, the developed equation was fitted to the data for the study described by Elam and colleagues [18] after appropriate choice of a scaling constant. For curve fitting, it should be noted that the time-constant equals C and approaches M at the two limiting MACS volumes (0 or → ∞; to confirm this, substitute these values for X into EQ8c and solve for KeN2O).
RESULTS
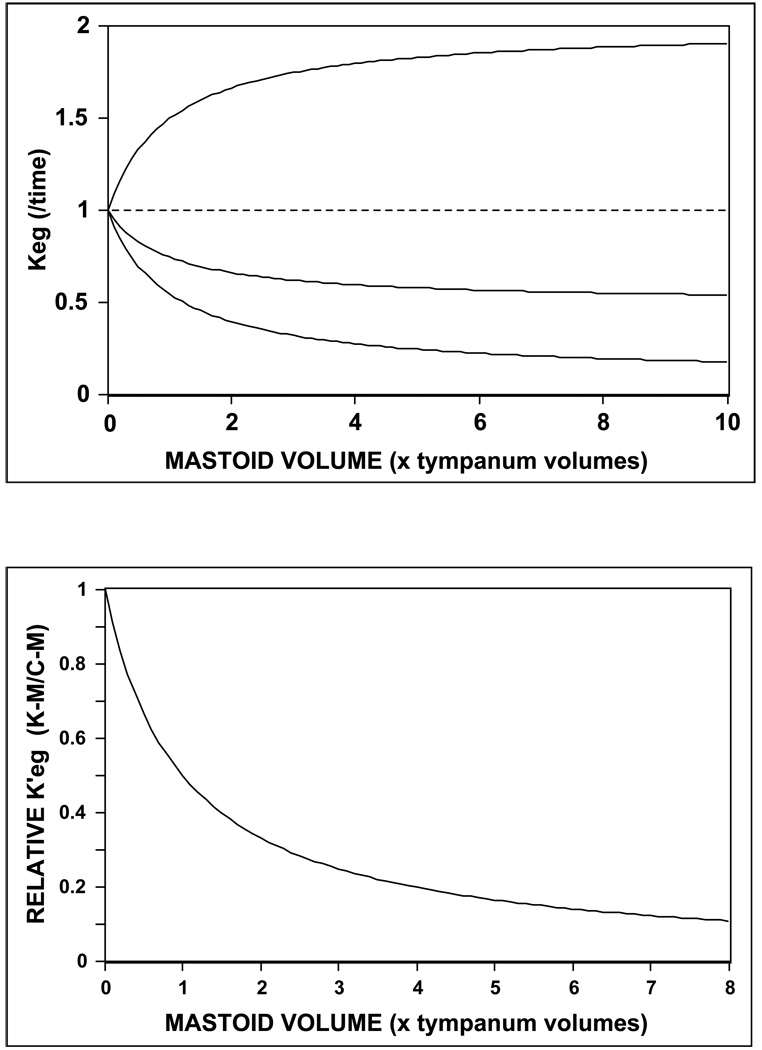
Using the simple model equation (EQ8c), the effect of MACS volume on the time-constant for transmucosal exchange of an inert gas, g was simulated at different values of the MACS buffering coefficient, M. For all simulations, the gas gradient (Gg, a constant) and the scaling constant (Ce) were assigned a value of 1 and the predicted transmucosal time-constant for the gas (Keg) was plotted as a function of relative MACS volume (multiples of tympanum volume). The results presented in Figure 1a show that: for M=1, MACS volume has no effect on the time-constant; for M>1, increasing MACS volume causes increasing values of the time-constant, and for M<1, increasing MACS volume causes decreasing values of the time-constant, i.e. lesser rates of transmucosal gas transfers at specified partial-pressure gradients, the characteristic of a pressure rate-buffer. As shown in Figure 1b, the curve for the function relating a normalized measure of the time-constant (Keg’, calculated as (Keg−M)/(Ce’−M)) to normalized MACS volume is identical at all M<1. Importantly, for any value of M, 50% of the maximum effect of MACS volume on the time-constant is achieved at 1, 66% at 2 and 75% at 3 equivalent tympanum volumes. This shows that the MACS rate-buffering effect on ME pressure change is a diminishing function of increasing MACS volume such that a high fraction of maximal buffering effect is realized at relatively small MACS volume (vis a vis that of the tympanum).
Figure 1.
a) Time-constant for ME pressure change effected by inert transmucosal gas exchange as a function of relative MACS volume for M = 2, 1, .5 and .1; and b) Relative time-constant for ME pressure change effected by diffusive gas exchange as a function of relative MACS volume for any ear with M<1 (See text for explanation).
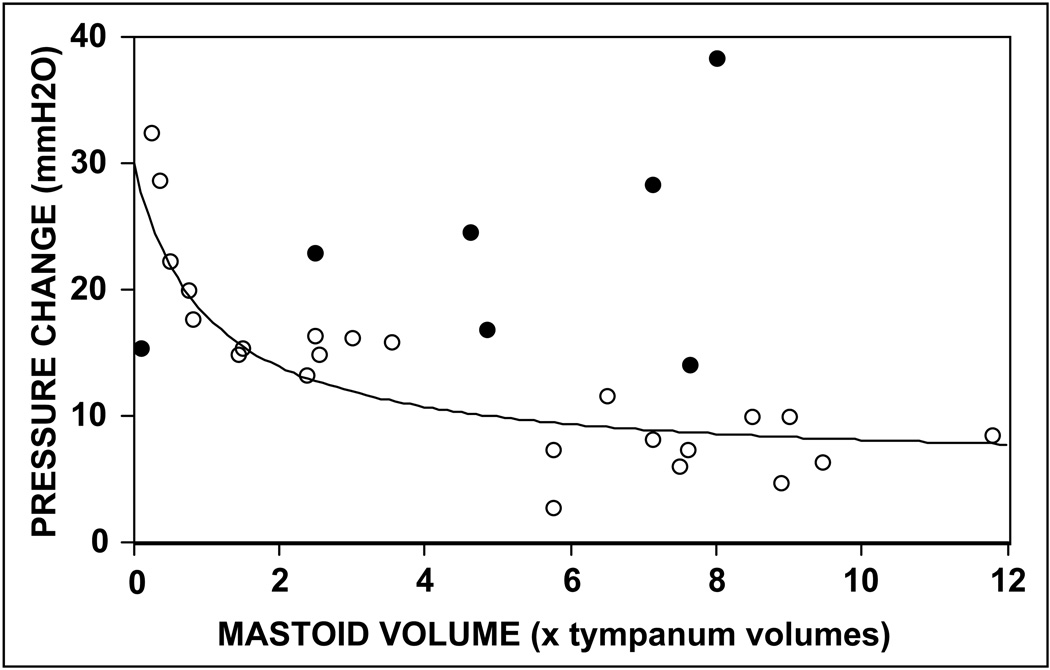
To provide an estimate of M for adult human MEs, the model equation was fitted to the reported data for the study by Elam and colleagues under the assumption that all ears were sampled from a population with the same value of M. There, three free parameters needed to be estimated: the value of the scaling constant (Ce’), the functional relation between pneumatized MACS area (measured) and volume, and the value of M. As a first approximation, the N2O time-constants (KeN2O) calculated for the two extremes of pneumatized MACS area in the population were identified and the scaling constant was estimated by the value of time-constant at a MACS volume of (approaches in the limit) 0 units (see previous section) and M was estimated as the absolute value of the difference between those time-constants divided by the estimate of scaling constant (i.e. maximum MACS volume effect on KeN2O divided by the measured KeN2O at 0 volume). The transform (MACS area to volume) multiplier was then chosen to alter the abscissa scale for best fit to the study data. Note that because the equation has more than one free parameter, an exact solution cannot be obtained, though, a family of related solutions can be defined. Shown in Figure 2 are a scatterplot of the data collected by Elam and colleagues and one fitted model solution (parameters: Ce’=30, multiplier=0.5, M=0.20). While there are clearly a number of outliers (demarcated as filled circles), the overall fit of the model equation was good. Importantly, because the form of the data distribution is not consistent with model predictions for M≥1 (see Figure 1a), the hypothesis that the MACS acts to buffer the rate of ME pressure change caused by diffusive gas exchange, i.e. is a functional pressure rate-limiter, is supported. While an exact value of M cannot be derived from the existing data, such a determination would be useful in specifying the MACS buffering “efficiency” for the population, with lesser values corresponding to higher efficiencies.
Figure 2.
Scatterplot of the rate of ME pressure change versus estimated MACS volume for the data reported by Elam and colleagues and one solution for the predicted function described by EQ8.
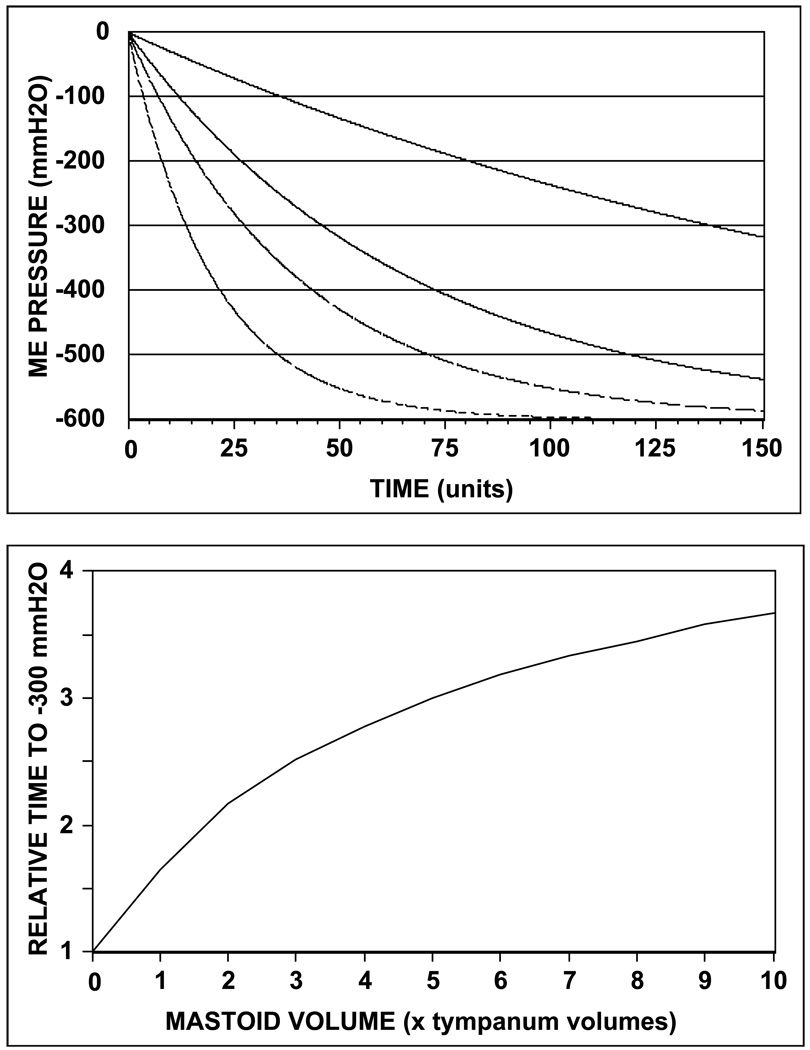
A functional interpretation of the value of M with respect to ME pressure-regulation requires an appreciation of the scaling relationship between the N2O (KeN2O, surrogate inert gas used for measurement of M) and N2 time-constants (KeN2, physiological inert gas that controls the rate of ME pressure change) and of the effect of the N2 time-constant on ME pressure change for time intervals between Eustachian tube openings. Regarding the former, EQ8a shows that for a given ME with a specified geometry, the N2 time-constant can be written as a linear function of the N2O constant [(KeN2=KeN2OSbN2FtN2(SbN2OFtN2O)−1 or KeN2=RKeN2O, where R is a scaling constant]. Thus, any linear operation on either of the two time-constants is true for the other. As to the latter, EQ7 shows that the rate of change in ME pressure is equal to the N2 time-constant multiplied by the extant ME-blood N2 pressure gradient. Using this equation, predicted ME pressure (ref. ambient) as a function of time was calculated for 4 representative values of the N2 time-constant (.05, .025, .015 and .005/time; ratio 1, .5, .3 and .1) and the results are shown in Figure 3a. All four functions have a similar general form (curvilinear decrease to −600 mm H2O in the limit where PeN2=PaN2), but lesser values of the time-constant are associated with lesser rates of ME pressure change. Consequently, for M<1, increased mastoid volume causes decreased values of the N2 time-constant, which in turn, causes lesser rates of ME pressure change.
Figure 3.
a) Effect of varying the N2 time-constant, KeN2 on the predicted ME pressure (referenced to ambient) -time function for periods without Eustachian tube opening (from top to bottom, KeN2 = .05, .025, .015 and .005/time); and b) Relative time to achieve a ME underpressure of −300 mm H2O (critical time) as a function of relative MACS volume for M=0.2.
This interpretive model may explain the protective effect of larger MACS volumes with respect to otitis media. There, past studies showed that at a specified ME underpressure (≤≈−300 mm H2O, ref ambient), the capillaries within the ME mucosa hemorrhage, the ME mucosa becomes permeable to passive fluid exchange and submucosal fluids are transferred to the ME airspace where they accumulate as an effusion [31–33]. The critical time (interval between successive Eustachian tube openings required to achieve pathological underpressure by transmucosal N2 exchange) was calculated from the model equation using the N2 time-constant estimated for M=0.2 and was plotted as a function of relative MACS volume (See Figure 3b). The form of the function shows that: the critical time is increased with increasing MACS volume; the slope of the function (change in critical time per change in MACS volume) decreases with increasing MACS volumes, and the critical time at the upper limit of MACS volume (= ∞) is 5 times greater than that for the lower limit of MACS volume (0 units), or, more generally, the maximum critical time is 1/M times the minimal critical time. Because the critical time is an inverse measure of the required frequency of effective Eustachian tube openings needed to resupply gas to the ME, these results show that the parameter M for a population contains information related to the minimal frequency of effective Eustachian tube openings required to prevent pathology.
DISCUSSION
Over more than 40 years, evidence has accumulated showing a linkage between a small MACS volume and a positive history for ME disease, but the responsible mechanism is still debated [1–5, 7–9, 11, 13, 15, 34, 35] While some have argued with experimental support that ME infection, otitis media and/or poor Eustachian tube function prevent the normal growth and development of the MACS, others suggested that small MACS predispose to otitis media by disturbing ME pressure-regulation (i.e. generating conditions that favor the development of underpressures sufficient to cause mucosal pathology). While the results presented here cannot be used to support or refute the former position, they do provide a mathematical foundation for understanding the effect of MACS volume on ME pressure-regulation.
In that regard, the possible effects of MACS volume on ME pressure has had confusing presentations in the literature with most describing a limiting effect of large MACS volume on the total magnitude of ME pressure change. For example, in explaining the protective role for a large MACS in preventing otitis media, Sade and Fuchs wrote: “The physiological role of the pneumatized MACS can be seen to be a function of its volume, which according to Boyle’s law, may share and damp ME pressure aberrations”, p39 [9]. As discussed in the Methods Section, Boyle’s law is applicable to the change in pressure for a given ear during fluctuations in cavity volume but is not relevant to comparisons across MEs with different total volumes and where ME pressure change is primarily effected by diffusive gas exchange. A MACS magnitude-limitation on ME pressure change erroneously implies that larger MACS volumes will maintain stable ME pressures at lesser deviations from ambient when compared to smaller MACS volumes. This expectation is not consistent with known physiological mechanisms of diffusive gas exchange where, in the absence of gas supply from an external source, ME pressure will be stable only when the blood-ME pressure gradients for all represented gases are equal to 0 mm H2O. Because the sum of the blood gas partial-pressures is less than that of the ambient environment by approximately 600 mm H20 [24, 25], ME pressure will be stabile at that underpressure irrespective of MACS volume (See Figure 3a).
Alternatively, the MACS could buffer the rate of ME pressure change effected by diffusive, transmucosal gas transfers. Because the total ME-blood pressure gradient under physiological conditions is attributable to the difference in N2 pressure, a rate-limiting function for the MACS would be manifest as a lesser rate of transmucosal N2 exchange for larger MACS. The developed equations show that this is true only true for ears derived from a population where the tympanum/MACS ratio of effective blood perfusion (related to surface area) per unit volume, M, is less than 1. Geometrical considerations show that the surface area per unit volume ratio of the MACS is much greater than that for the tympanum, and consequently, if M<1, blood perfusion per unit surface area must be much less for the MACS when compared to the tympanum [1, 14, 37–39].
In vivo measurement of the parametric contributors to M is not easily done, but the developed model equation makes verifiable predictions for the effect of M on the form of the function relating the rate of ME pressure change at established arterial-ME inert gas pressure gradients (the time-constant) to relative MACS volume (See Figure 1). The results of an earlier experiment designed to estimate the value of M in cynomologus monkeys, a species characterized by a MACS similar to humans, could not exclude M=1, i.e. no effect of MACS volume on the rate of ME pressure change [16]. In this report, the relevant equation was fitted to a data set published for adult humans breathing gas mixtures containing 50% N2O and one solution included an estimated M of 0.2 (See Figure 2) [18]. Moreover, the overall form of the data distribution for the experiment appeared to exclude M≥1 implying a rate-limiting function for the MACS with respect to ME pressure change caused by diffusive gas exchange. If the estimate of .2 (or any value of M<1) is reproducible in future experiments, MEs with larger MACS will be characterized by lesser rates of pressure decrease secondary to transmucosal N2 exchange and will require lesser frequencies of effective Eustachian tube openings for gas resupply to the ME, i.e. more efficient pressure-regulation.
The value of M estimated here assumes that M is fixed for sequentially added MACS volumes, that M is a constant in the population of adult ears sampled and that tympanum blood flow is independent of MACS volume (See EQ8 for effect of changing Q on Keg). Violation of the first two assumptions could introduce large errors into the estimate but would also significantly alter the form of the predicted function relating ME pressure change to relative MACS volume. The general agreement between the predicted from of the function and the experimental data suggests that those assumptions are valid for the modeled population of adults. However, because these relationships must be maintained during the period of growth and development, they are too restrictive to allow for extension of any conclusions to the pediatric population.
The last assumption is more troublesome to data interpretation since increased mucosal perfusion which is directly related to the value of the time-constant for inert gas exchange is a common feature of inflammation and MACS volume has been related to diseases characterized by ME inflammation [33, 40]. Thus, when distributed within a population of ears, increased mucosal perfusion associated with residual inflammation or subclinical disease in ears with small MACS would impose a relationship between the time-constant and MACS volume that mimics the function predicted for M<1, i.e. higher values for the time-constants at lower relative MACS volumes and lower values at greater MACS volumes. Indeed, the outliers in Figure 2 lying above the model prediction may represent a variant of this phenomenon wherein undiagnosed, perhaps transient, ME mucosal inflammation increased the N2O time-constant relative to that for the more usual, uninflamed state. For these reasons, while suggestive of a rate-liming effect of MACS volume on ME pressure change, the study reported by Elam and colleagues should be repeated with better control over the extant inflammatory state of the mucosa (e.g. by MRI validation of a disease-free state) for all MEs to eliminate this possible source of error.
If indeed the value of M is less than one for the human ME, does this provide an explanation for the relationship between MACS volume and otitis media? As noted above, in the absence of Eustachian tube openings, the value of M has no relevance to the terminal ME pressure at equilibrium with blood, but does affect the rate of ME pressure change in response to established ME-blood pressure gradients. For M<1, the absolute value of M defines the maximum effect of MACS volume on the rate of ME pressure change, while for any given value of M, the major impact of increasing MACS volume is confined to relatively few volume multiples of the tympanum (i.e. efficient rate-buffering does not require exceedingly large mastoid volumes vis a vis tympanum volume). Accepting that ME pathology develops at ME underpressures on the order of −300 mm H20, M<1 predicts that larger MACS volumes will allow for longer times without effective Eustachian tube openings before disease is provoked. Such an effect is supported by past reports of a protective effect of large MACS volumes with respect to otitis media in adults during viral upper respiratory tract infections [9]. There, it could be argued that the viral infection caused a transient Eustachian tube dysfunction in most ears, but otitis media only in those ears where the underpressure associated with mucosal pathology was achieved prior to resolution of the Eustachian tube dysfunction, an expectation for confined to ears with a high required frequency of Eustachian tube openings for gas resupply (i.e. those with smaller MACS volumes). This and related hypotheses that define the functional characteristics of ME pressure-regulation and the relationship between pressure-dysregulation and ME disease are being explored further in our laboratories.
Acknowledgments
Supported in part by a grant from the National Institutes of Health (DC007667)
REFERENCES
- 1.Koc A, et al. Evaluation of the mastoid air cell system by high resolution computed tomography: three-dimensional multiplanar volume rendering technique. J Laryngol Otol. 2003;117(8):595–598. doi: 10.1258/002221503768199906. [DOI] [PubMed] [Google Scholar]
- 2.Lindeman P, Holmquist J. Mastoid volume and eustachian tube function in ears with cholesteatoma. Am J Otol. 1987;8(1):5–7. [PubMed] [Google Scholar]
- 3.Aoki K, et al. Relationship between middle ear pressure, mucosal lesion, and mastoid pneumatization. Laryngoscope. 1998;108(12):1840–1845. doi: 10.1097/00005537-199812000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Sade J. The correlation of middle ear aeration with mastoid pneumatization. The mastoid as a pressure buffer. Eur Arch Otorhinolaryngol. 1992;249(6):301–304. doi: 10.1007/BF00179376. [DOI] [PubMed] [Google Scholar]
- 5.Magnuson B. Functions of the mastoid cell system: auto-regulation of temperature and gas pressure. J Laryngol Otol. 2003;117(2):99–103. doi: 10.1258/002221503762624512. [DOI] [PubMed] [Google Scholar]
- 6.Raveh E, et al. Mastoid buffering properties: I. Gas partial pressures. Ann Otol Rhinol Laryngol. 1999;108(8):750–755. doi: 10.1177/000348949910800807. [DOI] [PubMed] [Google Scholar]
- 7.Valtonen HJ, et al. Development of mastoid air cell system in children treated with ventilation tubes for early-onset otitis media: a prospective radiographic 5-year follow-up study. Laryngoscope. 2005;115(2):268–273. doi: 10.1097/01.mlg.0000154731.08410.b8. [DOI] [PubMed] [Google Scholar]
- 8.Sade J, Luntz M. Modified radical mastoidectomies as gas pockets. Acta Otolaryngol. 1989;107(5–6):456–459. doi: 10.3109/00016488909127540. [DOI] [PubMed] [Google Scholar]
- 9.Sade J, Fuchs C. Secretory otitis media in adults: II. The role of mastoid pneumatization as a prognostic factor. Ann Otol Rhinol Laryngol. 1997;106(1):37–40. doi: 10.1177/000348949710600107. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, et al. Gas exchange function through the mastoid mucosa in ears after surgery. Laryngoscope. 1997;107(8):1117–1121. doi: 10.1097/00005537-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Sade J. Surgical planning of the treatment of cholesteatoma and postoperative follow-up. Ann Otol Rhinol Laryngol. 2000;109(4):372–376. doi: 10.1177/000348940010900406. [DOI] [PubMed] [Google Scholar]
- 12.Saad MM, Doyle WJ, Gest TR. Morphology of the middle ear in the rhesus monkey (Macaca mulatta) Acta Anat (Basel) 1982;112(2):117–130. doi: 10.1159/000145503. [DOI] [PubMed] [Google Scholar]
- 13.Sade J. Hyperectasis: the hyperinflated tympanic membrane: the middle ear as an actively controlled system. Otol Neurotol. 2001;22(2):133–139. doi: 10.1097/00129492-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Doyle WJ. Mucosal surface area determines the middle ear pressure response following establishment of sniff-induced underpressures. Acta Otolaryngol. 1999;119(6):695–702. doi: 10.1080/00016489950180658. [DOI] [PubMed] [Google Scholar]
- 15.Kanick SC, Doyle WJ. Barotrauma during Air Travel: Predictions of a Mathematical Model. J Appl Physiol. 2004 doi: 10.1152/japplphysiol.00974.2004. [DOI] [PubMed] [Google Scholar]
- 16.Doyle WJ, et al. Rate of nitrous oxide exchange across the middle ear mucosa in monkeys before and after blockage of the mastoid antrum. Otolaryngol Head Neck Surg. 2003;128(5):732–741. doi: 10.1016/S0194-59980223309-4. [DOI] [PubMed] [Google Scholar]
- 17.Sade J, Ar A, Fuchs C. Barotrauma vis-a-vis the "chronic otitis media syndrome": two conditions with middle ear gas deficiency Is secretory otitis media a contraindication to air travel? Ann Otol Rhinol Laryngol. 2003;112(3):230–235. doi: 10.1177/000348940311200307. [DOI] [PubMed] [Google Scholar]
- 18.Elam M, et al. Middle ear pressure variations during 50% N2O anesthesia as a function of mastoid pneumatization. Am J Otol. 1998;19(6):709–711. [PubMed] [Google Scholar]
- 19.Alper CM, et al. Tympanometry accurately measures middle ear underpressures in monkeys. Ann Otol Rhinol Laryngol. 2003;112(10):877–884. doi: 10.1177/000348940311201009. [DOI] [PubMed] [Google Scholar]
- 20.Ghadiali SN, Swarts JD, Doyle WJ. Effect of tensor veli palatini muscle paralysis on eustachian tube mechanics. Ann Otol Rhinol Laryngol. 2003;112(8):704–711. doi: 10.1177/000348940311200810. [DOI] [PubMed] [Google Scholar]
- 21.Honjo I, et al. Pumping and clearance function of the eustachian tube. Am J Otolaryngol. 1985;6(3):241–244. doi: 10.1016/s0196-0709(85)80095-8. [DOI] [PubMed] [Google Scholar]
- 22.Doyle WJ, et al. Exchange rates of gases across the tympanic membrane in rhesus monkeys. Acta Otolaryngol. 1998;118(4):567–573. doi: 10.1080/00016489850154748. [DOI] [PubMed] [Google Scholar]
- 23.Felding UN, Banks JM, Doyle WJ. Gas diffusion across the tympanic membrane in chinchillas: effect of repeated perforations. Auris Nasus Larynx. 2004;31(4):353–359. doi: 10.1016/j.anl.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Sade J, Luntz M. Dynamic measurement of gas composition in the middle ear. II: Steady state values. Acta Otolaryngol. 1993;113(3):353–357. doi: 10.3109/00016489309135824. [DOI] [PubMed] [Google Scholar]
- 25.Hergils L, Magnuson B. Middle ear gas composition in pathologic conditions: mass spectrometry in otitis media with effusion and atelectasis. Ann Otol Rhinol Laryngol. 1997;106(9):743–745. doi: 10.1177/000348949710600905. [DOI] [PubMed] [Google Scholar]
- 26.Sade J, Luntz M, Levy D. Middle ear gas composition and middle ear aeration. Ann Otol Rhinol Laryngol. 1995;104(5):369–373. doi: 10.1177/000348949510400506. [DOI] [PubMed] [Google Scholar]
- 27.Felding UN, Banks JM, Doyle WJ. Middle ear gas exchange in the air phase. Acta Otolaryngol. 2003;123(7):808–811. doi: 10.1080/00016480310015416. [DOI] [PubMed] [Google Scholar]
- 28.Doyle WJ, Alper CM, Seroky JT. Trans-mucosal inert gas exchange constants for the monkey middle ear. Auris Nasus Larynx. 1999;26(1):5–12. doi: 10.1016/s0385-8146(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 29.Doyle WJ. Experimental results do not support a gas reserve function for the mastoid. Int J Pediatr Otorhinolaryngol. 2000;52(3):229–238. doi: 10.1016/s0165-5876(00)00292-5. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe M, et al. Gas exchange function of the middle ear in patients with otitis media with effusion. Eur Arch Otorhinolaryngol. 1997;254(9–10):453–455. doi: 10.1007/BF02439979. [DOI] [PubMed] [Google Scholar]
- 31.Flisberg K. The effects of vacuum on the tympanic cavity. Otolaryngol Clin North Am. 1970;3(1):3–13. [PubMed] [Google Scholar]
- 32.Swarts JD, et al. In vivo observation with magnetic resonance imaging of middle ear effusion in response to experimental underpressures. Ann Otol Rhinol Laryngol. 1995;104(7):522–528. doi: 10.1177/000348949510400704. [DOI] [PubMed] [Google Scholar]
- 33.Alper CM, Swarts JD, Doyle WJ. Middle ear inflation for diagnosis and treatment of otitis media with effusion. Auris Nasus Larynx. 1999;26(4):479–486. doi: 10.1016/s0385-8146(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 34.Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003;30(1):7–14. doi: 10.1016/s0385-8146(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 35.Miura M, et al. Influence of the gas exchange function through the middle ear mucosa on the development of sniff-induced middle ear diseases. Laryngoscope. 1998;108(5):683–686. doi: 10.1097/00005537-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Alper CM, et al. Magnetic resonance imaging of the development of otitis media with effusion caused by functional obstruction of the eustachian tube. Ann Otol Rhinol Laryngol. 1997;106(5):422–431. doi: 10.1177/000348949710600511. [DOI] [PubMed] [Google Scholar]
- 37.Isono M, et al. Computerized assessment of the mastoid air cell system. Auris Nasus Larynx. 1999;26(2):139–145. doi: 10.1016/s0385-8146(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 38.Park MS, Yoo SH, Lee DH. Measurement of surface area in human mastoid air cell system. J Laryngol Otol. 2000;114(2):93–96. doi: 10.1258/0022215001904969. [DOI] [PubMed] [Google Scholar]
- 39.Luntz M, et al. Volume of mastoid pneumatization: three-dimensional reconstruction with ultrahigh-resolution computed tomography. Ann Otol Rhinol Laryngol. 2001;110(5 Pt 1):486–490. doi: 10.1177/000348940111000516. [DOI] [PubMed] [Google Scholar]
- 40.Alper CM, Doyle WJ, Seroky JT. Higher rates of pressure decrease in inflamed compared with noninflamed middle ears. Otolaryngol Head Neck Surg. 1999;121(1):98–102. doi: 10.1016/S0194-5998(99)70133-6. [DOI] [PubMed] [Google Scholar]